Abstract
Context
Jinlong capsule (JLC) is an animal-derived traditional Chinese medical preparation for advanced hepatocellular carcinoma (HCC). However, its clinical efficacy is still not well investigated.
Objective
This study summarizes the efficacy and safety of JLC combined with trans-arterial chemoembolization (TACE) for patients with HCC.
Methods
The databases PubMed, Cochrane Library, Web of Science, EMBASE, Medline, China National Knowledge Infrastructure, Wanfang Database, Chinese Scientific Journal Database and Chinese Biological Medicine Database were systematically searched from the date of their inception until February 2020. Jinlong capsule, trans-arterial chemoembolization, and hepatocellular carcinoma were the key terms searched. Randomized controlled trials and high-quality prospective cohort trials comparing the combined use of JLC and TACE versus TACE for HCC were included. Data were pooled using random or fixed effect models depending on heterogeneity.
Results
Data from 19 articles with 1,725 HCC patients were analysed. Compared with TACE treatment alone, the combination of TACE and JLC significantly prolonged patients’ 6–36 month overall survival (p < 0.05), and markedly improved the overall response rate (RR = 1.37, 95% CI = 1.24–1.52, p < 0.00001) and disease control rate (RR = 1.11, 95% CI = 1.06–1.17, p < 0.0001) of patients. The liver function, quality of life, and immune function of patients were significantly improved; the partial adverse events related to TACE were also effectively relieved after the combination treatment.
Conclusion
This meta-analysis suggests that the combination of TACE and JLC is more effective in the treatment of HCC than treatment with TACE alone.
Introduction
Hepatocellular carcinoma (HCC) ranks as the sixth most common cancer and the third leading cause of cancer-related death (Shen et al. Citation2019). According to global cancer statistics, about 840,000 newly diagnosed cases and 780,000 deaths occurred worldwide in 2018 (Bray et al. Citation2018; Shen et al. Citation2019). China is a high-risk region for HCC, and accounts for approximately 55% of the HCC-related deaths worldwide (Ma et al. Citation2016). Trans-arterial chemoembolization (TACE) is the preferred locoregional treatment method for unresectable HCC (Fan et al. Citation2019). However, TACE treatment alone is limited in its ability to eliminate tumour tissue and typically has a high recurrence rate (Zhou et al. Citation2007; Fan et al. Citation2019). In addition, it can also further influence the liver functions and damage the patient’s hepatic arterial system (Sergio et al. Citation2008; Cabrera et al. Citation2011; Kim and Han Citation2016). In view of these limitations of TACE therapy in HCC, complementary and alternative medicine has been increasingly used in the treatment of advanced HCC.
Jinlong capsule (JLC) is a Chinese patent medicine, which contains at least 17 types of amino acids including histidine, arginine, serine, aspartic acid, glycine, etc., and is extracted by modern cryogenic and biochemical separation technologies from the entire body of three fresh animals (animals recently killed) including Agkistrodon acutus (Deinagkistrodon acutus Günther [Viperidae]), Multibanded krait (Bungarus multicinctus Blyth [Elapidae]) and Gecko (Gekkosuinhouna Gunther) (Wu et al. Citation2010; Li and Xiao Citation2014; Li et al. Citation2018). It was approved by the Ministry of Health of China for the treatment of HCC in April 1998, and was selected as ‘Chinese characteristic medicine’ by the National Pharmacopoeia Committee in 2001 (https://baike.so.com/doc/5344965-5885498.html). Preclinical studies indicate that JLC can inhibit proliferation and induces apoptosis in a variety of cancer cells (Wu et al. Citation2010; Li et al. Citation2013, Citation2018). Shi et al. (Citation2019) demonstrated that JLC can suppress migration and invasion of tumour cell via the modulation of mTOR/S6 signalling pathway. Other studies suggest that JLC can also significantly improve the lymphocyte function of patients with primary liver cancer after TACE treatment, by regulating the expression level of IL-2 and sIL-2R and balancing the immune state of the body (Zhang et al. Citation2008).
Several clinical trials have indicated that TACE combined with JLC exhibit more prominent therapeutic effects in advanced HCC than does TACE alone (Zhang et al. Citation2008; Wu et al. Citation2010). Despite the wide use of JLC in HCC treatment for many years, its clinical efficacy remains controversial. Therefore, in this study, we conducted a meta-analysis to investigate the clinical efficacy and safety of TACE combined with JLC in comparison with TACE alone for the treatment of end-stage HCC to provide a scientific basis for the design of future clinical trials.
Materials and methods
This systematic review and meta-analysis were performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al. Citation2009; Panic et al. Citation2013). Ethical approval was not necessary because this study was a meta-analysis.
Search strategy
Nine electronic databases, including PubMed, Cochrane Library, Web of Science, EMBASE, Medline, China National Knowledge Infrastructure (CNKI), Wanfang Database, Chinese Scientific Journal Database (CSJD) and Chinese Biological Medicine Database (CBM), were searched for the relevant original articles published before February 2020, with the key terms ‘Jinlong’ or ‘Jinlong Jiaonang’ or ‘Jinlong capsule’ and ‘liver carcinoma’ or ‘hepatocellular carcinoma’ or ‘liver cancer’ or ‘hepatocellular cancer’ or ‘HC’ or ‘HCC’ combined with ‘transarterial chemoembolization’ or ‘transcatheter hepatic arterial chemoembolization’ or ‘trans-arterial chemoembolization’ or ‘TACE’ (Supplementary Table 1).
Table 1. Clinical information from the eligible trials in the meta-analysis.
Inclusion and exclusion criteria
Studies that met the following inclusion criteria were considered eligible for this meta-analysis: (I) Randomized controlled trials (RCTs) and high-quality prospective cohort trials with advanced HCC patients; (II) Studies involving more than 20 advanced HCC patients; and (III) Studies comparing the clinical outcomes of TACE plus JLC adjuvant therapy (combination group) with those of TACE alone (control group).
Trials were excluded if they met the following criteria: (I) Reviews, case report, noncontrast articles, or trials unrelated with the topics; (II) Patients with mixed malignancies; (III) Articles without sufficient available data; and (IV) The intervention of HCC patients was not based on TACE and JLC combined treatment.
Data extraction and quality assessment
Data were independently extracted by two reviewers (Jia SZ and Fu Y) according to the same inclusion and exclusion criteria; disagreements were settled by the third investigator (Tao HM). The following data was extracted from eligible literatures: (a) the first author’s name; (b) year of publication; (c) tumour stages or Karnofsky performance score (KPS); (d) number of cases; (e) ages of patients; (f) intervening methods; (g) dosage of JLC and (h) study parameter types. The quality of the included trials was evaluated according to the Cochrane Handbook tool (Zeng et al. Citation2015).
Outcome definition
The clinical responses included treatment efficacy, quality of life (QoL), immune function and adverse events. Treatment efficacy was evaluated in terms of the overall survival (OS) rate, overall response rate (ORR) and disease control rate (DCR). QoL was assessed using the quality of life improved rate (QIR) and KPS. The immune function indicators (percentages of CD3+, CD4+, CD8+, and NK cells and CD4+/CD8+ ratio), liver function and the decrease rate of alpha-fetoprotein (AFP) in HCC patients were determined and compared between the TACE&JLC and JLC groups. Adverse events including leucopoenia, gastrointestinal adverse effects, hepatotoxicity and myelosuppression were also assessed.
Statistical analysis
The RevMan 5.3 (Nordic Cochran Centre, Copenhagen, Denmark) and Stata 13.0 (Stata Corp., College Station, TX, USA) were used for statistical analyses. Dichotomous data were represented by the risk ratio (RR) with the respective 95% confidence interval (CI), whereas continuous variables were expressed as mean difference (MD) with 95% CI. A two-tailed p value < 0.05 was considered statistically significant. Cochrane’s Q-test and I2 statistics were used to assess the heterogeneity between studies; if p > 0.1 or I2 < 50%, the fixed effects model was used for the meta-analysis; otherwise, the random effects model was used according to the DerSimonian and Laird method (Jackson et al. Citation2012; George and Aban Citation2016). The presence of publication bias was investigated using Begg’s and Egger’s test (Lin and Chu Citation2018). If publication bias existed, a trim-and-fill method was applied to coordinate the estimates from unpublished studies, and the adjusted results were compared with the original pooled RR (Duval and Tweedie Citation2000; Shi and Lin Citation2019). Sensitivity analysis was further conducted to assess the stability of results and evaluate the variation of pooled data.
Results
Search results
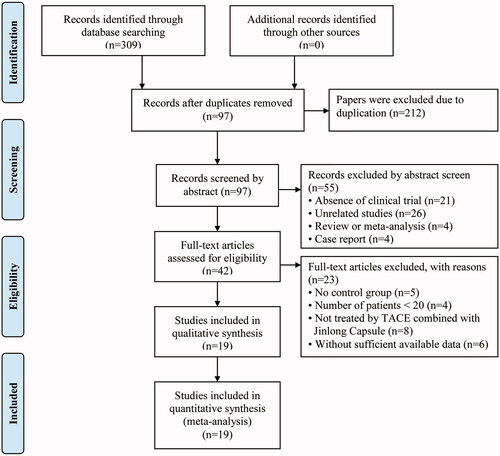
Three hundred and nine articles were initially identified. Of those, 212 papers were excluded because they were duplicates. After reviewing the title and abstracts, 55 articles were further excluded because they were not clinical trials (n = 21), were unrelated studies (n = 26), were reviews or meta-analyses (n = 4), or were case reports (n = 4), leaving 42 studies as potentially relevant. After a detailed assessment of the full text articles, those without a control group (n = 5), involving less than 20 HCC patients (n = 4), in which the intervention of HCC patients did not involve TACE and JLC combined treatment (n = 8), and studies with insufficient data (n = 6) were excluded. Finally, 19 trials (Xie et al. Citation2003; Liang et al. Citation2005; Li et al. Citation2007; Dong et al. Citation2008; Jia et al. Citation2008; Wang et al. Citation2009; Wu et al. Citation2010; Zeng et al. Citation2010; Citation2012, Citation2014; Zhang Citation2012; Zhang et al. Citation2012; Jiang et al. Citation2013; Li et al. Citation2013; Yang et al. Citation2013; Yuan et al. Citation2013; Liu et al. Citation2015; Meng Citation2016; Zheng et al. Citation2018) involving 1,725 advanced HCC patients were included in this analysis ().
Patient characteristics
All included trials were performed in different medical centres in China. In total, 885 advanced HCC patients were treated with TACE and JLC combined therapy, while 840 patients were treated with TACE alone. Detailed information on the involved studies and HCC patients is shown in .
Quality assessment
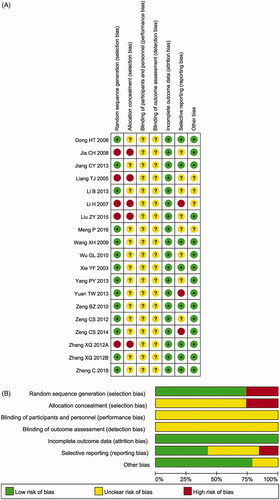
The assessment of the risk of bias is shown in . Fourteen studies (Xie et al. Citation2003; Dong et al. Citation2008; Wang et al. Citation2009; Wu et al. Citation2010; Zeng et al. Citation2010, Citation2012, Citation2014; Zhang et al. Citation2012; Jiang et al. Citation2013; Li et al. Citation2013; Yang et al. Citation2013; Yuan et al. Citation2013; Meng Citation2016; Zheng et al. Citation2018) were determined to have a low risk of bias, and the remaining five studies (Liang et al. Citation2005; Li et al. Citation2007; Jia et al. Citation2008; Zhang Citation2012; Liu et al. Citation2015) were not truly RCTs. All included trials did not provide a clear description of the performance and detection risks. The attrition risks of involved trials were low. Three trials (Li et al. Citation2007; Yuan et al. Citation2013; Zeng et al. Citation2014) were considered to have a high reporting risk owing to a lack of primary outcomes (ORR or DCR) and eight studies (Liang et al. Citation2005; Jia et al. Citation2008; Wu et al. Citation2010; Zeng et al. Citation2012; Li et al. Citation2013; Yang et al. Citation2013; Liu et al. Citation2015; Meng Citation2016) were considered as having an unclear reporting risk due to lack of safety assessment.
Figure 2. (A) Risk of bias summary: review of authors’ judgments about each risk of bias item for included studies. (B) Risk of bias graph: review of authors’ judgments about each risk of bias item presented as percentages across all included studies. Note. Each colour represents a different level of bias: red for high-risk, green for low-risk, and yellow for unclear-risk of bias.
Therapeutic efficacy assessments
ORR and DCR
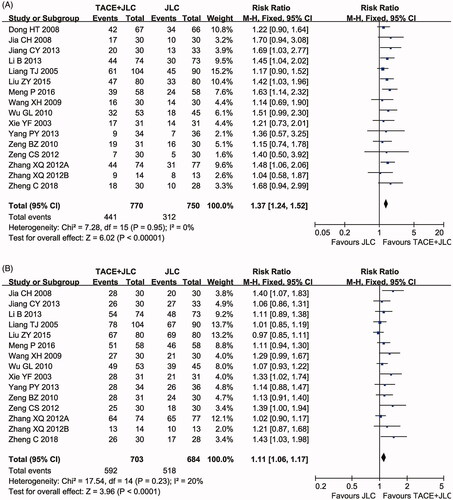
Sixteen clinical trials (Xie et al. Citation2003; Liang et al. Citation2005; Dong et al. Citation2008; Jia et al. Citation2008; Wang et al. Citation2009; Wu et al. Citation2010; Zeng et al. Citation2010, Citation2012; Zhang Citation2012; Zhang et al. Citation2012; Jiang et al. Citation2013; Li et al. Citation2013; Yang et al. Citation2013; Liu et al. Citation2015; Meng Citation2016; Zheng et al. Citation2018) involving 1,520 cases compared the ORR and DCR between the two groups. As shown in , the pooled results showed that patients who underwent combined therapy had significantly improved ORR (RR = 1.37, 95% CI = 1.24–1.52, p < 0.00001) and DCR (RR = 1.11, 95% CI = 1.06–1.17, p < 0.0001) compared to those on TACE treatment alone. No obvious heterogeneity was found among the included articles, so a fixed effects model was conducted to pool the data.
Long-term survival
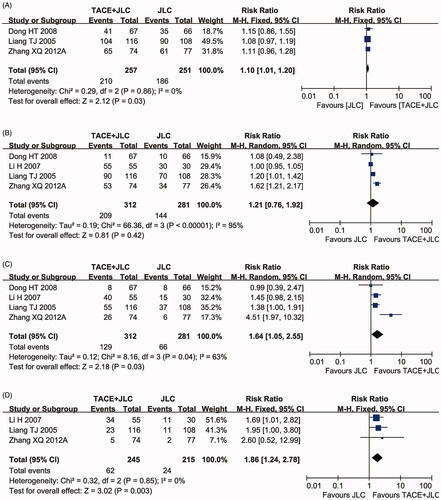
Four clinical trials (Liang et al. Citation2005; Li et al. Citation2007; Dong et al. Citation2008; Zhang Citation2012) with 593 HCC patients reported the OS (). The analysis results showed that patients underwent combined therapy had significantly improved 6-month (RR = 1.10, 95% CI = 1.01–1.20, p = 0.03), 24-month (RR = 1.64, 95% CI = 1.05–2.55, p = 0.03) and 36-month OS (RR = 1.86, 95% CI = 1.24–2.78, p < 0.003), whereas the 12-month OS (RR = 1.21, 95% CI = 0.76–1.92, p = 0.42) was not significantly different from that in patients who received TACE alone. There was statistical heterogeneity in the 12-month (p < 0.00001, I2 = 95%) and 24-month OS (p = 0.04, I2 = 63%) according to the heterogeneity test. Therefore, the random effects model was used to pool these meta-analyses. Otherwise, fixed-effect model was used.
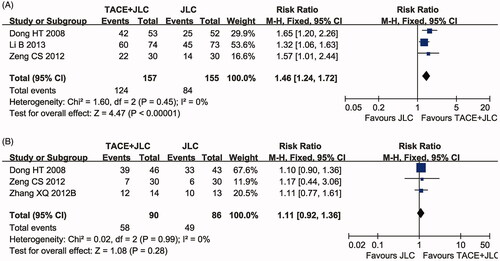
Liver function and AFP
Three clinical trials (Dong et al. Citation2008; Zeng et al. Citation2012; Li et al. Citation2013) with 312 patients reported an improvement of liver function (ILF) and three clinical trials (Dong et al. Citation2008; Zhang et al. Citation2012; Li et al. Citation2013) with 176 patients reported on AFP between the two groups. As shown in , the liver function of the HCC patients who received combined therapy was significantly improved compared with that of patients on TACE treatment alone (RR = 1.46, 95% CI = 1.24–1.72, p < 0.00001). No significant difference in AFP decrease rate (, RR = 1.11, 95% CI = 0.92–1.36, p = 0.28) was found between the TACE and TACE & JLC groups. Both heterogeneities of the two indexes were not remarkable (ILF: p = 0.45, I2 = 0%; AFP: p = 0.99, I2 = 0%) and the statistical model was the fixed effects model.
Figure 5. Forest plot of the comparison of improvement of liver function (ILF, A) and alpha-fetoprotein (AFP) decrease rate (B) between the TACE and TACE + JLC groups. TACE: trans-arterial chemoembolization; JLC: Jinlong capsule. A fixed effects meta-analysis model (Mantel-Haenszel method) was used.
QoL assessment
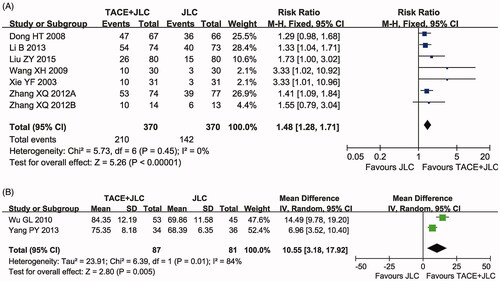
Seven trials (Xie et al. Citation2003; Dong et al. Citation2008; Wang et al. Citation2009; Zhang Citation2012; Zhang et al. Citation2012; Li et al. Citation2013; Liu et al. Citation2015) with 740 participants measured the QIR () and two trials (Wu et al. Citation2010; Yang et al. Citation2013) including 208 patients reported data on KPS (). In this analysis, our results showed that the QoL of HCC patients receiving combined therapy was significantly improved compared to those of the patients treated with TACE alone, indicated by obviously increased QIR (RR = 1.48, 95% CI = 1.28–1.71, p < 0.00001) and KPS (MD = 10.55, 95% CI = 3.18–17.92, p = 0.005). QIR (p = 0.45, I2 = 0%) was not heterogeneous among the studies, so the fixed-effect model was used to analyse its RR. Otherwise, the random-effect model was used.
Immune function evaluation
The immune status of patients was examined between the two groups in eight controlled studies (Xie et al. Citation2003; Jia et al. Citation2008; Zeng et al. Citation2010; Zhang et al. Citation2012; Yuan et al. Citation2013; Zeng et al. Citation2014; Meng Citation2016; Zheng et al. Citation2018) including 504 patients (). Compared with the percentages of CD3+, CD4+ and NK cells, and CD4+/CD8+ ratio, in the TACE group, those in the combined treatment group were significantly increased (CD3+: MD = 13.69, 95% CI = 7.40–19.98, p < 0.0001; CD4+: MD = 8.63, 95% CI = 4.94–12.32, p < 0.00001; NK: MD = 5.43, 95% CI = 1.58–9.28, p = 0.006; CD4+/CD8+: MD = 0.40, 95% CI = 0.23–0.56, p < 0.00001), whereas the proportions of CD8+ were obviously decreased (MD = −5.89, 95% CI = −8.56–3.22, p < 0.00001). The random effects model was used to pool this meta-analysis for the significant heterogeneity.
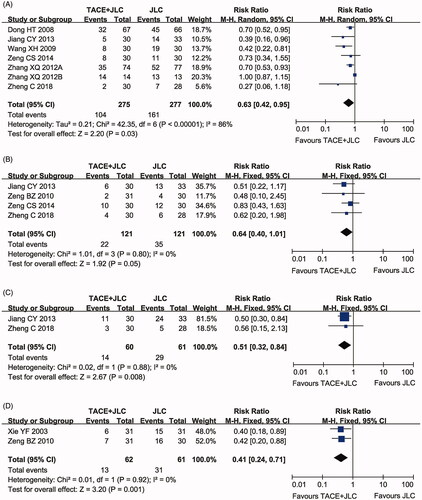
Assessment of adverse events
As shown in , compared with patients treated with TACE alone, patients treated with JLC and TACE displayed lower incidence rates of leucopoenia (RR = 0.63, 95% CI = 0.42–0.95, p = 0.03), gastrointestinal side effects (RR = 0.64, 95% CI = 0.40–1.01, p = 0.05), hepatotoxicity (RR = 0.51, 95% CI = 0.32–0.84, p = 0.008) and myelosuppression (RR = 0.41, 95% CI = 0.24–0.71, P = 0.001). Fixed-effect models were used to analyse the RR rate because of the low degree of heterogeneity.
Figure 8. Forest plot of the comparison of adverse effects, including leucopoenia (A), gastrointestinal adverse effects (B), hepatotoxicity (C) and myelosuppression (D) between the TACE and TACE + JLC groups. TACE: trans-arterial chemoembolization; JLC: Jinlong capsule. A fixed effects meta-analysis model (Mantel-Haenszel method) was used.
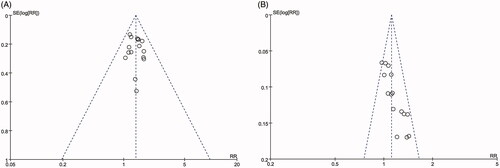
Publication bias
As shown in , the funnel plots were symmetrical for the ORR but asymmetrical for DCR. The Begg’s and Egger’s regression tests results showed that there was publication bias in DCR (Begg < 0.0001; Egger < 0.0001). No significant publication bias was found for ORR (Begg = 0.620; Egger = 0.545). To determine if the bias affected the pooled risk for DCR, we conducted a trim-and-fill analysis. The adjusted RR indicated the same trend as was indicated by the result of the primary analysis (before: p < 0.0001, after: p = 0.045), reflecting the reliability of our primary conclusions.
Sensitivity analysis
Sensitivity analysis was performed to explore the influence of individual studies on the pooled results by deleting one study at a time from the pooled analysis. As signified by and , the results revealed that no individual study significantly affected the ORR and DCR, which indicated statistically robust results.
Figure 10. Sensitivity analysis for overall response rate (ORR, A) and disease control rate (DCR, B).
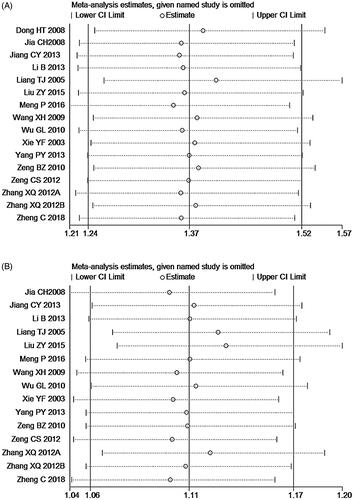
Table 2. The results of sensitivity analysis of clinical trials.
We also conducted subgroup analyses to explore the source of heterogeneity in the ORR and DCR with respect to sample sizes and types of studies involved. As shown in , in terms of ORR, our analysis results showed that no significant difference was found between different sample sizes of included studies. Moreover, the types of involved studies (RCTs) were associated with improved DCR.
Table 3. Subgroup analyses of ORR and DCR between the combination and control group.
Discussion
TACE is one of the few effective therapeutic treatments in the treatment of end-stage HCC (Ma et al. Citation2016). However, TACE only has a modest survival benefit and the disadvantages of TACE such as drug resistance and toxic side effects have become a substantial burden on HCC patients (Sergio et al. Citation2008; Kim and Han Citation2016; Ma et al. Citation2016; Zhu et al. Citation2018). Therefore, there is a pressing need to develop novel HCC therapies that may complement current conventional treatment strategies. Many studies have reported that the addition of JLC could be improve HCC patients’ survival time, QoL, and immune function and reduce side effects caused by TACE (Zhang et al. Citation2008; Wu et al. Citation2010). Although there had been statistical analyses in the published literature, the exact therapeutic effects had not been systematically investigated. In this analysis, we conducted a wide-ranging online search with strict inclusion and exclusion criteria to provide clear and systematic conclusions.
After final screening, data from 19 trials (Xie et al. Citation2003; Liang et al. Citation2005; Li et al. Citation2007; Dong et al. Citation2008; Jia et al. Citation2008; Wang et al. Citation2009; Wu et al. Citation2010; Zeng et al. Citation2010, Citation2012, Citation2014; Zhang Citation2012; Zhang et al. Citation2012; Jiang et al. Citation2013; Li et al. Citation2013; Yang et al. Citation2013; Yuan et al. Citation2013; Liu et al. Citation2015; Meng Citation2016; Zheng et al. Citation2018) involving 1,725 advanced HCC patients were included in our meta-analysis. The JLC in all the included studies was manufactured by the Beijing Jiansheng Pharmaceutical Co., Ltd. The Quality Standards of JLC in this study have been approved by the Chinese State Food and Drug Administration (SFDA), and granted the Manufacturing Approve Number accordingly (Z10980041). The dosages of JLC were 1.0 ± 0.25 g given three times per day via oral administration. Through statistical analysis of the extracted data, our results found that the combination of JLC and TACE displayed better therapeutic effects than TACE alone. Compared to TACE treatment alone, JLC could significantly improve the ORR, DCR, liver function and QoL in patients with HCC (p < 0.05). Moreover, the long-term survival rates (6-, 24- and 36-month OS) of the HCC patients in the TACE & JLC group were significantly prolonged compared with the TACE alone group, which indicated that using JLC not only improved the short-term curative effects, but also enhanced the long-term clinical efficacy of TACE in the treatment of end-stage HCC. AFP is commonly used to predict the metastasis, recurrence, and prognosis of HCC after comprehensive treatments (Wong et al. Citation2015; He et al. Citation2019). Our analysis showed that the AFP reduction rate was increased after treatment with the combination of TACE and JLC, but it did not reach a statistical significance. All these results indicated that using JLC could improve the therapeutic effects of TACE in advanced HCC.
Immune system reconstruction is one of the critical methods for effective treatment of malignant tumours. Our analysis showed that the percentages of CD3+, CD4+, and NK cells and the CD4+/CD8+ ratio were significantly increased when JLC was administered to HCC patients, indicating that the immune function of HCC patients was improved after receiving JLC-mediated therapy.
With respect to safety, our analysis showed that some of the adverse events caused by TACE, including leucopoenia, gastrointestinal adverse effects, hepatotoxicity and myelosuppression were obviously alleviated when JLC was applied to patients with HCC. Therefore, JLC may be a well-tolerated treatment for HCC and can effectively alleviate partial adverse events associated with TACE.
To verify the reliability of the pooled results, sensitivity analysis was performed to explore an individual study’s influence on the pooled results; subgroup analysis was conducted to assess the impact of sample sizes and research types on the ORR and DCR. We found that the types of involved studies (RCT) were associated with improved DCR. However, recent studies on the impacts of these factors on the curative effect of JLC adjuvant therapy remain insufficient, and further investigations should be performed.
There are some limitations in our analysis. First, there exists a publication bias for DCR, which might be because some authors tended to deliver the positive results of articles to editors. Therefore, any conclusions need to be made with caution. Second, included clinical studies were all conducted in different centres in China and JLC was not approved in any other FDA except SDFA, which may cause an unavoidable regional bias and subsequently influence the clinical application of JLC worldwide. Moreover, the effective component in JLC for HCC was not sure because JLC contained at least 17 types of amino acids from three fresh animals including Agkistrodon, Bungarus and Gecko. Finally, different trials evaluated the treatment efficacy with different outcomes, resulting in a reduction in the size of the statistical sample, making it difficult to summarize the results at the same scale.
Conclusions
Our meta-analysis showed that JLC combined with TACE was more effective than TACE treatment alone in advanced HCC. The application of JLC not only clearly enhanced the therapeutic effects of TACE but also effectively improved the QoL and immune function of HCC patients. However, the low quality of some included trials increases risks and bias, therefore the findings from our study should be dealt with some caution. The clinical efficacy and safety of JLC-mediate therapy for HCC still needs more high-quality, multi-centre large randomized trials to verify.
Author contributions
Tao HM and Jia SZ contributed in conception, design, statistical analysis and drafting of the manuscript. Jia SZ and Fu Y contributed in data collection and manuscript drafting. Tao HM supervised the study. All authors approved the final version for submission.
Supplementary_Table_1.doc
Download MS Word (36.5 KB)Availability of data and material
All data generated or analysed during this study are included in this published article.
Disclosure statement
The authors report no conflict of interest.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. 2018. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 68(6):394–424.
- Cabrera R, Pannu DS, Caridi J, Firpi RJ, Soldevila-Pico C, Morelli G, Clark V, Suman A, George TJ, Nelson DR. 2011. The combination of sorafenib with transarterial chemoembolisation for hepatocellular carcinoma. Aliment Pharmacol Ther. 34(2):205–213.
- Dong HT, Zhao W, Lu WP, Chen LZ, Yin QZ, Zhang Y, He YH. 2008. Clinical observation on 133 cases of primary hepatocellular carcinoma treated by jinlong capsule and hepatic artery intervention therapy. Chin J Clin Oncol. 35:378–380.
- Duval S, Tweedie R. 2000. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 56(2):455–463.
- Fan W, Wu Y, Lu M, Yao W, Cui W, Zhao Y, Wang Y, Li J. 2019. A meta-analysis of the efficacy and safety of iodine [131I] metuximab infusion combined with TACE for treatment of hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 43(4):451–459.
- George BJ, Aban IB. 2016. An application of meta-analysis based on DerSimonian and Laird method. J Nucl Cardiol. 23(4):690–692.
- He C, Peng W, Liu X, Li C, Li X, Wen TF. 2019. Post-treatment alpha-fetoprotein response predicts prognosis of patients with hepatocellular carcinoma: a meta-analysis. Medicine. 98(31):e16557.
- Jackson D, White IR, Riley RD. 2012. Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat Med. 31(29):3805–3820.
- Jia CH, Wang WY, Kang Y. 2008. Clinical studies on combination therapy of jinlong capsule and transarterial chemoembolization in treatment of primary hepatic carcinoma. Chin J Cancer Prev Treat. 15:1416–1418.
- Jiang CY, Cao JJ, Cheng Q. 2013. Clinical evaluation of Jinlong capsule combined with TACE in the treatment of primary hepatocellular carcinoma. Med Innov China. 10:110–111.
- Kim DY, Han KH. 2016. Transarterial chemoembolization versus transarterial radioembolization in hepatocellular carcinoma: optimization of selecting treatment modality. Hepatol Int. 10(6):883–892.
- Li B, Zhao LX, Liu ZW, Li L, Ma LB, Zhou ZX, Hu WN. 2013. Jinlong capsule combined with interventional therapy for primary hepatocellular carcinomas: a clinical analysis on 150 patients. Chin J Hepatobiliary Surg. 19:530–533.
- Li D, Ni T, Tao L, Jin F, Wang H, Feng J, Zhu G, Qian Y, Ding Y, Sunagagwa M, et al. 2018. Jinlong capsule (JLC) inhibits proliferation and induces apoptosis in human gastric cancer cells in vivo and in vitro. Biomed Pharmacother. 107:738–745.
- Li H, Zhang B, Yu GY. 2007. Preventive effect of Jinlong capsule on recurrence and metastasis of resectable hepatocellular carcinoma after operation. Capit Med. 24:35–36.
- Li J, Xiao ST. 2014. Fingerprint establishment of amino acids composition of Jinlong capsule. Chin J Inf Tradit Chin Med. 21:62–64.
- Li Y, Hu J, Huang H, He Y. 2013. Effect of Jinlong capsule on proliferation and apoptosis of human pancreatic cancer cells BxPC-3. J Tradit Chin Med. 33(2):205–210.
- Liang TJ, Qin CY, Zhang CQ, Zhao XQ. 2005. Clinical studies on the combination therapy with jinlong capsule and chemotherapy plus embolization by hepatic artery catheterization on primary hepatic carcinoma. Chin J Clin Oncol. 32:641–643.
- Lin L, Chu H. 2018. Quantifying publication bias in meta-analysis. Biometrics. 74(3):785–794.
- Liu ZY, Zhang XH, Zhang XS, Li JY, Ji XW. 2015. Clinical observation of jinlong capsule combined with TACE in the treatment of primary hepatocellular carcinoma. Heilongjiang Med Pharm. 38:75–76.
- Ma X, Li RS, Wang J, Huang YQ, Li PY, Su HB, Wang RL, Zhang YM, Liu HH, Zhang CE, et al. 2016. The therapeutic efficacy and safety of compound kushen injection combined with transarterial chemoembolization in unresectable hepatocellular carcinoma: an update systematic review and meta-Analysis. Front Pharmacol. 7:70.
- Meng P. 2016. Clinical efficacy and mechanism of jinlong capsule plus hepatic artery embolism for primary liver cancer. Pract J Cancer. 31:403–406.
- Moher D, Liberati A, Tetzlaff J, Altman DG. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 62(10):1006–1012.
- Panic N, Leoncini E, de Belvis G, Ricciardi W, Boccia S. 2013. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS One. 8(12):e83138.
- Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R, Baldan A, Girardi L, Cillo U, Burra P, Giacomin A, et al. 2008. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 103(4):914–921.
- Shen A, Liu M, Zheng D, Chen Q, Wu Z. 2019. Adjuvant transarterial chemoembolization after curative hepatectomy for hepatocellular carcinoma with microvascular invasion: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 19:30137–30138.
- Shi J, Zhang W, He L, Kong F, Pan M, Guo J, Xu X, Guo J, Wang H, Wang Y. 2019. Jinlong capsule inhibits migration and invasion in human glioblastoma cells via the modulation of mTOR/S6 signaling pathway. Drug Des Devel Ther. 13:1023–1032.
- Shi L, Lin L. 2019. The trim-and-fill method for publication bias: practical guidelines and recommendations based on a large database of meta-analyses. Medicine (Baltimore). 98(23):e15987.
- Wang XH, Yang JQ, Li YH. 2009. Clinical observation of jinlong capsule combined with interventional therapy for hepatocellular carcinoma. Chin J Integr Trad West Med. 29:273–274.
- Wong RJ, Ahmed A, Gish RG. 2015. Elevated alpha-fetoprotein: differential diagnosis – hepatocellular carcinoma and other disorders. Clin Liver Dis. 19(2):309–323.
- Wu GL, Zhang L, Li TY, Chen J, Yu GY, Li JP. 2010. Short-term effect of combined therapy with Jinlong capsule and transcatheter arterial chemoembolization on patients with primary hepatic carcinoma and its influence on serum osteopontin expression. Chin J Integr Med. 16(2):109–113.
- Xie YF, Tan XF, Ma QH, Liu SZ. 2003. Clinical observation of jinlong capsule combined with interventional therapy for hepatocellular carcinoma. Chin J Clin Oncol. 30:297–298.
- Yang PY, Sun YY, Zhang YC, Zhang X, Sun BX, Jia YJ. 2013. Effectiveness of early intervention with Jin-long capsules and transarterial chemoembolization for the treatment of primary liver cancer. Chin J Clin Oncol. 40:45–49.
- Yuan TW, Wen SW, Dang ZJ, Zhang XQ, Chang JP, Xue YQ. 2013. Regulation of immune functions by combined Jinlong capsule and interventional therapy in patients with primary liver cancer. Chin J Clin Oncol. 40:1116–1118.
- Zeng BZ, Yang YQ, Han XW. 2010. Jinlong capsule combined with TACE in the treatment of 31 cases of primary liver cancer. Trad Chin Med Res. 23:35–37.
- Zeng CS, Cai LM, Huang ZC, Xu QY. 2014. The effect of Jinlong capsule on the immune function for intervened patients with primary liver cancer. J Clin Oncol. 13:80–83.
- Zeng CS, Cai LM, Li JW, Huang ZC, Xiao YH, Zhang CY, Wang XM, Luo HJ. 2012. Influence of jinlong capsule combined with transarterial chemo-embolization on quality of life of patients with primary liver cancer. Chin J Clin Oncol. 39:1839–1842.
- Zeng X, Zhang Y, Kwong JS, Zhang C, Li S, Sun F, Niu Y, Du L. 2015. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 8(1):2–10.
- Zhang HJ, Yang JJ, Wang WX, Jiang X, Mao YJ, Yang CA, Guo JX. 2008. Effects of Jinlong capsule on expressions of interleukin-2 and soluble interleukin-2 receptor in patients with primary liver cancer after transarterial chemoembolization therapy. J Chin Integr Med. 6(9):907–910.
- Zhang XQ. 2012. Jinlong capsule combined with chemotherapy for 14 cases of primary liver cancer with pulmonary metastasis. Jiangxi J Trad Chin Med. 43:35–37.
- Zhang XQ, Guo P, Dang ZJ, Wen SW. 2012. Jinlong capsule combined with interventional therapy for hepatocellular carcinomas: clinical observation. J Intervent Radiol. 21:249–251.
- Zheng C, Zhang RS, Pan Y, Liu HL. 2018. Effects of jinlong capsule combined with interventional therapy on patients with primary hepatocellular carcinoma and its influence on T lymphocyte subsets and tumor immune factors. Modern Digest Interv. 23:506–509.
- Zhou ZH, Liu LM, Chen WW, Men ZQ, Lin JH, Chen Z, Zhang XJ, Jiang GL. 2007. Combined therapy of transcatheter arterial chemoembolisation and three-dimensional conformal radiotherapy for hepatocellular carcinoma. Br J Radiol. 80(951):194–201.
- Zhu Y, Cui PJ, Yao J, Zhang ZY, Yang J. 2018. Efficacy of transarterial chemoembolisation with or without antiviral therapy for patients with hepatocellular carcinoma after radical hepatectomy. Gastroenterol Res Pract. 2018:6414759.

![Figure 7. Forest plot of the comparison of immune function indicators [CD3+ (A); CD4+ (B); CD8+ (C); CD4+/CD8+ (D) and NK (E)] between the TACE and TACE + JLC groups. TACE: trans-arterial chemoembolization; JLC: Jinlong capsule.](/cms/asset/190b00a4-1faa-4bea-a766-8a4356b1474c/iphb_a_1799040_f0007_c.jpg)