Abstract
Context
As a major active iridoid glycoside from Gardenia jasminoides J. Ellis (Rubiaceae), geniposide possesses various pharmacological activities, including anti-platelet aggregation and anti-inflammatory action.
Objectives
This study explores the effect of geniposide in diabetic wound model by anti-inflammatory action.
Materials and methods
Diabetic rodent model in Wistar rats was induced by streptozotocin combined with high-fat feed. The selected rats were divided into control group, the diabetic model group and geniposide subgroups (200, 400 and 500 mg/kg), and orally administrated once daily with saline or geniposide. Wound area and histochemical indicators were measured on day 7 after continuous administration, to assess lesion retraction, inflammatory cells and fibroblasts.
Results
Geniposide notably enhanced lesion retraction by 1.06–1.84 times on day 7 after surgical onset in diabetic rats (p < 0.05). In the pathological experiment by HE staining, geniposide significantly reduced inflammatory cell infiltration and proliferation of fibroblasts in the central lesion regions. In diabetic rats treated with geniposide, the levels of pro-inflammatory factors (tumour necrosis factor-α (TNF-α), interleukin-1β (IL-1β)) and IL-6 were significantly reduced (p < 0.05), followed with the increment of IL-10 in a dose-dependent manner. The IC50 of geniposide on TNF-α, IL-1β and IL-6 could be calculated as 1.36, 1.02 and 1.23 g/kg, respectively. It assumed that geniposide-induced IL-10 expression contributed to inhibiting the expression of pro-inflammatory factors.
Discussion and conclusions
Geniposide promoted diabetic wound healing by anti-inflammation and adjusting blood glucose. Further topical studies are required to evaluate effects on antibacterial activity and skin regeneration.
Introduction
Diabetic skin ulcer (DSU) is a common complication of diabetes patients induced by high glucose (Capodanno and Angiolillo Citation2010). Most DSU patients will eventually progress to chronic and incurable ulcers. Different from ordinary skin lesion, nerve damage and the lesions of the surrounding blood vessels in diabetes patients make DSU difficult to heal, and complicated with repeated infections, all of which seriously affects quality of life (Lim et al. Citation2017). Due to many factors such as high costs, treatment tolerance and side effects, the treatment options of DSU on the market are still very limited.
Gardenia jasminoides J. Ellis (Rubiaceae) is a classic Chinese herb used for medicine and food; it is the main composition of many Chinese medicine prescriptions, such as Huanglian Jiedu decoction, Zhihong ointment and Zhi-zi-chi decoction. In Chinese medical theory, Gardenia jasminoides could reach meridians of heart, lung, and triple burner and suppress the evil fire, relieve internal heat, and cool blood in the body (Liu et al. Citation2013). Geniposide is a major iridoid glycoside in Gardenia jasminoides (Chen, Chen et al. Citation2020; Chen, Li et al. Citation2020). It has been reported in the literature that geniposide performed anti-inflammation and neuroprotective activities via modulating mitogen-activated protein kinase (MAPK)/nuclear factor kappa-B (NF-κB) and phosphatidylinositide 3-kinases-protein kinase B (PI3K-Akt) signalling pathways (Suzuki et al. Citation2001; Wu et al. Citation2019; Yuan et al. Citation2020; Zhou et al. Citation2020). In our earlier studies, the antithrombotic effect and anti-platelet aggregation of geniposide and genipin were also evaluated in animal models (Zhang et al. Citation2013; Liu, Chen, et al. Citation2021; Liu, Wang, et al. Citation2021). However, the potential effect of geniposide in wound healing of diabetic rats was not studied. This study evaluates the effect of geniposide in diabetic wound model by anti-inflammatory action.
Materials and methods
Animal and chemical agents
Healthy male Wistar rats 8 weeks old, body weight 250 ± 20 g were obtained from the Experimental Animal Center of Jiangxi University of Traditional Chinese Medicine; geniposide (HPLC > 95%, catalogue no. syz090911 from the Chengdu Dicotyledon Chinese Medicine Resources Co., Ltd., Chengdu, China); streptozotocin (catalogue no. S0130, Sigma-Aldrich, St. Louis, MO); and IL-6, interleukin-1β (IL-1β), tumour necrosis factor-α (TNF-α), IL-10 detection kit from R&D Systems (Minneapolis, MN) (IL-6: catalogue no. DY506; IL-1β: catalogue no. DY501; TNF-α: catalogue no. DY510; IL-10: catalogue no. SR1000). All animal experiment operations were approved and implemented by the Ethics Committee of Jiangxi University of Science and Technology (SCXK-2018-103).
Preparation of diabetic wound model
All rats were continuously fed with high-fat diets (w/60% kcal from fat, 5.10 kcal/g, mainly containing 31.7% lard, 25.8% casein, 16.2% maltodextrin, 8.9% sucrose, 6.5% powdered cellulose, 3.2% soybean oil, 2.1% potassium citrate and others, catalogue no. 58Y1; TestDiet, St. Louis, MO) for 10 weeks, with free excess to food and water. Subsequently, rats were administrated intraperitoneally with a single injection of streptozotocin (55 mg/kg STZ in buffered-solution (pH 4.5) according to our experience). This protocol was modified according to former literature (Magalhães et al. Citation2019; Chen TJ et al. Citation2020). Two weeks after injection, the level of blood glucose was tested. Rats with fasting plasma glucose (FPG) more than 16.7 mmol/L were included as successful diabetic model (Beserra et al. Citation2019). The blood sugar levels of the rats were measured by using a OneTouch® blood glucose meter (LifeScan, Wayne, PA). Glycated haemoglobin (HbA1c) was tested by kits from Chongqing Biospes Co., Ltd. (Chongqing, China).
For modelling of diabetic wound, diabetic rats were anaesthetized intraperitoneally with 1% sodium pentobarbital (40 mg/kg). After carefully cleaning the depilated area with 75% sterile medical alcohol, a hole puncher was used to create a 2 cm diameter wound reaching the lower fascia layer on the right side of back, and the sterile gauze was used to wrap the wound. The percentage of the wound area was calculated by the following formula: retraction (%)=(initial lesion area – area of lesion when measuring)/initial lesion area × 100 (Champeau et al. Citation2021).
Experimental grouping and intervention
The selected rats were divided into control group (normal rats), the model group (diabetic rats treated with saline) and geniposide subgroups (diabetic rats treated with 200 mg/kg geniposide (Gen-L), 400 mg/kg geniposide (Gen-M) and 500 mg/kg geniposide (Gen-H)) for the study of skin lesion in diabetic rats. A total of 95 diabetic rats were used in these experiments. The dose range of geniposide was selected according to the maximum resolution of geniposide and our previous experience in animal studies. They were orally administrated with saline or geniposide solution (dissolved in saline) once daily for seven days after preparing the rat model of diabetic wound. The levels of FPG and HbA1c in each rat were measured on day 7 after continuous administration. The sampling collection was performed 1 h after last administration.
HE staining
The method was performed according to the previous report with a few modification (Haghshenas et al. Citation2019). After intervention of geniposide for seven days, the marginal and intermediate tissues of skin lesion were taken, cleaned and fixed with 4% paraformaldehyde. After fixing in 4% paraformaldehyde at 4 °C for 24 h, the tissues were subjected to dehydration, embedding and sectioning with 5 μm thickness before HE staining. The indicators were consisted of the infiltration of inflammatory cell and the number of fibroblasts on the longitudinal section of the wound tissue under the microscope. Four fields of view at the central lesion regions were randomly selected for each sample to observe the changes under a 400-fold microscope. Since many neutrophils will move to the lesion region within first a few days, the number of inflammatory cells was identified by the nucleus stained in deep blue. The fibroblasts were recognized by cell morphology in dark purple.
Detection of inflammatory factors in wound tissue
On day 7 after surgery, the wound tissue was extracted and homogenized on ice to prepare the homogenate, centrifuged at 12,000 rpm for 15 min and supernatant was collected for ELISA assay. ELISA kits (R&D Systems, Inc., Minneapolis, MN) were used to detect the levels of inflammatory factors such as IL-6, IL-10, IL-1β and TNF-α in the tissue homogenate.
Statistical methods
The GraphPad Prism software 7.0 (La Jolla, CA) was used for statistical analysis, and the data were expressed in the form of mean ± standard error of the mean (SEM), and the statistical results p < 0.05 were considered as statistically significant.
Results
Effect of geniposide on blood glucose level in diabetic rats
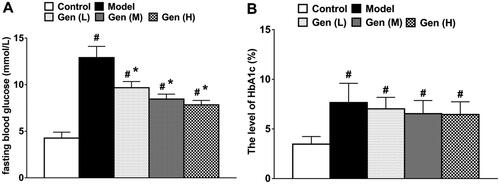
In all diabetic rats, the blood glucose levels increased significantly. After oral administration of geniposide, the blood glucose level significantly decreased on day 7 after continuous administration compared to the model group (, p < 0.05), whilst the level of HbA1c was not changed in treatment subgroups.
Figure 1. The levels of FBG (A) and HbA1c (B) in non-diabetic and diabetic rats were determined on day 7 after continuous administration. Data are shown as means ± SEM (n = 6 in each group with total of 30 rats). #p< 0.05 and *p< 0.05 denote statistical significance compared with normal control group and the model group, respectively, analysed by one-way ANOVA followed by the post hoc Student–Newman–Keuls test.
Assessment of wound healing in diabetic rats
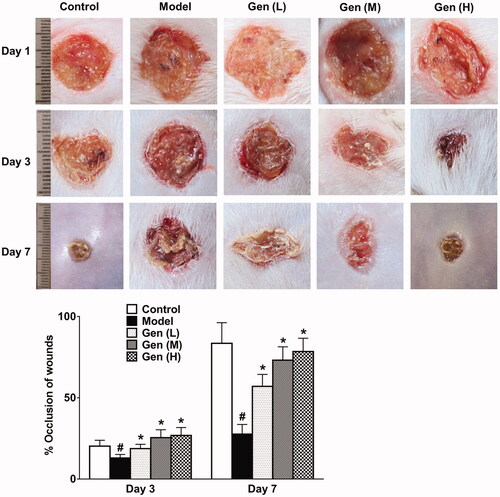
Results showed that diabetic rats received geniposide by gavage could significantly promote lesion retraction compared to the model group on day 3 and day 7 after surgery, respectively (, p < 0.05). This improvement continued until the termination of treatment strategy.
Figure 2. Geniposide promoted wound healing dose-dependently on day 1, 3 and 7 after continuous administration. Data are shown as means ± SEM (n = 4–5 in each group with total of 23 rats). #p< 0.05 and *p< 0.05 denote statistical significance compared with normal control group and the model group, respectively, analysed by one-way ANOVA followed by the post hoc Student–Newman–Keuls test.
Assessment of skin lesion in diabetic rats by HE staining
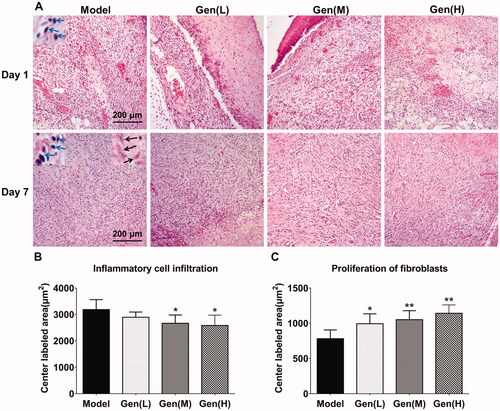
As shown in HE staining, the inflammatory cells and the proliferation of fibroblasts in the central lesion regions were quantified on day 7 in each sample (). The results showed that oral administration of geniposide (Gen-M and Gen-H) could significantly restrain the expression of inflammatory cells in the tissue by 16.4% and 18.9% (p < 0.05), and notably promote the proliferation of fibroblasts by 27.0%, 34.5% and 46.0% (p < 0.05).
Figure 3. Geniposide promoted wound healing in HE staining via the regulation of inflammatory cell infiltration and proliferation of fibroblasts in the central lesion regions. (A) HE staining of skin wound in diabetic rats in each group. (B, C) The quantification of inflammatory cell infiltration and proliferation of fibroblasts was performed on day 7 after surgery and analysed by GraphPad Prism software (La Jolla, CA). The blue arrows indicated the infiltrated inflammatory cells, whilst the black arrows represented fibroblasts. Data are shown as means ± SEM (n = 4–5 in each group with total of 19 rats). *p< 0.05 and **p< 0.01 denote statistical significance compared with the model group, analysed by one-way ANOVA followed by the post hoc Student–Newman–Keuls test.
The changes of inflammatory factors in wound tissues
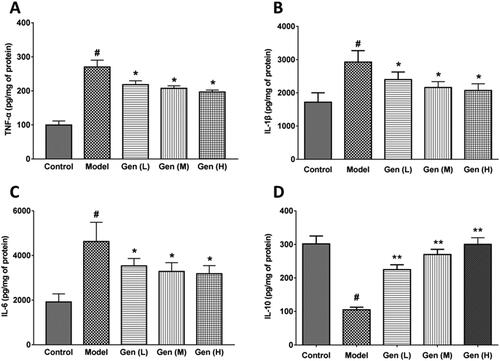
The anti-inflammatory and pro-inflammatory factors in the wound tissue were detected by the ELISA method. We found that the skin lesion in diabetic rats was susceptible to infection due to the wound exposure, and the levels of TNF-α, IL-1β and IL-6 in wound tissues were significantly increased on day 7 after surgery. After oral administration of geniposide, the levels of pro-inflammatory factors (TNF-α, IL-1β) and IL-6 were significantly reduced (p < 0.05, ), followed with the increment of anti-inflammatory factor IL-10 in a dose-dependent manner (). The IC50 values of geniposide on TNF-α, IL-1β and IL-6 were calculated as 1.36, 1.02 and 1.23 g/kg, respectively. The expression of IL-10 was enhanced by geniposide dose-dependently, with the increase of 1.14, 1.57 and 1.86 times (p < 0.01). We assumed that the anti-inflammatory action of geniposide mainly attributed to the increment of IL-10 level, which suppressed the expression of pro-inflammatory factors.
Figure 4. Geniposide inhibited the inflammatory factors in wound tissue. (A–D) The levels of pro-inflammatory factors (TNF-α, IL-1β), IL-6 and anti-inflammatory factor IL-10 were measured on day 7 in each group by ELISA kits. Data are shown as means ± SEM (n = 4–5 in each group with total of 23 rats). #p< 0.05, *p< 0.05, and **p< 0.01 denote statistical significance compared with normal control group and the model group, respectively, analysed by one-way ANOVA followed by the post hoc Student–Newman–Keuls test.
Discussion
The large number of inflammatory cells produced in wound tissue of diabetic patients may strongly limit the process of wound closure. It has been reported in the literature that wounds under hyperglycaemic conditions could prohibit the expression of anti-inflammatory cytokines (such as IL-10) and intensify the expression of pro-inflammatory cytokines (such as TNF-α, IL-1β and IL-6) (Feng et al. Citation2016). In addition, pro-inflammatory cytokines not only participate in the inflammatory process, but also are considered as mediators of cell proliferation and differentiation. In activated stratum corneum cells, IL-6 stimulates the formation of granulation tissue, and promotes the occurrence of epithelial mechanisms and angiogenesis (Finnerty et al. Citation2006). TNF-α is shown to possess a dual role in reducing the formation of granulation tissue and the arrangement of collagen fibre (Breder et al. Citation2020). The anti-inflammatory factor IL-10 is a pleiotropic cytokine that exerts immunosuppressive or immunostimulatory effects in a variety of cell types. In addition, IL-10 can indirectly promote fibroblast proliferation and collagen deposition at the wound tissue (Wei et al. Citation2020).
Our results demonstrated that the inflammatory cells in the wound of diabetic rats were inhibited after oral administration of geniposide, especially in the central area of the lesion. Geniposide regulated these inflammatory mediators by reducing pro-inflammatory cytokines (such as IL-6) and up-regulating the level of anti-inflammatory cytokine IL-10. Inhibitor of nuclear factor kappa-B kinase (IKKs)/NF-κB pathway is the classical inflammatory pathway to produce TNF-α, IL-6 and IL-1β. Geniposide-induced IL-10 could also prohibit inflammatory factors by promoting the B-cell lymphoma factor 3 (Bcl-3) expression to inhibit the activity of IKKs (Wu et al. Citation2018). Besides, Li et al. reported that geniposide alleviated renal dysfunction in diabetic nephropathy mice through adenosine 5′-monophosphate-activated protein kinase (AMPK)/sirtuin1 (SIRT1)/NF-κB pathway, followed with the decreased levels of TNF-α, IL-6 and IL-1β (Li et al. Citation2020). In the disorders of atherosclerosis and osteoarthritis, geniposide was also proved to alleviate inflammation by regulating miR-101/mitogen activated protein kinase phosphatase-1 (MKP-1)/p38 pathway (Chen et al. Citation2018; Cheng et al. Citation2019). The expressions of IL-1, TNF-α and NO were also greatly suppressed by geniposide.
Poor wound healing is one of the common complications of diabetes in clinical practice. The skin lesion of diabetic patients is susceptible to inflammation due to high sugar levels and dysfunction of native immunity, all of which easily contribute to DSU. The delayed wound healing in DSU commonly attribute to the changes of endogenous factors, including collagen synthesis, fibroblast proliferation and immunological regulation, etc. (Monteiro-Soares et al. Citation2020). Our results showed that oral administration of geniposide could negatively regulate the blood glucose level of diabetic rats and enhance wound healing of DSU by suppressing inflammation and promoting fibroblast proliferation. In further study, the potential promoting effect of geniposide in wound healing should be evaluated in topical preparations.
Conclusions
Geniposide promoted diabetic wound healing by anti-inflammation and adjusting blood glucose. Further topical studies are required to evaluate effects on antibacterial activity and skin regeneration.
Author contributions
JWW, LYL, MZX, and DJQ carried out the experiments and data interpretation. CXY wrote the manuscript, performed statistical analysis. JWW and CXY obtained funding for the research project.
Disclosure statement
All authors declared no competing interests in this work.
Additional information
Funding
References
- Beserra FP, Vieira AJ, Gushiken LFS, de Souza EO, Hussni MF, Hussni CA, Nobrega RH, Martinez ERM, Jackson CJ, de Azevedo Maia GL, et al. 2019. Lupeol, a dietary triterpene, enhances wound healing in streptozotocin-induced hyperglycemic rats with modulatory effects on inflammation, oxidative stress, and angiogenesis. Oxid Med Cell Longev. 2019:3182627.
- Breder JSC, Pires ALR, Azevedo FF, Apolinario PP, Cantaruti T, Jiwani SI, Moraes AM, Consonni SR, Araujo EP, Adams GG. 2020. Enhancement of cellular activity in hyperglycemic mice dermal wounds dressed with chitosan–alginate membranes. Braz J Med Biol Res. 53(1):e8621.
- Capodanno D, Angiolillo DJ. 2010. Diabetes mellitus: the scary killer haunting silently. EuroIntervention. 5(8):879–881.
- Champeau M, Jary D, Vignion-Dewalle AS, Mordon S, de Lassalle EM, Vignoud S, Mortier L. 2021. Introduction of a model of skin lesions on rats and testing of dissolving microneedles containing 5-aminolevulinic acid. Int J Pharm. 594:120115.
- Chen L, Li M, Yang Z, Tao W, Wang P, Tian X, Li X, Wang W. 2020. Gardenia jasminoides Ellis: ethnopharmacology, phytochemistry, and pharmacological and industrial applications of an important traditional Chinese medicine. J Ethnopharmacol. 257:112829.
- Chen TJ, Chen SS, Wang DC, Hung HS. 2020. High-fat diet reduces novelty-induced expression of activity-regulated cytoskeleton-associated protein. J Cell Physiol. 235(2):1065–1075.
- Chen Y, Shou K, Gong C, Yang H, Yang Y, Bao T. 2018. Anti-inflammatory effect of geniposide on osteoarthritis by suppressing the activation of p38 MAPK signaling pathway. Biomed Res Int. 2018:8384576.
- Cheng SB, Zhou FH, Xu YL, Liu XY, Zhang Y, Gu MH, Su ZJ, Zhao DD, Zhang L, Jia YH. 2019. Geniposide regulates the miR-101/MKP-1/p38 pathway and alleviates atherosclerosis inflammatory injury in ApoE–/– mice. Immunobiology. 224(2):296–306.
- Feng Y, Sanders AJ, Morgan LD, Harding KG, Jiang WG. 2016. Potential roles of suppressor of cytokine signaling in wound healing. Regen Med. 11(2):193–209.
- Finnerty CC, Herndon DN, Przkora R, Pereira CT, Oliveira HM, Queiroz DM, Rocha AM, Jeschke MG. 2006. Cytokine expression profile over time in severely burned pediatric patients. Shock. 26(1):13–19.
- Haghshenas M, Hoveizi E, Mohammadi T, Kazemi Nezhad SR. 2019. Use of embryonic fibroblasts associated with graphene quantum dots for burn wound healing in Wistar rats. In Vitro Cell Dev Biol Anim. 55(4):312–322.
- Li FT, Chen Y, Li YJ, Huang MZ, Zhao WC. 2020. Geniposide alleviates diabetic nephropathy of mice through AMPK/SIRT1/NF-κB pathway. Eur J Pharmacol. 886:173449.
- Lim JZ, Ng NS, Thomas C. 2017. Prevention and treatment of diabetic foot ulcers. J R Soc Med. 110(3):104–109.
- Liu H, Chen XY, Liu YL, Fang CJ, Chen SF. 2021. Antithrombotic effects of Huanglian Jiedu decoction in a rat model of ischaemia–reperfusion-induced cerebral stroke. Pharm Biol. 59(1):823–827.
- Liu H, Chen YF, Li F, Zhang HY. 2013. Fructus Gardenia (Gardenia jasminoides J. Ellis) phytochemistry, pharmacology of cardiovascular, and safety with the perspective of new drugs development. J Asian Nat Prod Res. 15(1):94–110.
- Liu H, Wang ZY, Zhou YC, Song W, Ali U, Sze DM. 2021. Immunomodulation of Chinese herbal medicines on NK cell populations for cancer therapy: a systematic review. J Ethnopharmacol. 268:113561.
- Magalhães DA, Kume WT, Correia FS, Queiroz TS, Allebrandt Neto EW, Santos MPD, Kawashita NH, França SA. 2019. High-fat diet and streptozotocin in the induction of type 2 diabetes mellitus: a new proposal. An Acad Bras Cienc. 91(1):e20180314.
- Monteiro-Soares M, Boyko EJ, Jeffcoate W, Mills JL, Russell D, Morbach S, Game F. 2020. Diabetic foot ulcer classifications: a critical review. Diabetes Metab Res Rev. 36(Suppl. 1):e3272.
- Suzuki Y, Kondo K, Ikeda Y, Umemura K. 2001. Antithrombotic effect of geniposide and genipin in the mouse thrombosis model. Planta Med. 67(9):807–810.
- Wei HX, Wang B, Li B. 2020. IL-10 and IL-22 in mucosal immunity: driving protection and pathology. Front Immunol. 11:1315.
- Wu HY, Mao XF, Tang XQ, Ali U, Apryani E, Liu H, Li XY, Wang YX. 2018. Spinal interleukin-10 produces antinociception in neuropathy through microglial β-endorphin expression, separated from antineuroinflammation. Brain Behav Immun. 73:504–519.
- Wu J, Wang B, Li M, Shi YH, Wang C, Kang YG. 2019. Network pharmacology identification of mechanisms of cerebral ischemia injury amelioration by baicalin and geniposide. Eur J Pharmacol. 859:172484.
- Yuan J, Zhang J, Cao J, Wang G, Bai H. 2020. Geniposide alleviates traumatic brain injury in rats via anti-inflammatory effect and MAPK/NF-kB inhibition. Cell Mol Neurobiol. 40(4):511–520.
- Zhang HY, Liu H, Yang M, Wei SF. 2013. Antithrombotic activities of aqueous extract from Gardenia jasminoides and its main constituent. Pharm Biol. 51(2):221–225.
- Zhou S, Sun Y, Zhao K, Gao Y, Cui J, Qi L, Huang L. 2020. miR‑21/PTEN pathway mediates the cardioprotection of geniposide against oxidized low‑density lipoprotein‑induced endothelial injury via suppressing oxidative stress and inflammatory response. Int J Mol Med. 45(5):1305–1316.
