Abstract
Context
Alpinetin, the major active constitutes of Alpinia katsumata Hayata (Zingiberaceae), has been demonstrated to possess the activity of anti-breast cancer. Cytochrome P450 enzymes (CYP450s) plays vital roles in the biotransformation of various drugs.
Objective
To assess the effect of alpinetin on the activity of CYP450s and estimate the inhibition characteristics.
Materials and methods
The activity of CYP450s was evaluated in pooled human liver microsomes with corresponding substrates and marker reactions. The effect of alpinetin was compared with blank control (negative control) and corresponding inhibitors (positive control). The dose-dependent and time-dependent experiments were conducted in the presence of 0, 2.5, 5, 10, 25, 50, and 100 μM alpinetin and incubated for 0, 5, 10, 15, and 30 min.
Results
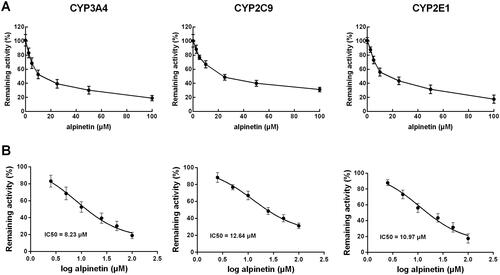
Alpinetin suppressed CYP3A4, 2C9, and 2E1 activity. All the inhibitions were significantly influenced by alpinetin contration with the IC50 values of 8.23 μM (CYP3A4), 12.64 μM (CYP2C9), and 10.97 μM (CYP2E1), respectively. The inhibition of CYP3A4 was fitted with the non-competitive model with a Ki value of 4.09 μM and was time-dependent with KI and Kinact values of 4.67 min and 0.041 μM−1, respectively. While CYP2C9 and 2E1 were inhibited by alpinetin competitively with Ki values of 6.42 (CYP2C9) and 5.40 μM (CYP2E1), respectively, in a time-independent manner.
Discussion and conclusion
The in vitro inhibitory effect of alpineticn on CYP3A, 2C9, and 2E1 implied the potential interaction of alpinetin or its origin herbs with the drugs metabolised by those CYP450s, which needs further in vivo validation.
Introduction
Breast cancer is one of the leading causes of cancer-related death in women. Although the clinical management of breast cancer has been greatly improved, metastasis and recurrency still frequently occur (Scully et al. Citation2012). Chemotherapy and radiotherapy gradually account for a critical position in the therapeutic strategies of breast cancer (Zhang and Li Citation2018). However, both these two treatments are not able to distinguish cancer cells (Staff et al. Citation2017). As expected, Chinese traditional medicine has been widely accepted in the therapy of various human diseases, which could avoid the side effects on normal cells. Alpinia katsumata Hayata (Zingiberaceae) seeds have been commonly used in the treatment of inflammation and gastric disorders (Lee et al. Citation2003), but its extractions have distinguished pharmacological activities (Wang et al. Citation2008). For example, (E)-methyl-cinnamate, an extraction of A. katsumata, were revealed to suppress pre-osteoblasts growth, migration, and differentiation (Park et al. Citation2020). Alpinetin, a major active ingredient of A. katsumata, has been reported to possess a significant antitumor activity in various human cancers, including breast cancer (Wang et al. Citation2016; Wu et al. Citation2016; Zhao et al. Citation2018; Hou et al. Citation2021; Zhang et al. Citation2021). It was reported that alpinetin showed an inhibitory effect on the progression of breast cancer via the ROS/NF-κB/HIF-1α axis (Zhang, Guo, et al. Citation2020). The interaction between active ingredients of different herbs would induce therapy failure or even drug toxicity.
As the prescription is always a mixture that includes various herbs with similar or complementary indications in traditional Chinese medicine, it makes the drug–drug interaction easier during the combination of various herbs. Cytochrome P450 enzymes (CYP450s) are a superfamily that partakes in the biotransformation of various xenobiotics, such as drugs and toxins (Manikandan and Nagini Citation2018). CYP450s is also a vital factor mediating the interaction between co-administrated drugs or compounds. Therefore, the influence of herb major active constituent on the activity of CYP450s is a critical basis for the clinical application of the original herbs and drugs, especially for the co-administration of different drugs (Lynch and Price Citation2007; Hakkola et al. Citation2020). Due to the miscellaneous pharmacological effects of alpinetin, especially for its anticancer activity, it is of great possibility that alpinetin would be co-administrated with other anticancer herbs. Hence, disclosing the effect of alpinetin on the activity of CYP450s would help understand the potential of alpinetin and its origin herb A. katsumata in inducing adverse drug-drug interaction.
Here, the interaction of alpinetin with eight major CYP450s isoenzymes was investigated in human liver microsomes, in order to guide the clinical co-administrated prescription of alpinetin and its origin herbs.
Materials and methods
Human liver microsomes assay
The pooled human liver microsomes (HLMs) were obtained from BD Biosciences Discovery Labware. The HLMs were incubated with specific substrates of corresponding CYP450 isoenzymes under the reaction conditions in according to previous reports (Dong et al. Citation2018; Zhang et al. Citation2019; Zhang, Feng, et al. Citation2020). The incubation system also included alpinetin (, 99%, Chengdu Munster Biothechnology Co, China, or specific inhibitors) and an β-NADPH generating system as previously reported in the potassium phosphate buffer with a final volume of 200 μL. The β-NADPH generating system contained NADP+, glucose-6-phosphate, glucose-6-phosphate dehydrogenase, and MgCl2, which was used to initiate the reaction after a pre-incubation of 3 min. At the end of the reactions, the acetonitrile or trichloroacetic acid (for the evaluation of CYP2A6) was added for the termination of the reactions. All reagents were of at least analytical reagent grade obtained from Sigma Aldrich (USA). All incubations were performed in triplicate and the concentrations of metabolites were analysed with HPLC.
Table 1. The reaction conditions of corresponding CYP450 isoforms.
Inhibition kinetic study
The effect of alpinetin on the activity of CYP1A2, 2A6, 3A4, 2C8, 2C9, 2C19, 2D6, and 2E1 was first evaluated with a concentration of 100 μM. The isoenzymes that were inhibited by alpinetin were further incubated with 0, 2.5, 5, 10, 25, 50, and 100 μM alpinetin to obtain the values of IC50.
Meanwhile, the kinetic studies were conducted in the presence of various substrate concentrations to obtain the values of Ki. The inhibition model was evaluated according to the following equations: v = (VmaxS)/[Km(1 + I/Ki) + S] for the competitive inhibition and v = (VmaxS)/[(Km + S(1 + I/KiKI)] for the non-competitive inhibition. The I is the concentration of the compound, Ki is the inhibition constant, S is the concentration of the substrate, and Km is the substrate concentration at half the maximum velocity (Vmax) of the reaction.
Time-dependent evaluation
The time-dependent inhibition assay was conducted with an incubation time of 0, 5, 10, 15, and 30 min after a 30 min pre-incubation with 1 mg/mL HLMs in the presence of NADPH-generating system at 37 °C. The concentration of corresponding substrates was close to the value of Km, while a higher concentration of approximate to 4-fold Km was used in the evaluation of the values of KI and Kinact.
Statistical analysis
All data were presented as mean ± SD and analysed by the Student’s t-test or one-way ANOVA followed by the Turkey post hoc test with SPSS 20.0.
Results
Effect of alpinetin on the activity of CYP450s
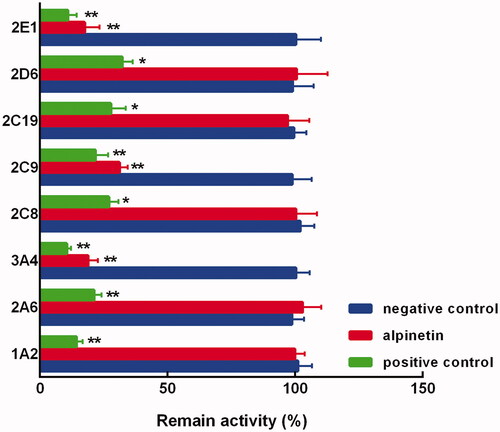
In the pooled liver microsomes, the activity of all CYP450 isoenzymes was obviously suppressed by their specific inhibitors (p < 0.01, ). While among the major isoenzymes, alpinetin showed a dramatically inhibitory effect on CYP3A4, 2C9, and 2E1 (p < 0.05, p < 0.01, ). Notably, the inhibition of these CYP450s was found to be concentration-dependent, which was enhanced with the increasing concentration of alpinetin (). The values of IC50 were obtained as 8.23 μM (CYP3A4) 12.64 μM (CYP2C9), and 10.97 μM (CYP2E1), respectively ().
Figure 2. The activity of CYP1A2, 2A6, 3A4, 2C8, 2C9, 2C19, 2D6, and 2E1 in the presence of alpinetin or specific inhibitors. Negative control: without any treatments, alpinetin: with 100 μM alpinetin, positive control: with specific inhibitors. *p < 0.05, **p < 0.01 relative to the negative control.
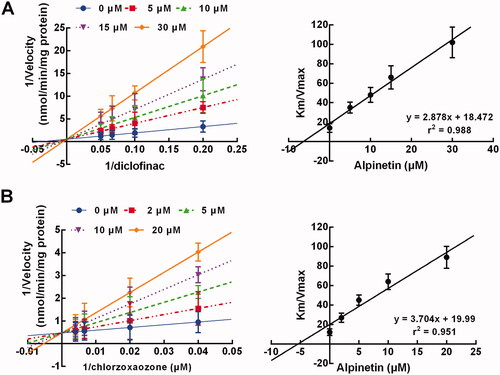
The inhibition model of CYP3A4
In the presence of 0, 2, 5, 10, and 20 μM alpinetin and various concentrations of testosterone, the inhibition of CYP3A4 was best fitted with the non-competitive model with a stable Km, where the velocity of CYP3A4 inhibition was found to be reduced with the increase of alpinetin concentration (). The Ki value of CYP3A4 inhibition by alpinetin was calculated as 4.09 μM ().
Figure 4. The inhibition model of CYP3A4. (A, B) The inhibition of CYP3A4 was non-competitive (A) with the Ki value of 4.09 μM (B). (C, D) The inhibition of CYP3A4 was time-dependent (C) with the KI and Kinact values of 4.67 min and 0.041 μM−1, respectively (D).
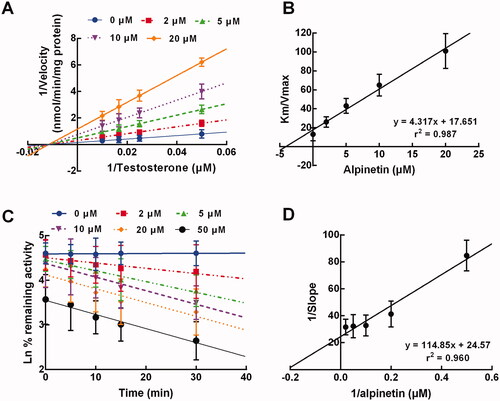
In addition, the inhibition of CYP3A4 by alpinetin also showed a time-dependent manner, which was enhanced by the elevating concentration of alpinetin (). The KI and Kinact value were obtained as 4.67 min and 0.041 μM−1, respectively ().
The inhibition model of CYP2C9 and 2E1
The inhibition of CYP2C9 and 2E1 by alpinetin was observed to be displayed competitively with a stable Vmax (), with the Ki values of 6.42 and 5.40 μM, respectively (). Meanwhile, no time-dependent characteristics were observed during the inhibition of CYP2C9 and 2E1 (data not shown).
Discussion
The alleviated effects of alpinetin on various human diseases have been reported in previous studies. The anti-inflammation effect of alpinetin has been illustrated in endometritis, bowel disease, and allergic asthma in mice (Liang et al. Citation2018; Wu, Li, et al. Citation2020; Yu et al. Citation2020), and its antitumor effect was observed in breast cancer and other malignant tumours (Wang et al. Citation2016; Zhao et al. Citation2018; Guo et al. Citation2020; Zhang, Guo, et al. Citation2020; Hou et al. Citation2021). With the widespread application of traditional Chinese herbs, the clinical treatment of breast cancer usually included herbal prescription, especially in the prevention and postoperative adjuvant therapies (Liu et al. Citation2019; Wang, Long, et al. Citation2020; Wang, Zhang, et al. Citation2020). The activity of CYP450s is a critical factor responsible for the therapeutic efficiency and is susceptible to external environmental conditions. Previously, several studies have unearthed the inhibitory or induced effect of various herbal extractions on the activity of different CYP450 isoenzymes. For example, lysionotin that is extracted from Lysionotus pauciflorus Maxim (Gesneriaceae) was revealed to inhibit the activity of CYP2C8, 2C19, and 3A4 in a dose-dependent manner (Li et al. Citation2020). Terminalia chebula Retz. dramatically suppressed the activity of CYP2E1 and 2C19, which induced its interaction with chlorzoxazone and omeprazole, which were metabolised by these two CYP450s (Wu, Dong, et al. Citation2020). As CYP450s could mediate the pharmacokinetic interactions between various herbs or drugs. Therefore, the effect of active extractions of herbs on CYP450 activity is of great significance for clinical prescriptions.
Herein, the inhibition of CYP3A4, 2C9, and 2E1 by alpinetin was observed, which was displayed in a concentration-dependent manner with various IC50 values. The inhibitory effect of alpinetin was demonstrated to be non-competitive in the present study, indicating that alpinetin did not affect the interaction between CYP3A4 and its substrates. Moreover, the inhibitory effect of alpinetin on CYP3A4 was enhanced by the incubation time. CYP3A4 is a vital member of the CYP450 family, which participates in the metabolism of numerous drugs, and was also involved in the adverse drug–drug interactions (Pal and Mitra Citation2006; Martinez-Jimenez et al. Citation2007). For instance, the inhibitory effect of Maha yogaraja guggulu and its major ingredients (E-guggulsterone and Z-guggulsterone) on CYP3A4 led to its interaction with conventional CYP3A4 substrates (Sabarathinam et al. Citation2021). Moreover, due the anti-breast cancer activity of alpinetin, its co-adminsitration with other antitumor drugs should draw special attention. According to reports, the latest small molecule targeted drug for breast cancer, palbociclib, a CDK4/6 inhibitor, its metabolism is closely related to CYP3A (Yu et al. Citation2017; Braal et al. Citation2021). Strong CYP3A inhibitor (itraconazole), inducer (Rifampicin), and sensitive CYP3A substrates (midazolam) have drug interactions with palbociclib, while moderate CYP3A inhibitors (diltiazem and verapamil) may increase blood palbociclib AUC by approximately 40% (Yu et al. Citation2017). Therefore, to avoid the risk of reduced effectiveness of palbociclib, the use of potent CYP3A4 inducers, including rifampicin, should be avoided. Similarly, if patients with certain diseases need to use CYP3A4 inhibitors including itraconazole and verapamil, the dose of palbociclib should be reasonably reduced. Hence, the interaction between alpinetin or its origin herbs and the drugs metabolised by CYP3A4 is of great potential to occur during their combination, which should draw special attention. Although CYP2C9 and 2E1 account for a small proportion in all CYP450 subtypes, they are also responsible for the metabolism of a huge number of drugs (Guengerich Citation2020; Wang, Gao, et al. Citation2020; Waring Citation2020). The observed inhibitory effects of alpinetin on CYP2C9 and 2E1 were found to be competitive, suggesting that alpinetin suppressed CYP2C9 and 2E1 activity via competing for the binding sites with substrates.
Previously, the role of genetic polymorphism in drug–drug interactions has been reported as a key factor that might affect the catalytic activity of CYP450 and therefore induce adverse drug–drug interaction (Bozina et al. Citation2009). The molecular mechanism underlying the effect of alpinetin on CYP450s activity needs further investigation. What’s more, the in vivo effect of alpinetin on CYP450s activity might be affected by the metabolites of alpinetin. A previous study has investigated the metabolites of alpinetin in rat plasma, urine, bile, and faeces, and identified a series of compounds with various functional groups, which was considered as the other potential mechanism underlying the inhibition of CYP3A4, 2C9, and 2E1 by alpinetin. On the other hand, the obtained in vitro findings directly evidenced the suppression of CYP3A4, 2C9, and 2E1 by alpinetin. However, the specific drug-drug interaction between alpinetin or its original source and their co-administrated drugs needs further in vivo validations.
Taken together, alpinetin dramatically inhibited CYP3A4, 2C9, and 2E1 in vitro. The administrated dose and incubation time were demonstrated to be two key factors that affected the degree of inhibition.
Acknowledgments
We thank the members of our laboratories for their contributions and helpful discussions. The authors gratefully acknowledge the support of staff and faculties at the Medical Research Center on processing the experimental samples.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Bozina N, Bradamante V, Lovric M. 2009. Genetic polymorphism of metabolic enzymes p450 (CYP) as a susceptibility factor for drug response, toxicity, and cancer risk. Arh Hig Rada Toksikol. 60(2):1032–242.
- Braal CL, Jongbloed EM, Wilting SM, Mathijssen RHJ, Koolen SLW, Jager A. 2021. Inhibiting cdk4/6 in breast cancer with palbociclib, ribociclib, and abemaciclib: similarities and differences. Drugs. 81(3):317–331.
- Dong G, Zhou Y, Song X. 2018. In vitro inhibitory effects of bergenin on human liver cytochrome p450 enzymes. Pharm Biol. 56(1):620–625.
- Guengerich FP. 2020. Cytochrome P450 2E1 and its roles in disease. Chem Biol Interact. 322:109056.
- Guo Y, Chen Y, Liu H, Yan W. 2020. Alpinetin inhibits oral squamous cell carcinoma proliferation via mir-211-5p upregulation and notch pathway deactivation. Nutr Cancer. 72(5):757–767.
- Hakkola J, Hukkanen J, Turpeinen M, Pelkonen O. 2020. Inhibition and induction of CYP enzymes in humans: An update. Arch Toxicol. 94(11):3671–3722.
- Hou S, Yuan Q, Cheng C, Zhang Z, Guo B, Yuan X. 2021. Alpinetin delays high-fat diet-aggravated lung carcinogenesis. Basic Clin Pharmacol Toxicol. 128(3):410–418.
- Lee SE, Shin HT, Hwang HJ, Kim JH. 2003. Antioxidant activity of extracts from Alpinia katsumadai seed. Phytother Res. 17(9):1041–1047.
- Li Y, Qin J, Wu H, Xu Y, Zhang L, Su K, Cui Y, Wang H. 2020. In vitro inhibitory effect of lysionotin on the activity of cytochrome P450 enzymes. Pharm Biol. 58(1):695–700.
- Liang Y, Shen T, Ming Q, Han G, Zhang Y, Liang J, Zhu D. 2018. Alpinetin ameliorates inflammatory response in LPS-induced endometritis in mice. Int Immunopharmacol. 62:309–312.
- Liu J, Mao JJ, Wang XS, Lin H. 2019. Evaluation of traditional Chinese medicine herbs in oncology clinical trials. Cancer J. 25(5):367–371.
- Lynch T, Price A. 2007. The effect of cytochrome p450 metabolism on drug response, interactions, and adverse effects. Am Fam Physician. 76(3):391–396.
- Manikandan P, Nagini S. 2018. Cytochrome p450 structure, function and clinical significance: A review. Curr Drug Targets. 19:38–54.
- Martinez-Jimenez CP, Jover R, Donato MT, Castell JV, Gomez-Lechon MJ. 2007. Transcriptional regulation and expression of CYP3A4 in hepatocytes. Curr Drug Metab. 8(2):185–194.
- Pal D, Mitra AK. 2006. Mdr- and CYP3A4-mediated drug-herbal interactions. Life Sci. 78(18):2131–2145.
- Park KR, Lee H, Cho M, Yun HM. 2020. A phytochemical constituent, (E)-methyl-cinnamate isolated from Alpinia katsumadai hayata suppresses cell survival, migration, and differentiation in pre-osteoblasts. Int J Mol Sci. 21(10):3700.
- Sabarathinam S, Rajappan Chandra SK, Thangavel Mahalingam V. 2021. Cyp3A4 mediated pharmacokinetics drug interaction potential of maha-yogaraj gugglu and E, Z guggulsterone. Sci Rep. 11(1):715.
- Scully OJ, Bay BH, Yip G, Yu Y. 2012. Breast cancer metastasis. Cancer Genomics Proteomics. 9(5):311–320.
- Staff NP, Grisold A, Grisold W, Windebank AJ. 2017. Chemotherapy-induced peripheral neuropathy: a current review. Ann Neurol. 81(6):772–781.
- Wang XQ, Yang XJ, Li JS. 2008. Studies on chemical constituents of Alpinia katsumadai. Zhong Yao Cai. 31(6):853–855.
- Wang J, Yan Z, Liu X, Che S, Wang C, Yao W. 2016. Alpinetin targets glioma stem cells by suppressing notch pathway. Tumour Biol. 37(7):9243–9248.
- Wang K, Gao Q, Zhang T, Rao J, Ding L, Qiu F. 2020. Inhibition of CYP2C9 by natural products: Insight into the potential risk of herb-drug interactions. Drug Metab Rev. 52(2):235–257.
- Wang S, Long S, Deng Z, Wu W. 2020. Positive role of Chinese herbal medicine in cancer immune regulation. Am J Chin Med. 48(7):1577–1592.
- Wang Y, Zhang Q, Chen Y, Liang CL, Liu H, Qiu F, Dai Z. 2020. Antitumor effects of immunity-enhancing traditional Chinese medicine. Biomed Pharmacother. 121:109570.
- Waring RH. 2020. Cytochrome p450: genotype to phenotype. Xenobiotica. 50(1):9–18.
- Wu G, Dong Z, Dong J, Wei L, Shi R, Kang S, Zhang D. 2020. Effects of mongolian medicine Terminalia chebula Retz. On 6 CYP450 enzymes in rats. Int J Clin Exp Pathol. 13(12):3128–3138.
- Wu D, Li S, Liu X, Xu J, Jiang A, Zhang Y, Liu Z, Wang J, Zhou E, Wei Z, et al. 2020. Alpinetin prevents inflammatory responses in ova-induced allergic asthma through modulating PI3K/AKT/NF-κB and HO-1 signaling pathways in mice. Int Immunopharmacol. 89(Pt A):107073.
- Wu XL, Zhao ZZ, Wu D, Chen GY, Liu H, He MX, Ding GH, He WY. 2016. Determination of main active components and metal elements of hainan Alpinia katsumadai by spectral analysis. Guang Pu Xue Yu Guang Pu Fen Xi. 36(4):1191–1196.
- Yu Y, Loi CM, Hoffman J, Wang D. 2017. Physiologically based pharmacokinetic modeling of palbociclib. J Clin Pharmacol. 57(2):173–184.
- Yu Z, Yue B, Ding L, Luo X, Ren Y, Zhang J, Mani S, Wang Z, Dou W. 2020. Activation of PXR by alpinetin contributes to abrogate chemically induced inflammatory bowel disease. Front Pharmacol. 11:474.
- Zhang Q, Li L. 2018. Photodynamic combinational therapy in cancer treatment. J BUON. 23(3):561–567.
- Zhang N, Liu J, Chen Z, Dou W. 2019. In vitro inhibitory effects of kaempferitrin on human liver cytochrome p450 enzymes. Pharm Biol. 57(1):571–576.
- Zhang X, Feng P, Gao X, Wang B, Gou C, Bian R. 2020. In vitro inhibitory effects of cepharanthine on human liver cytochrome p450 enzymes. Pharm Biol. 58(1):247–252.
- Zhang T, Guo S, Zhu X, Qiu J, Deng G, Qiu C. 2020. Alpinetin inhibits breast cancer growth by ROS/NF-κB/HIF-1α axis. J Cell Mol Med. 24(15):8430–8440.
- Zhang Y, Zhang Y, Li Y, Zhang L, Yu S. 2021. Preclinical investigation of alpinetin in the treatment of cancer-induced cachexia via activating pparγ. Front Pharmacol. 12:687491.
- Zhao X, Guo X, Shen J, Hua D. 2018. Alpinetin inhibits proliferation and migration of ovarian cancer cells via suppression of stat3 signaling. Mol Med Rep. 18(4):4030–4036.