Abstract
Context
The effects of Rhodiola rosea L. (Crassulaceae) polysaccharides (RRPs) on haematopoiesis are poorly understood.
Objective
To determine the effects of RRPs on haematopoiesis in mice with aplastic anaemia.
Materials and methods
Aplastic anaemia was induced in Kunming mice by 60Coγ (2.0 Gy) irradiation and cyclophosphamide administration (50 mg/kg/day for 3 consecutive days; intraperitoneal injection). The in vivo effects of RRPs (10, 20, and 40 mg/kg; intraperitoneal injection) on haematopoiesis were analyzed using peripheral blood tests, histopathological examination of haematopoietic tissues, culture of haematopoietic progenitors and bone marrow stromal cells (BMSCs), and Western blotting of Fas and Fas ligand (FasL). The in vitro effects of RRPs on bone-marrow haematopoietic progenitors and BMSCs were also evaluated.
Results
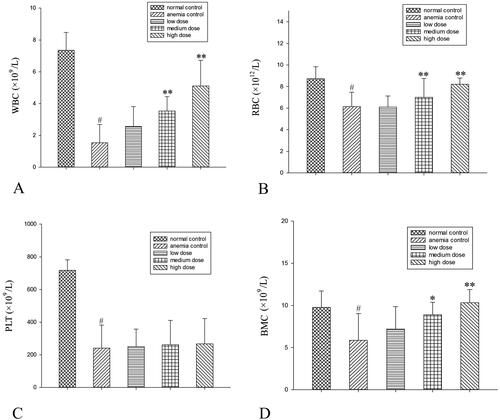
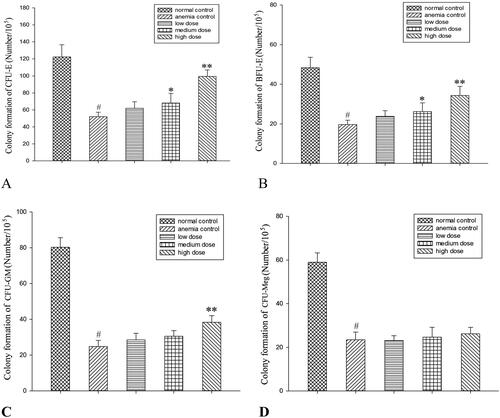
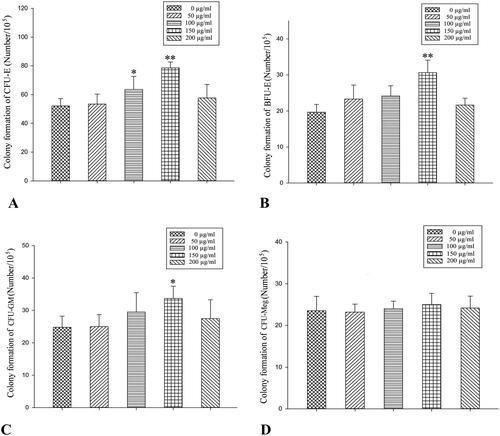
Compared to anaemic controls, high-dose RRPs (40 mg/kg) significantly increased red blood cells (8.21 ± 0.57835 versus 6.13 ± 1.34623 × 1012/L), white blood cells (5.11 ± 1.6141 versus l.54 ± 1.1539 × 109/L), and BMSCs (10.33 ± 1.5542 versus 5.87 ± 3.1567 × 1012/L) in mice with aplastic anaemia (all p < 0.01). High-dose RRPs significantly increased the formation of colony-forming unit-granulocyte macrophage (CFU-GM), burst-forming unit-erythroid (BFU-E), and colony-forming unit-erythroid (CFU-E; p < 0.01). Fas and FasL protein expression in BMSCs decreased after RRPs administration. Especially at the high dose, RRPs (150 μg/mL) significantly promoted in vitro CFUs-E, BFUs-E, and CFUs-GM formation. RRPs (150–300 μg/mL) also promoted BMSC proliferation.
Discussion and conclusions
RRPs helped to promote haematopoietic recovery in mice with aplastic anaemia, facilitating haematopoietic tissue recovery. This study indicated some mechanisms of the haematopoietic regulatory effects of RRPs. Our findings provide a laboratory basis for clinical research on RRPs.
Introduction
Rhodiola rosea L. (Crassulaceae) is a perennial herb or subshrub (i.e., dwarf shrub) with a long history of being used as a dietary supplement and a traditional medicine in China, Russia, and other countries. In China, R. rosea is widely used to eliminate fatigue, improve physical activity, and alleviate altitude sickness in high-altitude areas (Petkov et al. Citation1986). Recent studies have identified more than 150 biologically active compounds in R. rosea (Shikov et al. Citation2020). Pharmacological research has revealed that R. rosea preparations exhibit an adaptogenic effect (Panossian et al. Citation2010; Panossian Citation2017). In addition, R. rosea has been shown to have anti-fatigue, anti-cold, anti-inflammatory, anti-radiation, anti-stress, and antidepressant (Amsterdam and Panossian Citation2016; Li et al. Citation2017; Tao et al. Citation2019) properties; it can also provide resistance to hypoxia, inhibit tumours, and control blood glucose levels (Shi et al. Citation2012; Lee et al. Citation2013; Leung et al. Citation2013; Zhang et al. Citation2013). In addition, extracts of R. rosea have certain positive effects on haematopoietic regulation (Udintsev and Schakhov Citation1991; Qian et al. Citation2011). Thus far, most pharmacological studies on R. rosea have focussed on its alcohol-soluble ingredients such as salidroside, rhodosin, and p-tyrosol (Hu et al. Citation2010; Li et al. Citation2011; Xie and Zhu Citation2012).
Rhodiola rosea polysaccharides (RRPs) are one of the main water-soluble ingredients of R. rosea, and primarily include glucose, galactose, mannose, rhamnose, and arabinose. Recent studies have demonstrated that RRPs are abundant in R. rosea and have special physiological activities and pharmacological effects.
Studies have shown that RRPs have significant hypoglycaemic effects (Chem et al. Citation1996; Ding Citation2011), antioxidative activities, and hepatoprotective effects, and could prevent and treat liver damage induced by toxic chemicals. The antioxidative activity of RRPs is probably mediated through an increase in serum superoxide dismutase and glutathione peroxidase levels along with a decrease in serum reactive oxygen species and malondialdehyde levels (Nan et al. Citation2003; Huang et al. Citation2012; Song et al. Citation2015; Xu et al. Citation2018). In vitro and in vivo experiments have shown that RRPs have a potent tumour-growth inhibition effect on Sarcoma 180 cells. The underlying mechanism includes increase in the production of serum interleukin-2, tumour necrosis factor-α, and interferon-γ, which elevates the ratio of CD4+/CD8+ T lymphocytes in tumour-bearing mice (Cai et al. Citation2012). Rhodiola rosea has also been demonstrated to have an anti-radiation effect and could promote the recovery of haematopoietic function (Gao et al. Citation1994; Huang and Jiang Citation2003; Li and Luo Citation2012; Lei et al. Citation2014). Genotoxicity tests, including the Ames test, mouse BMC micronucleus test, and mouse sperm abnormality test, demonstrated that RRPs are safe, with no toxicity or mutagenicity (Liu et al. Citation2022).
However, systematic research on RRPs, particularly from the perspective of haematopoietic regulation, is rare. Therefore, the present study aimed to determine the influence of RRPs on bone-marrow haematopoietic function in a mouse model of aplastic anaemia. The purpose of this study was to enhance the exploitation and utilization of R. rosea.
Materials and methods
Experimental materials
RRPs were provided by Mingbo Company (Chengdu, China). Cyclophosphamide injections were purchased from Shanghai Hualian Pharmaceutical Co. Ltd. (Shanghai, China). Iscove’s modified Dulbecco’s medium (IMDM) substratum, granulocyte-macrophage colony-stimulating factor, thrombopoietin, and erythropoietin were obtained from Jingmei Co. (Chengdu, China). The following equipment was used: a fully automatic blood-cell analyzer (Hema-Screen18, Hospitex Diagnostics, Italy), a flow cytometer (Beckman Co., Brea, CA), an inverted-phase contrast microscope (Chongqing Optical Instrument Factory, Chongqing, China), a CO2 incubator (MCD-15A, Japan), and a fully automatic enzyme-linked immunosorbent assay (ELISA) metre (Thermo Fisher Scientific, Waltham, MA). The radiation source (60Coγ) was procured from the Sichuan Academy of Agricultural Sciences (Chengdu, China).
Animals
A total of 105 healthy Kunming male mice (weight, 18–22 g each; age, 6–8 weeks) were obtained from the Experimental Animal Centre, Chengdu University of Traditional Chinese Medicine (Certification No. CSDZG-7). The study was approved by the Animal Ethics Committee of Chengdu University of Traditional Chinese Medicine, China. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Preparation of RRP solution
RRPs were diluted in normal saline to obtain solutions with concentrations of 1, 2, and 4 mg/mL. These solutions were stored at 4 °C until analysis.
Establishment of mouse model of aplastic anaemia
The mice were randomly divided into five groups as follows: normal-control group (n = 20), aplastic anaemia control group (n = 25; to account for early deaths), and three RRP groups with low, medium, and high doses of RRPs (10, 20, and 40 mg/kg, respectively; n = 20 each). The aplastic anaemia model was established using the method described by Zhang et al. (Citation2006). The mice in the aplastic anaemia and RRP groups were irradiated with 2.0 Gy 60Coγ and then intraperitoneally injected with cyclophosphamide at a dose of 50 mg/kg/day for 3 consecutive days. After the modelling, the mice in the RRP groups received daily 0.2 mL intraperitoneal injections of high-, medium-, and low-dose RRPs for 7 consecutive days. During this time, the mice in the normal- and anaemic control groups were intraperitoneally injected with the same volume of normal saline (i.e., 0.2 mL/day).
Peripheral blood cell and bone marrow cell counts
Routine blood tests were performed 24 h after the last RRP/saline injection in each group. From each group, 10 mice were sacrificed, and their femurs harvested. The harvested bones were used to prepare BMC suspensions according to a routine method (Zhang et al. Citation2005), and the BMC counts were noted.
Histopathological examination
From each group, six randomly selected mice were sacrificed. Their femurs were harvested, fixed in 4% neutral formalin, decalcified, desiccated, paraffin embedded, cut into semi-thin sections, stained with haematoxylin and eosin, and examined using light microscopy to evaluate the histopathological changes in the bone marrow.
In vivo effects of RRPs on colony formation by bone-marrow haematopoietic progenitors
A portion of the BMC suspensions prepared for the cell count assessments was used for in vitro cultivation. The BMC suspension was diluted to a concentration of 1 × 105 cells/mL, and then cultured at 37 °C under 5% CO2 with saturated humidity. The culture system was consistent with the method described by Chen et al. (Citation2009). After 3 days, we counted the number of colony-forming units-erythroid (CFUs-E), and after 7 days, we counted the number of CFUs-E, burst-forming units-erythroid (BFUs-E), colony-forming units-granulocyte-macrophage (CFUs-GM), and colony-forming units-megakaryocyte (CFUs-Meg).
Western blot analysis of Fas and Fas ligand expressions
Protein concentrations were determined using the Bradford method. The total protein extraction was conducted by adding a protein isolation solution to the BMC suspension. Samples of the protein extracts were boiled for 3 min in a water bath, and 50 μg total proteins were separated using 10% sodium dodecyl sulphate polyacrylamide gel electrophoresis and then transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with 5% skim milk for 1 h and then incubated overnight with the primary antibodies at 4 °C. Next, the membranes were rinsed three times with Tris-buffered saline supplemented with Tween 20 (TBST), incubated for 1 h with the appropriate secondary antibodies (anti-Fas and anti-Fas ligand [FasL]) at room temperature, and again washed three times with TBST. Finally, Fas and FasL expressions were measured using enhanced chemiluminescence and ImageQuant LAS 4000 (GE, New York, NY, USA).
In vitro effects of RRPs on colony formation by bone-marrow haematopoietic progenitors
Six anaemic mice were sacrificed, and their femurs were harvested and used to prepare BMC suspensions (concentration, 1 × 105 cells/mL). The suspensions were divided into five groups with differing RRP concentrations. The basal culture medium was the same as that used to test the in vivo effects of RRPs, except that it was supplemented with IMDM or different concentrations of RRPs. The final RRP concentrations in the five in vitro groups were 0, 50, 100, 150, and 200 μg/mL. The cells were cultured for 7 days at 37 °C under 5% CO2 and saturated humidity, and colony formation was observed using inverted-phase contrast microscopy.
In vitro effects of RRPs on the proliferation of bone marrow stromal cells
Six normal mice and six anaemic mice were sacrificed, and BMC suspensions were prepared as described above. Their concentrations were adjusted to 2 × 107 cells/mL, and the suspensions were divided into 12 groups: 6 normal-RRP groups and 6 model-RRP groups. Each group contained six 200 μL samples of BMC suspensions. After 24 h of culture, non-adherent cells were eliminated. The culture fluid was then removed, and fresh culture fluid containing different concentrations of RRPs was added, to obtain final concentrations of 0, 50, 100, 150, 200, and 300 μg/mL. The proliferation of bone marrow stromal cells (BMSCs) was observed after 24, 48, and 72 h of culture at 37 °C under 5% CO2 with saturated humidity. BMSC proliferation was analyzed using MTT assays (Moura et al. Citation2012). At 4 h before the end of each culture period, most of the culture fluid was removed, and MTT was added to each well. Then, the culture plate was placed back into the incubator. After 4 h, the MTT was removed, and the cells were washed twice with phosphate-buffered saline. Next, 100 μL isopropyl alcohol was added to lyse the cells. Finally, ELISA was performed to detect the absorbance of each well at 570 nm, and cell proliferation was measured as follows:
Cell proliferation rate = (absorbance value of the experimental groups − average absorbance value of the control groups)/average absorbance value of the control groups × 100%.
Statistical analysis
Data are presented as means and standard deviations. Analysis of variance was used to assess between-group differences, and p = 0.05 was deemed to indicate statistical significance.
Results
Effects of RRPs on peripheral blood
RRPs elevated the red and white blood cell counts in a dose-dependent manner in mice with aplastic anaemia, but failed to elevate the platelet count. RRPs also increased the number of BMCs ().
Effects of RRPs on histopathological appearance of bone marrow
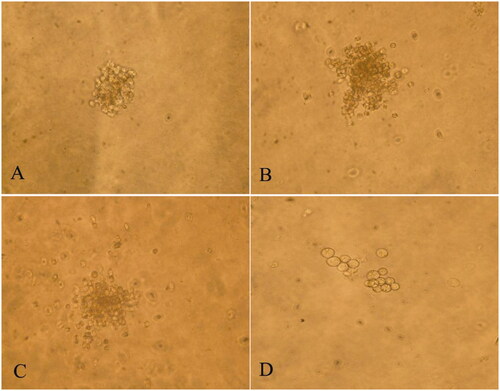
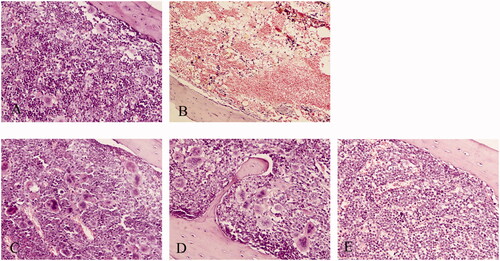
Compared to normal mice, anaemic mice showed decreased bone-marrow hyperplasia, decreased haematopoietic tissue area, decreased haematopoietic cells, increased adipocytes, and decreased megakaryocytes, accompanied with interstitial edoema. At 7 days after the intraperitoneal injection of different doses of RRPs, the haematopoietic cells in the bone marrow gradually increased, and non-haematopoietic cells decreased; interstitial edoema was relieved, and the number of megakaryocytes and the area of haematopoietic tissue increased ().
Figure 2. Influence of RRPs on histopathological changes in bone marrow in mice with aplastic anaemia. (A) Bone marrow, normal control (100×); (B) bone marrow, anaemic control (100×); (C) bone marrow, high-dose RRPs (100×); (D) bone marrow, medium-dose RRPs (100×); and (E) bone marrow, low-dose RRPs (100×). Stain, haematoxylin and eosin.
In vivo effects of RRPs on proliferation of bone-marrow haematopoietic progenitors
There were more than 50 BFUs-E and CFUs-GM, more than 8 CFUs-E, and more than 3 CFUs-Meg () in the normal control group. Compared to the anaemic control group, the medium-dose RRP group showed significantly increased formation of BFUs-E and CFUs-E (p < 0.05), while the high-dose RRP group showed significantly increased formation of CFUs-GM, BFUs-E, and CFUs-E (p < 0.01; ).
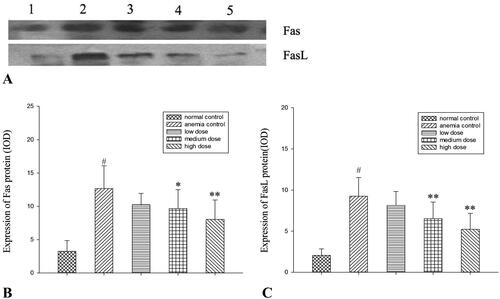
Effects of RRPs on Fas and FasL protein expressions
Western blotting revealed that the protein expression levels of Fas and FasL in BMCs were decreased in all RRP groups, and the decreases were significant in the high-dose group (p < 0.01; ).
Figure 5. Protein expression of Fas and FasL. (A) Western blot analysis of Fas and FasL. Lane 1, normal controls; lane 2, anaemic controls; lane 3, low-dose RRPs; lane 4, medium-dose RRPs; and lane 5, high-dose RRPs. (B) Integrated optical density (IOD) of Fas. (C) IOD of FasL. #p<0.01 versus normal controls; *p<0.05 and **p<0.01 versus anaemic controls.
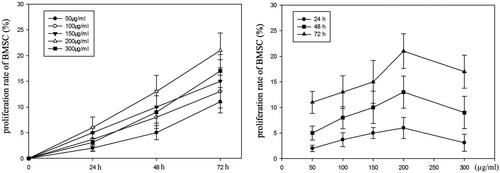
In vitro effects of RRPs on proliferation of bone-marrow haematopoietic progenitors
The optimal in vitro concentration of RRPs for promoting the formation of CFUs-E, BFUs-E, and CFUs-GM was 150 μg/mL. RRPs did not significantly promote the formation of CFUs-Meg ().
Effects of RRPs on BMSC proliferation
After 48 h of culture, stromal cells in the bone marrow were arranged in a fusiform shape. With the extension of the culture time, the BMSC proliferation rates increased in both the normal and model groups, especially within the concentration range of 150–300 μg/mL ().
Discussion
Rhodiola rosea is a rare medicinal plant that grows in cold, high-altitude regions. The main components of R. rosea are salidroside, p-tyrosol, and polysaccharides. Recent pharmacological research has revealed that these components help promote the plant’s adaptability to environmental stress and regulate its physiological functions. These components also have antihepatotoxic activities and anti-fatigue and anti-ageing functions (Udintsev and Schakhov Citation1991; Hu et al. Citation2010; Li et al. Citation2011; Qian et al. Citation2011; Lee et al. Citation2013; Leung et al. Citation2013). The present research focussed on the influence of RRPs on bone-marrow haematopoietic function in mice with aplastic anaemia. The purpose of this research was to enhance the exploitation and utilization of R. rosea.
This experiment induced aplastic anaemia in mice through the combined use of 60Coγ irradiation and cyclophosphamide. The model groups showed evident reductions in white blood cells, red blood cells, platelets, and BMCs. In addition, the bone marrow showed a decrease in the haematopoietic tissue area and an increase in adipocytes. These changes indicated that the aplastic anaemia model had been successfully constructed. Seven days after the intraperitoneal injection of different doses of RRPs, the peripheral blood cells of the mice in the model groups recovered to differing degrees. High doses of RRPs had an evident effect on leukocytes, erythrocytes, and haemoglobin. Bone-marrow haematopoietic cells gradually increased, and non-haematopoietic cells decreased. Interstitial edoema was relieved, and the haematopoietic tissue area increased. These findings suggested that RRPs potentially promoted the recovery of peripheral blood cell counts and haematopoietic tissues.
The in vivo experiments showed that medium and high doses of RRPs could promote the formation of CFUs-E and BFUs-E; low, medium, and high doses of RRPs all promoted the formation of CFUs-GM, but only the high dose produced a statistically significant effect. RRPs showed no considerable effect on CFUs-Meg. These findings indicate that in mice with myelosuppression, RRPs improved haematopoietic function by promoting the proliferation of erythroid cells and granulocytes. These results were consistent with the changes observed in the peripheral blood cell counts. The in vitro experiments indicated that the pro-proliferation effects of RRPs on bone-marrow haematopoietic progenitor cells were lower under in vitro conditions than under in vivo conditions. The proliferation-promoting effects of RRPs were mainly focussed on erythroid progenitor cells in the in vitro experiments. Our research indicated that RRPs had direct and indirect effects on haematopoietic progenitor cells, and that this effect was selective.
BMSCs are important components of the haematopoietic microenvironment. They greatly influence the growth, differentiation, and self-renewal of haematopoietic stem cells, and play a crucial role in haematopoietic regulation (Chu and Berek Citation2013; Chen et al. Citation2017, Citation2020). To reveal the influence of RRPs on stromal cells in the bone marrow, we cultured these cells with different doses of RRPs, and analyzed their proliferation using MTT assays. The results showed that the cell proliferation rates were improved in both the normal and model groups in a time-dependent manner. This suggested that RRPs had a good pro-proliferation effect on BMSCs in mice with myelosuppression. Hence, it is conceivable that RRPs could regulate the haematopoietic microenvironment to enhance the recovery of haematopoietic function in bone marrow damaged by radio- and chemotherapy.
FasL is secreted by natural killer cells and activated T cells, and induces apoptosis of target cells via the death receptor Fas/Apo1/CD95; both FasL and Fas mediate the immunocytotoxic death of harmful cells such as tumour cells and virus-infected cells (Pitti et al. Citation1998). Studies have shown that Fas/FasL-mediated excessive apoptosis of haematopoietic stem/progenitor cells may be an important mechanism of bone-marrow haematopoietic disorders (Maciejewski et al. Citation1995a, Citation1995b; Brazil and Gupta Citation2002; Chen et al. Citation2015). Our previous experiments involving the streptavidin–biotin complex immunohistochemical method indicated that RRPs can reduce the apoptosis of BMCs by affecting the Fas/FasL-caspase-3 apoptosis signalling pathway in rats with myelosuppression (Li et al. Citation2008). In the present experiment, we noticed that 7 days after the intraperitoneal injection of different doses of RRPs, the protein expressions of Fas and FasL were decreased in BMCs, indicating that under the present modelling conditions, RRPs could inhibit BMC apoptosis by influencing the Fas/FasL apoptotic pathway and thereby promote the restoration of haematogenesis in mice with aplastic anaemia.
Conclusions
The present research found that in mice with aplastic anaemia, RRPs at specific concentrations could promote haematopoietic recovery by inhibiting Fas and FasL, increasing peripheral blood cells, facilitating haematopoietic tissue recovery, and promoting haematopoietic progenitor cell and BMSC proliferation. This study indicated some mechanisms underlying the haematopoietic regulatory effects of RRPs. Our research provides a valuable laboratory basis for deeper research on the clinical application of RRPs.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets generated and analyzed in the present study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Amsterdam JD, Panossian AG. 2016. Rhodiola rosea L. as a putative botanical antidepressant. Phytomedicine. 23(7):1160–783.
- Brazil JJ, Gupta P. 2002. Constitutive expression of the Fas receptor and its ligand in adult human bone marrow: a regulatory feedback loop for the homeostatic control of hematopoiesis. Blood Cells Mol Dis. 29(1):94–103.
- Cai Z, Li W, Wang H, Yan W, Zhou Y, Wang G, Cui J, Wang F. 2012. Antitumor effects of a purified polysaccharide from Rhodiola rosea and its action mechanism. Carbohydr Polym. 90(1):296–300.
- Chem X, Di L, Wu Y, Liu X, Ren Q. 1996. Hypoglycemic effect of Rhodiola sachalinensis A. Bor. polysaccharides: comparison of administration in different ways (Chinese). Zhongguo Zhong Yao Za Zhi. 21(11):685–687.
- Chen Y, Luo X, Zou Z, Liang Y. 2020. The role of reactive oxygen species in tumor treatment and its impact on bone marrow hematopoiesis. Curr Drug Targets. 21(5):477–498.
- Chen Y, Zhu B, Zhang L, Yan S, Li J. 2009. Experimental study of the bone marrow protective effect of a traditional Chinese compound preparation. Phytother Res. 23(6):823–826.
- Chen Y, Zou Z, Wu Z, Zhao Z, Luo X, Xie C, Liang Y. 2015. TNF-α-induced programmed cell death in the pathogenesis of acquired aplastic anemia. Expert Rev Hematol. 8(4):515–526.
- Chen YF, Liu H, Luo XJ, Zhao Z, Zou ZY, Li J, Lin XJ, Liang Y. 2017. The roles of reactive oxygen species (ROS) and autophagy in the survival and death of leukemia cells. Crit Rev Oncol Hematol. 112:21–30.
- Chu VT, Berek C. 2013. The establishment of the plasma cell survival niche in the bone marrow. Immunol Rev. 251(1):177–188.
- Ding WF. 2011. Effect of Rhodiola polysaccharides on glycolipid metabolism induced by streptozotocin in diabetic mice (Chinese). J Guangxi Coll Tradit Chin Med. 14:4–5.
- Gao Y, Liu XZ, Ma CY, Ge ZL. 1994. Effect of Rhodiola on hematopoiesis in mice (Chinese). Chin J Radiol Med Prote. 14:391.
- Hu X, Lin S, Yu D, Qiu S, Zhang X, Mei R. 2010. A preliminary study: the anti-proliferation effect of salidroside on different human cancer cell lines. Cell Biol Toxicol. 26(6):499–507.
- Huang BY, Wei H, Zhou QM. 2012. Effect of Rhodiola rosea polysaccharide on UVA-induced oxidative damage in rats. J Environ Occup Med. 29:31–33.
- Huang XQ, Jiang YZ. 2003. Pharmacological effects of Rhodiola rasea on the hematopoietic system (Chinese). J Sichuan Continuing Educ Coll Med Sci. 22:198–199.
- Lee Y, Jung JC, Jang S, Kim J, Ali Z, Khan IA, Oh S. 2013. Anti-inflammatory and neuroprotective effects of constituents isolated from Rhodiola rosea. Evid Based Complement Alternat Med. 2013:514049.
- Lei YY, Guo XH, Liu L, Zhou F. 2014. Progress in the auxiliary anti-tumor effect of Rhodiola rasea (Chinese). Herald Med. 33:1344–1347.
- Leung SB, Zhang H, Lau CW, Huang Y, Lin Z. 2013. Salidroside improves homocysteine-induced endothelial dysfunction by reducing oxidative stress. Evid Based Complement Alternat Med. 2013:679635.
- Li F, Tang H, Xiao F, Gong J, Peng Y, Meng X. 2011. Protective effect of salidroside from Rhodiolae Radix on diabetes-induced oxidative stress in mice. Molecules. 16(12):9912–9924.
- Li J, Zhu BD, Huang AP. 2008. Rhodiola rosea polysaccharide: its effects on apoptosis related protein expression of rats with myelosuppression. J Shaanxi Coll Tradit Chin Med. 31:52–54.
- Li X, Luo J. 2012. Effect of Rhodiola and Rehmannia on immune function in myelosuppressive mice. Jilin J Tradit Chin Med. 32:499–501.
- Li Y, Pham V, Bui M, Song L, Wu C, Walia A, Uchio E, Smith-Liu F, Zi X. 2017. Rhodiola rosea L.: an herb with anti-stress, anti-aging, and immunostimulating properties for cancer chemoprevention. Curr Pharmacol Rep. 3(6):384–395.
- Liu CF, Wei LL, Wu R, Zhang Q, Ji JW, Tian GH. 2022. Toxicological tests on safety assessment of the polysaccharide from Rhodiola rosea (Chinese). Chin Arch Tradit Chin Med. 40(3):191–196.
- Maciejewski J, Selleri C, Anderson S, Young NS. 1995a. Fas antigen expression on CD34+ human marrow cells is induced by interferon gamma and tumor necrosis factor alpha and potentiates cytokine-mediated hematopoietic suppression in vitro. Blood. 85(11):3183–3190.
- Maciejewski JP, Selleri C, Sato T, Anderson S, Young NS. 1995b. Increased expression of Fas antigen on bone marrow CD34+ cells of patients with aplastic anaemia. Br J Haematol. 91(1):245–252.
- Moura CC, Soares PB, Reis MV, Fernandes Neto AJ, Soares CJ. 2012. Soy milk as a storage medium to preserve human fibroblast cell viability: an in vitro study. Braz Dent J. 23(5):559–563.
- Nan JX, Jiang YZ, Park EJ, Ko G, Kim YC, Sohn DH. 2003. Protective effect of Rhodiola sachalinensis extract on carbon tetrachloride-induced liver injury in rats. J Ethnopharmacol. 84(2–3):143–148.
- Panossian A, Wikman G, Sarris J. 2010. Rosenroot (Rhodiola rosea): traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine. 17(7):481–493.
- Panossian A. 2017. Understanding adaptogenic activity: specificity of the pharmacological action of adaptogens and other phytochemicals. Ann N Y Acad Sci. 1401(1):49–64.
- Petkov VD, Yonkov D, Mosharoff A, Kambourova T, Alova L, Petkov VV, Todorov I. 1986. Effects of alcohol aqueous extract from Rhodiola rosea L. roots on learning and memory. Acta Physiol Pharmacol Bulg. 12(1):3–16.
- Pitti RM, Marsters SA, Lawrence DA, Roy M, Kischkel FC, Dowd P, Huang A, Donahue CJ, Sherwood SW, Baldwin DT, et al. 1998. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature. 396(6712):699–703.
- Qian EW, Ge DT, Kong SK. 2011. Salidroside promotes erythropoiesis and protects erythroblasts against oxidative stress by up-regulating glutathione peroxidase and thioredoxin. J Ethnopharmacol. 133(2):308–314.
- Shi TY, Feng SF, Xing JH, Wu YM, Li XQ, Zhang N, Tian Z, Liu SB, Zhao MG. 2012. Neuroprotective effects of salidroside and its analogue tyrosol galactoside against focal cerebral ischemia in vivo and H2O2-induced neurotoxicity in vitro. Neurotox Res. 21(4):358–367.
- Shikov AN, Kosman VM, Flissyuk EV, Smekhova IE, Elameen A, Pozharitskaya ON. 2020. Natural deep eutectic solvents for the extraction of phenyletanes and phenylpropanoids of Rhodiola rosea L. Molecules. 25(8):1826.
- Song XY, Huang BY, Li XM, Lin Y, Liu DJ, Li L. 2015. Protective effects of Rhodiola rosea polysaccharides on passive smoking induced oxidative injury in rats. J Environ Occup Med. 32:1062–1066.
- Tao H, Wu X, Cao J, Peng Y, Wang A, Pei J, Xiao J, Wang S, Wang Y. 2019. Rhodiola species: a comprehensive review of traditional use, phytochemistry, pharmacology, toxicity, and clinical study. Med Res Rev. 39(5):1779–1850.
- Udintsev SN, Schakhov VP. 1991. Decrease of cyclophosphamide haematotoxicity by Rhodiola rosea root extract in mice with Ehrlich and Lewis transplantable tumors. Eur J Cancer. 27(9):1182.
- Xie H, Zhu DH. 2012. Advance in studies on pharmacological effect of salidroside on nervous system diseases (Chinese). Zhongguo Zhong Yao Za Zhi. 37(17):2505–2509.
- Xu Y, Jiang H, Sun C, Adu-Frimpong M, Deng W, Yu J, Xu X. 2018. Antioxidant and hepatoprotective effects of purified Rhodiola rosea polysaccharides. Int J Biol Macromol. 117:167–178.
- Zhang X, Zhu B, Jin S, Yan S, Chen Z. 2006. Effects of salidroside on bone marrow matrix metalloproteinases of bone marrow depressed anemic mice (Chinese). Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 23:1314–1319.
- Zhang XS, Zhu BD, Hung XQ, Chen YF. 2005. Effect of salidroside on bone marrow cell cycle and expression of apoptosis-related proteins in bone marrow cells of bone marrow depressed anemia mice (Chinese). Sichuan Da Xue Xue Bao Yi Xue Ban. 36:820–823, 846.
- Zhang Y, Yao Y, Wang H, Guo Y, Zhang H, Chen L. 2013. Effects of salidroside on glioma formation and growth inhibition together with improvement of tumor microenvironment. Chin J Cancer Res. 25(5):520–526.