?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Context
Thais savignyi Deshayes (Muricidae) is widely distributed in the Red Sea. Its abundance and the history of Muricidae in traditional medicine make it a tempting target for investigation.
Objective
To investigate the chemical profile and biological activities of T. savignyi tissue extracts.
Materials and methods
Methanol, ethanol, acetone, and ethyl acetate extracts from T. savignyi tissue were compared in their antioxidant by total antioxidant capacity, DPPH free radical scavenging, and total phenolic content. In addition, the antimicrobial, and antibiofilm properties (at 250 µg/mL) of the extracts were tested against Escherichia coli, Pseudomonas aeruginosa, Proteus vulgaris, Klebsiella pneumoniae, Staphylococcus aureus, and Candida albicans. The antioxidant extract with greatest activity was assessed for cytotoxicity (range 0.4–100 µg/mL) against 3 human cancer cell lines (UO-31, A549 and A431), and its chemical composition was investigated using GC-MS. Moreover, docking simulation was performed to predict its constituents’ binding modes/scores to the active sites of thymidylate kinase.
Results
The ethyl acetate extract (Ts-EtOAc) showed the highest total antioxidant capacity (551.33 mg AAE/g dry weight), total phenolics (254.46 mg GAE/g dry weight), and DPPH scavenging (IC50= 24.0 µg/mL). Ts-EtOAc exhibited strong antibacterial (MIC: 3.9 µg/mL against K. pneumoniae), antibiofilm (MIC: 7.81 µg/mL against S. aureus), and antifungal (MIC: 3.9 µg/mL against C. albicans) activities and considerable cytotoxicity against cancer cells (UO-31: IC50= 19.96 ± 0.93, A549: IC50= 25.04 ± 1.15 μg/mL). GC-MS identified multiple bioactive metabolites in Ts-EtOAc extract belonging to miscellaneous chemical classes. Molecular docking studies revealed that the constituents of Ts-EtOAc have antibacterial potential.
Discussion and conclusions
T. savignyi extract has considerable antimicrobial and cytotoxic activities. Further studies are needed to isolate the active constituents of this snail for comprehensive drug discovery tests.
Introduction
New efficient drugs and bioactive chemicals for treating human disorders such as cancer, inflammation, and microbial infection are sorely needed (Newman and Cragg Citation2020; Atanasov et al. Citation2021). The marine ecosystem is characterised by the high biodiversity of mollusc species, with more than 50,000 identified species (Benkendorff Citation2010), making it an excellent source for discovering novel bioactive compounds and pharmaceuticals (de Vries and Beart Citation1995).
Molluscs are notable for surviving in a challenging marine environment teeming with harmful viruses and bacteria. At the same time, they lack acquired immunity and rely only on innate immunity with alternate strategies for defending themselves against predators and successfully healing wounds in the presence of various microorganisms (Bachere et al. Citation1995; Derby et al. Citation2007). This protection is mediated by the production of secondary metabolites, which are also used in communication and predatory behaviours by molluscs (Cimino and Sodano Citation1993; Craig Citation2000; Kanda et al. Citation2003; Cummins et al. Citation2006; Hooper et al. Citation2007). Indeed, many secondary metabolites have been isolated from various marine mollusc species (Benkendorff Citation2010). Although not all molluscan natural products were pharmacologically tested, some have demonstrated considerable biological activity, including antibacterial and cytotoxic activities (Odeleye et al. Citation2019).
Screening for cytotoxic activities has led to several molluscan products being investigated as anticancer treatments, while some molluscan natural products have shown promising anti-coagulant, immune-modulating, antioxidant, anti-inflammatory, and antihypertensive properties (Jimeno et al. Citation2004; Senthilkumar and Kim Citation2013; Ahmad et al. Citation2018). Thousands of bioactive compounds have been isolated from marine molluscs. These include peptides, sterols, terpenes, polypropinate, nitrogenous compounds, macrolides, fatty acid derivatives, alkaloids, and other miscellaneous compounds (Blunt et al. Citation2006; Chakraborty and Joy Citation2020). Some of these have been the subject of clinical trials, such as dolastatin 10 from the opisthobranch Dolabella Auricularia Lightfoot (Aplysiidae), aplyronine A from the sea hare Aplysia kurodai Baba (Aplysiidae), aplysistatin from A. angasi G.B. Sowerby II (Aplysiidae), and kahalalide F from the sacoglossan Elysia rufescens Pease (Plakobranchidae) (Proksch et al. Citation2002). Two well-known natural molluscan products have been used in the development of novel drugs: ziconotide, isolated from the venom of the cone snail Conus magus L. (Conidae), which is used as an analgesic to treat chronic pains (Schroeder et al. Citation2004; Mayer et al. Citation2010), and brentuximab vedotin, derived from dolastatin 10 from D. auricularia which is used for the treatment of Hodgkin lymphoma and systemic anaplastic large cell lymphoma (Mayer et al. Citation2010). Both drugs are approved by the US Food and Drug Administration (FDA) (Cheung et al. Citation2015).
Thais savignyi Deshayes (Muricidae) is a carnivorous gastropod snail usually found attached to rocks. Muricid gastropods have a history of use in traditional medicine, and this snail has been the subject of numerous investigations related to its distribution, identification, and molecular phylogeny (Hayashi Citation2005; Li et al. Citation2010; Benkendorff et al. Citation2015). T. savignyi is distributed along the intertidal zones of the Red Sea (Abu El-Einin et al. 2021). The present study investigates the antioxidant, antimicrobial, and cytotoxic activities of crude tissue extract from T. savignyi and its chemical composition. In addition, in silico docking studies were performed to identify the possible binding interactions of the identified compounds in T. savignyi extract with thymidylate kinase, a key enzyme in bacterial DNA biosynthesis, bacterial survival, and one of the attractive therapeutic targets for the development of new antibacterial agents (Choi et al. Citation2012; Keating et al. Citation2012).
Materials and methods
Samples collection
Thais savignyi was collected from the Ain El-Sokhna region on the Red Sea, Egypt, during October 2020. Details of sample collection and identification are described in Abu El-Einin et al. (Citation2021). Snails were cleaned using distilled water, the shells were broken, and the soft parts were dissected. The freshly collected tissues were divided into four 50 g portions. For organic extraction, each portion was blended and soaked separately in a volume of 500 mL each of methanol (Ts-MeOH), ethanol (Ts-EtOH), acetone (Ts-Acetone), and ethyl acetate (Ts-EtOAc), and kept in conical flasks for 3 days in a dark place. The solutions were filtered through Whatman filter paper (No. 1) and concentrated using a rotary evaporator (Buchi, Switzerland). The obtained extracts were completely air-dried, to remove any solvent residues, and stored at 4 °C until used in the analysis.
DPPH free radical scavenging activity
The free radical scavenging activity of the four snail extracts was determined based on the scavenging activity of 1,1-diphenyl-2-picrylhydrazyl (DPPH) (Sigma-Aldrich, Germany) according to Brand-Williams et al. (Citation1995) and Ullah et al. (Citation2018). Various concentrations from each extract (50–500 µg/mL) were prepared in methanol and 0.1 mL from each concentration was added to 3.9 mL of 6 10−5 mol/L DPPH in methanol. The mixture was shaken vigorously. A blank sample containing the same volume of methanol and DPPH was prepared to serve as the control. Ascorbic acid (Merck, Germany) was used as a standard. After 30 min of incubation in the dark, the absorbance of the control and test samples was measured at 517 nm. The experiment was carried out in triplicate. The radical scavenging activity was expressed as IC50 values, which indicate the concentration of sample required to scavenge 50% DPPH free radicals (Braca et al. Citation2003; Gülçın et al. Citation2007). IC50 was calculated according to the following equation:
(1)
(1)
Total antioxidant capacity (TAC) by phosphomolybdenum method
The total antioxidant capacity of each extract was evaluated according to the phosphomolybdenum method using ascorbic acid as a standard (Prieto et al. Citation1999). Briefly, 0.5 mL of each sample extract and ascorbic acid (200 µg/mL) in methanol were combined in dry tubes with 5 mL of molybdate reagent solution (1 mL of 0.6 M sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate were added to 20 mL of distilled water and the mixture was made up to 50 mL using distilled water). The reaction mixture tubes were then firmly capped and incubated in a thermal block at 95 °C for 90 min. Following incubation, samples were left to cool at room temperature for 20–30 min, and the absorbance was measured at 695 nm using a spectrophotometer against a blank sample containing all reagents, solvents and methanol (0.5 mL) instead of sample extract. All experiments were carried out in triplicate. The total antioxidant activity was expressed as the number of ascorbic acid (AAE) gram equivalents.
Total phenolic content (TPC)
The total phenolic content of each extract was estimated using the Folin-Ciocalteu’s reagent according to Singleton and Rossi (Citation1965). A volume of 0.1 mL from each extract in methanol (200 µg/mL) was mixed with 0.5 mL of the Folin-Ciocalteu’s reagent and 1.5 mL of sodium carbonate (20 g/100 mL water). A final volume of 10 mL was achieved by adding distilled water. Following incubation for 2 h at room temperature, the absorbance of the mixture was measured at 765 nm using a spectrophotometer (UVmini-1240, Shimadzu Corp., Kyoto, Japan). Gallic acid was used as a standard for the calibration. All tests were performed in triplicate. The total phenolic content was expressed as mg of gallic acid equivalent (GAE) per g of extract (Alhakmani et al. Citation2013; Ghareeb et al. Citation2014).
Antibacterial and antifungal activity
Antimicrobial activity for each extract of T. savignyi was assessed against six human pathogenic bacterial strains (four Gram-negative bacteria; Escherichia coli ATCC 25955, Pseudomonas aeruginosa ATCC 10145, Proteus vulgaris, and Klebsiella pneumoniae, and one Gram-positive bacteria; Staphylococcus aureus NRRL B-767) and one pathogenic fungal strain (the yeast Candida albicans ATCC 10231) according to the National Committee for Clinical Laboratory Standards (Wayne 2002). The minimum inhibitory concentration (MIC) was determined and Ciprofloxacin (control drug) was used for comparison as described by Hamed et al. (Citation2020). Each strain of bacteria and fungi was cultured overnight in lysogeny broth (LB) medium at 30 °C. The culture was then adjusted to an optical density (OD) of 0.6 at a wavelength of 600 nm. Sample extracts were dissolved in methanol and 10 µL of test extracts (250 µg/mL) were added to a sterile 96-well flat polystyrene plate containing 80 µL of LB. Finally, 10 µL of bacterial culture suspension (log phase), were added to obtain a final volume of 100 µL/well. Wells containing only LB medium with inoculum served as a blank. Microplates were incubated overnight at 37 °C. After incubation, 20 μL of 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) at a concentration of 1 mg/mL was prepared in water, filtered and added to each well, and the plates were stirred for 20 min in the dark. The viable bacteria were detected by the change of the yellow MTT colour to purple. The absorbance was measured using a Spectrostar Nano Microplate Reader (BMG LABTECH GmbH, Allmendgrun, Germany).
Biofilm inhibition assay
The biofilm inhibitory activity of sample extracts against four clinical microbes compromising S. aureus, Bacillus subtilis, P. aeruginosa, and E. coli was measured using a sterile 96-well polystyrene (flat bottom) microtitre tissue culture plate as described by Kalishwaralal et al. (Citation2010) with modification by Balasamy et al. (Citation2019). Briefly, bacterial strains were cultured overnight in LB broth medium, and 10 µL of this culture was added to 180 µL of LB broth, followed by 10 µL (final concentration of 250 g/mL) of samples and control (without test sample). The plates were incubated at 37 °C for 24 h. Following incubation, the content of each well was removed and washed twice with 200 μL phosphate buffer saline (PBS, pH 7.2) to remove any floating bacteria, and the wells were left to dry. After fixation with 2% sodium acetate, the test samples were stained with 200 μL crystal violet solution (0.1% w/v) for 1 h. The excess dyes were removed with sterile water, and the wells were washed with PBS and left to dry. Further, the stain was solubilised in 95% ethanol and biofilm formation was quantified by measuring the absorbance at OD at 570 nm wavelength using a Spectrostar Nano Microplate Reader (BMG LABTECH GmbH, Allmendgrun, Germany). The percent of biofilm inhibition was calculated using the following equation:
(2)
(2)
Cytotoxicity assay
Cell lines for human kidney renal cell carcinoma UO-31, adenocarcinomic human alveolar basal epithelial cells A549 and human epidermoid carcinoma A431 were obtained from ATCC via the holding company for biological products and vaccines (VACSERA), Cairo, Egypt. Staurosporine was used as a positive control for comparison. The cell lines were freshly cultivated as monolayers in RPMI-1640 medium (Sigma Co., St. Louis, MO, USA), supplemented with 10% heat-inactivated fetal bovine serum (GIBCO, UK), 1% glutamine, 100 units/mL penicillin and 100 µg/mL streptomycin, and incubated at 37 °C in a 5% CO2 incubator. The cytotoxicity of the tested extract was evaluated using a colorimetric MTT assay. Cancer cell lines (1 104 cells/mL) were seeded in 96 flat-bottom well plates for screening. Different concentrations of Ts-EtOAc extract (0.4–100 µg/mL) were added to the cultures (in triplicates) and incubated for 24 h in 5% CO2 incubator. After 24 h, the cells were washed with PBS, mixed with 20 µL of MTT (Sigma Co., St. Louis, MO, USA) at a 5 mg/mL concentration, and subsequently incubated for 4 h at 37 °C in a 5% CO2 incubator. The purple formazan crystals formed were washed with 100 µL of DMSO, and each well’s optical density was observed. The colorimetric assay is measured and recorded at an absorbance of 570 nm using a plate reader (EXL 800, USA). The relative cell viability expressed as a percentage was calculated as follows:
(3)
(3)
Gas chromatography-mass spectrometry (GC-MS) analysis
GC-MS investigation of Ts-EtOAc extract was performed utilising a Thermo Scientific, Trace GC Ultra/ISQ interfaced with a Single Quadrupole MS, TG-5MS fused silica capillary column (30 m, 0.251 mm, 0.1 mm film thickness) as follows: an electron ionisation system was run in electron impact mode with ionisation energy of 70 eV. Helium gas (99.999%) was used as the mobile phase at a constant flow rate of 1 mL/min, and an injection volume of 2 μL was utilised (a split ratio of 10:1). The injector temperature and the ion-source temperature were maintained at 250 °C. In the GC part, the oven temperature was set up at 110 °C (isothermal for 2 min), with an increase of 10 °C/min until reaching 200 °C, followed by a 5 °C/min increase to score 280 °C, at which the oven temperature was kept isothermal for 9 min. Mass spectra were taken at 70 eV; a scan interval of 0.5 s and fragments from 45 to 450 Da. The solvent delay was 0–2 min, and the total GC-MS running time was 36 min. The relative percentage amount of each component was calculated by comparing its average peak area to the total area. Processing of data was carried out using GC-MS solution software. The compounds were identified by comparing their mass spectra to Wiley and the National Institute of Standards and Technology (NIST) Libraries (Adams Citation2007).
Molecular docking against thymidylate kinase (TMK)
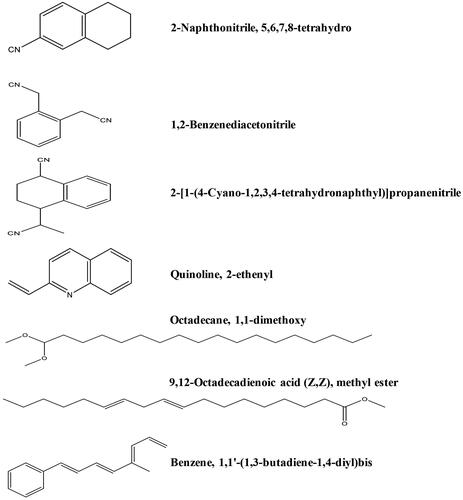
The major identified compounds, 2-naphthonitrile, 5,6,7,8-tetrahydro (1), 2-benzenediacetonitrile (2), quinoline, 2-ethenyl (3), 2-[1-(4-cyano-1,2,3,4-tetrahydronaphthyl)] propanenitrile (4), octadecane, 1,1-dimethoxy (5), 9,12-octadecadienoic acid (Z,Z), methyl ester (6), and benzene, 1,1′-(1,3-butadiene-1,4-diyl)bis (7), from the Ts-EtOAc GC-MS analysis, were docked against the active sites of the thymidylate kinase enzyme (TMK) from S. aureus using the Molecular Operating Environment (MOE; Chemical Computing Group ULC, Montreal, QC, Canada). The co-crystal structure of TMK was obtained from the Protein Data Bank (PDB ID 4QGG; www.rcsb.org/pdb). For validation, redocking of the co-crystallized ligand was first carried out, displaying a docking score value of −9.632 kcal/mol with a Root Mean Square Deviation (RMSD) of 1.7 Å. The chemical structures of identified compounds were built and saved in their 2 D and 3 D conformations by the Builder tool incorporated into MOE 2014.0901. Then, the energy of the docked structures was minimised using the MMF94FX force field with a gradient Root Mean Square (RMS) of 0.0001 kcal/mol. A docking simulation of examined compounds in the active binding site of TMK was then performed. Water molecules were deleted during the docking process, and the missing hydrogen atoms were retained to correct ionisation states to be assigned to the protein structure. The ‘Docking’ module in MOE was run to achieve molecular docking. The docking process was applied with the default settings. The top 30 poses, as ranked by London dG, were saved, and then GBVI/WSA dG (Generalized-Born Volume Integral/Weighted Surface Area) scoring function was applied to score the resultant poses. The ‘Ligand Interactions’ MOE tool for analysis of docking results was utilised by visualisation of the protein-ligand interactions inside the active site (Barakat et al. Citation2018).
Statistical analysis
The data are presented as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) test was used for statistical analyses in SPSS v.20.0 (Statistical Program for Social Sciences software; SPSS Inc. USA). The significance of the data was determined using Duncan’s multiple range test. p Values less than 0.05 were considered statistically significant.
Results
Total antioxidant capacity, total phenolics and free radical scavenging activity
Screening of different solvent extracts of T. savignyi for their antioxidant potential showed that ethyl acetate extract (Ts-EtOAc) was the highest in total antioxidant capacity, followed by acetone extract, with 551.33 and 532.0 mg AAE/g dry weight of extract, respectively. However, the methanol extract was the lowest in total antioxidant capacity compared to the reference antioxidant (ascorbic acid), acetone, ethanol, and Ts-EtOAc extracts. The same order of antioxidant powerfulness was also observed when comparing the total phenolic contents (TPC) of each extract. For example, Ts-EtOAc gave the highest yield of total phenolics with a TPC value of 254.46 mg GAE/g dry weight of extract, and methanol extract had the lowest TPC (150.28 mg GAE/g). Moreover, the free radical scavenging activity, measured by the DPPH assay, of Ts-EtOAc extract was the highest, with a 50% inhibition (IC50) at 24.0 µg/mL concentration. Overall, Ts-EtOAc extract has the most potent antioxidant activity among all the extracts tested ().
Table 1. Total antioxidant capacity (TAC) and total phenolic content (TPC), and free radical scavenging activity (DPPH) values of different solvent extracts of Thais savignyi snails.
Antimicrobial activity
The in vitro antimicrobial activity of the crude solvent extracts of T. savignyi was tested against several human pathogenic microorganisms, including five Gram-negative bacteria, one Gram-positive bacterium, and one pathogenic fungal strain. The results showed that, among the tested extracts, only Ts-EtOAc exhibited broad antimicrobial activity with strong antibacterial activity against K. pneumoniae with antibacterial activity up to 87.65% inhibition, and moderate antibacterial activity towards each of P. vulgaris, P. aeruginosa, and S. aureus with inhibition activities of 64.94, 59.44, and 67.19%, respectively. Concerning the extract activity against the fungal strain tested, it showed a pronounced antifungal activity against C. albicans with an inhibition percentage of 84.02%. The other tested extracts showed low or no activity against the tested microbes (). Additionally, the MIC of the Ts-EtOAc extract was also evaluated and presented in . Ts-EtOAc demonstrated the highest activity against K. pneumoniae and C. albicans with MIC of 3.90 μg/mL.
Table 2. Antibacterial inhibition (%) of Thais savignyi snail extracts against various bacteria and fungi.
Table 3. Minimum inhibitory concentration (MIC) of Ts-EtOAc extract and a standard drug against various bacterial pathogens.
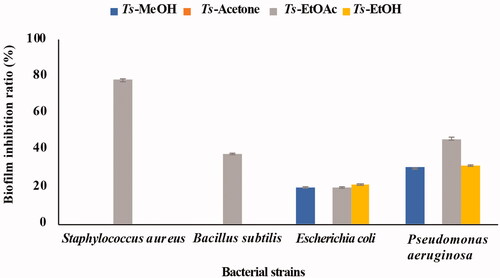
The ability of the T. savignyi organic extracts to eradicate bacterial biofilm formation by four pathogenic bacteria (S. aureus, P. aeruginosa, B. subtilis, and E. coli) was measured using the MTT assay. Ts-EtOAc inhibited biofilm formation by S. aureus with an inhibition ratio close to 80% and showed moderate biofilm inhibition against B. subtilis and P. aeruginosa. However, it showed low biofilm inhibition activity towards E. coli. Ts-EtOH extract showed only low biofilm inhibition activity against E. coli and P. aeruginosa ().
Cytotoxicity
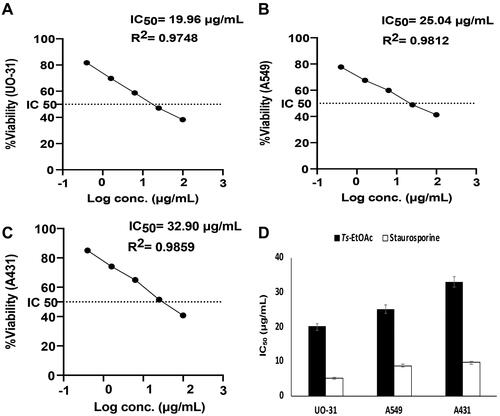
The in vitro cytotoxic effects of Ts-EtOAc extract against human kidney renal cell carcinoma UO-31, adenocarcinomic human alveolar basal epithelial cells A549 and human epidermoid carcinoma cells A431 were examined using the MTT assay and compared to control. The Ts-EtOAc extract showed variable toxicity towards UO-31, A549, and A431 cell lines. A dose dependent decrease in cell viability was observed (). The highest observed effect was against UO-31 with an IC50 value of 19.96 ± 0.93 μg/mL compared to 5.165 ± 0.24 μg/mL for the control drug (). The lowest effect was observed against the A431 cell line. shows the IC50 values for Ts-EtOAc extract vs. staurosporine as a positive control against cancer cell lines investigated. Overall, the extract showed moderate toxicity against all cell lines examined ().
Figure 2. Cytotoxicity of Ts-EtOAc extract against different human cancer cell lines after 24 h of treatment. (A) UO-31: Human kidney renal cell carcinoma; (B) A549: Adenocarcinomic human alveolar basal epithelial cells; (C) A431: Human epidermoid carcinoma cells (X-axis: Log concentrations of the extract from 0.4 to 100 μg/mL and Y-axis: the percentage of cell viability). (D): The comparison of average IC50 of the extract vs. staurosporine as a positive control. Data represent the mean ± SD of three independent experiments.
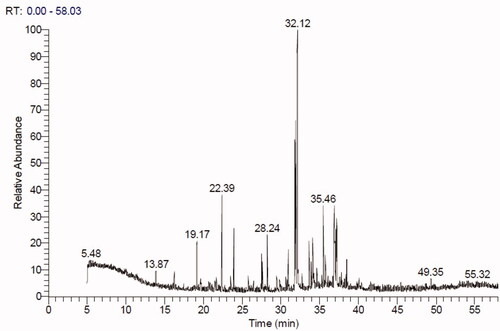
GC-MS investigation of Ts-EtOAc extract
The GC-MS chromatogram of ethyl acetate extract of T. savignyi (Ts-EtOAc) is shown in . GC-MS analysis of Ts-EtOAc extract identified 45 compounds. The total peak areas of the identified ingredients constitute 95.82% of the total extract composition. The proposed chemical structures of the identified compounds are recorded in . The chemical composition of Ts-EtOAc extract is characterised by a high representation of 2-naphthonitrile, 5,6,7,8-tetrahydro with 17.19% followed by 1,2-benzenediacetonitrile (8.67%) and 2-[1-(4-cyano-1,2,3,4-tetrahydronaphthyl)] propanenitrile (8.38%). Other major compounds identified include quinoline, 2-ethenyl (6.39%), (1a′,4a′,4aa′,10aa′)-1,4,4a,5,6,7,8,9,10,10a-decahydro-1,4,11,11-tetramethyl-1,4 methanocycloocta[d]pyridazine (4.0%), octadecane, 1,1-dimethoxy (3.79%), 9,12-octadecadienoic acid (Z,Z), methyl ester (3.60%), and benzene, 1,1′-(1,3-butadiene-1,4-diyl)bis (2.79%). The aforementioned compounds collectively represented 54.81% of the overall peak areas ().
Table 4. Chemical composition of Ts-EtOAc extract.
Molecular docking analysis
Molecular docking results of spectrometrically identified compounds (1-7) inside the active site of thymidylate kinase (PDB: 4QGG) are represented in and . The anticipated active site residues of the target protein were Arg36, Arg48, Arg70, Arg92, Gln37, and Gln101 amino acids. Redocking of the co-crystallized ligand yielded a docking score value of −9.632 kcal/mol with a RMSD of 1.7 Å. Four hydrogen bonds were implicated in the interaction of the ligand with TMK residues; one bond with each of Arg36 and Arg70 and two bonds with the crucial residue Gln101 (). Compounds under examination demonstrated binding energies scores ranging from −3.004 to −9.117 kcal/mol. Compounds 1 (), 2 (), 4 (), 5 (), 6 () demonstrated good binding interactions with the most critical residues in the active site, as predicted by the docking scores and their binding poses. Compound 4 (2-[1-(4-cyano-1,2,3,4-tetrahydronaphthyl) propanenitrile) with the best binding energy score, recording −9.117 kcal/mol, demonstrated strong binding interaction inside the active binding region via the creation of four hydrogen binds with Arg36, Arg48, Arg92, and Gln37 ().
Figure 5. The two-dimensional (A) and three-dimensional (B) suggested binding modes of redocked ligand within the binding pocket of TMK (PDB: 4QGG).
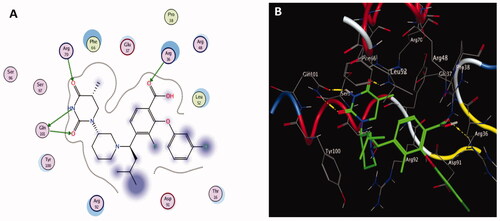
Figure 6. The two-dimensional (left panel) and three-dimensional (right panel) suggested binding modes of compounds 1 (A), 2 (B) and 3 (C) within the binding pocket of TMK (PDB: 4QGG).
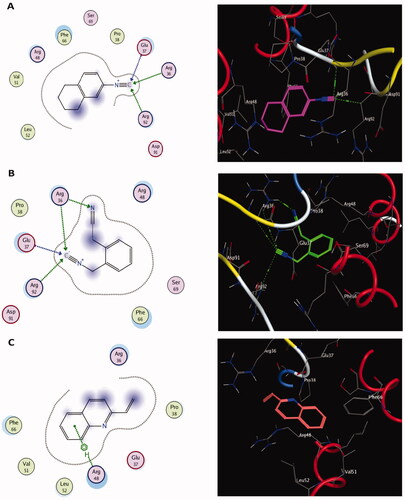
Figure 7. The two-dimensional (A) and three-dimensional (B) suggested binding modes of compound 4 within the binding pocket of TMK (PDB: 4QGG).
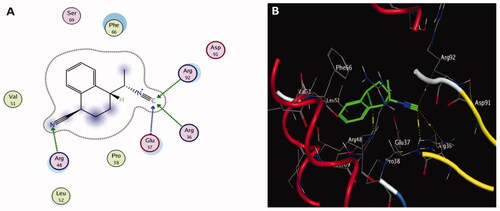
Figure 8. The two-dimensional (left panel) and three-dimensional (right panel) suggested binding modes of compounds 5 (D), 6 (E) and 7 (F) within the binding pocket of TMK (PDB: 4QGG).
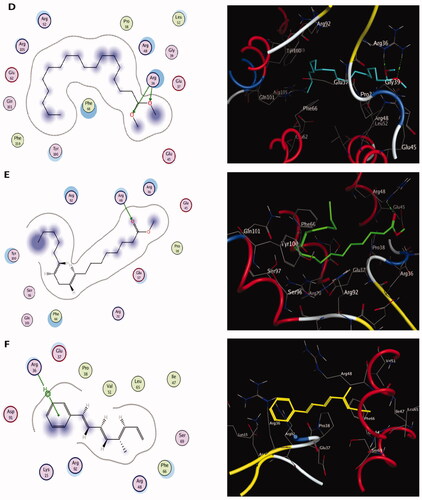
Table 5. The docking scores (kcal/mol) and binding interactions of compounds (1–7) from Ts-EtOAc extract within the active binding sites of TMK (PDB: 4QGG).
Discussion
Marine organisms are rich in structurally diverse bioactive materials with drug development potential. Bioactive substances isolated from marine fauna have antiviral, antimicrobial, antiprotozoal, antifungal, anthelmintic, and anticancer activities (Pangestuti and Kim Citation2017). Secondary metabolites derived from marine molluscs have a wide range of pharmaceutical applications (Benkendorff Citation2010; Odeleye et al. Citation2019). In the present investigation, the gastropod snail, Thais savignyi, collected from the Red Sea, was extracted with different organic solvents and its antioxidant, antimicrobial, and cytotoxic activities were evaluated.
Antioxidant activity of Ts-EtOAc extract
Reactive oxygen species (ROS) are implicated in many disorders and ailments (Brieger et al. Citation2012). Maintaining the homeostasis of ROS production and antioxidants within the cells is crucial for disease prevention. Complementary antioxidants (dietary components or medications) are recommended to overcome the antioxidant deficiency. In this regard, medicinal plants and marine organisms have been extensively studied for their antioxidant power (Xu et al. Citation2017; Chakraborty and Joy Citation2020). In the present investigation, the tissue extracts of T. savignyi were compared for their antioxidant capacity. The results showed that Ts-EtOAc was the best extract in total antioxidant capacity and DPPH free radical scavenging activities. Although EtOAc is a semi-polar solvent, it yielded the highest phenolic content among the other extracts tested, including methanol. This may be due to the specific nature of T. savignyi tissues. The IC50 for Ts-EtOAc was 24.0 µg/mL. The smaller the IC50 value (concentration required to inhibit 50% of DPPH free radicals), the higher the free radical scavenging activity of the extract (Molyneux Citation2004). Other mollusc extracts showed lower antioxidant activities compared to Ts-EtOAc. For example, the IC50 value for the methanol extract of the marine snail, Pleuroploca trapezium L. (Fasciolariidae), was 4021 μg/mL (Prem Anand et al. Citation2010). Moreover, crude peptide extract of the Patella rustica L. (Patellidae) snail showed a scavenging ability of 79.77% at 0.39 mg/mL against the DPPH radical (Borquaye et al. Citation2015). The antioxidant activities (expressed as IC50) of the methanolic extract of two marine bivalves, Perna viridis L. (Mytilidae) and Meretrix meretrix L. (Veneridae), were 247.85 µg/mL and 84.46 µg/mL, respectively (Krishnamoorthy et al. Citation2019; Minsas et al. Citation2020).
Many bioactive secondary metabolites bearing phenolic moieties have been identified in marine molluscs (Salas and Chakraborty Citation2020). Phenolic compounds possess potent antioxidant and radical scavenging characteristics and can pre-empt the progress of many diseases (Dias et al. Citation2021). Therefore, the antioxidant potency of Ts-EtOAc compared to other solvent extracts investigated may be due to its high content of phenolics (254.46 mg/g). Similarly, ethyl acetate-methanol extracts of the muricid gastropod Chicoreus ramosus L. and Babylonia spirata L. (Babyloniidae) had higher phenolic content than chloroform extract of the same species. Moreover, the high phenolic content of ethyl acetate-methanol was associated with antioxidant preferences in scavenging DPPH radicals (Salas and Chakraborty Citation2020).
Antimicrobial activity
The emergence and propagation of antimicrobial resistance are today’s most significant health concerns. The marine environment is a rich source of new compounds critical for the treatment of drug-resistant bacterial diseases (Liu et al. Citation2019). Molluscs are largely benthic organisms that live clinging to surfaces, making them vulnerable to microbial and viral invasions. Due to the absence of an acquired immune system, these animals must have developed alternate defense mechanisms against the assault of microbial invasions. Molluscs may have evolved a variety of antibacterial, antifungal, antiparasitic, and antiviral secondary metabolites for circulation in the haemolymph and inclusion in mucus secretions on the body surface (Benkendorff Citation2010). As a result, molluscs are a rich source of antibacterial chemicals (Benkendorff et al. Citation2004). The results of antibacterial screenings of different extracts of T. savignyi indicated that Ts-EtOAc was the most powerful against all tested microbes, with MIC values ranging from 15.62 μg/mL in the case of P. aeruginosa up to 3.90 μg/mL against K. pneumoniae. Methanol, ethanol, and acetone extracts were of either low or negative antibacterial activity against the tested pathogens. Kumaran et al. (Citation2011) discovered that the ethyl acetate extract of Thais tissoti Petit de la Saussaye (Muricidae) was more effective against K. pneumoniae and S. aureus than the methanol extract. Similarly, ethyl acetate of the marine snail, B. spirata, was more effective than methanol or acetone extracts against E. coli, K. pneumoniae, and S. aureus (Kumaran et al. Citation2011). Likewise, Prem Anand and Edward (Citation2002) found that the ethyl aetate extract of the marine gastropod species Cypraea errones L. (Cypraeidae) had antimicrobial activity against P. aeruginosa and S. aureus compared to a heptane extract that had no activity against the same microbes.
The present results also showed that Ts-EtOAc was active against the fungus C. albicans with MIC value of 3.90 μg/mL. Crude peptide extract of P. rustica was also effective in inhibiting the growth of C. albicans at concentrations comparable to the positive control (37 mm for P. rustica against 40 for the standard drug ciprofloxacin) (Borquaye et al. Citation2015).
The advantage of EtOAc extract over methanol extract could be attributed to the former’s lower polarity, which allows for the presence of fatty acids and lipids in the extract due to their lipophilic nature. According to Karthikeyan et al. (Citation2014), ethyl acetate extract of the oyster Saccostrea glomerata Gould (Ostreidae) is a high source of fatty acids, which could explain its antibacterial potential. However, microbial responses to diverse solvent extracts are mediated by a unique interaction with specific chemicals in the studied extract, which could explain why one solvent extract can inhibit some microorganisms while others cannot. Because the types and amounts of bioactive chemicals differ from one species to another, variances in antibacterial potentials between mollusc species extracted with comparable or variable solvents are expected. Unlike the present finding that EtOAc is the best solvent for the extraction of T. savignyi, producing significant antibiotic activity, the antibacterial activities of ethanol extracts of other species, such as the gastropods B. spirata and Turbo brunneus Röding (Turbinidae), were the strongest against E. coli, K. pneumoniae, P. vulgaris, and Salmonella typhi (Prem Anand et al. Citation1997). Furthermore, Cypraea errones crude methanol extract had stronger antibacterial and antifungal activity (Prem Anand and Edward Citation2002).
The majority of bacterial organisms can form biofilms on different surfaces in food production facilities and medical settings, leading to a persistent source of infections (Dongari-Bagtzoglou Citation2008; Percival et al. Citation2015; Galie et al. Citation2018; Flemming and Wuertz Citation2019). The communities of bacteria in the biofilm are held together by different biomolecules such as polysaccharides, secreted proteins, and extracellular DNA (Muhammad et al. Citation2020). Mollusc species that interact or live on surfaces manifested with biofilms have evolved means by which they prevent fouling of their shells by bacteria (Wahl et al. Citation2012). In the present investigation, the antibiofilm experiments showed that Ts-EtOAc has considerable biofilm inhibition activities against S. aureus, P. aeruginosa, B. subtilis, and E. coli. The highest biofilm inhibitory ratio was recorded against S. aureus (80%). Natural products from other marine organisms showed a considerable antibiofilm activities. For example, OctoPartenopin, a peptide extracted from the suckers of Octopus vulgaris, was used to synthesise four analogues that have proven functional in inhibiting the antibiofilm formation by S. aureus, P. aeruginosa, and C. albicans (Maselli et al. Citation2020). Moreover, the methanol/water tissue extracts of Nerita albicilla L. (Neritidae) and N. oryzarum Récluz (Neritidae) inhibited 93% and 95% of 40 different marine biofilm bacteria, respectively (Ramasamy and Murugan Citation2005).
Cytotoxicity
Cancer is one of the most common causes of death worldwide (Bray et al. Citation2018). According to the International Agency for Research on Cancer (IARC), over 20 million new cancer cases are expected to be diagnosed in developing countries by 2025, most of them in low-and middle-income countries (Shah et al. Citation2019). Therefore, the search for new pharmaceuticals with distinct mechanisms of action against cancer is an urgent prerequisite. Chemical investigation of marine molluscs has resulted in isolating a broad spectrum of bioactive metabolites with anticancer properties (Ciavatta et al. Citation2017). Here we investigated the in vitro cytotoxic activity of Ts-EtOAc extract against three cancer cell lines: human kidney renal cell carcinoma UO-31, adenocarcinomic human alveolar basal epithelial cells A549, and human epidermoid carcinoma cells A431 representing kidney, lung, and skin cancers, respectively. These cancer types have a tremendous economic impact and a high mortality rate worldwide (Ljungberg et al. Citation2011; Siegel et al. Citation2018; Iqbal et al. Citation2019; Sung et al. Citation2021). The Ts-EtOAc extract was particularly chosen for cytotoxic investigation because it was the highest antioxidant extract among the T. savignyi extracts tested. Cancer initiation, progression, and resistance to therapy are all influenced by oxidative stress (Snezhkina et al. Citation2019). Natural antioxidants such as Ts-EtOAc may help inhibit tumour initiation by protecting DNA from oxidation and the resulting DNA damage caused by ROS, causing premalignant lesions to retreat and prevent them from progressing to cancer (Sithranga Boopathy and Kathiresan Citation2010). Indeed, Ts-EtOAc showed moderate in vitro toxicity towards UO-31, A549, and A431 cell lines. The best-observed effect was against UO-31 with an IC50 value of 19.96 ± 0.93 μg/mL compared to 5.165 ± 0.24 μg/mL for the control drug. However, the lowest effect was observed against the A431 cell line. These data suggest that T. savignyi can be used as an anticancer nutraceutical and, with proper modifications of extraction and purification, may lead to many anticancer drugs.
Other mollusc-derived compounds showed cytotoxic activities against different cancer cell lines. The isoquinoline alkaloid, jorumycin, from the nudibranch slug, Jorunna funebris Kelaart (Discodorididae), showed potent anticancer activity against lung adenocarcinoma cell line A549 with an IC50 = 12.5 ng/mL (Fontana et al. Citation2000). This compound also exhibited considerable cytotoxicity against other cell lines, including murine leukaemia cell line P388, human colon cancer cell line HT29, and human melanoma cell line MEL28 (Fontana et al. Citation2000; Saito et al. Citation2004). Zalypsis is another DNA-binding alkaloid isolated from J. funebris that shows potent antitumor activities against prostate, renal, breast, and colon cancers (Scott and Williams Citation2002; Chakraborty and Joy Citation2020). Kahalalide F is a cyclic depsipeptide isolated from the marine mollusc E. rufescens (Hamann and Scheuer Citation1993; Hamann et al. Citation1996). Kahalalide F was effective against several cell lines with solid multidrug resistance, such as human breast cancer cell lines H5578T and Hs-578T, human lung adenocarcinoma cell line A549 (IC50 = 0.135 μM) and other cell lines resistant to topoisomerase II inhibitors (Faircloth and Cuevas Citation2006). Serova et al. (Citation2013) showed that elisidepsin (a synthetic derivative from kahalalide F) was effective against various human breast cancer cell lines, human colon cancer cell lines, human head and neck cancer cell lines, and human hepatocarcinoma (Serova et al. Citation2013). The depsipeptide kulokekahilide-2 from the sea slug, Philinopsis speciosa Pease (Aglajidae), was also efficient against murine leukaemia cell lines P338, human ovarian cancer cell line SK-OV-3, and human melanoma cell line MDA-MB-435 (Nakao et al. Citation2004).
GC-MS analysis
The previous results of Ts-EtOAc extract bioactivity as an antioxidant, antimicrobial, and cytotoxic agent prompted additional chemical analysis to identify the chemical compound classes present in this extract. GC-MS analysis of Ts-EtOAc extract identified 45 compounds belonging to various chemical groups, including naphthalene, quinoline, alkane, alkyne, pyridazine, phenanthrene, cyclic ketones, cycloalkene, and aldehyde derivatives, ketones, monoterpenes, nitriles, fatty acid esters, stilbenoids, and sesquiterpenoids. Naphthalene derivatives were the most prevalent category, accounting for 25.57% of the total peak areas in the extract (95.82%). This is the first report of naphthalene derivatives in a marine gastropod. These metabolites could be produced due to the absorption of aromatic hydrocarbons by T. savignyi. The antioxidant, antimicrobial, and cytotoxic activities of Ts-EtOAc extract may be attributed to identified active metabolites. Previous literature data, for example, demonstrated that naphthalene is a cytotoxic moiety with a range of biological applications, including anticancer, antimicrobial, anti-inflammatory, and antiviral properties (Makar et al. Citation2019). Plant-derived naphthalene derivatives were reported to have DPPH radical scavenging activity as well as cytotoxic activity against cancer cell lines such as the human cholangiocarcinoma cancer cell line HuCCA-1, the lung adenocarcinoma cell line A549, the human hepatocellular liver carcinoma cell line HepG2, and the T-lymphoblast or acute lymphoblastic leukaemia cell line MOLT-3 (Molee et al. Citation2018). Furthermore, naphthalene derivatives from the endophytic fungus Phomopsis fukushii inhibited the growth of S. aureus (Li et al. Citation2021). A synthetic naphthalene compound, (E)-3-(2,3,4-trimethoxyphenyl)-1-(naphth-2-yl)-prop-2-en-1-one, inhibited the growth of four cancer cell lines: PC-3 human prostate cancer cell line, OVCAR human ovarian cancer cell line, IMR-32 human neuroblastoma cell line, and HEP-2 human hepatocellular liver carcinoma cell line (Budhiraja et al. Citation2012). Besides, the same compound demonstrated potent antimicrobial activity against four bacterial strains: S. aureus, B. subtilis, P. aeruginosa, E. coli, and S. typhi, and two fungal strains, Aspergillus niger and C. albicans (Budhiraja et al. Citation2012).
Furthermore, pyridazine derivatives have been reported to have diverse biological activities, including antiviral, anticancer, and antimicrobial properties (Butnariu and Mangalagiu Citation2009). Quinoline derivatives have diverse biological activities and constitute an important class of compounds for new drug development (Orhan Puskullu et al. Citation2013). Various synthetic quinoline derivatives were screened for their biological activities. Some derivatives showed antibacterial (Narender et al. Citation2006; Reddy et al. Citation2009; Matada et al. Citation2021), antifungal (Musiol et al. Citation2006), and cytotoxic activity (Costa et al. Citation2020). Phenanthrenes are a relatively small group of natural products derived primarily from plants. Almost all of the phenanthrene compounds isolated from plants demonstrated a variety of biological activities, including antioxidants (Behery et al. Citation2013; Woo et al. Citation2014), antimicrobial (Guo et al. Citation2016; Tóth et al. 2016), and cytotoxicity (Ma et al. Citation2016). Other chemical classes represented in Ts-EtOAc extract, such as ketones, monoterpenes, fatty acid esters, stilbenoids, and sesquiterpenoids, have been shown to have beneficial biological activities (Mallesha et al. Citation2012; Abdel-Aziz et al. Citation2018; Akinwumi et al. Citation2018; Mothana et al. Citation2018; Ghareeb et al. Citation2019; Elkhouly et al. Citation2020). Hamed et al. Citation2020;
Molecular docking
Thymidylate kinase (TMK) is considered the key enzyme in the production of thymidine triphosphate and acts by catalysing the conversion of thymidine monophosphate into thymidine diphosphate (dTDP), which is subsequently phosphorylated by nucleoside diphosphate kinase to produce thymidine triphosphate (Hu et al. Citation2005; Cui et al. Citation2013). The thymidylate kinase enzyme plays a critical role in bacterial DNA biosynthesis. Therefore, TMK is a promising target for antibacterial drugs (Kawatkar et al. Citation2014). Indeed, TMK was validated as a potent antibacterial target for drugs designed to treat gram-positive bacterial infections (Keating et al. Citation2012). Docking studies using the MOE program (Barakat et al. Citation2018; Mustafa and Mostafa Citation2020) were carried out to predict the most suitable binding pose of identified compounds (1–7) in Ts-EtOAc extract using the bacterial enzyme; thymidylate kinase (PDB: 4QGG). The potencies of these identified compounds were evaluated computationally based on their docking scores (energy scores). This score represents the strength of the non-covalent interactions among numerous molecules within the binding pocket of a target protein. The higher the negative score is, the more beneficial interactions between the chemical and the target protein are. Most of the investigated compounds showed considerable binding interactions with important residues in the active site TMK (ID: 4QGG). However, compound 4 (2-[1-(4-cyano-1,2,3,4-tetrahydronaphthyl)] propanenitrile) showed the highest negative score of −9.117 kcal/mol based on performing binding interactions inside the active binding site via the formation of four hydrogen bonds with Arg36, Arg48, Arg92, and Gln37. Moreover, targeting the same bacterial enzyme, Barakat et al. (Citation2018) found docking score of a synthetic pyrazole-dimedone derivative to be −6.86 kcal/mol through binding interactions with the amino groups of Arg70 and Gln101 and the crucial residues Phe66 and Arg92 of TMK. Moreover, Saminathan et al. (Citation2020) docked two pyrazoline-thiocyanatoethanone derivatives; 1-(5-[4-fluorophenyl]-3-phenyl-4,5-dihydro-1H-pyrazol-1-yl)-2-thiocyanatoethanone (FSCN) and 1-(5-[4-chlorophenyl]-3-phenyl-4,5-dihydro-1H-pyrazol-1-yl)-2-thiocyanatoethanone (ClSCN) against the thymidylate kinase (ID: 4QGG). Their results showed that the two compounds formed different bonded interactions between compounds and TMK binding site residues with binding energies of −7.8 and −7.3 kJ/mol, respectively, for FSCN and ClSCN. The interactions of the FSCN involved six hydrogen bonds with the Arg36, Arg48, Arg92, and Thr16 residues of TMK, while the compound ClSCN interacted with protein 4QGG through five hydrogen bonds with the residues Arg36, Arg92, and Thr16 (Saminathan et al. Citation2020).
In the present investigation, the docking score of compound 4 is better than that of synthetic compounds and the standard drug ciprofloxacin, at 6.9 kcal/mol (Barakat et al. Citation2018). Thus, it can be a promising source of antibacterial drugs. The molecular docking results confirm those of in vitro antibacterial and antibiofilm analysis, where Ts-EtOAc showed better activities against S. aureus than other tested T. savignyi extracts.
Conclusions
Marine molluscs represent an abundant source of metabolites with diverse biological activities. The preceding results suggest that the marine gastropod snail, Thais savignyi, is an excellent source of bioactive metabolites. The ethyl acetate extract from this snail possessed high antioxidant capacities and free radical scavenging activities. Moreover, the extract had considerable antimicrobial activity against pathogenic bacteria and fungi, and cytotoxicity against different cancer cell lines. Chemical analysis of the extract identified 45 compounds belonging to diverse classes of chemicals. The most abundant classes were naphthalene (predominant; 25.57%), pyridazine, quinoline alkane, and phenanthrene derivatives, nitriles, and fatty acid esters. Molecular docking results of the highly abundant compounds suggest their substantial potential to occupy the active sites of TMK, inhibiting the activity of this critical bacterial enzyme. These crucial interactions support the potential antibacterial activity of these compounds. In particular compound 4 (2-[1-(4-cyano-1,2,3,4-tetrahydronaphthyl)] propanenitrile), belongs to naphthalene derivatives, possesses a better drug-likeness nature and good inhibition behaviour with TMK protein. Therefore, more studies are needed to isolate active constituents of Ts-EtOAc extract for comprehensive drug discovery tests.
Acknowledgements
We thank Dr. Aidan Emery from the Natural History Museum of London for his critical reading and linguistic revision of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abdel-Aziz MS, Ghareeb MA, Saad AM, Refahy LA, Hamed AA. 2018. Chromatographic isolation and structural elucidation of secondary metabolites from the soil-inhabiting fungus Aspergillus fumigatus 3T-EGY. Acta Chromatogr. 30(4):243–249.
- Abu El-Einin AA, Gad El-Karim RM, Habib MR, Zayed KM, Ali REM. 2021. Identification of the gastropod snails and shells collected from Ain El-Sokhna region, Red Sea, Egypt. Egypt J Aquat Biol Fisher. 25:101–117.
- Adams RP. 2007. Identification of essential oil components by gas chromatography/mass spectrometry (vol. 456). Carol Stream (IL): Allured Publishing Corporation.
- Ahmad TB, Liu L, Kotiw M, Benkendorff K. 2018. Review of anti-inflammatory, immune-modulatory and wound healing properties of molluscs. J Ethnopharmacol. 210:156–178.
- Akinwumi BC, Bordun KAM, Anderson HD. 2018. Biological activities of stilbenoids. IJMS. 19(3):792–817.
- Alhakmani F, Kumar S, Khan SA. 2013. Estimation of total phenolic content, in vitro antioxidant and anti–inflammatory activity of flowers of Moringa oleifera. Asian Pac J Trop Biomed. 3(8):623–627.
- Atanasov AG, Zotchev SB, Dirsch VM; International Natural Product Sciences Taskforce, Supuran CT. 2021. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov. 20(3):200–216.
- Bachere E, Mialhe E, Noel D, Boulo V, Morvan A, Rodriguez J. 1995. Knowledge and research prospects in marine mollusc and crustacean immunology. Aquaculture. 132(1-2):17–32.
- Balasamy RJ, Ravinayagam V, Alomari M, Ansari MA, Almofty SA, Rehman S, Dafalla H, Marimuthu PR, Akhtar S, Al Hamad M. 2019. Cisplatin delivery, anticancer and antibacterial properties of Fe/SBA-16/ZIF-8 nanocomposite. RSC Adv. 9(72):42395–42408.
- Barakat A, Al-Majid AM, Al-Qahtany BM, Ali M, Teleb M, Al-Agamy MH, Naz S, Ul-Haq Z. 2018. Synthesis, antimicrobial activity, pharmacophore modeling and molecular docking studies of new pyrazole-dimedone hybrid architectures. Chem Cent J. 12(1):29–42.
- Behery FA, Naeem ZEM, Maatooq GT, Amer MM, Ahmed AF. 2013. A novel antioxidant phenanthrenoid dimer from Juncus acutus L. Nat Prod Res. 27(2):155–163.
- Benkendorff K, Pillai R, Bremner JB. 2004. 2,4,5-Tribromo-1H-imidazole in the egg masses of three muricid molluscs. Nat Prod Res. 18(5):427–431.
- Benkendorff K, Rudd D, Nongmaithem BD, Liu L, Young F, Edwards V, Avila C, Abbott CA. 2015. Are the traditional medical uses of muricidae molluscs substantiated by their pharmacological properties and bioactive compounds? Mar Drugs. 13(8):5237–5275.
- Benkendorff K. 2010. Molluscan biological and chemical diversity: secondary metabolites and medicinal resources produced by marine molluscs. Biol Rev Camb Philos Soc. 85:757–775.
- Blunt JW, Copp BR, Munro MH, Northcote PT, Prinsep MR. 2006. Marine natural products. Nat Prod Rep. 23(1):26–78.
- Borquaye LS, Darko G, Ocansey E, Ankomah E. 2015. Antimicrobial and antioxidant properties of the crude peptide extracts of Galatea paradoxa and Patella rustica. SpringerPlus. 4(1):1–6.
- Braca A, Fico G, Morelli I, De Simone F, Tomè F, De Tommasi N. 2003. Antioxidant and free radical scavenging activity of flavonol glycosides from different Aconitum species. J Ethnopharmacol. 86(1):63–67.
- Brand-Williams W, Cuvelier ME, Berset CL. 1995. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. 28(1):25–30.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. 2018. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 68(6):394–424.
- Brieger K, Schiavone S, Miller FJ, Krause KH. 2012. Reactive oxygen species: from health to disease. Swiss Med Wkly. 142:w13659.
- Budhiraja A, Kadian K, Kaur M, Aggarwal V, Garg A, Sapra S, Nepali K, Suri OP, Dhar KL. 2012. Synthesis and biological evaluation of naphthalene, furan and pyrrole based chalcones as cytotoxic and antimicrobial agents. Med Chem Res. 21(9):2133–2140.
- Butnariu RM, Mangalagiu II. 2009. New pyridazine derivatives: synthesis, chemistry and biological activity. Bioorg Med Chem. 17(7):2823–2829.
- Chakraborty K, Joy M. 2020. High-value compounds from the molluscs of marine and estuarine ecosystems as prospective functional food ingredients: an overview. Food Res Int. 137:109637.
- Cheung RCF, Ng TB, Wong JH. 2015. Marine peptides: bioactivities and applications. Mar Drugs. 13(7):4006–4043.
- Choi JY, Plummer MS, Starr J, Desbonnet CR, Soutter H, Chang J, Miller JR, Dillman K, Miller AA, Roush WR. 2012. Structure guided development of novel thymidine mimetics targeting Pseudomonas aeruginosa thymidylate kinase: from hit to lead generation. J Med Chem. 55(2):852–870.
- Ciavatta ML, Lefranc F, Carbone M, Mollo E, Gavagnin M, Betancourt T, Dasari R, Kornienko A, Kiss R. 2017. Marine mollusk‐derived agents with antiproliferative activity as promising anticancer agents to overcome chemotherapy resistance. Med Res Rev. 37(4):702–801.
- Cimino G, Sodano G. 1993. Biosynthesis of secondary metabolites in marine molluscs. In: Scheuer PJ, ed. Marine natural products - diversity and biosynthesis. Topics in Current Chemistry. Berlin, Heidelberg: Springer; p. 77–115.
- Costa CA, Lopes RM, Ferraz LS, Esteves GN, Di Iorio JF, Souza AA, de Oliveira IM, Manarin F, Judice WAS, Stefani HA, et al. 2020. Cytotoxicity of 4-substituted quinoline derivatives: anticancer and antileishmanial potential. Bioorg Med Chem. 28(11):115511.
- Craig AG. 2000. The characterization of conotoxins. J Toxicol Toxin Rev. 19:55–93.
- Cui Q, Shin W, Luo Y, Tian J, Cui H, Yin D. 2013. Thymidylate kinase: an old topic brings new perspectives. Curr Med Chem. 20(10):1286–1305.
- Cummins SF, Nichols AE, Schein CH, Nagle GT. 2006. Newly identified water-borne protein pheromones interact with attractin to stimulate mate attraction in Aplysia. Peptides. 27(3):597–606.
- de Vries DJ, Beart PM. 1995. Fishing for drugs from the sea: Status and strategies. Trends Pharmacol Sci. 16(8):275–279.
- Derby CD, Kicklighter CE, Johnson PM, Zhang X. 2007. Chemical composition of inks of diverse marine molluscs suggests convergent chemical defenses. J Chem Ecol. 33(5):1105–1113.
- Dias R, Oliveira H, Fernandes I, Simal-Gandara J, Perez-Gregorio R. 2021. Recent advances in extracting phenolic compounds from food and their use in disease prevention and as cosmetics. Crit Rev Food Sci Nutr. 61(7):1130–1151.
- Dongari-Bagtzoglou A. 2008. Pathogenesis of mucosal biofilm infections: challenges and progress. Expert Rev anti Infect Ther. 6(2):201–208.
- Elkhouly HI, Hamed AA, El Hosainy AM, Ghareeb MA, Sidkey NM. 2020. Bioactive secondary metabolite from endophytic Aspergillus tubenginses ASH4 isolated from Hyoscyamus muticus: antimicrobial, antibiofilm, antioxidant and anticancer activity. PJ. 13(2):434–442.
- Faircloth G, Cuevas C. 2006. Kahalalide F and ES285: Potent anticancer agents from marine molluscs. Prog Mol Subcell Biol. 43:363–379.
- Flemming HC, Wuertz S. 2019. Bacteria and archaea on Earth and their abundance in biofilms. Nat Rev Microbiol. 17(4):247–260.
- Fontana A, Cavaliere P, Wahidulla S, Naik CG, Cimino G. 2000. A new antitumor isoquinoline alkaloid from the marine nudibranch Jorunna funebris. Tetrahedron. 56(37):7305–7308.
- Galie S, García-Gutiérrez C, Miguélez EM, Villar CJ, Lombó F. 2018. Biofilms in the food industry: Health aspects and control methods. Front Microbiol. 9:898.
- Ghareeb MA, Shoeb HA, Madkour HMF, Refaey LA, Mohamed MA, Saad AM. 2014. Antioxidant and cytotoxic activities of Tectona grandis Linn leaves. Int J Phytopharmacol. 5:143–157.
- Ghareeb MA, Sobeh M, El-Maadawy WH, Mohammed HS, Khalil H, Botros S, Wink M. 2019. Chemical profiling of polyphenolics in Eucalyptus globulus and evaluation of its hepato–renal protective potential against cyclophosphamide induced toxicity in mice. Antioxidants. 8(9):415.
- Gülçin I, Elmastaş M, Aboul‐Enein HY. 2007. Determination of antioxidant and radical scavenging activity of basil (Ocimum basilicum L. Family Lamiaceae) assayed by different methodologies. Phytother Res. 21(4):354–361.
- Guo JJ, Dai BL, Chen NP, Jin LX, Jiang FS, Ding ZS, Qian CD. 2016. The anti-Staphylococcus aureus activity of the phenanthrene fraction from fibrous roots of Bletilla striata. BMC Complement Altern Med. 16(1):1–7.
- Hamann MT, Otto CS, Scheuer PJ, Dunbar DC. 1996. Kahalalides: Bioactive peptides from a marine mollusk Elysia rufescens and its algal diet Bryopsis sp. J Org Chem. 61(19):6594–6600.
- Hamann MT, Scheuer PJ. 1993. Kahalalide F: A bioactive depsipeptide from the sacoglossan mollusk Elysia rufescens and the green alga Bryopsis sp. J Am Chem Soc. 115(13):5825–5826.
- Hamed AA, Kabary H, Khedr M, Emam AN. 2020. Antibiofilm, antimicrobial and cytotoxic activity of extracellular green-synthesized silver nanoparticles by two marine-derived actinomycete. RSC Adv. 10(17):10361–10367.
- Hamed AA, Soldatou S, Qader MM, Arjunan S, Miranda KJ, Casolari F, Pavesi C, Diyaolu OA, Thissera B, Eshelli M, et al.,. 2020. Screening fungal endophytes derived from under-explored Egyptian marine habitats for antimicrobial and antioxidant properties in factionalised textiles. Microorganisms. 8(10):1617.
- Hayashi SE. 2005. The molecular phylogeny of the Buccinidae (Caenogastropoda: Neogastropoda) as inferred from the complete mitochondrial 16S rRNA gene sequences of selected representatives. Moll Res. 25:85–98.
- Hooper C, Day R, Slocombe R, Handlinger J, Benkendorff K. 2007. Stress and immune responses in abalone: limitations in current knowledge and investigative methods based on other models. Fish Shellfish Immunol. 22(4):363–379.
- Hu R, Li L, Degreve B, Dutschman GE, Lam W, Cheng YC. 2005. Behavior of thymidylate kinase toward monophosphate metabolites and its role in the metabolism of 1-(2′-deoxy-2′-fluoro-β-l-arabinofuranosyl)-5-methyluracil (Clevudine) and 2′, 3′-didehydro-2′, 3′-dideoxythymidine in cells. Antimicrob Agents Chemother. 49(5):2044–2049.
- Iqbal MA, Arora S, Prakasam G, Calin GA, Syed MA. 2019. MicroRNA in lung cancer: Role, mechanisms, pathways and therapeutic relevance. Mol Aspects Med. 70:3–20.
- Jimeno J, Faircloth G, Sousa-Faro JM, Scheuer P, Rinehart K. 2004. New marine derived anticancer therapeutics─ a journey from the sea to clinical trials. Mar Drugs. 2(1):14–29.
- Kalishwaralal K, BarathManiKanth S, Pandian SR, Deepak V, Gurunathan S. 2010. Silver nanoparticles impede the biofilm formation by Pseudomonas aeruginosa and Staphylococcus epidermidis. Colloids Surf B Biointerfaces. 79(2):340–344.
- Kanda A, Iwakoshi-Ukena E, Takuwa-Kuroda K, Minakata H. 2003. Isolation and characterization of novel tachykinins from the posterior salivary gland of the common octopus Octopus vulgaris. Peptides. 24(1):35–43.
- Karthikeyan SC, Velmurugan S, Donio MB, Michaelbabu M, Citarasu T. 2014. Studies on the antimicrobial potential and structural characterization of fatty acids extracted from Sydney rock oyster Saccostrea glomerata. Ann Clin Microbiol Antimicrob. 13:332.
- Kawatkar SP, Keating TA, Olivier NB, Breen JN, Green OM, Guler SY, Hentemann MF, Loch JT, McKenzie AR, Newman JV, et al. 2014. Antibacterial inhibitors of Gram-positive thymidylate kinase: structure-activity relationships and chiral preference of a new hydrophobic binding region. J Med Chem. 57(11):4584–4597.
- Keating TA, Newman JV, Olivier NB, Otterson LG, Andrews B, Boriack-Sjodin PA, Breen JN, Doig P, Dumas J, Gangl E, et al. 2012. In vivo validation of thymidylate kinase (TMK) with a rationally designed, selective antibacterial compound. ACS Chem Biol. 7(11):1866–1872.
- Krishnamoorthy V, Chuen L, Sivayogi V, Kathiresan S, Bahari M, Raju G, Parasuraman S. 2019. Exploration of antioxidant capacity of extracts of Perna viridis, a marine bivalve. Phcog Mag. 15(66):402–409.
- Kumaran NS, Bragadeeswaran S, Thangaraj S. 2011. Screening for antimicrobial activities of marine molluscs Thais tissoti (Petit, 1852) and Babylonia spirata (Linnaeus, 1758) against human, fish and biofilm pathogenic microorganisms. Afr J Microbiol Res. 5:4155–4161.
- Li H, Lin D, Fang H, Zhu A, Gao Y. 2010. Species identification and phylogenetic analysis of genus Nassarius (Nassariidae) based on mitochondrial genes. Chin J Ocean Limnol. 28(3):565–572.
- Li X-M, Mi Q-L, Gao Q, Li J, Song C-M, Zeng W-L, Xiang H-Y, Liu X, Chen J-H, Zhang C-M, et al. 2021. Antibacterial naphthalene derivatives from the fermentation products of the endophytic fungus Phomopsis fukushii. Chem Nat Compd. 57(2):293–296.
- Liu M, El-Hossary EM, Oelschlaeger TA, Donia MS, Quinn RJ, Abdelmohsen UR. 2019. Potential of marine natural products against drug-resistant bacterial infections. Lancet Infect Dis. 19(7):e237–e245.
- Ljungberg B, Campbell SC, Choi HY, Cho HY, Jacqmin D, Lee JE, Weikert S, Kiemeney LA. 2011. The epidemiology of renal cell carcinoma. Eur Urol. 60(4):615–621.
- Ma W, Zhang Y, Ding YY, Liu F, Li N. 2016. Cytotoxic and anti-inflammatory activities of phenanthrenes from the medullae of Juncus effusus L. Arch Pharm Res. 39(2):154–160.
- Makar S, Saha T, Singh SK. 2019. Naphthalene, a versatile platform in medicinal chemistry: sky-high perspective. Eur J Med Chem. 161:252–276.
- Mallesha L, Mohana KN, Veeresh B. 2012. Synthesis and biological activities of Schiff bases of gabapentin with different aldehydes and ketones: a structure–activity relationship study. Med Chem Res. 21(1):1–9.
- Maselli V, Galdiero E, Salzano AM, Scaloni A, Maione A, Falanga A, Naviglio D, Guida M, Di Cosmo A, Galdiero S. 2020. OctoPartenopin: identification and preliminary characterization of a novel antimicrobial peptide from the suckers of Octopus vulgaris. Mar Drugs. 18(8):380.
- Matada BS, Pattanashettar R, Yernale NG. 2021. A comprehensive review on the biological interest of quinoline and its derivatives. Bioorg Med Chem. 32:115973.
- Mayer AM, Glaser KB, Cuevas C, Jacobs RS, Kem W, Little RD, McIntosh JM, Newman DJ, Potts BC, Shuster DE. 2010. The odyssey of marine pharmaceuticals: A current pipeline perspective. Trends Pharmacol Sci. 31(6):255–265.
- Minsas S, Nurdiansyah SI, Prayitno DI, Sofiana MSJ, Kalija TA, Fadly D. 2020. Screening of bioactive compounds and antioxidant activity of ale-ale shellfish (Meretrix meretrix) crude extracts from West Kalimantan, Indonesia. Sys Rev Pharm. 11:222–227.
- Molee W, Phanumartwiwath A, Kesornpun C, Sureram S, Ngamrojanavanich N, Ingkaninan K, Mahidol C, Ruchirawat S, Kittakoop P. 2018. Naphthalene derivatives and quinones from Ventilago denticulata and their nitric oxide radical scavenging, antioxidant, cytotoxic, antibacterial, and phosphodiesterase inhibitory activities. Chem Biodiversity. 15(3):1700537.
- Molyneux P. 2004. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J Sci Technol. 26:211–219.
- Mothana RA, Khaled JM, Noman OM, Kumar A, Alajmi MF, Al-Rehaily AJ, Kurkcuoglu M. 2018. Phytochemical analysis and evaluation of the cytotoxic, antimicrobial and antioxidant activities of essential oils from three Plectranthus species grown in Saudi Arabia. BMC Complement Altern Med. 18(1):237.
- Muhammad MH, Idris AL, Fan X, Guo Y, Yu Y, Jin X, Qiu J, Guan X, Huang T. 2020. Beyond risk: bacterial biofilms and their regulating approaches. Front Microbiol. 21:928.
- Musiol R, Jampilek J, Buchta V, Silva L, Niedbala H, Podeszwa B, Palka A, Majerz-Maniecka K, Oleksyn B, Polanski J. 2006. Antifungal properties of new series of quinoline derivatives. Bioorg Med Chem. 14(10):3592–3598.
- Mustafa M, Mostafa YA. 2020. Antimicrobial pyridazines: synthesis, characterization, cytotoxicity, substrate promiscuity, and molecular docking. Chem Biodivers. 17(6):e2000100.
- Nakao Y, Yoshida WY, Takada Y, Kimura J, Yang L, Mooberry SL, Scheuer PJ. 2004. Kulokekahilide-2, a cytotoxic depsipeptide from a cephalaspidean mollusk Philinopsis speciosa. J Nat Prod. 67(8):1332–1340.
- Narender P, Srinivas U, Ravinder M, Rao BA, Ramesh C, Harakishore K, Gangadasu B, Murthy U, Rao VJ. 2006. Synthesis of multisubstituted quinolines from Baylis–Hillman adducts obtained from substituted 2-chloronicotinaldehydes and their antimicrobial activity. Bioorg Med Chem. 14(13):4600–4609.
- Newman DJ, Cragg GM. 2020. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod. 83(3):770–803.
- Odeleye T, White WL, Lu J. 2019. Extraction techniques and potential health benefits of bioactive compounds from marine molluscs: A review. Food Funct. 10(5):2278–2289.
- Orhan Puskullu M, Tekiner B, Suzen S. 2013. Recent studies of antioxidant quinoline derivatives. Mini Rev Med Chem. 13(3):365–372.
- Pangestuti R, Kim SK. 2017. Bioactive peptide of marine origin for the prevention and treatment of non-communicable diseases. Mar Drugs. 15(3):67.
- Percival SL, Suleman L, Vuotto C, Donelli G. 2015. Healthcare-associated infections, medical devices and biofilms: risk, tolerance and control. J Med Microbiol. 64(Pt 4):323–334.
- Prem Anand T, Chellaram C, Kumaran S, Felicia Shanthini C. 2010. Biochemical composition and antioxidant activity of Pleuroploca trapezium meat. J Chem Pharmaceut Res. 2:526–535.
- Prem Anand T, Edward JK. 2002. Antimicrobial activity in the tissue extracts of five species of cowries Cypraea spp. (Mollusca: Gastropoda) and an ascidian Didemnum psammathodes (Tunicata: Didemnidae). Indian J Mar Sci. 31:239–242.
- Prem Anand T, Rajaganapathi J, Edward JK. 1997. Antibacterial activity of marine molluscs from Portonovo region. Indian J Mar Sci. 26:206–208.
- Prieto P, Pineda M, Aguilar M. 1999. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 269(2):337–341.
- Proksch P, Edrada RA, Ebel R. 2002. Drugs from the seas - current status and microbiological implications. Appl Microbiol Biotechnol. 59(2-3):125–134.
- Ramasamy MS, Murugan A. 2005. Potential antimicrobial activity of marine molluscs from Tuticorin, southeast coast of India against 40 biofilm bacteria. J Shellfish Res. 24:243–251.
- Reddy EA, Islam A, Mukkanti K, Bandameedi V, Bhowmik DR, Pal M. 2009. Regioselective alkynylation followed by Suzuki coupling of 2,4-dichloroquinoline: synthesis of 2-alkynyl-4-arylquinolines. Beilstein J Org Chem. 5:32.
- Saito N, Tanaka C, Satomi T, Oyama C, Kubo A. 2004. Chemistry of renieramycins. Part 4. Synthesis of a simple natural marine product, 6-hydroxy-7-methoxyisoquinolinemethanol. Chem Pharm Bull (Tokyo). 52(2):282–286.
- Salas S, Chakraborty K. 2020. Functional properties of the marine gastropod molluscs Chicoreus ramosus and Babylonia spirata collected from the southern coast of India. J Aquat Food Prod Technol. 29(3):264–278.
- Saminathan M, Kanagarajan S, Chandrasekaran R, Sivasubramaniyan A, Raja R, Alagusundaram P. 2020. Synthesis, structural, DFT investigations and antibacterial activity assessment of pyrazoline‐thiocyanatoethanone derivatives as thymidylate kinase inhibitors. J Chin Chem Soc. 67(6):1100–1112.
- Schroeder CI, Smythe ML, Lewis RJ. 2004. Development of small molecules that mimic the binding of ω-conotoxins at the N-type voltage-gated calcium channel. Mol Divers. 8(2):127–134.
- Scott JD, Williams RM. 2002. Chemistry and biology of the tetrahydroisoquinoline antitumor antibiotics. Chem Rev. 102(5):1669–1730.
- Senthilkumar K, Kim SK. 2013. Marine invertebrate natural products for anti-inflammatory and chronic diseases. Evid Based Complement Alternat Med. 2013:572859.
- Serova M, de Gramont A, Bieche I, Riveiro ME, Galmarini CM, Aracil M, Jimeno J, Faivre S, Raymond E. 2013. Predictive factors of sensitivity to elisidepsin, a novel kahalalide F-derived marine compound. Mar Drugs. 11(3):944–959.
- Shah SC, Kayamba V, Peek RM, Jr, Heimburger D. 2019. Cancer control in low- and middle-income countries: is it time to consider screening? J Glob Oncol. 5:1–8.
- Siegel J, Totonchy M, Damsky W, Berk-Krauss J, Castiglione F, Sznol M, Petrylak DP, Fischbach N, Goldberg SB, Decker RH, et al. 2018. Bullous disorders associated with anti-PD-1 and anti-PD-L1 therapy: a retrospective analysis evaluating the clinical and histopathologic features, frequency, and impact on cancer therapy. J Am Acad Dermatol. 79(6):1081–1088.
- Singleton VL, Rossi JA. 1965. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Amer J Enol Viticulture. 16:144–158.
- Sithranga Boopathy N, Kathiresan K. 2010. Anticancer drugs from marine flora: an overview. J Oncol. 2010:214186.
- Snezhkina AV, Kudryavtseva AV, Kardymon OL, Savvateeva MV, Melnikova NV, Krasnov GS, Dmitriev AA. 2019. ROS generation and antioxidant defense systems in normal and malignant cells. Oxid Med Cell Longev. 2019:6175804.
- Sung WW, Ko PY, Chen WJ, Wang SC, Chen SL. 2021. Trends in the kidney cancer mortality-to-incidence ratios according to health care expenditures of 56 countries. Sci Rep. 11(1):1479.
- Toth B, Liktor-Busa E, Kusz N, Szappanos A, Mandi A, Kurtan T, Urban E, Hohmann J, Chang FR, Vasas A. 2016. Phenanthrenes from Juncus inflexus with antimicrobial activity against methicillin-resistant Staphylococcus aureus. J Nat Prod. 79(11):2814–2823.
- Ullah R, Alsaid MS, Shahat AA, Naser AA, Al-Mishari AA, Adnan M, Tariq A. 2018. Antioxidant and hepatoprotective effects of methanolic extracts of Zilla spinosa and Hammada elegans against carbon tetrachloride induced hepatotoxicity in rats. Open Chem. 16(1):133–140.
- Wahl M, Goecke F, Labes A, Dobretsov S, Weinberger F. 2012. The second skin: Ecological role of epibiotic biofilms on marine organisms. Front Microbiol. 3:292.
- Wayne P. 2002. National committee for clinical laboratory standards. Perform Stand Antimicrob Disc Susceptibility Test. 12:1–53.
- Woo KW, Kwon OW, Kim SY, Choi SZ, Son MW, Kim KH, Lee KR. 2014. Phenolic derivatives from the rhizomes of Dioscorea nipponica and their anti-neuroinflammatory and neuroprotective activities. J Ethnopharmacol. 155(2):1164–1170.
- Xu DP, Li Y, Meng X, Zhou T, Zhou Y, Zheng J, Zhang JJ, Li HB. 2017. Natural antioxidants in foods and medicinal plants: extraction, assessment and resources. IJMS. 18(1):96.