?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Context
Bombax ceiba Linnaeus (Bombacaceae) is known as silk cotton tree, the flowers of which are used in many medicinal applications.
Objective
To investigate the therapeutic effect of B. ceiba flower aqueous extracts (BCE) against loperamide-induced constipation and characterize the chemical composition of BCE.
Materials and methods
Sixty male Kunming mice were divided into control (saline), model (10 mg/kg loperamide + saline), phenolphthalein (10 mg/kg loperamide + 10 mg/kg phenolphthalein) and different dosage of BCE (10 mg/kg loperamide + 40, 80 and 160 mg/kg BCE, respectively) groups, and received intragastric administrations for eight days. Faecal water content, number of faeces, first black-stool defecation time and gastrointestinal transit rates were evaluated. Various biochemical and molecular biomarkers were assessed in blood and colon. UPLC-ESI-QTOF-MS/MS was used to tentatively identify the composition of the BCE.
Results
BCE treatment (160 mg/kg) could increase faecal water (15.75%), faeces number (11.65%), gastrointestinal transit rate (25.37%) and decrease first black-stool defecation time (24.04%). The BCE (80 mg/kg) increased the serum level of motilin (30.62%), gastrin (54.46%) and substance P (18.99%), and decreased somatostatin (19.47%). Additionally, the BCE (160 mg/kg) reduced the mucosal damage, restored colonic goblet cell function, down-regulated the protein expression of AQP3 (33.60%) and increased c-kit protein expression (11.63%). Twelve known compounds, including protocatechuic acid, chlorogenic acid and rutin, previously reported in B. ceiba, were identified in the BCE.
Discussion and conclusions
This study suggested that BCE is a promising agent for the treatment of constipation.
Introduction
Constipation is a clinically common digestive disease in modern society (Chen et al. Citation2019; Lu et al. Citation2021), characterized by difficult excretion, low-frequency defecation, hard-dry stool and prolonged gastrointestinal emptying time (Li et al. Citation2020). Previous surveys showed that the prevalence of constipation ranged from approximately 2–30%, especially in elderly populations and children (Zhao et al. Citation2019; Gan et al. Citation2020). Long-term constipation may cause not only feelings of discomfort (Lu et al. Citation2021), but also gastrointestinal nerve dysfunction, intestinal flora disturbances (Wang et al. Citation2017), hepatic encephalopathy, cardiocerebrovascular disease (Payne and Grimm Citation2017), and even fatal pulmonary embolism (Yin et al. Citation2018) or colorectal cancer (Gan et al. Citation2020). The cause of constipation may have various factors, including a low-fibre diet, low physical activity, low water intake, medicine abuse (Hayeeawaema et al. Citation2020), lifestyle changes (Wang et al. Citation2017). Constipation is also one of the most common non-motor symptoms of Parkinson’s disease, and the most common gastrointestinal symptoms in alimentary tract tumour, inflammatory bowel disease, diabetes, metabolic and endocrine disorders, hyperparathyroidism, hypothyroidism and other neurological diseases (Gao et al. Citation2021). At present, the pathogenesis of constipation may include changes in gastrointestinal peptides hormones and aquaporins, colonic dysfunction, and abnormal interstitial cells of Cajal (ICC) (Liu et al. Citation2020). The gastrointestinal tract can produce a variety of gastrointestinal peptides hormones, including inhibitory transmitters represented by somatostatin (SS) and excitatory transmitters represented by motilin (MTL) (Liu et al. Citation2020), gastrin (Gas), substance P (SP), etc. (Han Citation2013; Gan et al. Citation2020). Aquaporin 3 (AQP3) is an important aquaporin located in the colon, which play an important role in the water transport effect (Ikarashi et al. Citation2016). In addition, some studies have shown that ICC is a pacemaker of the intestine (Han et al. Citation2010; Liu et al. Citation2020) and constipation is associated with loss and injury to ICC in the gastrointestinal tract (Huizinga and Chen Citation2014). The stem cell factor and its receptor tyrosine kinase (SCF/C-kit) signalling pathway could affect the phenotype of ICC and it may provide an important target for the therapy of constipation (Li et al. Citation2019).
More than 50% of patients fail to be cured, though the high global expenditure of constipation therapy annually (Zhang et al. Citation2021). Administration of laxatives and prokinetic agents (MTL agonists, 5-hydroxytryptamine modulators, opioid antagonists and chloride-channel activators) are common clinical treatments for constipation (Shin et al. Citation2014). However, long-term intake of these agents can be associated with complications, such as drug dependence, severe diarrhoea (Lu et al. Citation2021) hypotension, tachycardia, postural dizziness, melanosis coli, etc. (Ma et al. Citation2019). Therefore, it is urgently needed to find a new drug of hazard-free treatment for constipation. As an alternative treatment for constipation, traditional Chinese medicine is universally applied, safe and effective (Sun et al. Citation2020). Moreover, some studies suggest that multiple plant extracts could be applied to control constipation and there is hardly any side effect (Gilani et al. Citation2000; Han 2015; Lu et al. Citation2021).
Bombax ceiba Linnaeus (Bombacaceae) is known as silk cotton tree (Arafa et al. Citation2019) and it is mainly cultivated in Southern China, India, Pakistan, Egypt and Northern Australia (Xu et al. Citation2017). Phytochemical investigations have revealed that this plant contains health-promoting phytopharmaceuticals such as naphthoquinones, flavonoids, sesquiterpenoids, phenolics, neolignans and steroids, possessing the medicinal properties (Xu et al. Citation2017). Furthermore, this plant has a strong ethnobotanical background. It has been used extensively in both Indian and Chinese traditional herbal medicines for the treatment of fever, inflammatory conditions, catarrhal affection (Yu et al. Citation2011), diuretic, oedema, hepatotoxicity and ulcer (Shahat et al. 2003; Xu et al. Citation2017). Moreover, according to the traditional Chinese medicine theory, the flower of B. ceiba is cool-natured (Zhang et al. Citation2015), and is regarded as having laxative properties (Shahat et al. Citation2003).
However, there have been no reports on the therapeutic effects of B. ceiba flower aqueous extracts (BCE) in constipation and its relevant mechanisms. Loperamide has been widely used for introducing spastic constipation model (Mori et al. Citation2013; Li et al. Citation2015). Therefore, we investigated the curative effect of BCE on loperamide-induced mice and revealed its mechanism by measuring the serum gastrointestinal peptides hormones concentration and the expression of AQP3 and receptor tyrosine kinase (c-kit) in the colon. In addition, an ultra-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry (UPLC-ESI-QTOF-MS/MS) has been applied to characterize the chemical composition of BCE.
Materials and methods
Preparation of B. ceiba flower aqueous extracts
Bombax ceiba flowers were obtained from Baise, Guangxi, China, in September 2020, and identified by Bin Yang. A voucher specimen (20200902) was deposited in Guangxi Medical University. The pure water was used to extract the shade dried B. ceiba flowers (1 kg). The filtrates were combined and then concentrated using a rotary evaporator. The excess water was removed by lyophilization with a freeze-dryer to obtain crude dark-brown sticky BCE for subsequent experiments. The yield of BCE from the extract was 10%.
The analysis of BCE by UPLC-ESI-QTOF-MS/MS
BCE were analysed in negative and positive ionization mode by UPLC-ESI-QTOF-MS. Approximately, 0.5 g of the BCE were accurately weighed and ultrasonically extracted with 50 mL methanol/water (50%, v/v) for 30 min. Then, the extract was filtered through a 0.22 μm membrane prior to analysis. Identification of compounds in BCE was conducted on an Acquity UPLCH-Class system (Waters, Milford, MA) that was coupled to a Waters XevoG2-S QTof mass spectrometer (Waters, Milford, MA). Chromatographic separation was performed on a Waters ACQUITY UPLC column (BEH C18: 2.1 × 50 mm, 1.7 μm) by gradient elution at a flow rate of 0.3 mL/min. The mobile phase consisted of water containing 0.1% formic acid (A) and acetonitrile (B) with the following gradient procedure: 0–5 min, 5–20% B; 5–20 min, 20–65% B; 20–20.5 min, 65–90% B; 20.5–22 min, 90–5% B; then hold at 5% B for 3 min. The injection volume was 10 μL.
Mass spectrometry with an electrospray ionization (ESI) source was conducted in both the positive and negative ion modes. The analysis was performed using full scan mode, and the mass range was set at m/z 100–1000. The MS settings were the following: capillary voltage, 2.5 kV; sampling cone, 40 V; source temperature, 120 °C and desolvation temperature, 300 °C. A 10 eV collision energy was used during the MS acquisition, while 30 eV was employed during the MSE acquisition. The setting for cone gas was 50 L/h. The data were gathered using MassLynxV4.1 software (Waters, Milford, MA).
Animals
Male Kunming mice (20 ± 2 g) were provided by the Experimental Animal Center of Guangxi Medical University (Guangxi, China). All mice were housed in normal cages with relative humidity of 60 ± 10%, room air changes 12–18 times/h, temperature of 25 ± 2 °C and a 12 h light/dark cycle. All mice were allowed to have free access to standard chow pellets and water ad libitum. The animal experimental procedure was approved by the Institutional Animal Ethical Committee of Guangxi Medical University. The certificate number of animal care is SYXK GUI 2020-0004.
Experimental design
Sixty mice were randomly divided into the following study groups (n = 10 each): a normal group (control): normal saline solution (0.25 mL)+normal saline solution (0.25 mL); a constipation model group (model): loperamide (lot: JEJ0944, Xian Janssen Pharmaceutical Ltd., Xi’an, China) (10 mg/kg; 0.25 mL)+normal saline solution (0.25 mL); a phenolphthalein: loperamide (10 mg/kg; 0.25 mL)+phenolphthalein (lot: 200502, Shandong Renhetang Pharmaceutical Co., Ltd., Shandong, China) (10 mg/kg; 0.25 mL); a low-dose BCE group (L-BCE): loperamide (10 mg/kg; 0.25 mL)+BCE (40 mg/kg; 0.25 mL); a medium-dose BCE group (M-BCE): loperamide (10 mg/kg; 0.25 mL)+BCE (80 mg/kg; 0.25 mL); a high-dose BCE group (H-BCE; 0.25 mL): loperamide (10 mg/kg; 0.25 mL)+BCE (160 mg/kg; 0.25 mL); the specific arrangements of the animal experiments are shown in .
Table 1. Specific arrangements of animal experiments.
The experiment was designed as described previously (Wang et al. Citation2017). All mice were fasted overnight (approximately 12 h) before the first experiment (with free access to water). Constipation was induced in animals via gavage of loperamide (10 mg per kg body weight loperamide hydrochloride in a volume of 0.25 mL) once per day for eight continuous days; 1 h later, the animals were administered with a corresponding concentration of BCE and phenolphthalein in the same manner. The animals in the normal group only received normal saline solution (a volume of 0.25 mL) by the same method.
Faecal water content and number of faeces
The faecal water content and number of faeces were measured on day 7. At the end of the intragastric administration period, mice were moved into a clean separate cage. At 3 h, the faeces samples were collected in individual tubes on ice, counted and weighed; and then the samples thoroughly dried in an oven to obtain the dry weight. Faecal water content was calculated as:
Measurement of first black faeces
At day 8, after fasting overnight 12 h with water provided, the mice in the normal group were received normal saline solution (0.25 mL) and those in other groups received an intragastric administration containing loperamide (10 mg/kg; 0.25 mL); 1 h later, all mice received an activated carbon meal by the same method. The mice were placed individually in a clean cage and allowed water and food ad libitum. The length of time from activated carbon meal to the appearance of first black faeces was recorded.
Measurement of gastrointestinal transit rate and collection of blood and tissue sample
At day 9, the mice fasted 12 h but with free access to water and then fed 0.25 mL of activated carbon meal. At 1 h, serum samples were stored in −80 °C before further assays. The animal’s abdomen was opened. The total length of the small intestine (from the pyloric sphincter to the cecum) and the activated carbon transport distance were measured to characterize the gastrointestinal transit rate of the mice. Proximal colon tissues were collected and fixed in 10% formalin for subsequent histology evaluation and immunohistochemical (IHC) analysis. The intestinal transit rate was calculated based on the following equation:
Histology evaluation
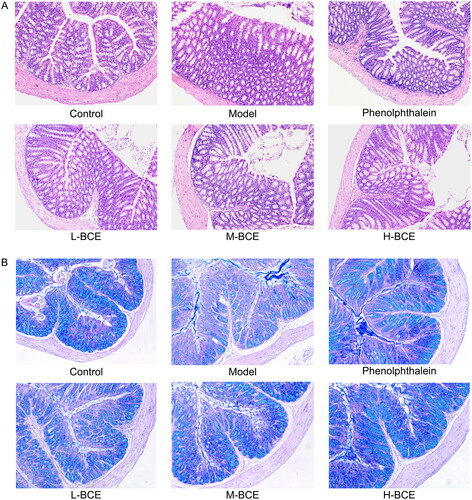
Colon tissues were fixed in 10% formalin and embedded in paraffin. Then, they were cut 5 µm thick sections, deparaffinized in xylene and rehydrated in graded concentrations of ethanol. The sections were placed on glass slides. Then, they stained with haematoxylin–eosin (HE) and Alcian Blue/periodic acid Schiff (AB/PAS) according to the standard procedure. Pathological changes in the colon were examined under a light microscope (×100), and images were recorded.
Assessment of MTL, gas, SP and SS levels in serum
The concentrations of MTL (lot: 2021-01, Jiangsu Jingmei Biological Technology Co., Ltd., Nanjing, China), Gas (lot: SB9JYJGPMA, Elabscience Biotechnology Co., Ltd., Wuhan, China), SP (lot: 2021-01, Jiangsu Jingmei Biological Technology Co., Ltd., Nanjing, China) and SS (lot: 2021-01, Jiangsu Jingmei Biological Technology Co., Ltd., Nanjing, China) in serum were estimated by enzyme-linked immunosorbent assay (ELISA) using commercially available kits.
Immunohistochemical analysis
The pre-treatment of colon tissues for IHC examination of proteins expression (AQP3 and C-kit) was essentially the same as for histology evaluation. H2O2 (0.1%) was used to remove the endogenous peroxidase activity in the colon sections. Then, we incubated them with primary antibodies (1:200) (AQP3, lot: ab125219, Abcam, Cambridge, UK; C-kit, lot: ab256345, Abcam, Cambridge, UK) overnight at 4 °C and incubated them with a secondary antibody for 1 h. Finally, we counterstained the colon sections with haematoxylin. The sections were examined under a light microscope (×400). We calculated the expression area of AQP3 and C-kit by Image-Pro Plus 6.0 (Version X, Media Cybernetics, Silver Springs, MD).
Statistical analysis
All statistical analyses were performed with GraphPad Prism 5 (GraphPad Software, La Jolla, CA). The data are presented as the mean ± standard deviation (SD) for each group. One-way analysis of variance (ANOVA) with Duncan’s multiple range tests was used to analyse the differences between the mean values of the groups. A p < 0.05 was considered to indicate statistical significance. The analyses were performed through SPSS 16.0 software (SPSS, Chicago, IL).
Results
Identification of the chemical composition in BCE
In this study, the base peak chromatogram obtained is illustrated in . The samples in negative ion mode showed stronger peak signals and rich mass information, so the peaks in negative ion mode we analysed to identification of the chemical composition in BCE. Based on the retention times, molecular formula, the MS/MS data, reference standards and literature data, 12 compounds were identified which include: protocatechuic acid, 1-caffeoylquinic acid, 5-coumaroylquinic acid, neochlorogenic acid, chlorogenic acid, 4-coumaroylquinic acid, 3-coumaroylquinic acid, clovamide, rutin, isoquercetin, quercetin 3-glucuronide and kaempferol-3-glucuronide (). Among them, three compounds, including protocatechuic acid, chlorogenic acid and rutin were unambiguously identified by comparing with the retention time and MS data of reference standards. In addition to the above compounds, some peaks of fatty acids were found in the chromatogram of BCE after 10 min.
Figure 1. Base peak ion chromatogram (BPI). (A) Positive mode BPI. (B) Negative mode BPI of BCE by UPLC-ESI-QTOF-MS/MS.
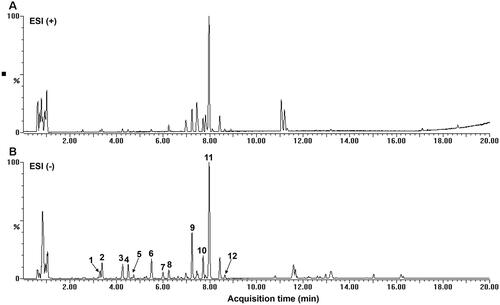
Table 2. UPLC-QTOF-MS/MS data for the identification of components from BCE.
Effect of BCE on faeces number and moisture content in loperamide-induced constipation mice
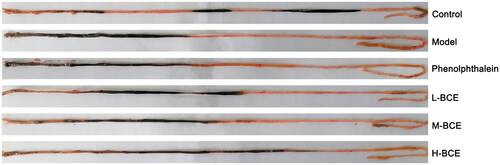
Compared with the control group, the faeces number and moisture content within 3 h in the model group were significantly decreased (p < 0.01) (). When treated by BCE, the faeces number in the L-BCE group and M-BCE group were increased (p < 0.05). Moreover, after treatment with L-BCE, M-BCE, H-BCE and phenolphthalein, the water content levels were increased compared with the model group (p < 0.05).
Table 3. The faecal water content, number of faeces, defecating time of first black faeces and intestinal transit rate in loperamide-induced constipation mice after treatment.
Effect of BCE on first black excretion time in loperamide-induced constipation mice
The time to the first black excretion time was significantly prolonged in the model group compared with the control group (p < 0.01). After treatment with M-BCE and H-BCE, the first black excretion time was decreased compared with the model group (p < 0.05) ().
Effect of BCE on intestinal transit rate in loperamide-induced constipation mice
As shown in and , the intestinal transit ratio significantly decreased in the model group compared with the control group (p < 0.01). After treatment with BCE, the intestinal transit ratio increased in the H-BCE group compared with the model group (p < 0.05).
Histopathological alterations of colon
shows the histological alterations of the mouse colonic tissue with loperamide-induced constipation by H&E and AB/PAS staining. The animals treated with loperamide alone exhibited a loss of epithelium and goblet cells depletion of the colon compared with the control group (). Following treatment, mucosal damage was reduced when compared with the model group (). The colons of mice in the phenolphthalein and BCE (L-BCE, M-BCE and H-BCE) group restored the secretory activity of goblet cells, which were comparable to those of the model group ().
Parameters of serum
To evaluate the effects of BCE on serum biochemical components in the loperamide-induced constipation mice, alterations of several components related to gastrointestinal motility-related biomarkers in serum were assessed by ELISA. As shown in , the levels of MTL, Gas and SP were remarkably decreased in the model group compared with the control group (p < 0.01), while SS was increased although the effect was not noteworthy. With the intervention of BCE or phenolphthalein, MTL, Gas and SP were increased but SS was decreased compared with the model group (p < 0.05 or not noteworthy) (). Therefore, these results show that BCE treatment may regulate the biomarkers related to gastrointestinal motility to relieve loperamide-induced constipation in Kunming mice.
Table 4. The concentrations of MTL, Gas, SP and SS in loperamide-induced constipation mice after treatment.
Effect of BCE on the expression of AQP3 and C-kit in loperamide-induced constipation mice
AQP3 plays an important role in regulating water transport effect in the colon. Moreover, ICC is a pacemaker of the intestine that plays an important role in controlling intestinal movement. Thus, we investigated the effect of BCE on the expression of AQP3 and C-kit in the colons of Kunming mice with loperamide-induced constipation. As shown in and , the levels of AQP3 were increased in the model group compared with the control group (p < 0.05), whereas the levels of AQP3 were decreased in the BCE and phenolphthalein group compared with the model group (p < 0.05 or p < 0.01). C-kit expression was decreased in the mice of model group compared with the control group (p < 0.05). Interestingly, the C-kit expression was remarkably increased in H-BCE group (p < 0.01) ( and ).
Figure 4. BCE reduced the expression of AQP3 in colon. Protein expression of AQP3 in the colon was identified by immunohistochemical analysis, ×400. n = 10 per group.
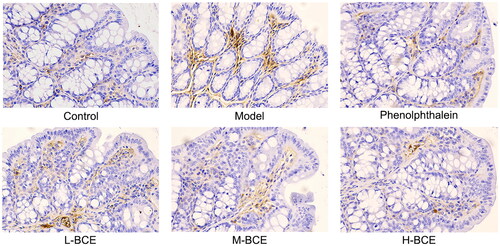
Figure 5. BCE improved the expression of C-kit in colon. Protein expression of C-kit in the colon was identified by immunohistochemical analysis, ×400. n = 10 per group.
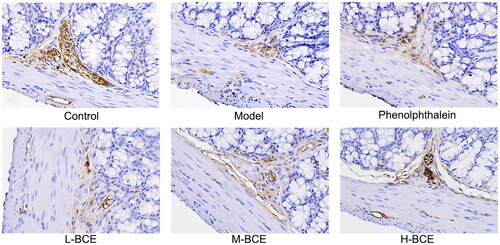
Table 5. The protein expression of AQP3 and C-kit in the colon of loperamide-induced constipation mice.
Discussion
Constipation is a common digestive tract disease (Liu et al. Citation2020) mainly caused by intestinal disorders, which is associated with infrequent bowel movements (Yin et al. Citation2018), altered bowel habits, difficulty during defecation and disappearance of defecation intention that causes discomfort and seriously affect the quality of life of patients (Wang et al. Citation2017). Some studies suggest that multiple plant extracts are attracting attention nowadays because of their laxative and there is hardly any side effect on constipation (Gilani et al. Citation2000; Han 2015; Lu et al. Citation2021). Moreover, according to the traditional Chinese medicine theory, the B. ceiba flower is cool-natured (Zhang et al. Citation2015), and is regarded as having laxative property (Shahat et al. Citation2003). BCE is an aqueous extract from B. ceiba flower, and our study suggests that BCE has a positive effect on relieving the symptoms of loperamide-induced constipation mice.
Loperamide, as an opioid receptor agonist (Hayeeawaema et al. Citation2020), is well known to decrease smooth muscle contraction and relaxation during intestinal peristalsis and delay gastrointestinal transit time (Wintola et al. Citation2010; Nelson and Camilleri Citation2015). Furthermore, loperamide has been used to induce spastic constipation (Wintola et al. Citation2010) in a variety of experiments to reveal the cause of constipation and determine novel compounds with therapeutic effects (Kakino et al. Citation2010; Lee et al. Citation2012; Yin et al. Citation2018). In the present study, the loperamide (model) group showed evident constipation symptoms, including significant decreases in faecal water content, faecal pellets number and small intestine transit rate and significant increases the time to the first blank faeces defecation in comparison to the control group. Moreover, there was no death in animals during the process of the study. This proves that loperamide could successfully induce the model of constipation which was relatively safe.
A common factor of constipation is that stool take longer to pass through the gastrointestinal tract, which cause stool to lose water and become hard-dry stool (Li et al. Citation2014). The transport function of the whole intestine could be reflected by the detection of the first black-stool defecation time (Lu et al. Citation2021), which is the sum time of the large intestine transit and the small intestine transit (Wang et al. Citation2017). A shorter time to the first black-stool defecation indicates a better effect of treatment and the prognosis; otherwise, the effect will be worse (Wang et al. Citation2017). Moreover, the intestinal transit rates were used to measure the small intestine transit time (Lu et al. Citation2021). A smaller value to the intestinal transit rates shows a better effect of treatment and the prognosis. The present study suggests that amount of defecation, moisture content of faeces, the intestinal transit rates, and the first black-stool defecation time have significantly changed in model of loperamide-induced constipation mice, while BCE could improve those parameters. In addition, phenolphthalein could only improve moisture content of faeces.
As it is known that mucins play an important role in the protection of the gastrointestinal epithelium, and quantitative change in mucin secretion might modify gastrointestinal defensive barrier (Wu et al. Citation2019). Moreover, most mucin is secreted by goblet cells in the gastrointestinal tract, which is a diverse family of densely glycosylated proteins with its characteristic ability to form gels, protect gastrointestinal tract and facilitate intestinal movement (Furness et al. Citation2013; Van Spaendonk et al. Citation2017). The results of our study indicated that BCE could restore the secretory activity of goblet cells. However, the precise mechanisms remain to be further researched.
The gastrointestinal motility-related biomarkers secreted by the intestinal nerve network act as neuromodulators and neurotransmitters to promote intestinal peristalsis and transportation, including excitatory factors (MTL, SP, Gas, etc.) (Yin et al. Citation2018) and inhibitory factor (SS, NO, etc.) (Gan et al. Citation2020). Abnormal secretion of enteric motility-related biomarkers may be the main pathogenesis of constipation (Liu et al. Citation2020). MTL is the intestinal hormone which can increase the migrating motor complex of gastrointestinal motility (Yin et al. Citation2018), but it has lower serum MTL levels in constipation children (Sun et al. Citation2020). As an excitatory neurotransmitter of intestinal nerve network, SP strongly promotes smooth muscle contraction, promotes gastrointestinal peristalsis and stimulates intestinal water and electrolyte secretion (Li et al. Citation2020). Gas, mainly secreted by G cells in the pyloric antrum of the stomach (Iijima et al. Citation2014), promotes gastrointestinal motility and stimulates the secretion of gastric acid by the parietal cells of the stomach (Gan et al. Citation2020). However, the secretion of Gas is inhibited by SS (Jiang et al. Citation2020). SS could suppress the movement of gastrointestinal smooth muscle and inhibit the secretion of gastrointestinal hormones (Han Citation2013). According to current ELISA results, with the intervention of phenolphthalein, MTL was increased but SS was decreased compared with the model group. In addition, compared with the model group, BCE significantly increased levels of serum MTL, SP, Gas, while inhibited serum SS. Nevertheless, the concrete relationship and mechanism between them still need further research.
Aquaporins are mainly expressed in the intestinal canal (Sun et al. Citation2020), which mediate the passive transport of free water across biofilm, thereby maintaining the homeostasis of the intracellular and extracellular environment (Matsuzaki et al. Citation2004). Moreover, the abnormal expression of aquaporins in the gastrointestinal tract is related to the occurrence of some diseases, such as constipation, gastritis, diarrhoea and gastric cancer (Liu et al. Citation2020). AQP3 is an important aquaporin located in the colon and is permeable to water (Li and Wang Citation2017). Previously studies have revealed the relationship between AQP3 and constipation, particularly morphine-induced constipation (Kon et al. Citation2015), promotes AQP3 expression level in the colon and subsequently increases water absorption from the luminal side to vasculature, which dries and hardens stool (Ikarashi et al. Citation2016). In the present study, the expression level of AQP3 was detected and the results showed that phenolphthalein and BCE relieve the symptoms of loperamide-induced constipation by decreasing the level of AQP3 in the colon of mice. However, the precise mechanisms remain to be further elucidated.
Interstitial cells of Cajal, as the gastrointestinal pacemaker cells (Yin et al. Citation2018), may play an important role in regulating neurotransmitters (Lees-Green et al. Citation2011), producing the smooth muscle electrical slow wave and conducting slow-wave potential, thereby coordinating intestinal motility (Xu et al. Citation2013). Moreover, the slow wave appears to be generated by submucosal ICC in the colon, which determines smooth muscle contractile activity (Li et al. Citation2015). The significant decrease of ICC may produce abnormal slow wave (Li et al. Citation2015), which can inhibit smooth muscle contractile activity and disrupt normal colonic motility, resulting in constipation (Su et al. Citation2019). As tyrosine kinase receptors of ICC, C-kit is critical in the maintenance of the ICC network and loss of C-kit expression may suggest the disruption of the ICC network in patients with constipation (Li et al. Citation2015). C-kit can be reliably identified by IHC techniques (Yin et al. Citation2018), which indirectly reflects the quantity and density of ICC (Liu et al. Citation2020). The results of our study indicated that BCE could increase the expression of c-kit protein, which protect the expression of ICC. But its mechanism might need further research in the future.
Nowadays, researchers have isolated and identified a variety of monomeric compounds from B. ceiba flower, including flavonoids, phenylpropanoid and phenolic acid compounds. Such as alanopine, angoletin (Komati et al. Citation2022), quercetin 3-glucuronide, rutin, neochlorogenic acid, 4-coumaroylquinic acid, caffeic acid, ferulic acid, syringic acid, protocatechuic acid, etc. (El-Hagrassi et al. Citation2011; Joshi et al. Citation2013; Zhang et al. Citation2015). In this article, 12 components in BCE were detected by UPLC-ESI-QTOF-MS. Among the 12 components, protocatechuic acid, chlorogenic acid, rutin and isoquercetin showed promising pharmacological activities in constipation (Rtibi et al. Citation2019), smooth muscle-relaxing (Tóth et al. Citation2015) and anti-inflammatory (Ezenyi et al. Citation2018).
Notably, except for constipation, B. ceiba is also traditionally used for the treatment of diarrhoea (Zhang et al. Citation2015), but scientific basis and study for this effect have not been provided (Yu et al. 2011; Arafa et al. Citation2019). Similar dual efficacy for diarrhoea and constipation has been reported for some popular herbs, such as Ginger and Psyllium husk (Mehmood et al. Citation2011), which is probably meant by the nature to offset the side-effects, such as diarrhoea and constipation usually observed with antidiarrheal chemical agents and laxative respectively when used alone at high doses (Palla and Gilani Citation2015). It may fit in the philosophy that natural products should be valued as a whole (Ahmed and Gilani Citation2014), as they have multiple targets to address disease along with having side-effect neutralizing potential (Gilani and Rahman Citation2005). However, further research on the antidiarrheal efficacy of BCE is needed.
Conclusions
BCE significantly increased the water content of faeces after constipation modelling, and BCE at a high‐dose exhibited the best effect for constipation relief. BCE could increase the number of faeces and promote small intestinal transit. The same tendency was observed for the time to the first black stool defecation. The potential mechanism may be associated with restoring the secretory activity of goblet cells, regulating the content of intestinal hormones and reducing the expression of AQP3 in the colon. In addition, BCE might improve the slow-wave potential of colon and regulate smooth muscle contractile activity by up-regulating c-kit in ICC. The extracts contained valuable flavonoids, and phenylpropanoids, they were likely the active compounds contributing to the therapeutic effect of BCE. These results suggested that BCE may be effective as a candidate in patients suffering from constipation.
Disclosure statement
The authors have no conflict of interest to report.
Additional information
Funding
References
- Ahmed T, Gilani AH. 2014. Therapeutic potential of turmeric in Alzheimer’s disease: curcumin or curcuminoids? Phytother Res. 28(4):517–525.
- Arafa AF, Foda DS, Mahmoud AH, Metwally NS, Farrag ARH. 2019. Bombax ceiba flowers extract ameliorates hepatosteatosis induced by ethanol and relatively moderate fat diet in rats. Toxicol Rep. 6:401–408.
- Chen Z, Lin S, Jiang Y, Liu L, Jiang J, Chen S, Tong Y, Wang P. 2019. Effects of bread yeast cell wall beta-glucans on mice with loperamide-induced constipation. J Med Food. 22(10):1009–1021.
- El-Hagrassi AM, Ali MM, Osman AF, Shaaban M. 2011. Phytochemical investigation and biological studies of Bombax malabaricum flowers. Nat Prod Res. 25(2):141–151.
- Ezenyi IC, Onavbavba G, Adzu B, Okhale S, Adeola SO. 2018. Sesamum indicum leaf extract therapy for symptoms of acute malaria: evaluation of anti-inflammatory, antipyretic and analgesic effects. J Phytomed Ther. 17:167–179.
- Furness JB, Rivera LR, Cho HJ, Bravo DM, Callaghan B. 2013. The gut as a sensory organ. Nat Rev Gastroenterol Hepatol. 10(12):729–740.
- Gan Y, Liang J, Diao W, Zhou X, Mu J, Pang L, Tan F, Zhao X. 2020. Lactobacillus plantarum KSFY06 and geniposide counteract montmorillonite-induced constipation in Kunming mice. Food Sci Nutr. 8(9):5128–5137.
- Gao CC, Li GW, Wang TT, Gao L, Wang FF, Shang HW, Yang ZJ, Guo YX, Wang BY, Xu JD. 2021. Rhubarb extract relieves constipation by stimulating mucus production in the colon and altering the intestinal flora. Biomed Pharmacother. 138:111479.
- Gilani AH, Aziz N, Ali SM, Saeed M. 2000. Pharmacological basis for the use of peach leaves in constipation. J Ethnopharmacol. 73(1–2):87–93.
- Gilani AH, Rahman A. 2005. Trends in ethnopharmacology. J Ethnopharmacol. 100(1–2):43–49.
- Han B. 2013. Correlation between gastrointestinal hormones and anxiety-depressive states in irritable bowel syndrome. Exp Ther Med. 6(3):715–720.
- Han J, Shen WH, Jiang YZ, Yu B, He YT, Li N, Mei F. 2010. Distribution, development and proliferation of interstitial cells of Cajal in murine colon: an immunohistochemical study from neonatal to adult life. Histochem Cell Biol. 133(2):163–175.
- Han W, Xu JD, Wei FX, Zheng YD, Ma JZ, Xu XD, Wei ZG, Wang W, Zhang YC. 2015. Prokinetic activity of Prunus persica (L.) Batsch flowers extract and its possible mechanism of action in rats. Biomed Res Int. 2015:569853.
- Hayeeawaema F, Wichienchot S, Khuituan P. 2020. Amelioration of gut dysbiosis and gastrointestinal motility by konjac oligo-glucomannan on loperamide-induced constipation in mice. Nutrition. 73:110715.
- Huizinga JD, Chen JH. 2014. Interstitial cells of Cajal: update on basic and clinical science. Curr Gastroenterol Rep. 16(1):363.
- Iijima K, Koike T, Abe Y, Shimosegawa T. 2014. Cutoff serum pepsinogen values for predicting gastric acid secretion status. Tohoku J Exp Med. 232(4):293–300.
- Ikarashi N, Kon R, Sugiyama K. 2016. Aquaporins in the colon as a new therapeutic target in diarrhea and constipation. Int J Mol Sci. 17(7):1172.
- Jiang H, Dong J, Jiang S, Liang Q, Zhang Y, Liu Z, Ma C, Wang J, Kang W. 2020. Effect of Durio zibethinus rind polysaccharide on functional constipation and intestinal microbiota in rats. Food Res Int. 136:109316.
- Joshi KR, Devkota HP, Yahara S. 2013. Chemical analysis of flowers of Bombax ceiba from Nepal. Nat Prod Commun. 8:583–584.
- Kakino M, Izuta H, Ito T, Tsuruma K, Araki Y, Shimazawa M, Oyama M, Iinuma M, Hara H. 2010. Agarwood induced laxative effects via acetylcholine receptors on loperamide-induced constipation in mice. Biosci Biotechnol Biochem. 74(8):1550–1555.
- Komati A, Anand A, Nagendla NK, Madhusudana K, Mudiam MKR, Babu KS, Tiwari AK. 2022. Bombax ceiba calyx displays antihyperglycemic activity via improving insulin secretion and sensitivity: identification of bioactive phytometabolomes by UPLC-QTof-MS/MS. J Food Sci. 87(4):1865–1881.
- Kon R, Ikarashi N, Hayakawa A, Haga Y, Fueki A, Kusunoki Y, Tajima M, Ochiai W, Machida Y, Sugiyama K. 2015. Morphine-induced constipation develops with increased aquaporin-3 expression in the colon via increased serotonin secretion. Toxicol Sci. 145(2):337–347.
- Lee HY, Kim JH, Jeung HW, Lee CU, Kim DS, Li B, Lee GH, Sung MS, Ha KC, Back HI, et al. 2012. Effects of Ficus carica paste on loperamide-induced constipation in rats. Food Chem Toxicol. 50(3–4):895–902.
- Lees-Green R, Du P, O'Grady G, Beyder A, Farrugia G, Pullan AJ. 2011. Biophysically based modeling of the interstitial cells of Cajal: current status and future perspectives. Front Physiol. 2:29.
- Li C, Nie SP, Zhu KX, Xiong T, Li C, Gong J, Xie MY. 2015. Effect of Lactobacillus plantarum NCU116 on loperamide-induced constipation in mice. Int J Food Sci Nutr. 66(5):533–538.
- Li C, Wang W. 2017. Molecular biology of aquaporins. Adv Exp Med Biol. 969:1–34.
- Li G, Wang Q, Qian Y, Zhou Y, Wang R, Zhao X. 2014. Component analysis of Pu-erh and its anti-constipation effects. Mol Med Rep. 9(5):2003–2009.
- Li X, Liu Y, Guan W, Xia Y, Zhou Y, Yang B, Kuang H. 2019. Physicochemical properties and laxative effects of polysaccharides from Anemarrhena asphodeloides Bge. in loperamide-induced rats. J Ethnopharmacol. 240:111961.
- Li Y, Long S, Liu Q, Ma H, Li J, Wei X, Yuan J, Li M, Hou B. 2020. Gut microbiota is involved in the alleviation of loperamide-induced constipation by honey supplementation in mice. Food Sci Nutr. 8(8):4388–4398.
- Liu S, Sui D, Fu W, Yu X, Li Y, Wu X, Hou Y, Guo M, Xu H. 2020. Laxative effects of Yangyin Tongmi capsule on a model of diphenoxylate-induced constipation in mice. Evid Based Complement Alternat Med. 2020:1471824.
- Lu Y, Zhang J, Zhang Z, Liang X, Liu T, Yi H, Gong P, Wang L, Yang W, Zhang X, et al. 2021. Konjac glucomannan with probiotics acts as a combination laxative to improve relieve constipation in mice by increasing short-chain fatty acid metabolism and 5-hydroxytryptamine hormone release. Nutrition. 84:111112.
- Ma H, Xiong H, Zhu X, Ji C, Xue J, Li R, Ge B, Cui H. 2019. Polysaccharide from Spirulina platensis ameliorates diphenoxylate-induced constipation symptoms in mice. Int J Biol Macromol. 133:1090–1101.
- Matsuzaki T, Tajika Y, Ablimit A, Aoki T, Hagiwara H, Takata K. 2004. Aquaporins in the digestive system. Med Electron Microsc. 37(2):71–80.
- Mehmood MH, Aziz N, Ghayur MN, Gilani AH. 2011. Pharmacological basis for the medicinal use of Psyllium husk (Ispaghula) in constipation and diarrhea. Dig Dis Sci. 56(5):1460–1471.
- Mori T, Shibasaki Y, Matsumoto K, Shibasaki M, Hasegawa M, Wang E, Masukawa D, Yoshizawa K, Horie S, Suzuki T. 2013. Mechanisms that underlie μ-opioid receptor agonist-induced constipation: differential involvement of μ-opioid receptor sites and responsible regions. J Pharmacol Exp Ther. 347(1):91–99.
- Nelson AD, Camilleri M. 2015. Chronic opioid induced constipation in patients with nonmalignant pain: challenges and opportunities. Therap Adv Gastroenterol. 8(4):206–220.
- Palla AH, Gilani AH. 2015. Dual effectiveness of flaxseed in constipation and diarrhea: possible mechanism. J Ethnopharmacol. 169:60–68.
- Payne I, Grimm LM. 2017. Functional disorders of constipation: paradoxical puborectalis contraction and increased perineal descent. Clin Colon Rectal Surg. 30(1):22–29.
- Rtibi K, Selmi S, Wannes D, Jridi M, Marzouki L, Sebai H. 2019. The potential of Thymus vulgaris aqueous extract to protect against delayed gastric emptying and colonic constipation in rats. RSC Adv. 9(36):20593–20602.
- Shahat AA, Hassan RA, Nazif NM, Van Miert S, Pieters L, Hammuda FM, Vlietinck AJ. 2003. Isolation of mangiferin from Bombax malabaricum and structure revision of shamimin. Planta Med. 69(11):1068–1070.
- Shin A, Camilleri M, Kolar G, Erwin P, West CP, Murad MH. 2014. Systematic review with meta-analysis: highly selective 5-HT4 agonists (prucalopride, velusetrag or naronapride) in chronic constipation. Aliment Pharmacol Ther. 39(3):239–253.
- Su H, Chen J, Miao S, Deng K, Liu J, Zeng S, Zheng B, Lu X. 2019. Lotus seed oligosaccharides at various dosages with prebiotic activity regulate gut microbiota and relieve constipation in mice. Food Chem Toxicol. 134:110838.
- Sun Y, Yan C, Jin S, Shi C, Zhao J, Li G. 2020. Curative effect and mechanism of Guiren Runchang granules on morphine-induced slow transit constipation in mice. Evid Based Complement Alternat Med. 2020:5493192.
- Tóth B, Bartho L, Vasas A, Sándor Z, Jedlinszki N, Pinke G, Hohmann J. 2015. Dual excitatory and smooth muscle-relaxing effect of Sideritis montana extract on guinea-pig ileum. Nat Prod Commun. 10(3):487–490.
- Van Spaendonk H, Ceuleers H, Witters L, Patteet E, Joossens J, Augustyns K, Lambeir AM, De Meester I, De Man JG, De Winter BY. 2017. Regulation of intestinal permeability: the role of proteases. World J Gastroenterol. 23(12):2106–2123.
- Wang L, Hu L, Xu Q, Jiang T, Fang S, Wang G, Zhao J, Zhang H, Chen W. 2017. Bifidobacteria exert species-specific effects on constipation in BALB/c mice. Food Funct. 8(10):3587–3600.
- Wintola OA, Sunmonu TO, Afolayan AJ. 2010. The effect of Aloe ferox Mill. in the treatment of loperamide-induced constipation in Wistar rats. BMC Gastroenterol. 10:95.
- Wu D, Xue X, Gao C, Liu Y, Wang T, Li L, Tong X, Li F, Xu J. 2019. Rhubarb-evoke mucus secretion through aggregation and degranulation of mast cell in the colon of rat: in vivo and ex vivo studies. Sci Rep. 9(1):19375.
- Xu GK, Qin XY, Wang GK, Xie GY, Li XS, Sun CY, Liu BL, Qin MJ. 2017. Antihyperglycemic, antihyperlipidemic and antioxidant effects of standard ethanol extract of Bombax ceiba leaves in high-fat-diet- and streptozotocin-induced type 2 diabetic rats. Chin J Nat Med. 15(3):168–177.
- Xu J, Chen Y, Liu S, Hou X. 2013. Electroacupuncture regulates apoptosis/proliferation of intramuscular interstitial cells of Cajal and restores colonic motility in diabetic constipation rats. Evid Based Complement Alternat Med. 2013:584179.
- Yin J, Liang Y, Wang D, Yan Z, Yin H, Wu D, Su Q. 2018. Naringenin induces laxative effects by upregulating the expression levels of c-Kit and SCF, as well as those of aquaporin 3 in mice with loperamide-induced constipation. Int J Mol Med. 41(2):649–658.
- Yu YG, He QT, Yuan K, Xiao XL, Li XF, Liu DM, Wu H. 2011. In vitro antioxidant activity of Bombax malabaricum flower extracts. Pharm Biol. 49(6):569–576.
- Zhang X, Yang H, Zheng J, Jiang N, Sun G, Bao X, Lin A, Liu H. 2021. Chitosan oligosaccharides attenuate loperamide-induced constipation through regulation of gut microbiota in mice. Carbohydr Polym. 253:117218.
- Zhang Y-B, Wu P, Zhang X-L, Xia C, Li G-Q, Ye W-C, Wang G-C, Li Y-L. 2015. Phenolic compounds from the flowers of Bombax malabaricum and their antioxidant and antiviral activities. Molecules. 20(11):19947–19957.
- Zhao X, Qian Y, Li G, Yi R, Park KY, Song JL. 2019. Lactobacillus plantarum YS2 (yak yogurt Lactobacillus) exhibited an activity to attenuate activated carbon-induced constipation in male Kunming mice. J Dairy Sci. 102(1):26–36.