Abstract
Context
Previously, we found Alisma orientalis beverage (AOB), a classic traditional Chinese medicine (TCM) formulation, had the potential effect of treating atherosclerosis (AS). The underlying mechanism was still unclear.
Objective
As an extention of our previous work, to investigate the underlying mechanism of action of AOB in the treatment for AS.
Materials and methods
Network pharmacology was conducted using SwissTargetPrediction, GeneCards, DrugBank, Metascape, etc., to construct component-target-pathway networks. In vivo, AS models were induced by a high-fat diet (HFD) for 8 consecutive weeks in APOE−/− mice. After the administration of AOB (3.8 g/kg, i.g.) for 8 weeks, we assessed the aortic plaque, four indicators of blood lipids, and expression of the PI3K/AKT/SREBP-1 pathway in liver.
Results
Network pharmacology showed that PI3K/AKT/SREBP-1 played a role in AOB’s treatment for AS (PI3K: degree = 18; AKT: degree = 17). Moreover, we found that the arterial plaque area and four indicators of blood lipids were all significantly reversed by AOB treatment in APOE−/− mice fed with HFD (plaque area reduced by about 37.75%). In addition, phosphorylated expression of PI3K/AKT and expression of SREBP-1 were obviously increased in APOE−/− mice fed with HFD, which were all improved by AOB (PI3K: 51.6%; AKT: 23.6%; SREBP-1: 40.0%).
Conclusions
AOB had therapeutic effects for AS by improving blood lipids and inhibition of the PI3K/AKT/SERBP-1 pathway in the liver. This study provides new ideas for the treatment of AS, as well as new evidence for the clinical application of AOB.
Introduction
The latest data from the Global Burden of Disease (GBD) showed that approximately 18.6 million people worldwide died of cardiovascular disease in 2019, which has surpassed infectious diseases as the leading cause of death and disability worldwide (Roth et al. Citation2020). Atherosclerosis (AS) is the main factor leading to the global epidemic of cardiovascular and cerebrovascular diseases, which is mainly characterized by lipid deposition and chronic inflammation in the arterial wall (Roth et al. Citation2020). Atherosclerosis (AS) is characterized by fibrofatty lesions formed on the inner wall of arteries and is the primary pathological basis of cardiovascular and cerebrovascular diseases (Kobiyama and Ley Citation2018). Increasing evidence has indicated that hypercholesterolemia-induced vascular inflammation and cholesterol deposition together constitute a risk factor for AS (Koeth et al. Citation2019). Statins, lipid-lowering drugs such as atorvastatin and rosuvastatin, are the first-line drugs for the treatment of AS in modern medicine and they have significant clinical efficacy in lowering blood lipids, but they can also cause adverse reactions such as liver damage and rhabdomyolysis (Soppert et al. Citation2020; Aryal et al. Citation2021). The latest study showed that a serine protease, PCSK9, actively targets LDL-R and causes its excessive accumulation, while PCKS9 inhibitors significantly reduce LDL-C levels and reverse plaque-like changes (Solanki et al. Citation2018). However, the high cost of the compound and lack of long-term safety and efficacy data limit its use in patients. Therefore, there is an urgent need to find drugs with safe effects and better efficacy.
Hypercholesterolemia is recognized as the main factor leading to AS; reducing blood cholesterol levels is an important way to prevent the development of AS (Francis Citation2010). Various studies suggested that lipid metabolism mechanisms play a key role in the pathophysiology of AS and that elevated LDL cholesterol leads to AS independent of inflammation, whereas residual cholesterol can drive the inflammatory component of AS (Geovanini and Libby Citation2018). However, this evidence was not capable of solving the root cause of treating AS. Alisma orientalis beverage (AOB) was first recorded in ‘Huangdi Neijing’, an ancient Chinese medical book, consisted of three herbs including Alismatis rhizoma (Sam.) Juzep. (Alismataceae) (Zexie), Atractylodis macrocephalae rhizoma Koidz. (Asteraceae) (Baizhu), and Pyrolae calliantha H. Andres (Pyrolaceae) (Luxiancao) based on the Chinese Pharmacopeia (2020 Edition). A previous study found that AOB can effectively inhibit the progression of atherosclerosis and improvement of blood lipid levels, and its mechanism of mitigating atherosclerosis may be related to gut microbiota and its metabolite (Zhu, Zhai, et al. Citation2020). However, how AOB influenced blood lipid levels was not identified. Due to the complex components of this formula, it is difficult to explore multiple targets in traditional Chinese medicine (TCM) formulation. Therefore, the underlying mechanism of AOB’s therapeutic actions have not been fully elucidated.
TCM has characteristics of multi-component, multi-target and integrity. Network pharmacology is based on theories of systems biology, genomics, proteomics and other disciplines, using high-throughput omics data analysis, computer simulation and network database retrieval (Hopkins Citation2008). The technology reveals the network relationship of drug-gene-target-disease interactions, predicts the mechanism of action of drugs through network relationships, evaluates drug efficacy, adverse reactions, etc., explores essential attributes of TCM by referring to the research ideas of network pharmacology, and has achieved good preliminary results in revealing the comprehensive overall effect of multiple pathways, multiple targets and multiple components of TCM (Zeng et al. Citation2019; Zhang et al. Citation2019; Zhu, Cai, et al. Citation2020). This study adopted network pharmacological results to pre-clinical experiments, starting from the material basis of AOB, analyzing and exploring the mechanism of action of AOB in the treatment for AS, and at the same time providing a certain theoretical basis for clinical application and follow-up research.
Materials and methods
Collection of chemical components for AOB and screening of active compounds
We used Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP, https://old.tcmsp-e.com/tcmsp.php) to screen the active ingredients of each herb (Ru et al. Citation2014). We then identified compounds with oral bioavailability (OB) ≥ 30% (Xu, Zhang, et al. Citation2012) and drug-likeness (DL) ≥ 0.18 (Jia et al. Citation2020) in AOB as compounds with pharmacological activity, based on absorption, distribution, metabolism and excretion (ADME) characteristics of the drugs in the body. After the preliminary screening of compounds, the PubChem database (https://pubchem.ncbi.nlm.nih.gov) was used to confirm their molecular structure and name, to improve the credibility of screening results. The identified molecules were entered into the SwissTargetPrediction website (swisstargetprediction.ch) to find the protein targets of the active compounds, and related targets were added based on published literature reports. Then, the screened protein targets were unified in the Uniprot protein database (https://www.uniprot.org) for specification and protein-gene docking for further prediction and analysis.
Prediction of potential targets of AOB for treatment
GeneCards is a searchable comprehensive database that automatically integrates gene-centric data from approximately 150 web sources, including genomics, transcriptomics, proteomics, genetics, clinical and functional information (Rebhan et al. Citation1997). With ‘Atherosclerosis’ as the keyword, relevant gene target information was searched in the GeneCards database (https://www.genecards.org) (Rebhan et al. Citation1997), and potential genes were supplemented using the TTD database (http://db.idrblab.net/ttd/) (Hamosh et al. Citation2005). When the number of targets is too large, the Score value in the Genecards database can be used for screening. The larger the score value, the closer the relationship between the target and the disease. The median of the Score value is used as the screening value. When there is too much data, multiple screening can be performed to obtain AS-related targets. The intersection of drug component-related targets and AS targets was operated by Venny2.1 (https://bioinfogp.cnb.csic.es/tools/venny/).
Construction of an active compound-disease-target network
Upload the intersection of targets to the STRING11.0 database (https://string-db.org) (Szklarczyk et al. Citation2017) to construct a protein-protein interaction (PPI) network model, set the biological species to ‘Homo sapiens’, and set ‘highest confidence’ > 0.9. The PPI network was obtained by screening, and the PPI network was further clustered by Cytoscape_v3.8.2 (Shannon et al. Citation2003) to obtain potential protein functional modules. The core targets are selected according to the comprehensive ranking of node connectivity (degree), node closeness (closeness) and node betweenness (betweenness).
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis
The core target genes obtained in the above steps were uploaded to the Metascape platform (http://metascape.org) (Zhou et al. Citation2019), and a threshold of p < 0.01 was set. The main biological processes and metabolic pathways were analyzed, including the KEGG pathway, GO biological process, Cell composition, and molecular function enrichment analysis. Then the data were saved and the results were visualized. Finally, a visualization network of ‘prescriptions – traditional Chinese medicine – chemical components – core targets – key pathways’ was built.
The preparation process of AOB
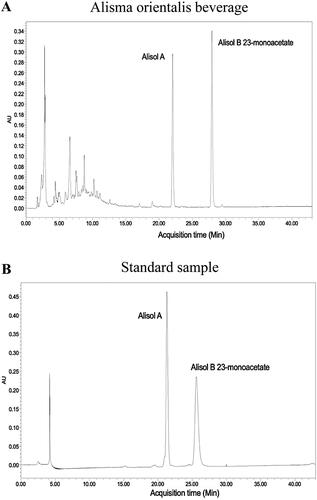
Alismatis rhizoma, Atractylodis macrocephalae rhizoma, and Pyrolae herba were purchased from Tong Ren Tang, Beijing. Alismatis rhizoma (100 ± 5 g), Atractylodis macrocephalae rhizoma (100 ± 5 g) and Pyrolae herba (50 ± 2 g) were placed into 5 L water to soak for 1 h. After soaking, we put the drug at 100 °C for condensation reflux extraction for 2 h and then recycled the medicine liquid and performed rotary evaporation and concentration at a constant temperature of 55 °C. After the concentrated medicinal liquid was recovered, it was freeze-dried at −60 °C and made into freeze-dried powder. The yield is 33.7%, which meets the requirements of drug preparation. The HPLC profile is shown in . Both the peak area and concentration of Alisol A and Alisol B 23-monoacetate in AOB are presented in .
Table 1. Components of AOB observed by HPLC.
Animals
Ten male C57BL/6J mice and 20 male APOE−/− mice were purchased from Changzhou Cavens Laboratory Animal Co., Ltd. (NO. SCXK (SU)-2016-0010). The weight of the mice was 22-24 g and the age of the mice was 4 weeks. All mice were acclimated to a standard rearing environment (Temperature: 18–22 °C, Humidity: 50–60%) for 1 week with 5 mice per cage before experiments carried out. All animal experiments were approved by the Animal Ethics Committee of Nanjing University of Chinese Medicine (NO. 202106A024). All animal experiments complied with animal ethics and all experiments were double-blind.
High-fat diet model (HFD), experimental design and AOB treatment
We designed three groups including control (CON), model (MOD), Alisma orientalis beverage (AOB). In the study, C57BL/6J mice were assigned to the control group, which were fed with a normal mouse diet; and APOE−/− were randomly assigned to the MOD group and AOB group. Mice in the MOD group were fed with a high-fat diet model for 8 weeks; AOB (3.8 g/kg, i.g.) was given daily while feeding with a high-fat diet for 8 weeks in the AOB group. All drugs followed the dosage of the Chinese Pharmacopoeia and did not impair liver and kidney function in mice after 8 weeks of AOB ().
Table 2. Liver function and renal function after AOB treatment for 8 weeks.
Analysis of blood lipids
All mice were anesthetized by using pentobarbital sodium (45 mg/kg; i.p.). After anesthetization, blood was collected by enucleating the eyeball. The collected blood was centrifuged at low speed (3000 rpm) at 4 °C for 10 min, then collected supernatant and stored in −20 °C. High-density lipoprotein cholesterol (HDL-C) and triglycerides (TG), the serum levels of CHO and low-density lipoprotein cholesterol (LDL-C) were measured using a Chemray 240 automatic biochemical analyzer (Wuhan Servicebio Technology, Co., Ltd., China). All experiments were performed as described by the manufacturer.
Aortic plaque analysis
The heart and the aortic arch were taken out at a low temperature of 4 °C, and the residual blood was washed with 0.01 M PBS. Tissues were placed in a cryostat (Leica, Germany) and serial sections (10 μm) from the aortic sinus to the aortic arch were made from the aortic root according to anatomical markers for histological examination of atherosclerotic aortic sinus lesions. Oil red O staining (ORO) and HE staining were subsequently performed. The plaque area was analyzed using Image Pro Plus 6.0 (Image analysis software, Media Cybernetics, Rockville, MD, USA).
Western blot
The mouse liver tissue (100 mg/kg) was taken out at a low temperature and placed in a lysate for sufficient grinding to a particle-free state, followed by the addition of protease inhibitors. We then centrifuged for 10 min and took the supernatant for a protein concentration test (protein concentration by BCA method). Moreover, the samples were equalized according to the protein concentration and cooked at 100 °C for 5 min with Loading buffer added until the protein was stable. After the target protein was separated by gel electrophoresis (80 v, 90 min), which was transferred to the PVDF membrane under constant flow (300 mA, 60 min, 4 °C). After 18 h of primary antibody including pPI3K (1:1000), PI3K (1:1000), pAKT (1:1000), AKT (1:1000), SREBP-1 (1:1000), GAPDH (1:5000) incubation at 4 °C, we made 2 h of secondary antibody (IgG-Rabbit, 1:4000) incubation at room temperature (20–26 °C), ECL imaging was performed. Visualization of the blot was performed with the chemiluminescent substrate SuperSignal West Pico (Thermo Fisher Science Inc.) and displayed as density relative to GAPDH. Experiments were performed at least 3 times.
Statistical analysis
All data were shown in the form of mean ± SEM. One-way ANOVA was used with the honestly important difference from Tukey or the post-hoc test from Dunnett. For all statistical tests, GraphPad Prism 8.0 was used, and one-way ANOVA was used in three groups. p < 0.05 was considered statistically significant.
Results
Identification of potential action targets of AOB
We collected 137 compounds in AOB from the TCMSP database, 46 of which belonged to Alismatis rhizoma, 55 belonged to Atractylodis macrocephalae rhizoma and 36 belonged to Pyrolae herba. After screening by ADME, a total of 7 active ingredients of Alismatis rhizoma, 4 from Atractylodis macrocephalae rhizoma, 5 from Pyrolae herba, and 1 common active ingredient from Alismatis rhizoma and Pyrolae herba were obtained, including Alisma alcohol, kaempferol, quercetin, gallic acid, atractylodes lactone, etc. (). Furthermore, we collected the targets of 17 active compounds in AOB from the TCMSP. After the integration of UniProt database entries and the deletion of duplicates, 601 targets were obtained ().
Table 3. The active compounds of AOB.
Table 4. The targets of active compounds in AOB.
We collected 4481 AS targets from the Genecards database. The median of the Score value was used as the screening value, so the target with a Score > 2.77 was set as the potential target of AS. We combined with OMIM, TTD, and DRUGBANK databases to supplement relevant targets, and deleted duplicate values after merging, and finally obtaines 1128 dyslipidemia-related targets. We took the intersection of the screened drug active ingredient targets and AS targets, and drew Venn diagrams through Venny2.1 to obtain 171 common targets of AOB and AS ( and ).
Figure 2. Targets screening involved in AOB for the treatment of AS. Venn diagram of disease targets.
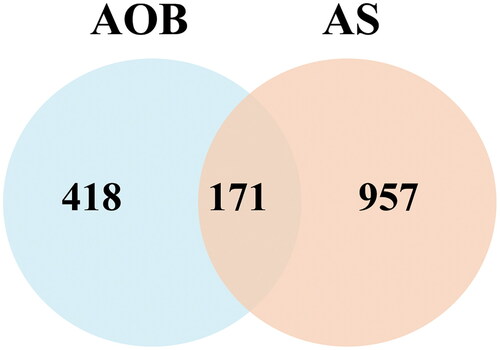
Table 5. The 171 common targets of AOB and AS.
The potential targets of AOB for the treatment of AS
To comprehensively elucidate the possible mechanism of AOB in the treatment of AS, 171 AOB anti-AS target gene names were imported into the STRING database to construct a PPI network. The required interaction score was 0.9 and the disconnected node network was hidden to draw a PPI network graph (). To achieve better visualization and identify core targets, we build a network using Cytoscape based on target degrees. With this network, core targets were obtained: PIK3R1, AKT1, PIK3CA, MAPK1, PTPN11, EGFR and MAPK4 ( and ). These targets may be considered as primary targets of action for AOB for AS treatment, and their identification suggests that AOB treats AS through multiple potential targets.
Figure 3. The potential targets of AOB for the treatment of AS. (A) The PPI network was constructed by the STRING database. (B) Drawing the PPI core network with Cytoscape 3.8.2 for visual display.
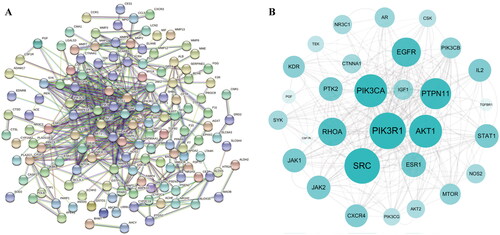
Table 6. The top 10 targets of the PPI Network.
GO and KEGG enrichment analysis for identification of the pathway mechanisms of AOB
The Metascape data platform was used to analyze the signal pathway of the related targets in the regulation of AS by AOB. AOB was mainly involved in the biological processes including regulation of cell adhesion, wound healing, positive regulation of protein phosphorylation, positive regulation of cell migration, etc. The main cellular components involved include membrane raft, the extrinsic component of the membrane, phosphatidylinositol 3-kinase complex, focal adhesion, etc. GO molecular functions of AOB involved include phosphotransferase activity, alcohol group as acceptor, protein kinase activity, kinase activity, kinase binding, protein kinase binding, phosphatase binding, transmembrane receptor protein tyrosine kinase activity, protein tyrosine kinase activity, transmembrane receptor protein kinase activity, protein phosphatase binding. The pathways involved mainly include cancer pathways, PI3K-AKT signaling pathway, EGFR tyrosine kinase inhibitor resistance, fluid shear stress and atherosclerosis, AGE-RAGE signaling pathway in diabetic complications, hepatitis B, etc. (). The top 10 significantly enriched (p < 0.01) terms in BP, CC and MF of GO analysis were selected (). The top 20 pathways with significant enrichment (p < 0.01) were selected ().
Figure 4. GO and KEGG enrichment analysis for identification of the pathway mechanisms of AOB. (A) The top 10 significantly enriched (p < 0.01) terms in BP, CC and MF of GO analysis were selected. (B) The top 20 pathways with significant enrichment (p < 0.01) were selected.
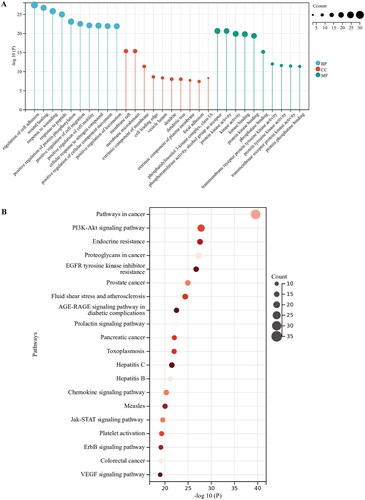
Table 7. KEGG enrichment analysis results.
Network-based revelation of Compound-Disease-Pathway-Target network correlations
Combined with the above analysis results, the connection between traditional Chinese medicine, disease, pathways and targets was established. CytoScape3.8.2 was used to construct a Compound-Disease-Pathway-Target network (). With the use of the built-in NetworkAnalyzer of CytoScape3.8.2, the network topology parameters of AOB treatment AS were analyzed, and the core components and core role targets were obtained. According to network analysis, 3 main components in the AOB treatment of AS: 16β-methoxyalisol B monoacetate (degree = 36), 3β-acetoxyatractylone (degree = 23), and 5, 2′-dihydroxy-6,7,8-trimethoxyflavone (degree = 19) (). Therefore, these compounds were regarded as the potential bioactive compounds of AOB against AS. Then, the top 10 core targets were selected according to the comprehensive ranking of degree, closeness and betweenness (). Interestingly, according to the network analysis results, the PIK3 family and AKT are the core targets of AOB in the treatment of AS, which is consistent with the previous PPI analysis results.
Figure 5. Compound-Disease-Pathway-Target Network. The orange hexagons represent AOB, the yellow hexagons represent AS, the green circles represent three traditional Chinese medicines, the blue diamonds around the green circles are the main components of the medicine, the red arrows represent the pathways, and the outermost blue circles are the targets. The darker the color, the more important the node is.
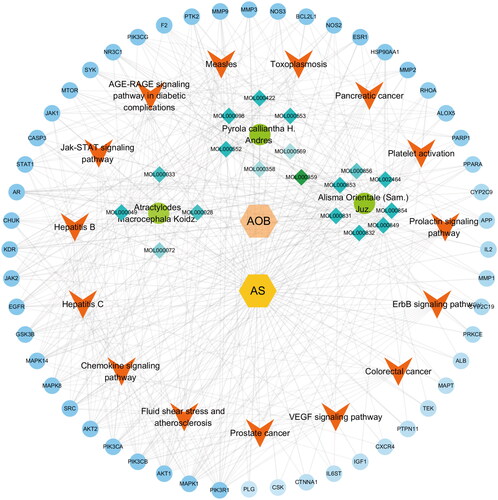
Table 8. Main components in the AOB treatment of AS.
Table 9. The top 10 targets of the Compound-Disease-Pathway-Target Network.
AOB reversed the aortic plaque area of as in APOE−/− mice stimulated with a high-fat diet
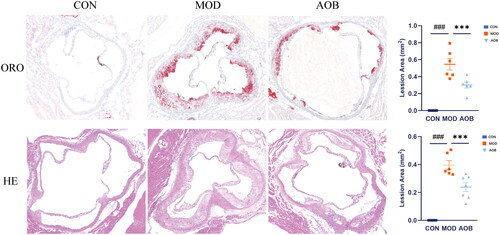
We performed an AS model with a high-fat diet (HFD). We found that C57BL/6J mice fed with a normal diet for 8 weeks showed no change in arterial plaque area and APOE−/− mice fed with HFD for 8 weeks showed a significant increase in arterial plaque area, which was compared with C57BL/6J mice fed with normal diet (). However, after AOB treatment for 8 weeks, the arterial plaque area was significantly reversed both in ORO and HE staining (; One-way ANOVA, ORO, F (2, 26) = 62.35, p < 0.001; HE, F (2, 26) = 86.91, p < 0.001). These data suggested that AOB had a potential effect to treat AS.
Figure 6. AOB reversed aortic plaque area of AS in APOE−/− mice. HE and ORO stained sections of aortic valve area in the control group, the model group and the AOB group. The atherosclerotic lesion area was quantitatively analyzed by Image J. Data show mean ± SEM values of 6 or more independent samples. # Represents comparison with the control group, ###represents p < 0.001; * represents comparison with the model group, *** represents p < 0.001.
AOB improved four indicators of blood lipids of as in APOE−/− mice stimulated with a high-fat diet
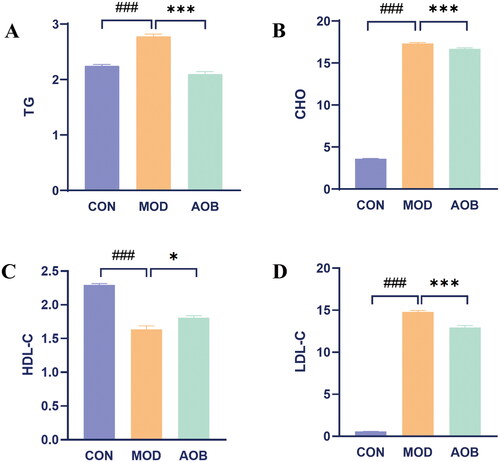
We then detected four indicators of blood lipids related to AS including TG, CHO, HDL and LDL. We found that TG, CHO, LDL were all increased after HFD feeding in APOE−/− mice for 8 weeks, which were all reversed obviously by AOB for 8 weeks [, One-way ANOVA, TG, F (2, 17) = 85.17, p < 0.001; CHO, F (2, 17) = 59.30, p < 0.001; LDL, F (2, 17) = 19.20, p < 0.001]. Moreover, HDL in blood serum was decreased after HFD feeding in APOE−/− mice for 8 weeks, which was also reversed obviously by AOB for 8 weeks [, One-way ANOVA, HDL, F (2, 17) = 76.44, p < 0.001].
Figure 7. AOB improved four indicators of blood lipid of AS in APOE−/− mice stimulated with high-fat diet. Data show mean ± SEM values of 6 independent samples. # Represents comparison with the control group, ### represents p < 0.001; * represents comparison with the model group, * represents p < 0.05, *** represents p < 0.001.
AOB alleviated as by regulating PI3K pathway
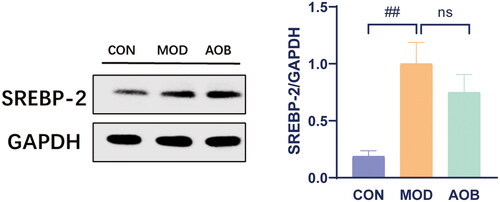
Based on our network pharmacological analysis, we chose the PI3K pathway to reveal the underlying mechanism of AOB treatment for AS. Then we measured the levels of PI3K/AKT/SREBP-1 in the liver. The results showed that HFD increased phosphorylated expressions of PI3K/AKT and expression of SREBP-1 in APOE−/− mice compared with C57BL/6J mice fed with a normal diet. Interestingly, AOB treatment for 8 weeks reversed all of them in the liver [, One-way ANOVA, pPI3K/PI3K, F (2, 17) = 6.997, p = 0.0147; pAKT/AKT, F (2, 17) = 7.925, p = 0.0087; SREBP-1, F (2, 17) = 18.14, p < 0.001]. Although the expression of SREBP-2 in the liver was significantly increased by HFD, which was not altered after AOB treatment for 8 weeks [, One-way ANOVA, F (2, 8) = 9.281 p = 0.0082].
Figure 8. AOB alleviated AS by regulating the PI3K pathway. The expression levels of the PI3K/AKT/SERBP-1 pathway proteins in each group were detected by western blots. The densitometric values of bands were quantitatively analyzed by Image J Densitometric values normalized to those in the model group and are presented as relative intensity. Data show mean ± SEM values of 6 independent samples. # Represents comparison with the control group, # represents p < 0.05, ## represents p < 0.01; * represents comparison with the model group, * represents p < 0.05, ** represents p < 0.01 *** represents p < 0.001.
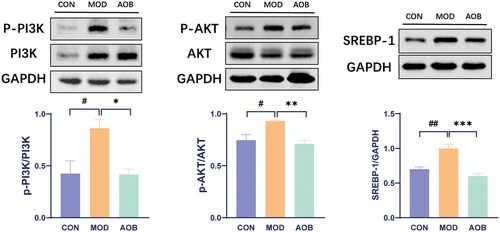
Figure 9. The expression of SREBP-2 in liver after AOB treatment for 8 weeks. The densitometric values of bands were quantitatively analyzed by Image J Densitometric values normalized to those in the model group and are presented as relative intensity. Data show mean ± SEM values of 6 independent samples. # Represents comparison with the control group, ## represents p < 0.01.
Discussion
The relationship between the lipid metabolism pathway of AOB and AS was still unclear. In this study, we used network pharmacology combining with experiments to reveal the role of AOB in lipid metabolism, which played a major role in the treatment for AS. We found 3 main components in the AOB treatment of AS: 16β-methoxyalisol B monoacetate (degree = 36), 3β-acetoxyatractylone (degree = 23), and 5, 2′-dihydroxy-6,7,8-trimethoxyflavone (degree = 19) according to network analysis. Moreover, core targets were obtained: PIK3R1, AKT1, PIK3CA, MAPK1, PTPN11, EGFR and MAPK4, which might participate in the treatment of AS. In addition, the top 10 significantly enriched (p < 0.01) terms in BP, CC and MF of GO analysis were selected and the top 20 pathways with significantly enriched were selected, including the PI3K/AKT/SREBP-1 pathway. Based on the results, we used experiments to identified the therapeutic actions of AOB in AS via the PI3K/AKT/SREBP-1 pathway. The results showed that AOB reversed the aortic plaque area of AS, and improved main indicators of blood lipids related to AS and alleviated AS by regulating PI3K/AKT/SREBP-1 pathway in APOE−/− mice stimulated with HFD. Taken together, we firstly demonstrated that AOB was capable of ameliorating AS by regulating the PI3K/AKT/SREBP-1 pathway.
Although a previous study has identified the effects of AOB on the treatment for AS (Zhu, Zhai, et al. Citation2020), the underlying molecular mechanism was still unclear. The aortic plaque was significantly increased in AS-related diseases and had been shown to be closely related to high-fat diets (HFD) (Pan et al. Citation2022). In our study, we found a significant increase in the aortic plaque area in APOE−/− mice fed with HFD for 8 weeks, which was reversed by AOB after 8 weeks of continuous treatment. In addition, abnormal blood lipid indicators including TG, CHO, HDL and LDL, which contributed to AS (Jaquish et al. Citation1996; Barboza et al. Citation2016), were all relieved by AOB. To sum up, AOB has the effect of alleviating AS in APOE−/− mice stimulated with HFD.
Network pharmacological analysis showed that 3 main components, 16β-methoxyalisol B monoacetate, 3β-acetoxyatractylone, and 5,2′-dihydroxy-6,7,8-trimethoxyflavone, are associated with AOB treatment of AS. 16β-Methoxyalisol B monoacetate from Alismatis rhizoma has been identified to have an antibacterial effect (Jin et al. Citation2012) and the pathophysiology of bacterial is associated with the development of inflammation (Ge et al. Citation2022; Keir and Chalmers Citation2022), risk factors for atherosclerosis. Meanwhile, the component was proved to have an inhibitory effect on phosphorylation of the PI3K/Akt pathway (Xu, Zhao, et al. Citation2009). Moreover, 3β-acetoxyatractylone from Atractylodis macrocephalae rhizoma has been indicated to have treatment-related effects of AS (Chen et al. Citation2017; Li et al. Citation2018). In addition, 5,2′-dihydroxy-6,7,8-trimethoxyflavone from Pyrolae herba, a natural flavonoid, plays a role in lipid decreasing (Lin et al. Citation2022) and the progression of treatment of AS (Kimura et al. Citation2022; Liu et al. Citation2022), which also participate in anti-inflammation (Huang et al. Citation2022; Liu et al. Citation2022). Network pharmacological results were further identified in our experimental studies, which showed that AOB was capable of releiving AS by regulating the PI3K/AKT/SREBP-1 pathway.
Hypercholesterolemia is recognized as a major contributor to AS, and lowering blood cholesterol levels is an important means in the treatment of AS. Sterol-regulating element-binding proteins (SREBPs) are a family of transcription factors involved in the biosynthesis of cholesterol, fatty acids, and triglycerides (Moslehi and Hamidi-Zad Citation2018), consisting of SREBP-1 and SREBP-2. SREBP-1 is responsible for the synthesis of fatty acids and cholesterol, while SREBP-2 only regulates the synthesis of cholesterol (Jeon and Osborne Citation2012). Studies showed that SREBPs in the liver plays a catalytic role in AS by increasing lipid synthesis (Karasawa et al. Citation2011; Pérez-Belmonte et al. Citation2017), and inhibiting SREBP-1 led to lower serum cholesterol levels, further alleviating AS (Karasawa et al. Citation2011). PI3K/AKT is the upstream signaling pathway of SREBP-1, whose activation increased the expression of SREBP-1 (Jeon and Osborne Citation2012). Recent studies showed that PI3K/AKT signaling is significantly upregulated in patients with nonalcoholic fatty liver disease, one of the risk factors for AS, and inhibitors against PI3K and AKT have potential regulatory effects on lipid metabolism (Aljabban et al. Citation2022). Our results showed that phosphorylation of the PI3K/AKT signaling pathway in the liver of AS model mice is significantly activated, resulting in elevated SREBPs. After 8 weeks of AOB administration, phosphorylation of the PI3K/AKT signaling pathway in the liver is restored, further lowering SREBP-1 signaling in the liver instead of SREBP-2. These results indicate that AOB regulated the PI3K/AKT/SERBP-1 pathway leading to therapeutic actions in AS by reducing lipid levels.
Conclusions
AOB has the therapeutic response of AS, which requires suppression of the PI3K/AKT/SERBP-1 pathway. These were our first findings on AOB’s treatment of AS and the underlying mechanism were associated with inhibition of the PI3K/AKT/SERBP-1 pathway, which suggests that traditional Chinese medicine has an obvious curative effect in the treatment of AS, and had a similar molecular mechanism as other western medicines (Mahtta et al. Citation2022), which provides strong evidence for our later development and extensive use of traditional Chinese medicine.
Consent form
All authors have approved the manuscript and agree with its submission.
Author contributions
Ruiyi Liu, Yan Sun, Boran Zhu and Haoxin Wu designed the study and wrote the manuscript. Ruiyi Liu and Yan Sun performed network pharmacology. Ruiyi Liu, Dong Di and Yan Sun performed the experiments. Ruiyi Liu, Boran Zhu and Yan Sun performed the analyzed the data. All data were generated inhouse, and no paper mill was used. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The raw data supporting the conclusions of this manuscript will be available from the corresponding author on reasonable request.
Additional information
Funding
References
- Aljabban J, Rohr M, Syed S, Khorfan K, Borkowski V, Aljabban H, Segal M, Mukhtar M, Mohammed M, Panahiazar M, et al. 2022. Transcriptome changes in stages of non-alcoholic fatty liver disease. World J Hepatol. 14(7):1382–1397.
- Aryal A, Harmon AC, Dugas TR. 2021. Particulate matter air pollutants and cardiovascular disease: strategies for intervention. Pharmacol Ther. 223:107890.
- Barboza LN, Lívero FA, Prando TB, Ribeiro Rde C, Lourenço EL, Budel JM, de Souza LM, Acco A, Dalsenter PR, Gasparotto AJ. 2016. Atheroprotective effects of Cuphea carthagenensis (Jacq.) J. F. Macbr. in New Zealand rabbits fed with cholesterol-rich diet. J Ethnopharmacol. 187:134–145.
- Chen Y, Yang W, Guo L, Wu X, Zhang T, Liu J, Zhang J. 2017. Atractylodes lactone compounds inhibit platelet activation. Platelets. 28(2):194–202.
- Francis GA. 2010. The complexity of HDL. Biochim Biophys Acta. 1801(12):1286–1293.
- Ge L, Liu D, Mao X, Liu S, Guo J, Hou L, Chen X, Huang K. 2022. Low dose of deoxynivalenol aggravates intestinal inflammation and barrier dysfunction induced by enterotoxigenic Escherichia coli infection through activating macroautophagy/NLRP3 inflammasomes. J Agric Food Chem. 70(9):3009–3022.
- Geovanini GR, Libby P. 2018. Atherosclerosis and inflammation: overview and updates. Clin Sci. 132(12):1243–1252.
- Hamosh A, Scott AF, Amberger JS, Bocchini CA, McKusick VA. 2005. Online Mendelian Inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 33(Database issue):D514–517.
- Hopkins AL. 2008. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 4(11):682–690.
- Huang H, He Y, Li Y, Gu M, Wu M, Ji L. 2022. Eriodictyol suppresses the malignant progression of colorectal cancer by downregulating tissue specific transplantation antigen P35B (TSTA3) expression to restrain fucosylation. Bioengineered. 13(3):5551–5563.
- Jaquish CE, Mahaney MC, Blangero J, Haffner SM, Stern MP, MacCluer JW. 1996. Genetic correlations between lipoprotein phenotypes and indicators of sex hormone levels in Mexican Americans. Atherosclerosis. 122(1):117–125.
- Jeon TI, Osborne TF. 2012. SREBPs: metabolic integrators in physiology and metabolism. Trends Endocrinol Metab. 23(2):65–72.
- Jia CY, Li JY, Hao GF, Yang GF. 2020. A drug-likeness toolbox facilitates ADMET study in drug discovery. Drug Discov Today. 25(1):248–258.
- Jin HG, Jin Q, Ryun Kim A, Choi H, Lee JH, Kim YS, Lee DG, Woo ER. 2012. A new triterpenoid from Alisma orientale and their antibacterial effect. Arch Pharm Res. 35(11):1919–1926.
- Karasawa T, Takahashi A, Saito R, Sekiya M, Igarashi M, Iwasaki H, Miyahara S, Koyasu S, Nakagawa Y, Ishii K, et al. 2011. Sterol regulatory element-binding protein-1 determines plasma remnant lipoproteins and accelerates atherosclerosis in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 31(8):1788–1795.
- Keir HR, Chalmers JD. 2022. Neutrophil extracellular traps in chronic lung disease: implications for pathogenesis and therapy. Eur Respir Rev. 31:210241.
- Kimura I, Kagawa S, Tsuneki H, Tanaka K, Nagashima F. 2022. Multitasking bamboo leaf-derived compounds in prevention of infectious, inflammatory, atherosclerotic, metabolic, and neuropsychiatric diseases. Pharmacol Ther. 235:108159.
- Kobiyama K, Ley K. 2018. Atherosclerosis. Circ Res. 123(10):1118–1120.
- Koeth RA, Lam-Galvez BR, Kirsop J, Wang Z, Levison BS, Gu X, Copeland MF, Bartlett D, Cody DB, Dai HJ, et al. 2019. l-Carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans. J Clin Invest. 129(1):373–387.
- Li W, Zhi W, Liu F, Zhao J, Yao Q, Niu X. 2018. Paeoniflorin inhibits VSMCs proliferation and migration by arresting cell cycle and activating HO-1 through MAPKs and NF-κB pathway. Int Immunopharmacol. 54:103–111.
- Lin YP, Fu SN, Li XP, Wang MM, Fang QL, Qiao X, Yan Q, Hua Y. 2022. Two novel flavonoids with lipid-lowering activity from Yi medicine Shekaqi. Chem Biodivers. 19:e202200363.
- Liu J, Zhang W, Li Y, Li X, Li Y, Guo F. 2022. Flavonoids extract from the seeds of Psoralea corylifolia L. (PFE) alleviates atherosclerosis in high-fat diet-induced LDLR(−/−) mice. Phytomedicine. 98:153983.
- Mahtta D, Lee MT, Ramsey DJ, Akeroyd JM, Krittanawong C, Khan SU, Sinh P, Alam M, Garratt KN, Schofield RS, et al. 2022. Significant facility-level variation in utilization of and adherence with secondary prevention therapies among patients with premature atherosclerotic cardiovascular disease: insights from the VITAL (Veterans wIth premaTure AtheroscLerosis) Registry7. Cardiovasc Drugs Ther. 36(1):93–102.
- Moslehi A, Hamidi-Zad Z. 2018. Role of SREBPs in liver diseases: a mini-review. J Clin Transl Hepatol. 6(3):332–338.
- Pan X, Wan R, Wang Y, Liu S, He Y, Deng B, Luo S, Chen Y, Wen L, Hong T, et al. 2022. Salvianolic acid B inhibiting chemically and mechanically activated Piezo1 channels as a mechanism for ameliorating atherosclerosis. Br J Pharmacol. 179(14):3778–3814.
- Pérez-Belmonte LM, Moreno-Santos I, Cabrera-Bueno F, Sánchez-Espín G, Castellano D, Such M, Crespo-Leiro MG, Carrasco-Chinchilla F, Alonso-Pulpón L, López-Garrido M, et al. 2017. Expression of sterol regulatory element-binding proteins in epicardial adipose tissue in patients with coronary artery disease and diabetes mellitus: preliminary study. Int J Med Sci. 14(3):268–274.
- Rebhan M, Chalifa-Caspi V, Prilusky J, Lancet D. 1997. GeneCards: integrating information about genes, proteins and diseases. Trends Genet. 13(4):163.
- Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, et al. 2020. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 76(25):2982–3021.
- Ru J, Li P, Wang J, Zhou W, Li B, Huang C, Li P, Guo Z, Tao W, Yang Y, et al. 2014. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 6(1):13.
- Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. 2003. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13(11):2498–2504.
- Solanki A, Bhatt LK, Johnston TP. 2018. Evolving targets for the treatment of atherosclerosis. Pharmacol Ther. 187:1–12.
- Soppert J, Lehrke M, Marx N, Jankowski J, Noels H. 2020. Lipoproteins and lipids in cardiovascular disease: from mechanistic insights to therapeutic targeting. Adv Drug Deliv Rev. 159:4–33.
- Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, et al. 2017. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 45(D1):D362–D368.
- Xu X, Zhang W, Huang C, Li Y, Yu H, Wang Y, Duan J, Ling Y. 2012. A novel chemometric method for the prediction of human oral bioavailability. Int J Mol Sci. 13(6):6964–6982.
- Xu YH, Zhao LJ, Li Y. 2009. Alisol B acetate induces apoptosis of SGC7901 cells via mitochondrial and phosphatidylinositol 3-kinases/Akt signaling pathways. World J Gastroenterol. 15(23):2870–2877.
- Zeng Q, Li L, Siu W, Jin Y, Cao M, Li W, Chen J, Cong W, Ma M, Chen K, et al. 2019. A combined molecular biology and network pharmacology approach to investigate the multi-target mechanisms of Chaihu Shugan San on Alzheimer’s disease. Biomed Pharmacother. 120:109370.
- Zhang R, Zhu X, Bai H, Ning K. 2019. Network pharmacology databases for traditional Chinese medicine: review and assessment. Front Pharmacol. 10:123.
- Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, Benner C, Chanda SK. 2019. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 10(1):1523.
- Zhu C, Cai T, Jin Y, Chen J, Liu G, Xu N, Shen R, Chen Y, Han L, Wang S, et al. 2020. Artificial intelligence and network pharmacology based investigation of pharmacological mechanism and substance basis of Xiaokewan in treating diabetes. Pharmacol Res. 159:104935.
- Zhu B, Zhai Y, Ji M, Wei Y, Wu J, Xue W, Tao WW, Wu H. 2020. Alisma orientalis beverage treats atherosclerosis by regulating gut microbiota in ApoE−/− mice. Front Pharmacol. 11:570555.