Abstract
Context
Non-alcoholic fatty liver disease (NAFLD) is a common liver disease, accompanied by liver lipid accumulation and inflammation. JianPi-QingHua formula (JPQH), a Chinese herbal formula, exhibits effects on obesity and T2DM. However, the hepatoprotective effect of JPQH has not been elucidated.
Objective
To investigate the hepatoprotective effect of JPQH in NAFLD induced by a high-fat diet (HFD) in mice.
Materials and methods
C57BL/6J mice were divided into four groups and fed a normal-fat diet (ND), high-fat diet (HFD), HFD + JPQH (2.5 g/kg), or HFD + metformin (300 mg/kg) for 6 weeks, respectively. Furthermore, the body weight, epididymal fat mass, blood glucose, and liver weight were measured. Serum total cholesterol (TC), triglycerides (TG), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were performed. Hematoxylin and eosin staining and Oil Red O staining were observed in hepatic histopathological changes. Western blotting and quantitative real-time polymerase chain reaction were utilized to assess the key protein expression of hepatic lipid metabolism and inflammation.
Results
Compared with the HFD group, JPQH could reduce body weight, epididymal fat mass, blood glucose and liver weight (p < 0.05), and markedly decreased the levels of serum TC, TG, ALT, AST (p < 0.05). Additionally, JPQH improved liver pathological changes. Consistent with the hepatic histological analysis, JPQH intervention suppressed lipid accumulation and inflammatory responses. Mechanistically, JPQH boosted SIRT1/AMPK signalling, and attenuated NF-κB pathway, which suppressed inflammatory responses.
Discussion and conclusions
These findings indicate that JPQH supplementation protected against HFD-induced NAFLD by regulating SIRT1/AMPK/NF-κB pathway, which provides a theoretical basis for the clinical treatment of patients with NAFLD.
Introduction
Nonalcoholic fatty liver disease (NAFLD), a common chronic liver disease, is characterized by hepatic fat accumulation without drinking alcohol, which may change into steatohepatitis, cirrhosis, and hepatocellular carcinoma (Duan et al. Citation2012; Powell et al. Citation2021). Increasing evidence reveals that obesity and diabetes can aggravate the development of NAFLD, which is mainly attributed to a high-caloric diet (Liu et al. Citation2020; Rohm et al. Citation2022). With the high morbidity of obesity and diabetes, NAFLD has become an increasingly prevalent liver disease. However, there are no approved drugs to prevent or treat NAFLD to date.
As shown by studies, the abnormal lipid metabolism in the liver has contributed to the progression of NAFLD (Buzzetti et al. Citation2016; Powell et al. Citation2021), which is consistent with the findings of high-fat-diet (HFD)-induced NAFLD in mice. Indeed, AMP-activated protein kinase (AMPK) plays a crucial role in abnormal lipid metabolism in the liver. Meanwhile, increasing evidence indicates that the reduced AMPK activity in the liver occurs in HFD-induced animals (Xu et al. Citation2022), which is bolstered by the antidiabetic drug metformin (Yan et al. Citation2019; Zamani-Garmsiri et al. Citation2021). Additionally, SIRT1, the metabolic sensor, is also devoted to lipid metabolism (Garcia et al. Citation2019; Li et al. Citation2020). Lipid metabolism accumulation increases lipogenesis, which is managed by sterol regulatory element binding protein 1 (SREBP1), fatty acid synthase (FASN) and acetyl CoA carboxylase (ACC), and inhibits lipolysis including hormone-sensitive lipase (HSL) and adipose triglyceride lipase (ATGL). It was reported that activating AMPK/SIRT1 pathway could inhibit the expression of genes and proteins of SREBP1 and FASN, promote lipolysis and improve the NAFLD (Li et al. Citation2020; Song et al. Citation2015). Hepatic inflammation is another important consideration in the progression of NAFLD (Buzzetti et al. Citation2016; Rohm et al. Citation2022). According to previous research, the activation of NF-κB is involved in the NAFLD animal models induced by HFD or patients with NAFLD (Cai et al. Citation2005; Grossini et al. Citation2021). NF-κB can be affected by IκB degradation induced by IκB phosphorylation (Cai et al. Citation2005). Moreover, some studies demonstrated that AMPK achieves anti-inflammatory effects in a SIRT1-dependent manner by suppressing the NF-κB pathway (Peixoto et al. Citation2017). It was reported that SIRT1 deficiency expedited the deterioration of hepatic inflammation by increasing NF-κB (Pfluger et al. Citation2008). Consequently, the AMPK/SIRT1/NF-κB pathway is of great value in the progression of NAFLD, due to the regulation of abnormal lipid metabolism and inflammation in the liver (Garcia et al. Citation2019; Smith et al. Citation2016).
Jianpi-Qinghua Formula (JPQH), which was derived from ‘Bupiwei Xieyinhuo Shengyang Decoction’, was proposed by Shuguang Hospital Affiliated with Shanghai University of Traditional Chinese Medicine (Shanghai, China) (Liu et al. Citation2022). The compositions of JPQH included Codonopsis pilosula (Franch.) Nannf (Campanulaceae), Astragalus membranaceus (Fisch.) Bunge (Leguminosae), Dioscorea opposita Thunb. (Dioscoreceae), Polygonatum sibiricum Red. (Liliaceae), Coptis chinensis Franch. (Ranunculaceae), Scutellaria baicalensis Georgi (Lamiaceae Martinov), Pueraria Lobata (Willd.) Ohwi. (Leguminosae) and Euonymus alatus (Thunb.) Sieb (Celastraceae). This formula has been used for more than two decades, and it is known for the treatment of lipid metabolism, intestinal inflammation, obesity, and insulin resistance (Gong et al. Citation2020; Liu et al. Citation2022). Our past study found that puerarin inhibited the hepatic gluconeogenesis via activation of PI3K/Akt pathway in diabetic rats (Liu et al. Citation2021). However, its therapeutic mechanism in the treatment of NAFLD has been unclear. Therefore, our study investigates whether JPQH will attenuate HFD-induced NAFLD by regulating hepatic lipid metabolism and inflammation.
Materials and methods
Drugs and reagents
JPQH were provided by Lei-Yun-Shang Pharmacy (Shanghai, China, batch number: 20210801) and the compositions of JPQH are presented in . The concentrated solution of JPQH was analyzed by a UPLC-MS system as previously reported (Cui et al. Citation2021). The results are shown in . The following reagents had been used, including Metformin (MET) (PHR1084, Sigma-Aldrich, St. Louis, MO), Oil red O dye (C0158M, Beyotime Biotechnology, Shanghai, China), RIPA Lysis Buffer (P0013B, Beyotime Biotechnology, Shanghai, China), protease inhibitor cocktails (P1005, Beyotime Biotechnology, Shanghai, China), phosphatase inhibitor cocktail (P1045, Beyotime Biotechnology, Shanghai, China), HiScript II Reverse Transcriptase Kit (with gDNase) (R223, Vazyme Biotech, Nanjing, China), ChamQ Universal SYBR qPCR Master Mix (Q711, Vazyme Biotech, Nanjing, China). The following antibodies were purchased from Cell Signaling Technology (Boston, MA): p-AMPK (4148S), AMPK (5832S), p-ACC (3661S), ACC (3676S), SIRT1 (8469S), p-IKK (2697S), IKKα (2682S), IKKβ (8943S), p-IκBα (5209S), IκBα (4812S), p-NF-κB p65 (3033S), NF-κB p65 (8242S), F4/80 (70076S). GAPDH (10494-1-AP, Proteintech, Shanghai, China), Goat anti-rabbit and Goat anti-mouse HRP conjugated secondary antibodies (A0208 and A0216, Beyotime Biotechnology, Shanghai, China) were used.
Figure 1. Main components of JPQH (20210801 batches) in negative (A) and positive (B) ion mode by using LC-MS. (1) Dulcitol; (2) Puerarin; (3) Magnoflorine; (4) Daidzin; (5) Berbamine hydrochloride; (6) Scutellarin; (7) Ferulic acid; (8) (R)-(+)-Corypalmine; (9) Lobetyolin; (10) Baicalin; (11) Epiberberine; (12) Jatrorrhizine; (13) Coptisine; (14) Daidzein; (15) Palmatine; (16) Wogonoside; (17) Berberin; (18) Baicalein; (19) Astragaloside IV; (20) Wogonin.
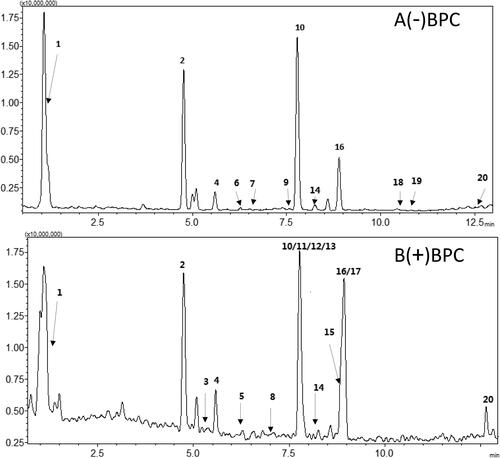
Table 1. Herbal medicines contained in JPQH.
Animals
In this study, six-week-old male C57BL/6J mice were obtained from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd. (SYXK(HU)2020–0009, China). The mice were placed in a room on a 12 h light/dark cycle with a constant temperature and humidity. All animal studies adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of Shanghai University of Traditional Chinese Medicine (Animal Ethics Number, PZSHUTCM 190,823,004). To establish the NAFLD models, the animals had been continuously raised on a high-fat diet (HFD) with 60% of calories from fat, 20% from carbohydrates, and 20% from protein (D12492; Research Diets, New Brunswick, NJ, USA) for 12 weeks based on a previous study (Liu et al. Citation2022). Meanwhile, the mice in the control group (ND, n = 10) were continuously given a normal diet (AIN-93G, Jiangsu Xietong Pharmaceutical Bio-engineering Co., Ltd., Nanjing, China). Then, the HFD-fed mice were randomly divided into three groups (n = 10, each group): HFD group, HFD + JPQH group and HFD + MET group. The HFD + JPQH group and HFD + MET group were administrated with JPQH (2.5 g/kg) or MET (300 mg/kg) by gavage for approximately 6 weeks. These remaining groups were given physiological saline for 6 weeks. The mice were sacrificed under pentobarbital sodium anesthesia, and visceral fat and liver tissue were collected for histopathological and molecular experiments.
Body weight and oral glucose tolerance test (OGTT)
The body weight (BW) of all the animals was recorded simultaneously (Friday morning 8:00–10:00) using the identical electronic scale. In order to complete the intraperitoneal glucose tolerance test (IPGTT) at the 6th week of administration, the mice intraperitoneally received glucose (1.5 g/kg) after fasting for 12 h. Besides, blood glucose was measured by glucometer (ACCU-CHEK Active, Roche, Mannheim, Germany) at different time points including 0, 30, 60, 90, 120 min and the area under the curve (AUC) of glucose was calculated.
Serum analysis
Serum cholesterol (TC), triglycerides (TG), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were determined using the automated biochemical analyzer (Hitachi High-Tech, Tokyo, Japan).
Histopathological examination
At the end of the experiment, a part of the liver tissues and adipose tissues were fixed, dehydrated in different concentrations of alcohol, and prepared as paraffin samples. These samples were cut into 5 µm slices, and then subjected to hematoxylin-eosin (H&E) and Sirius red staining. NAFLD activity scores were defined in accordance with liver section histology (Ding et al. Citation2020; Nelson et al. Citation2011. To detect lipid droplets of liver tissues, Oil-red O staining was used. These tissues were embedded by OCT, prepared at 8 μm thickness and stained with Oil Red O dye (C0158M, Beyotime Biotechnology, Shanghai, China). Finally, the images were photographed by an optical microscope with a digital camera (Nikon, Tokyo, Japan) and measured using Image J.
Immunofluorescence staining and immunohistochemical staining
For NF-κB staining, the liver tissue paraffin sections (5 µm), dewaxing to water, were implemented with heat-induced antigen retrieval and were incubated with PBS with 10% normal goat serum for 1h, and then NF-κB antibody (1:150 dilution) overnight at 4 °C. The next day, the sections were incubated with the secondary antibodies (8889s, Cell Signaling Technology, Boston, MA) and stained with DAPI. To assess for hepatic inflammation using macrophage biomarkers F4/80, the sections were incubated with F4/80 antibody overnight (1:1000 dilution), and then dealt with the secondary antibody (1:200 dilution) for 1 h. In addition, images were acquired by a fluorescence microscope (Nikon, Tokyo, Japan).
Quantitative reverse transcriptase-polymerase chainreaction (qRT-PCR)
Total RNAs from the liver tissues were extracted with the FastPure Tissue Total RNA Isolation Kit (Vazyme Biotech, Nanjing, China), and then they were reverse transcribed to cDNA by HiScript II Reverse Transcriptase Kit (Vazyme Biotech, Nanjing, China). Apart from that, the real-time PCR was completed by referring to the reagent instructions for ChamQ Universal SYBR qPCR Master Mix (Vazyme Biotech, Nanjing, China). The PCR primers are shown in .
Table 2. Primer sequences used for qRT-PCR in this study.
Western blotting
Liver tissues were homogenized with RIPA buffer and measured by BCA assay (#23225, Thermo Fisher Scientific, MA, USA). Besides, the extracted liver proteins were separated by SDS-PAGE and transferred to PVDF membranes. Furthermore, the blots were then incubated with primary antibodies against p-AMPK, AMPK, p-ACC, ACC, SIRT1, p-IKK, IKKα, IKKβ, p-IκBα, IκBα, p-NF-κB p65, NF-κB p65, GAPDH and used for the corresponding secondary antibody. Then, the results were acquired by an imaging system (Tonan, Shanghai, China) and analyzed by Image J.
Statistical analysis
All the results were analyzed by the software GraphPad Prism 5.0, and were presented as the means ± SD. Beyond that, the comparisons among the four groups were performed by one-way ANOVA p < 0.05 was considered statistical significance.
Results
JPQH improved metabolic parameters in the HFD-fed mice
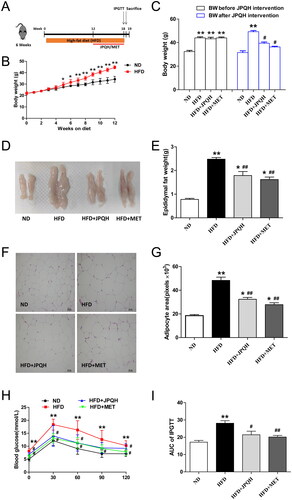
To determine whether JPQH affected the metabolic parameters in HFD-induced NAFLD mice, we measured the body weight, epididymal adipose mass, adipocyte size and blood glucose. As shown in , this was the schedule of our experiment. It could be observed that compared with normal diet (ND) mice, the body weight of these mice fed with an HFD for 12 weeks increased significantly (p < 0.01), while those with JPQH intervention for 6 weeks could lose weight (). Consistent with the above findings, JPQH supplementation significantly diminished epididymal fat mass and adipocyte size (p < 0.01) (). Furthermore, we found that JPQH could decrease blood glucose at 0, 30, 60 min and AUC of IPGTT (p < 0.05) (). Taken together, these data suggested that JPQH attenuated the metabolic disorder in the HFD-fed mice.
Figure 2. JPQH improved metabolic parameters in HFD-fed mice. (A) Experimental arrangement of NAFLD animal model and drug intervention. (B) Changes of Body weight in high-fat-fed (HFD) mice for 12 weeks (n = 6). (C) Body weight of mice before JPQH treatment or after JPQH intervention for 6 weeks (n = 6). (D-E) Weight of epididymal adipose tissue after JPQH treatment for 6 weeks (n = 6). (F) Representative pictures of H&E staining of epididymal adipose tissue (×200). (G) Quantification of adipocyte size (n = 3). (H-I) Intraperitoneal glucose-tolerance test (IPGTT) after 6 weeks of JPQH treatment (n = 6). The data are presented as means ± SD. ND: the normal-diet group, HFD: the high-fat-diet group, JPQH: HFD + JPQH group, MET: HFD + Metformin group. #p < 0.05, ##p < 0.01 vs. the ND group; *p < 0.05, **p < 0.01 vs. the HFD group.
JPQH treatment suppressed hepatic lipid accumulation in the HFD-fed mice
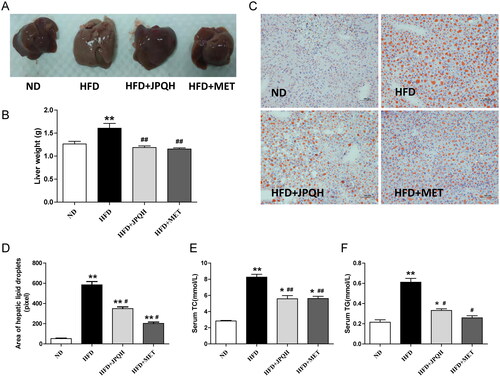
We further observed whether JPQH could protect mice against HFD-induced NAFLD. As displayed in , the size and weight of liver tissues in the HFD-fed mice were larger than the ND-fed ones (p < 0.01), and JPQH treatment suppressed this increase. Then, Oil red O staining revealed that JPQH obviously ameliorated hepatic lipid accumulation in the HFD-fed mice (p < 0.01) (). Similarly, JPQH administration reduced the levels of serum TC and TG ().
Figure 3. JPQH treatment suppressed hepatic lipid accumulation in the HFD-fed mice. (A) Representative pictures of liver tissue after JPQH intervention for 6 weeks (n = 3– 5). (B) Liver weights of each group after JPQH intervention for 6 weeks (n = 6). (C) Oil red O staining of liver sections (×200, n = 3). (D) Quantification of the stained lipid droplets by Image J. (E-F) The serum levels of TC and TG (n = 6). The data are presented as means ± SD, #p < 0.05, ##p < 0.01 vs. the ND group; *p < 0.05, **p < 0.01 vs. the HFD group.
JPQH attenuated liver injury induced by HFD
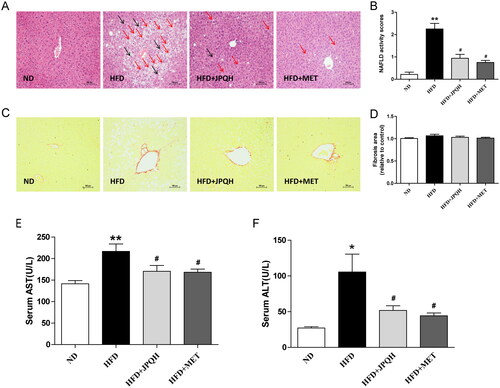
Subsequently, we examined whether JPQH ameliorated liver injury induced by HFD. In this study, H&E staining showed that the mice in the HFD group had excessive accumulation of lipid droplets (red arrow) and inflammatory cell infiltration (black arrow), and all of the changes were ameliorated after JPQH supplementation (). According to the pathological score of liver injury (Nelson et al. Citation2011; Ding et al. Citation2020), NAFLD lesion severity in the JPQH group was also significantly reduced (p < 0.01) (). However, Sirius red staining showed there was no significant difference between the groups (), suggesting that this stage had not yet developed into liver fibrosis. Elevated hepatic AST and ALT activity was also the hallmark of liver damage. JPQH attenuated hepatic AST and ALT activity in HFD group (). As indicated by these results, JPQH mitigated the NAFLD induced by HFD.
Figure 4. JPQH attenuated liver injury induced by HFD. (A) Representative images of H&E staining of liver (200×, n = 3) (red arrow: lipid droplets, black arrow: inflammatory cell infiltration). (B) NAFLD activity scores of each group on the basis of the general NAFLD scoring system examined in H&E. (C) Sirius red staining for estimating liver fibrosis area (×200, n = 3). (D) Quantitative evaluation of liver fibrosis area by Image J. (E-F) Determination of serum AST and ALT at 6th week of JPQH treatment (n = 6). The data are presented as means ± SD, #p < 0.05, ##p < 0.01 vs. the ND group; *p < 0.05, **p < 0.01 vs. the HFD group.
JPQH inhibited hepatic lipid accumulation via activating AMPK/SIRT1 pathway
Abnormal lipid metabolism in the liver participated in the progression of NAFLD (Buzzetti et al. Citation2016), which was regulated by the AMPK/SIRT1pathway (Garcia et al. Citation2019; Li et al. Citation2020). The above results prompted us to examine whether JPQH inhibited hepatic lipid accumulation by the AMPK/SIRT1 pathway. We observed the ratios of p-AMPK/AMPK in the liver induced by HFD mice was notably downregulated (p < 0.05). In contrast, those increased after JPQH treatment for 6 weeks (). The protein levels of SIRT1, the metabolic sensor regulating hepatic lipid metabolism, were higher in the HFD + JPQH group than those in the HFD group (). In comparison to mice induced by HFD, JPQH treatment manifested enhanced expression of p-ACC/ACC in the liver ().
Figure 5. JPQH inhibited hepatic lipid accumulation via activating AMPK/SIRT1 pathway. (A-B) Western blot observed the level of p-AMPK/AMPK in the liver (n = 3). (C-D) Effects of JPQH on protein expression of SIRT1in the liver measured by Western blot (n = 3). (E-F) The expression of p-ACC/ACC in the liver was measured by western blot (n = 3). (G-I) The mRNA levels on hepatic lipogenesis, lipolysis, and triglyceride synthesis-related genes assessed by real-time PCR (n = 4). The data are presented as means ± SD, #p < 0.05 vs. the ND group; *p < 0.05 vs. the HFD group.
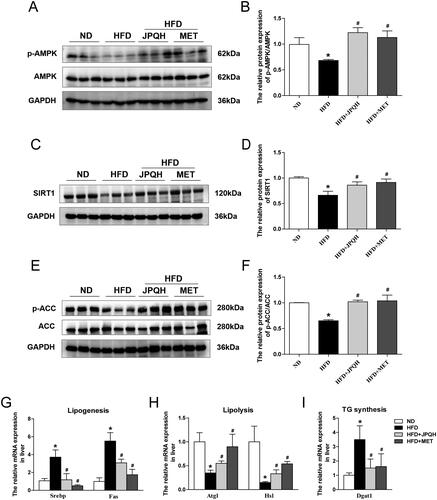
The AMPK/SIRT1 pathway affected lipid metabolism through the following genes including lipogenesis, lipolysis and triglyceride synthesis. As presented in , the expression of lipogenesis-related genes including Sterol Regulatory Element-Binding Protein (SREBP1) and fatty acid synthase (FASN) were remarkably elevated in HFD-fed mice (p < 0.05), and JPQH reduced the expression of SREBP1 and FASN (p < 0.05). In addition, JPQH lowered the mRNA level of adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL) in the liver, which are lipolysis-related genes (). In the wake of JPQH administration, diacylglycerol acyltransferase (DGAT), the triglyceride synthesis, was downregulated (). The above experiments indicated that JPQH inhibited hepatic lipid accumulation by activating the AMPK/SIRT1 pathway.
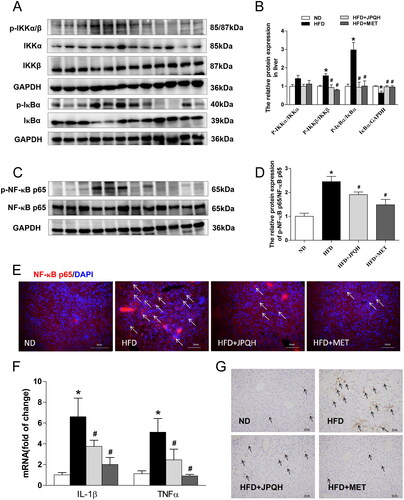
JPQH suppressed hepatic inflammation by inhibiting NF-κB pathway
The activation of IKK-NFκB pathways was also a major factor in the progress of NAFLD (Heida et al. Citation2021; Hotamisligil, Citation2006). Thus, we investigated whether JPQH suppressed hepatic inflammation by the NF-κB pathway. Compared with the ND group, the ratios of p-IKK/IKK and p-IκBα/IκBα, upstream proteins of NF-κB, significantly increased in the liver induced by HFD (p < 0.05), but those were decreased by JPQH treatment (). Moreover, the level of IκBα was upregulated in the JPQH + HFD group (p < 0.05) (), resisting NF-κB translocation to the nucleus. We also found the ratio of p-NF-κB p65/NF-κB p65 decreased after JPQH treatment for 6 weeks (p < 0.05) (). Consistent with these results, JPQH could inhibit NF-κB translocation to the nucleus (). By studying the expression of inflammatory cytokines in the liver, we found that JPQH blocked the mRNA level of IL-1β and TNF-α in the HFD mice (p < 0.05) (). As expected, the less F4/80 staining after the intervention of JPQH was further confirmed by the immunohistochemistry staining ()). These data demonstrated that JPQH suppressed hepatic inflammation, which might be related to NF-κB pathways.
Figure 6. JPQH suppressed hepatic inflammation by inhibiting the NF-κB pathway. (A-B) Western blot observed the level of p-IKK/IKK and p-IκBα/IκBα in the liver (n = 3). (C-D) The expression of p-NF-κB p65/NF-κB p65 in the liver was measured by western blot (n = 3). (E) The effects of JPQH on the nuclear translocation of NF-κB p65 were measured by Immunofluorescence (Red: NF-κB p65, Blue: DAPI, White arrows indicate NF-κB p65 positive cells, n = 3). (F) The mRNA levels of inflammatory genes were assessed by real-time PCR (n = 4). (G) F4/80 immunostaining of the liver (Arrows point to F4/80 positive cells, n = 3). The data are presented as means ± SD, #p < 0.05 vs. the ND group; *p < 0.05 vs. the HFD group.
Discussion
Patients with obesity and diabetes are increasing worldwide, which are often accompanied by NAFLD. Thus, the pathogenesis of NAFLD is associated with obesity and diabetes (Fujii and Kawada, Citation2020; Polyzos et al. Citation2019). The hypothesis of ‘multiple hit’ elucidates the pathogenesis of NAFLD, including the accumulation of lipids in the liver (Buzzetti et al. Citation2016; Tilg and Moschen, Citation2010). At the same time, hepatic lipid accumulation leads to insulin resistance and inflammation (Tilg and Moschen, Citation2010). These causative factors may be a significant approach to treating NAFLD. Currently, there are no special drugs to treatment with NAFLD. Therefore, drugs for ameliorating NAFLD are in urgent need.
Clinically, NAFLD patients are obese, with insulin resistance and glucose intolerance. The preclinical NAFLD animal models induced by HFD is similar to the pathological characteristics of human with NAFLD (Santhekadur et al. Citation2018). Thus, we chose the NAFLD animal model to feed mice an HFD for 12 weeks (Kashyap et al. Citation2019; Santhekadur et al. Citation2018). At the end of the last administration, the HFD mice had obesity, hyperglycemia and liver damage, which was similar to the phenotype of humans with NAFLD. According to the histopathological analysis, these mice had higher lipid content and inflammatory infiltrating cells in the liver tissue, as evidenced by markedly elevated serum ALT and AST. These results revealed that the NAFLD mouse models were successfully established. Notably, we did not find liver fibrosis in the HFD mice, indicating that the mice have not yet developed NASH.
Recently, accumulating evidence have shown that traditional Chinese medicine (TCM) plays an important role in the prevention and treatment of NAFLD (Han et al. Citation2021; Tang et al. Citation2020). JPQH, a traditional Chinese medicine formula, is considered to treat metabolic syndrome including obesity and diabetes (Gong et al. Citation2020; Liu et al. Citation2022), which are related to NAFLD. Previous studies had discovered that the phytochemical components of JPQH identified mainly include puerarin, baicalin, wogonoside and berberine (Xu et al. Citation2021). Importantly, our past study found that puerarin inhibited hepatic gluconeogenesis via activation of the PI3K/Akt pathway in diabetic rats (Liu et al. Citation2021). In addition, baicalin, wogonoside and berberine had the effect of improving NAFLD (Zhu et al. Citation2019; Jiang et al. Citation2020; Liu et al. Citation2020). Here, our results showed that JPQH not only markedly reduced blood glucose and the level of serum AST and ALT, but also decreased the numbers of lipid droplets and inflammatory cell infiltration, further showing that JPQH could improve NAFLD in the HFD mice.
Interestingly, the network pharmacology suggests that the active ingredients‐targets of JPQH are FASN and NFKB1A, which were related to lipid metabolism and inflammation respectively (Xu et al. Citation2021). These factors contribute to the development of NAFLD. In this report, we intended to prove that JPQH could inhibit abnormal lipid metabolism and inflammation in the liver, and improve NAFLD in HFD mice.
Abnormal lipid metabolism is a critical manifestation of NAFLD (Buzzetti et al. Citation2016; Duan et al. Citation2012; Powell et al. Citation2021). The AMPK/SIRT1 pathway has been considered a metabolic pathway to alleviate NAFLD (Garcia et al. Citation2019; Li et al. Citation2020; Xu et al. Citation2022). The expression of AMPK and SIRT1 in the liver is reduced in HFD-fed mice for 12 weeks (Xu et al. Citation2022). Activation of AMPK/SIRT1 pathway protects against NAFLD, accompanied by inhibition of lipogenesis (Garcia et al. Citation2019; Li et al. Citation2020), which is consistent with the results of metformin (Song et al. Citation2015; Zamani-Garmsiri et al. Citation2021). In the current study, we found that the HFD mice had large numbers of lipid droplets, and JPQH supplementation improved hepatic lipid accumulation. Furthermore, we tested whether AMPK/SIRT1 pathway got involved in the protective effect of NAFLD. As expected, JPQH increased the expression of p-AMPK and SIRT1, and reduced the mRNA levels related to lipogenesis including SREBP1 and FASN, which is in line with network pharmacology (Xu et al. Citation2021). Thus, JPQH intervention abated hepatic lipid accumulation and relieved NAFLD induced by HFD via AMPK/SIRT1 pathway.
Hepatic inflammation is another important feature of NAFLD (Buzzetti et al. Citation2016; Rohm et al. Citation2022). According to existing studies, abnormal lipid metabolism and inflammation contribute to NAFLD progression, which are mutual influences (Buzzetti et al. Citation2016). Besides, NF-κB is considered a key pro-inflammatory transcription factor, which produces IL-1β and TNF-α, and gets involved in the process of NAFLD (Heida et al. Citation2021; Hotamisligil, Citation2006). SIRT1, the regulator of lipid metabolism, activates the NF-κB pathway in the progression of NAFLD (Pfluger et al. Citation2008). Our studies elucidated that JPQH treatment reduced the levels of p-IKK, p-IκBα and p-NF-κB p65, and increased IκBα protein expression, along with lower IL-1β and TNF-α in the liver. In addition, we demonstrated that JPQH decreased the inflammatory cell infiltration in the liver detected by F4/80 staining. Therefore, JPQH could suppress hepatic inflammation induced by HFD, which is related to NF-κB pathway.
Conclusions
JPQH exhibits better therapeutic effect on lipid dysregulation and inflammation in NAFLD, which is related to regulation of AMPK/SIRT1/NF-κB pathway. These results suggest that JPQH has a protective effect on NAFLD. However, further clinical studies are required to develop potential therapeutic agents for NAFLD.
Author contributions
Xu Han and Hao Lu designed and supervised the study. Jing Tian and Mengjie Cai performed the experiments. Jing Tian, Shenyi Jin, Qingguang Chen, Jiahui Xu, Qiuyue Guo and Zihui Yan participated in analyzing data. Jing Tian and Mengjie Cai wrote the manuscript.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Buzzetti E, Pinzani M, Tsochatzis EA. 2016. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 65(8):1038–1048.
- Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. 2005. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 11:183–190.
- Liu Z, Zhang Y, Graham S, Wang X, Cai D, Huang M, Pique-Regi R, Dong XC, Chen YE, Willer C, et al. 2020. Causal relationships between NAFLD, T2D and obesity have implications for disease subphenotyping. J Hepatol. 73(2):263–276.
- Cui J, Shi Y, Xu X, Zhao F, Zhang J, Wei B. 2021. Identifying the cardioprotective mechanism of Danyu Tongmai granules against myocardial infarction by targeted metabolomics combined with network pharmacology. Phytomedicine. 98:153829.
- Ding X, Jian T, Li J, Lv H, Tong B, Li J, Meng X, Ren B, Chen J. 2020. κChicoric acid ameliorates nonalcoholic fatty liver disease via the AMPK/Nrf2/NFB signaling pathway and restores gut microbiota in high-fat-diet-fed mice. Oxid Med Cell Longev. 2020:9734560.
- Duan XY, Qiao L, Fan JG. 2012. Clinical features of nonalcoholic fatty liver disease-associated hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 11(1):18–27.
- Fujii H, Kawada N. 2020. The role of insulin resistance and diabetes in nonalcoholic fatty liver disease. Int J Mol Sci. 21:3863.
- Garcia D, Hellberg K, Chaix A, Wallace M, Herzig S, Badur MG, Lin T, Shokhirev MN, Pinto AFM, Ross DS, et al. 2019. Genetic liver-specific AMPK activation protects against diet-induced obesity and NAFLD. Cell Rep. 26(1):192–208.
- Gong F, Chen QG, Han X, Liu YH, Lu H. 2020. Effects of Jianpi Qinghua formula on glycolipid metabolism indexes and body mass in patients of type 2 diabetes mellitus with syndrome of deficiency of both qi and yin. Shanghai J Traditional Chinese Med. 54(S1):65–6676.
- Grossini E, Garhwal DP, Calamita G, Romito R, Rigamonti C, Minisini R, Smirne C, Surico D, Bellan M, Pirisi M. 2021. Exposure to plasma from non-alcoholic fatty liver disease patients affects hepatocyte viability, generates mitochondrial dysfunction, and modulates pathways involved in fat accumulation and inflammation. Front Med. 8:693997.
- Han R, Qiu H, Zhong J, Zheng N, Li B, Hong Y, Ma J, Wu G, Chen L, Sheng L. 2021. Si Miao Formula attenuates non-alcoholic fatty liver disease by modulating hepatic lipid metabolism and gut microbiota. Phytomedicine. 85:153544.
- Heida A, Gruben N, Catrysse L, Koehorst M, Koster M, Kloosterhuis NJ, Gerding A, Havinga R, Bloks VW, Bongiovanni L, et al. 2021. The hepatocyte IKK: NF-κB axis promotes liver steatosis by stimulating de novo lipogenesis and cholesterol synthesis. Mol Metab. 54:101349.
- Hotamisligil GS. 2006. Inflammation and metabolic disorders. Nature. 444(7121):860–867.
- Jiang G, Chen D, Li W, Liu C, Liu J, Guo Y. 2020. Effects of wogonoside on the inflammatory response and oxidative stress in mice with nonalcoholic fatty liver disease. Pharm Biol. 58:1177–1183.
- Kashyap ML, Ganji S, Nakra NK, Kamanna VS. 2019. Niacin for treatment of nonalcoholic fatty liver disease (NAFLD): novel use for an old drug? J Clin Lipidol. 13(6):873–879.
- Li S, Qian Q, Ying N, Lai J, Feng L, Zheng S, Jiang F, Song Q, Chai H, Dou X. 2020. Activation of the AMPK-SIRT1 pathway contributes to protective effects of salvianolic acid A against lipotoxicity in hepatocytes and NAFLD in mice. Front Pharmacol. 11:560905.
- Liu J, Yuan Y, Gong X, Zhang L, Zhou Q, Wu S, Zhang X, Hu J, Kuang G, Yin X, et al. 2020. Baicalin and its nanoliposomes ameliorates nonalcoholic fatty liver disease via suppression of TLR4 signaling cascade in mice. Int Immunopharmacol. 80:106208.
- Liu YH, Han X, Cai MJ, Jin SY, Yan ZH, Lu H, Chen QG. 2022. Jianpi Qinghua fomula alleviates insulin resistance via restraining of MAPK pathway to suppress inflammation of the small intestine in DIO mice. BMC Complement Med Ther. 22:129.
- Liu YH, Qiu Y, Chen QG, Han X, Cai MJ, Hao L. 2021. Puerarin suppresses the hepatic gluconeogenesis via activation of PI3K/Akt signaling pathway in diabetic rats and HepG cells. Biomed Pharmacother. 137:111325.
- Nelson JE, Wilson L, Brunt EM, Yeh MM, Kleiner DE, Unalp-Arida A, Kowdley KV, Nonalcoholic Steatohepatitis Clinical Research Network. 2011. Relationship between the pattern of hepatic iron deposition and histological severity in nonalcoholic fatty liver disease. Hepatology. 53(2):448–457.
- Peixoto CA, de Oliveira WH, da Araújo SMR, Nunes AKS. 2017. AMPK activation: role in the signaling pathways of neuroinflammation and neurodegeneration. Exp Neurol. 298:31–41.
- Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschöp MH. 2008. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A. 105(28):9793–9798.
- Polyzos SA, Kountouras J, Mantzoros CS. 2019. Obesity and nonalcoholic fatty liver disease: from pathophysiology to therapeutics. Metabolism. 92:82–97.
- Powell EE, Wong VW, Rinella M. 2021. Non-alcoholic fatty liver disease. Lancet. 397(10290):2212–2224.
- Rohm TV, Meier DT, Olefsky JM, Donath MY. 2022. Inflammation in obesity, diabetes, and related disorders. Immunity. 55:31–55.
- Santhekadur PK, Kumar DP, Sanyal AJ. 2018. Preclinical models of non-alcoholic fatty liver disease. J Hepatol. 68(2):230–237.
- Smith BK, Marcinko K, Desjardins EM, Lally JS, Ford RJ, Steinberg GR. 2016. Treatment of nonalcoholic fatty liver disease: role of AMPK. Am J Physiol Endocrinol Metab. 311(4):E730–E740.
- Song YM, Lee YH, Kim JW, Ham DS, Kang ES, Cha BS, Lee HC, Lee BW. 2015. Metformin alleviates hepatosteatosis by restoring SIRT1-mediated autophagy induction via an AMP-activated protein kinase-independent pathway. Autophagy. 11(1):46–59.
- Tang K, Deng Y, Zheng C, Nie H, Pan M, Chen R, Xie J, Yang Q, Zhang Y. 2020. Prevention of nonalcoholic hepatic steatosis by Shenling Baizhu powder: involvement of adiponectin-induced inhibition of hepatic SREBP-1c. Oxid Med Cell Longev. 2020:9701285.
- Tilg H, Moschen AR. 2010. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 52:1836–1846.
- Xu H, Lyu X, Guo X, Yang H, Duan L, Zhu H, Pan H, Gong F, Wang L. 2022. Distinct AMPK-mediated FAS/HSL pathway is implicated in the alleviating effect of nuciferine on obesity and hepatic steatosis in HFD-fed mice. Nutrients. 14:1898.
- Xu JH, Chen QG, Han X, Liu YH, Jin SY, Cai MJ, Liu SY, Lu H. 2021. Elucidation of the mechanisms and molecular targets of Jianpi Qinghua formula for treating insulin resistance in type 2 diabetes based on network pharmacology. Liaoning J Trad Chinese Med. 48:5-9 + 253–254.
- Yan J, Yao B, Kuang H, Yang X, Huang Q, Hong T, Li Y, Dou J, Yang W, Qin G, et al. 2019. Liraglutide, sitagliptin, and insulin glargine added to metformin: The effect on body Weight and intrahepatic lipid in patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease. Hepatology. 69(6):2414–2426.
- Zamani-Garmsiri F, Hashemnia SMR, Shabani M, Bagherieh M, Emamgholipour S, Meshkani R. 2021. Combination of metformin and genistein alleviates non-alcoholic fatty liver disease in high-fat diet-fed mice. J Nutr Biochem. 87:108505.
- Zhu X, Bian H, Wang L, Sun X, Xu X, Yan H, Xia M, Chang X, Lu Y, Li Y, et al. 2019. Berberine attenuates nonalcoholic hepatic steatosis through the AMPK-SREBP-1c-SCD1 pathway. Free Radic Biol Med. 141:192–204.
