Abstract
Context
A Chinese herbal formula, Tiaopi Xiezhuo decoction (TXD), is developed from a classical Chinese prescription Sanhuang Xiexin decoction.
Objective
To investigate the regulatory effect of TXD on gut dysbiosis, as a treatment of constipation in patients with peritoneal dialysis (PD).
Materials and methods
The chemical content of TXD was assessed by high-performance liquid chromatography. A total of 29 PD patients were enrolled and treated with TXD orally (3 g crude drug/each/twice/day) for 3 months. Blood and faecal samples were collected at the beginning and end, to determine the changes in biochemical characteristics and gut microbial composition. The stool conditions were asked to be scored. Additional 30 healthy individuals were recruited as a control for the analysis of gut microbiota.
Results
Although having no significant effects on serum biochemical characteristics, 3-month TXD intervention improved constipation in PD patients: decreased 80% abdominal distention (p < 0.01), increased 2.6-fold sloppy stools (p < 0.05) and eliminated hard stool completely (p < 0.01). The analysis of gut microbiota showed that, compared to the healthy group, the microbial richness was reduced in PD patients. After a 3-month TXD treatment, this reduced richness was raised, and Paraprevotella clara, Lachnospiraceae bacterium 2-146FA, Phascolarctobaterium succinatutens, Lachnospiraceae bacterium 2-1-58FAA, Fusobacterium mortiferum, and Prevotella copri were accumulated in the intestinal flora. Furthermore, the bacterial species enriched by TXD correlated with the improvement of constipation.
Discussion and conclusions
TXD treatment may improve constipation by modulating gut dysbiosis in PD patients. These findings provide data to support the further application of TXD in the adjuvant treatment of PD.
Introduction
Peritoneal dialysis (PD) is one of the three main alternative therapies for end-stage kidney disease (ESKD). PD uses the patient’s peritoneal tissue to exchange water, creatinine, urea, electrolytes, glucose, and other small and medium-sized toxins between the capillaries on either side of the peritoneum and dialysate (Teitelbaum Citation2021). However, certain complications discount the therapeutic effect of PD. For example, constipation is highly prevalent in these patients and affects their quality of life (Longstreth et al. Citation2006; Cano et al. Citation2007; Kosmadakis et al. Citation2019). Constipation is associated with an increased risk of bacterial translocation and eventual enteric peritonitis. Peritonitis results in considerable morbidity, mortality, and health care costs, as well as limitations in PD modality (Cho and Johnson Citation2014). Thus, there is an urgent need to develop strategies that reduce constipation and prolong the treatment time of PD.
Tiaopi Xiezhuo decoction (TXD), a Chinese herbal formula, is developed from the historical Sanhuang Xiexin decoction which was first described in the Synopsis of Golden Chamber by Zhongjing Zhang of the Eastern Han Dynasty. To improve gastrointestinal symptoms and limit liquid intake in patients with dialysis, the recipe of TXD was modified according to medical records and finally consists of Rheum palmatum L. (Polygonaceae), Coptis chinensis Franch (Ranunculaceae), and Zingiber officinale Roscoe (Zingiberaceae) at a ratio of 1:1:1. Among them, R. palmatum is a traditional herbal medicine for the treatment of constipation. Our clinical data showed that TXD significantly improved gastrointestinal symptoms in PD patients (Liang et al. Citation2020) but the underlying mechanism is still unknown. It is noticed that all three herbs in TXD are reported to have antibacterial properties (Park et al. Citation2008; Wong et al. Citation2010; Feng et al. Citation2011). Recently, R. palmatum and C. chinensis have been found to modulate gut microbiota in rodent studies (Ji et al. Citation2020; Lyu et al. Citation2021). Therefore, we hypothesized that TXD may improve constipation by modulating gut dysbiosis in patients with PD. In response, we designed this study, where we recruited 29 PD patients and treated them with a 3-month TXD intervention, to estimate the changes in constipation situation and gut microbial structures at the baseline and post-intervention. The faecal samples of additional 30 healthy individuals were used as a control for the analysis of gut microbiota.
Materials and methods
Preparation and fingerprint analysis of Tiaopi Xiezhuo decoction
Tiaopi Xiezhuo decoction (TXD) is made by commercial granules of R. palmatum (1 g/bag), C. chinensis (0.5 g/bag), and Z. officinale (0.5 g/bag) at one bag of each for a time, which were produced by Jiangyin Tianjiang Pharmaceutical Co., Ltd. According to the manufacturer’s protocols, the roots of each herb have been decocted, filtered, concentrated and dried, and made into granules, respectively; each bag of granules is equivalent to 3 g of a crude drug; qualities of granules, such as appearance, determination of content, size of granule, solubility, hygroscopicity, heavy metals, toxic elements, pesticide residues, and microbial limit, were controlled rigorously according to the 2015 Chinese pharmacopoeia.
The chemical content of TXD solution was assessed by high-performance liquid chromatography (HPLC) using a COSMOSIL 5C18-MS-II column (4.6 × 250 mm, 5 µm, Nacalai Tesque, Inc., Kyoto, Japan) on an Agilent 1290 Infinity II LC system (Agilent, Cheadle, UK). The granules of TXD were dissolved in 50 mL of deionized water. After being filtered through a 0.45 µm membrane, 5 μL of TXD solution was carefully transferred to sample vials for HPLC analysis. The mobile phase consisted of acetonitrile (ThermoFisher Scientific, Shanghai, China) as solvent A and 0.2% formic acid (Merck, Shanghai, China) in water as solvent B. Then the elution was performed in mobile phase gradient (A:B) of 2:98, 15:85, 30:70 and 98:2 at a rate of 1 mL/min with UV detection at 280 nm. Standard epiberberine or berberine (Sichuan Victory Biological Technology Co., Ltd., Chengdu, China) were prepared in methanol at a concentration of 1 mg/mL and eluted in the mobile phase.
Study design and subjects
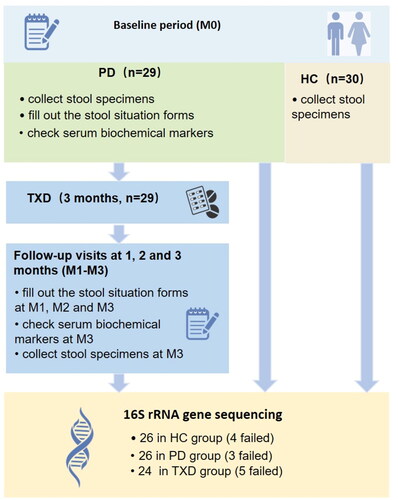
We recruited 29 PD patients and 30 healthy individuals for this study. Among them, 29 randomized PD patients aged 18–80 years old, who had received stable PD for longer than 2 months, were enrolled on the peritoneal dialysis clinic of Guangdong Provincial Hospital of Traditional Chinese Medicine between December 2017 to December 2018; 30 healthy individuals were recruited from the families of PD patients, 18–80 years old, who have no chronic kidney diseases, cardiovascular diseases or cancers. We excluded subjects who showed infection within 1 month, had diarrhoea within 2 weeks and used hormones, antibiotics, or intestinal flora-regulatory drugs in the previous 3 months. We also excluded those with inflammatory bowel disease, intestinal malignancies, and tuberculosis. All the participants completed the ‘Information Collection Form’ upon enrollment in the study, stated that they were willing to cooperate with this research, and signed an informed consent form. The protocol was approved by the ethics committee of the Guangdong Provincial Hospital of Traditional Chinese Medicine (B2017-052.2-02), and the project has been registered at the China Clinical Trial Registration Center (ChiCTR-INR-17013624). The trial was performed in accordance with Good Clinical Practice guidelines. The clinical trial flow diagram is shown in .
As shown in , the stool specimen and serum sample of 29 PD (PD group) were collected at the baseline (M0). Subsequently, all 29 PD patients were given 3-month TXD intervention (orally, twice a day, TXD group), and asked to attend follow-up visits at the end of one, two, and three months (termed as M1, M2 and M3). At each visit, every patient was asked to fill out the forms about stool conditions. The stool specimens and serum samples were collected only after 3-month TXD treatment (M3). The stool specimens of the healthy group (HC group) were obtained as a control for the analysis of gut microbiota.
Evaluation of constipation in PD patients
At the enrollment and follow-up visits (M0-M3), all 29 PD patients were asked to fill out forms ranking their stool experience, characterized as abdominal distention, sloppy stool, and hard stool, and provide a 0–4 score indicating light to heavy (Svedlund et al. Citation1988). The average scores were then calculated.
Stool specimen collection
Between 10–20 g of stool specimens were collected for the analysis of microbiota from all 29 PD patients before and after the 3-month TXD intervention. All faecal specimens were stored in a refrigerator at −80 °C until assay. During the development of the following microbiota profiles, three specimens in the PD group and five in the TXD group failed to be sequenced. Additionally, 30 stool specimens were obtained from healthy individuals, but four were not successfully sequenced.
Analysis of gut microbiota
All stool samples were sent to BGI Genomics Co., Ltd. for structural analysis of gut microbiota. Fecal bacterial DNA was extracted, and 16S rRNA gene sequence libraries were generated using the V3-V4 primer region on the Illumina MiSeq platform (Illumina, San Diego, CA, USA). The operational taxonomic units and α- or β-diversity indices were calculated based on the OTUs data using QIIME (version 1.8.0). Principal coordinates analysis (PCoA) was used to compare the community composition of gut bacteria between groups. Linear discriminant analysis Effect Size (LEfSe) was used to identify the specific species with the most significant differences. The HUMAnN2 method was used to directly compare metagenomics to the metabolic pathways of response genes. Metabolic pathways were mainly interpreted by referring to the MetaCYC database.
Statistical analysis
Statistical analyses were performed using SPSS 18.0 statistical software (SPSS Inc., Chicago, USA). Data are presented as mean value ± standard error for normally distributed variables, or as medians for non-normally distributed variables. Spearman rank correlation was determined, using GraphPad Prism software (GraphPad Software, San Diego, USA), to analyze the relationship between the enriched species and clinical data. Statistical significance was set at p < 0.05.
Results
Fingerprinting analysis of TXD
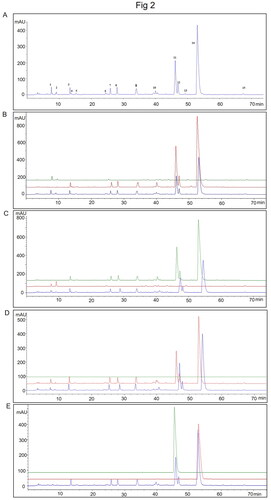
Representative HPLC chromatograms of TXD solution are shown in . Under our condition, 15 peaks are appearing in the solution. Among them, Peak 1, 2, 6, 10, 13, and 15 are from R. palmatum (); Peak 3, 4, 5, 7, 8, 9, 10, 11, 12, and 14 are from C. chinensis (). Under the same condition, we didn’t detect peaks from Z. officinale (). Additionally, compared to the standard Peaks of epiberberine and berberine, Peak 11 is epiberberine and Peak 14 is berberine ().
Figure 2. The ultra-high performance liquid chromatography fingerprints of TXD. (A) representative HPLC chromatograms of TXD. (B) chromatogram overlap of R. palmatum granules (green), TXD without R. palmatum granules (red) and TXD (blue). (C) chromatogram overlap of C. chinensis granules (green), TXD without C. chinensis granules (red) and TXD (blue). (D) chromatogram overlap of Z. officinale granules (green), TXD without Z. officinale granules (red) and TXD (blue). (E) chromatogram overlap of epiberberine (green), berberine (red) and TXD (blue).
Effects of TXD on clinical characteristics and constipation of PD patients
To estimate the effects of TXD on the clinical characteristics of PD patients, 22 biochemical markers were determined in the serum of PD patients before and after 3-month TXD intervention. As shown in , no significant changes were observed between the baseline and post-intervention.
Table 1. Clinical characteristics of PD patients before and after the TXD intervention.
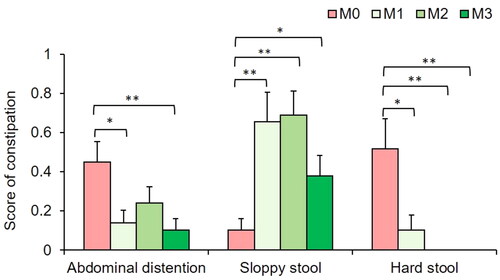
Next, the patient-recorded stool scores were analyzed at each visit. The results showed that, compared to that of PD patients, TXD greatly improved constipation in PD patients from one month of intervention, decreasing the occurrence of abdominal distention and hard stool, and increasing the occurrence of sloppy stool (). At the end of the 3-month intervention, TXD decreased by 80% abdominal distention (p < 0.01), increased 2.6-fold sloppy stools (p < 0.05) and eliminated hard stool completely (p < 0.01, seen in ).
Effects of TXD on gut microbiota in PD patients
Before estimating the effects of TXD on gut microbiota, the changes in microbial structure were determined between PD patients and healthy individuals. Compared with HC group, the richness index, not the Shannon index, showed a significant decrease in PD group’s gut microbiota (). PCoA assessment showed distinct clustering of intestinal microbe communities for both groups (). The ratio of Firmicutes to Bacteroidetes exhibited no difference in PD patients compared to that in healthy individuals (). In addition, compared with HC group, the species Bacteroides thetaiotaomicron, Bacteroides fragilis, Escherichia coli, Parabacteroides unclassified, and Ruminococcus gnavus were greatly enriched in the PD group (). According to metagenomic analysis, the biochemical metabolic functions of intestinal flora were predicted using sequencing results. The most statistically significant metabolic pathways are listed in . Among them, some lead to the disordered metabolism of creatinine and arginine, as well as excessive toxin synthesis (PWY-1269, PWY-5656, CRNFORCAT-PWY, PWY-4981), which further affect renal function through the gut-kidney axis. Together, these results demonstrate that gut dysbiosis developed in PD patients.
Figure 4. Gut dysbiosis developed in patients with peritoneal dialysis. (A) richness index of gut microbiota. (B) Shannon index of gut microbiota. (C) plots of unweighted unifac-based PCoA. (D) ratio of Firmicutes and Bacteroidetes. (E) LEfSe plot showing specific bacteria. HC: healthy group; PD: peritoneal dialysis patient group. *** p < 0.001; ns: non-significance.
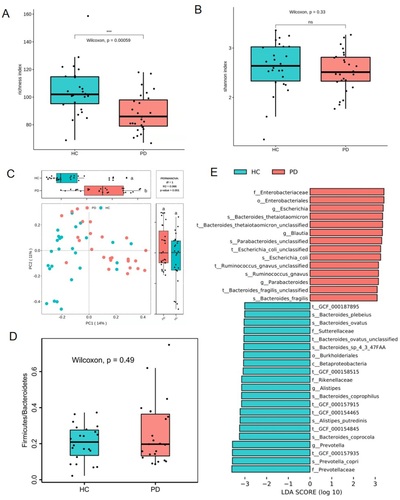
Table 2. LEfSe plot showing predicted metagenome pathways in PD patients.
Compared with those prior to TXD intervention, the richness index of intestinal flora was significantly increased in PD patients receiving 3-month TXD (p = 0.047, ). However, the Shannon index showed no difference in intestinal flora before and after intervention (p = 0.900, ). To further estimate the change in composition, LEfSe analysis was performed (). The enriched intestinal bacteria in the PD group included Bacteroides massiliensis and Enterobacter aerogenes. Following TXD intervention, the bacteria that accumulated were Prevotella copri, Fusobacterium mortiferum, Lachnospiraceae bacterium 2-1-46FFA, Lachnospiraceae bacterium 2-1-58FFA, Veillonella parvula, and Paraprevotella clara. Consistently, the most enriched metabolic pathways were those related to glucolipid metabolism (), but not disordered metabolism of creatinine/arginine and excessive toxin synthesis any more (). These data suggest that gut dysbiosis was greatly modulated by TXD intervention in patients with PD.
Figure 5. Effects of TXD on gut dysbiosis in PD patients. (A) richness index of gut microbiota. (B) Shannon index of gut microbiota. (C) LEfSe plot showing specific bacteria. TXD: Tiaopi Xiezhuo decoction; PD: peritoneal dialysis patient group; TXD: the group treated by TXD. * p < 0.05; NS: non-significance.
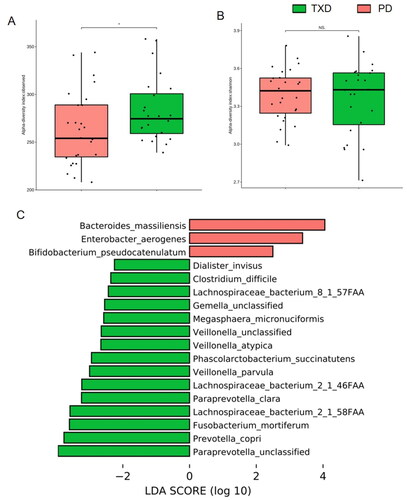
Table 3. LEfSe plot showing predicted metagenome pathways after the TXD intervention.
Correlation of microbial abundance with clinical characteristics and constipation
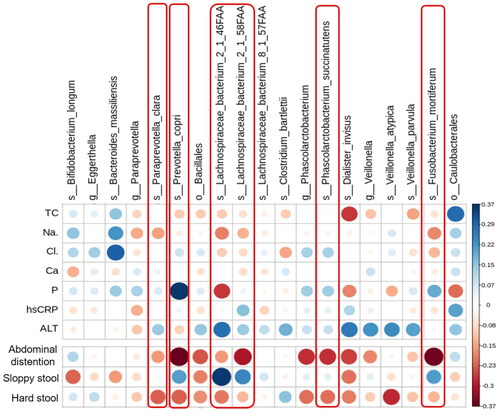
To investigate the association of specific bacteria enriched or diminished by TXD intervention with the effects of TXD on PD patients, the biochemical characteristics or stool scores were analyzed via Spearman analysis with specific bacteria in and the heat map was drawn (). In this heat map, blue indicates a positive correlation, while red indicates a negative correlation; the darker the colour, the greater the correlation, and the larger the circle, the higher the relative abundance. The p-values of all dots on the graph were less than 0.05, indicating statistical significance. As shown in , most of 3-month TXD-enriched species, including P. copri, F. mortiferum, L. bacterium 2-1-46FFA, and L. bacterium 2-1-58FFA, had a negative correlation with abdominal distention and hard stool, but positive correlation with sloppy stool. These suggested that gut bacteria modulated by TXD may play important roles in alleviating constipation in patients with PD. Differently, although the biochemical characteristics presented no difference between PD and TXD groups, we still found some of them were correlated with TXD-enriched species. For example, Dialister invisus, V. parvula, P. copri, F. mortiferum, L. bacterium 2-1-46FFA, and L. bacterium 2-1-58FFA were negatively correlated with total cholesterol, implying the extension of TXD treatment may have beneficial effects on reducing total cholesterol. L. bacterium 2-1-46FFA and D. invisus were negatively correlated with the serum levels of phosphate but P. copri exhibited a positive correlation.
Figure 6. Correlation of TXD-modulated microbial abundance with clinical characteristics and constipation in PD patients. Red frames mark TXD-enriched species with higher correlation with stool conditions. ALT: alanine aminotransferase; Ca: calcium; Cl: chloride; hsCRP: hypersensitive c reactive protein; Na: sodium; P: phosphate; PD: peritoneal dialysis; TC: total cholesterol; TXD: Tiaopi Xiezhuo decoction.
Collectively, our results suggest that TXD may modulate gut dysbiosis to improve constipation in patients with PD.
Discussion
Recently, the composition and richness of gut microbiota have been investigated in patients with PD (Stadlbauer et al. Citation2017; Yacoub et al. Citation2017; Jiang et al. Citation2021; Luo et al. Citation2021). In the present study, we consistently found that gut dysbiosis developed in PD patients in the form of accumulated harmful bacteria (E. coli and B. fragilis) and diminished beneficial bacteria (Bacteroides and Alistipes); the analysis of the biochemical metabolic functions of intestinal flora showed that their metabolic pathways lead to the disordered metabolism of creatinine and arginine, as well as excessive toxin synthesis (), which further affected renal function through the gut-kidney axis. E. coli is closely related to peritonitis in patients. A study reports that E. coli is the main cause of Gram-negative peritonitis (59.2%), which is often associated with a high probability of mortality and technique failure (Feng et al. Citation2014). In addition, the toxicity of E. coli is more serious than previously thought, leading to a worsened prognosis of E. coli peritonitis in PD patients (Valdes-Sotomayor et al. Citation2003). B. fragilis, a major disease-causing Bacteroides species, is a common bacterium of appendicitis and septicemia, and can also be isolated from urinary tract infection excretions and peritonitis-infected abdominal exudate (Choi et al. Citation2016; Valguarnera and Wardenburg Citation2020; Luo et al. Citation2021). Other Bacteroides species have opposing effects and produce short chain fatty acids (SCFAs), which are involved in gut barrier integrity maintenance, glucose and lipid metabolism, and immunity regulations (Koh et al. Citation2016). Further investigation into the function of gut bacteria will help understand their role in the pathology of PD.
TXD is developed from the historical Sanhuang Xiexin Decoction, and consists of R. palmatum, C. chinensis, and Z. officinale at a ratio of 1:1:1. Based on the HPLC analysis (), there are 15 peaks mainly from R. palmatum and C. chinensis. Due to the HPLC experimental difference (Tao et al. Citation2009), we didn’t detect peaks from Z. officinale. Among 15 peaks, two of the main compounds in TXD solution are epiberberine and berberine. Berberine is a natural pentacyclic isoquinoline alkaloid with multiple pharmacological effects. Abundant evidence suggests that berberine can modulate gut microbiota in animal and clinical studies, which reduces the composition of harmful bacteria (such as E. coli and Enterococci bacteria), enriches beneficial bacteria including Lactobacilli and Bifidobacteria, and elevates SCFA production in the colon (Habtemariam Citation2020). There is no study to support whether epiberberine has similar effects on the modulation of gut microbiota to berberine. In the present study, our results demonstrated that TXD intervention modulates the structure of gut dysbiosis in patients with PD (). Although not consistent with that accumulated by berberine in the references, most of the enriched species by TXD are considered as SCFA-producing bacteria and to promote human health (Thompson et al. Citation1992; Morotomi et al. Citation2009; Scheiman et al. Citation2019; Vacca et al. Citation2020). This difference may be attributed to other compounds in TXD which may have effects to regulate the composition of gut microbiota.
Among the species enriched by TXD, Prevotella is a common spore-free Gram-negative anaerobic bacterium. It is widely found in the oral cavity, intestinal tract, female genital tract, and other parts of the healthy human body, and constitutes the normal flora in these areas (Ley Citation2016). As members of the Bacteroidetes, Prevotella is more common in populations that consume a plant-rich diet, suggesting that it is a beneficial microbe (Wu GD et al. Citation2011; Martinez et al. Citation2015). Indeed, Prevotella can use xylan, xylose, and carboxymethylcellulose to produce high levels of short-chain fatty acids (Flint et al. Citation2008). In addition, live P. copri was found to improve glucose tolerance in a mouse model (Kovatcheva-Datchary et al. Citation2015). However, Prevotella has also been reported to be associated with immune diseases, especially rheumatoid arthritis, female genital tract inflammation, and oral infection (Kim and Kim Citation2016). Our data showed that P. copri was enriched by TXD and positively correlated with the improvement of constipation in PD patients, but we also found it was positively correlated with the serum levels of phosphate (), which are unexpected in patients with chronic kidney disease (CKD). Further studies are needed to understand the role of Prevotella in CKD.
Our data demonstrated that the 3-month intervention of TXD decreased the abundance of E. coli and enriched multiple beneficial species in patients with PD. It is important to note that intestinal dysbiosis, especially E. coli, is associated with an increased risk of peritonitis (Kosmadakis et al. Citation2018). However, in the present study, because of time limitations, we did not explore whether TXD intervention can reduce the incidence of peritonitis. Additionally, compounds of three herbs in TXD have been reported to reduce proteinuria, inhibit renal fibrosis and improve renal function in previous studies (Li et al. Citation1997; Wu JS et al. Citation2014; Ren et al. Citation2018; Lu et al. Citation2020; Xiao et al. Citation2021). Although no significant improvements were detected after 3-month TXD treatment, we still found some TXD-enriched species correlated with part of the biochemical characteristics, for example, total cholesterol. Thus, extending observations to determine the effects of clinical TXD treatment may be necessary for the future.
Clinical data have shown that constipation is one of the most common gastrointestinal disorders among patients with CKD (Kosmadakis et al. Citation2019). It has been increasingly recognized that chronic constipation in patients with CKD, particularly those receiving PD, affects the mechanical properties of dialysis techniques and predisposes to bacterial intestinal translocation and eventual enteric peritonitis as well as limitations in PD modality (Cano et al. Citation2007; Kosmadakis et al. Citation2018, Citation2019). Recently, increasing studies have suggested that gut microbiota may play important roles in the pathophysiology of constipation, but no clear consensus exists (Ohkusa et al. Citation2019). The culturing analysis of faecal samples from children with constipation revealed a significantly higher level of Clostridium and Bifidobacterium species (Zoppi et al. Citation1998). However, reduced levels of Bifidobacterium, Lactobacillus, Bacteroides, and Clostridium species, and increased levels of Enterobacteriaceae were observed in adult constipated patients (Khalif et al. Citation2005). The results of 16S rRNA gene sequencing demonstrated that constipated patients had a significantly lower level of Bacteroidetes, in particular, Prevotella, and an increased level of several species of Firmicutes, including Lactobacillus (Zhu et al. Citation2014). The role of specific intestinal bacteria in the development or improvement of constipation is still poorly understood. The potential mechanism may rely on the products of bacterial fermentation, such as SCFAs. One study in rats has shown that propionate, butyrate, and valerate induce concentration-dependent phasic contractions in the middle and distal colon (Yajima Citation1985). The effect of SCFAs may be mediated by stimulating the mucosal receptors and acting directly on the colonic smooth muscle (Rondeau et al. Citation2003). The data in the present study revealed that most of the bacteria enriched by TXD positively correlated with the treatment of constipation, including P. copri, F. mortiferum, Lachnospiraceae, V. parvula, and P. clara. Most of these bacteria are reported to produce SCFA. Whether the beneficial effect of TXD on constipation is associated with SCFA levels in the colon requires further exploration in patients with PD.
Conclusions
This study is an exploratory work to investigate the beneficial effects of TXD on patients with PD. Our data suggest that TXD treatment may improve constipation by modulating gut microbiota. These findings provide data to support the further application of TXD in the adjuvant treatment of PD.
Author contributions
During the research work, Dr Yu Peng, Dr Lei Wang and Dr Xusheng Liu designed the studies; Dr Yu Peng, Tingting Zheng, Xiaoning Xie, Jianfeng Wu, and Lizhe Fu recruited the individuals and collected clinical data; Yang Chen did the analysis of gut microbiota; Dr Lei Wang and Yuting Zeng drafted the manuscript; Fuhua Lu, La Zhang, Dr Yu Peng, Lei Wang and Xusheng Liu revised the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Cano AE, Neil AK, Kang JY, Barnabas A, Eastwood JB, Nelson SR, Hartley I, Maxwell D. 2007. Gastrointestinal symptoms in patients with end-stage renal disease undergoing treatment by hemodialysis or peritoneal dialysis. Am J Gastroenterol. 102(9):1990–1997.
- Cho Y, Johnson DW. 2014. Peritoneal dialysis-related peritonitis: towards improving evidence, practices, and outcomes. Am J Kidney Dis. 64(2):278–289.
- Choi VM, Herrou J, Hecht AL, Teoh WP, Turner JR, Crosson S, Bubeck Wardenburg J. 2016. Activation of Bacteroides fragilis toxin by a novel bacterial protease contributes to anaerobic sepsis in mice. Nat Med. 22:563–567.
- Feng X, Yan D, Zhao KJ, Luo JY, Ren YS, Kong WJ, Han YM, Xiao XH. 2011. Applications of microcalorimetry in the antibacterial activity evaluation of various Rhizoma coptidis. Pharm Biol. 49:348–353.
- Feng X, Yang X, Yi C, Guo Q, Mao H, Jiang Z, Li Z, Chen D, Cui Y, Yu X. 2014. Escherichia coli peritonitis in peritoneal dialysis: the prevalence, antibiotic resistance and clinical outcomes in a South China dialysis center. Perit Dial Int. 34:308–316.
- Flint HJ, Bayer EA, Rincon MT, Lamed R, White BA. 2008. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol. 6:121–131.
- Habtemariam S. 2020. Berberine pharmacology and the gut microbiota: a hidden therapeutic link. Pharmacol Res. 155:104722.
- Ji C, Deng Y, Yang A, Lu Z, Chen Y, Liu X, Han L, Zou C. 2020. Rhubarb enema improved colon mucosal barrier injury in 5/6 nephrectomy rats may associate with gut microbiota modification. Front Pharmacol. 11:1092.
- Jiang N, Zhang C, Feng H, Yuan J, Ding L, Fang W, Gu A, Huang J, Li N, Gu L, et al. 2021. Clinical characteristics associated with the properties of gut microbiota in peritoneal dialysis patients. Perit Dial Int. 41:298–306.
- Khalif IL, Quigley EM, Konovitch EA, Maximova ID. 2005. Alterations in the colonic flora and intestinal permeability and evidence of immune activation in chronic constipation. Dig Liver Dis. 37(11):838–849.
- Kim D, Kim WU. 2016. Editorial: can Prevotella copri be a causative pathobiont in rheumatoid arthritis? Arthritis Rheumatol. 68(11):2565–2567.
- Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F. 2016. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 165(6):1332–1345.
- Kosmadakis G, Albaret J, da Costa Correia E, Somda F, Aguilera D. 2018. Gastrointestinal disorders in peritoneal dialysis patients. Am J Nephrol. 48:319–325.
- Kosmadakis G, Albaret J, Da Costa Correia E, Somda F, Aguilera D. 2019. Constipation in peritoneal dialysis patients. Perit Dial Int. 39:399–404.
- Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, Hallen A, Martens E, Bjorck I, Backhed F. 2015. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab. 22:971–982.
- Ley RE. 2016. Gut microbiota in 2015: prevotella in the gut: choose carefully. Nat Rev Gastroenterol Hepatol. 13:69–70.
- Li P, Kawachi H, Morioka T, Orikasa M, Oite T, Shi ZS, Shimizu F. 1997. Suppressive effects of sairei-to on monoclonal antibody 1-22-3-induced glomerulonephritis: analysis of effective components. Pathol Int. 47(7):430–435.
- Liang JY, Zheng TT, Wu JF, Xie XN, Lu FH, Liu XS, Peng Y. 2020. A randomized controlled trial of Professor Huang Chunlin’s prescription for regulating spleen and relieving turbid to improve gastrointestinal function in peritoneal dialysis patients. Modernization of Traditional Chinese Medicine and Materia Materia-World Science and Technology. 22:498–503.
- Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. 2006. Functional bowel disorders. Gastroenterology. 130(5):1480–1491.
- Lu Z, Ji C, Luo X, Lan Y, Han L, Chen Y, Liu X, Lin Q, Lu F, Wu X, et al. 2020. Nanoparticle-mediated delivery of emodin via colonic irrigation attenuates renal injury in 5/6 nephrectomized rats. Front Pharmacol. 11:606227.
- Luo D, Zhao W, Lin Z, Wu J, Lin H, Li Y, Song J, Zhang J, Peng H. 2021. The effects of hemodialysis and peritoneal dialysis on the gut microbiota of end-stage renal disease patients, and the relationship between gut microbiota and patient prognoses. Front Cell Infect Microbiol. 11:579386.
- Lyu Y, Lin L, Xie Y, Li D, Xiao M, Zhang Y, Cheung SCK, Shaw PC, Yang X, Chan PKS, et al. 2021. Blood-glucose-lowering effect of Coptidis Rhizoma extracts from different origins via gut microbiota modulation in db/db mice. Front Pharmacol. 12:684358.
- Martinez I, Stegen JC, Maldonado-Gomez MX, Eren AM, Siba PM, Greenhill AR, Walter J. 2015. The gut microbiota of rural Papua New Guineans: composition, diversity patterns, and ecological processes. Cell Rep. 11:527–538.
- Morotomi M, Nagai F, Sakon H, Tanaka R. 2009. Paraprevotella clara gen. nov., sp. nov. and Paraprevotella xylaniphila sp. nov., members of the family 'Prevotellaceae’ isolated from human faeces. Int J Syst Evol Microbiol. 59(Pt 8):1895–1900.
- Ohkusa T, Koido S, Nishikawa Y, Sato N. 2019. Gut microbiota and chronic constipation: a review and update. Front Med. 6:19.
- Park M, Bae J, Lee DS. 2008. Antibacterial activity of [10]-gingerol and [12]-gingerol isolated from ginger rhizome against periodontal bacteria. Phytother Res. 22:1446–1449.
- Ren Y, Wang D, Lu F, Zou X, Xu L, Wang K, Huang W, Su H, Zhang C, Gao Y, et al. 2018. Coptidis Rhizoma inhibits NLRP3 inflammasome activation and alleviates renal damage in early obesity-related glomerulopathy. Phytomedicine. 49:52–65.
- Rondeau MP, Meltzer K, Michel KE, McManus CM, Washabau RJ. 2003. Short chain fatty acids stimulate feline colonic smooth muscle contraction. J Feline Med Surg. 5:167–173.
- Scheiman J, Luber JM, Chavkin TA, MacDonald T, Tung A, Pham LD, Wibowo MC, Wurth RC, Punthambaker S, Tierney BT, et al. 2019. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat Med. 25:1104–1109.
- Stadlbauer V, Horvath A, Ribitsch W, Schmerbock B, Schilcher G, Lemesch S, Stiegler P, Rosenkranz AR, Fickert P, Leber B. 2017. Structural and functional differences in gut microbiome composition in patients undergoing haemodialysis or peritoneal dialysis. Sci Rep. 7(1):15601.
- Svedlund J, Sjodin I, Dotevall G. 1988. GSRS–a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 33(2):129–134.
- Tao Y, Li W, Liang W, Van Breemen RB. 2009. Identification and quantification of gingerols and related compounds in ginger dietary supplements using high-performance liquid chromatography-tandem mass spectrometry. J Agric Food Chem. 57(21):10014–10021.
- Teitelbaum I. 2021. Peritoneal dialysis. N Engl J Med. 385(19):1786–1795.
- Thompson J, Nguyen NY, Robrish SA. 1992. Sucrose fermentation by Fusobacterium mortiferum ATCC 25557: transport, catabolism, and products. J Bacteriol. 174(10):3227–3235.
- Vacca M, Celano G, Calabrese FM, Portincasa P, Gobbetti M, De Angelis M. 2020. The controversial role of human gut Lachnospiraceae. Microorganisms. 8:537.
- Valdes-Sotomayor J, Cirugeda A, Bajo MA, del Peso G, Escudero E, Sanchez-Tomero JA, Selgas R, Grupo de Estudios Peritoneales de M. 2003. Increased severity of Escherichia coli peritonitis in peritoneal dialysis patients independent of changes in in vitro antimicrobial susceptibility testing. Perit Dial Int. 23:450–455.
- Valguarnera E, Wardenburg JB. 2020. Good gone bad: one toxin away from disease for Bacteroides fragilis. J Mol Biol. 432:765–785.
- Wong RW, Hagg U, Samaranayake L, Yuen MK, Seneviratne CJ, Kao R. 2010. Antimicrobial activity of Chinese medicine herbs against common bacteria in oral biofilm. A Pilot Study. Int J Oral Maxillofac Surg. 39:599–605.
- Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, et al. 2011. Linking long-term dietary patterns with gut microbial enterotypes. Science. 334:105–108.
- Wu JS, Liu Y, Shi R, Lu X, Ma YM, Cheng NN. 2014. Effects of combinations of Xiexin decoction constituents on diabetic nephropathy in rats. J Ethnopharmacol. 157:126–133.
- Xiao Y, Liu Y, Lai Z, Huang J, Li C, Zhang Y, Gong X, Deng J, Ye X, Li X. 2021. An integrated network pharmacology and transcriptomic method to explore the mechanism of the total Rhizoma Coptidis alkaloids in improving diabetic nephropathy. J Ethnopharmacol. 270:113806.
- Yacoub R, Nugent M, Cai W, Nadkarni GN, Chaves LD, Abyad S, Honan AM, Thomas SA, Zheng W, Valiyaparambil SA, et al. 2017. Advanced glycation end products dietary restriction effects on bacterial gut microbiota in peritoneal dialysis patients; a randomized open label controlled trial. PLoS One. 12(9):e0184789.
- Yajima T. 1985. Contractile effect of short-chain fatty acids on the isolated colon of the rat. J Physiol. 368:667–678.
- Zhu L, Liu W, Alkhouri R, Baker RD, Bard JE, Quigley EM, Baker SS. 2014. Structural changes in the gut microbiome of constipated patients. Physiol Genomics. 46(18):679–686.
- Zoppi G, Cinquetti M, Luciano A, Benini A, Muner A, Bertazzoni Minelli E. 1998. The intestinal ecosystem in chronic functional constipation. Acta Paediatr. 87:836–841.