Abstract
Context
Endometriosis (EMs) is a gynecological disorder. Ligustrazine has been reported to exert an anti-inflammatory effect on EMs. However, the underlying mechanisms are not completely understood.
Objective
To investigate the effects of ligustrazine on the progression of EMs and the underlying regulatory mechanisms.
Materials and methods
Human endometrial stromal cells (HESCs) were isolated from patients with EMs or control subjects. HESCs were treated with 25, 50, 100, or 200 μM ligustrazine for 1, 3, 6, or 12 h. Western blot and enzyme-linked immunosorbent assays were performed to determine the levels of proteins and inflammatory cytokines, respectively. The binding between STAT3 and insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1) was assessed by chromatin immunoprecipitation and dual-luciferase reporter assays. The relationship between IGF2BP1 and RELA was assessed by RNA immunoprecipitation and RNA pull-down assay.
Results
Phosphorylated STAT3, IGF2BP1, RELA, TNF-α, IL-6, and IL-1β were upregulated in EMs tissues compared with control tissues (by 1.79-, 2.55-, 1.58-, 3.01-, 2.55-, and 3.34-fold, respectively). Ligustrazine inhibited the expression of p-STAT3, IGF2BP1, RELA, IL-6, TNF-α, and IL-1β. Overexpression of STAT3 promoted RELA-mediated inflammatory responses, while ligustrazine (100 µM) notably reversed this phenomenon. Ligustrazine also alleviated RELA-induced inflammation via downregulating IGF2BP1. STAT3 bound to the promoter of IGF2BP1, and IGF2BP1 bound to the RELA mRNA.
Discussion and conclusion
Ligustrazine inhibited inflammation in EMs via regulating the STAT3/IGF2BP1/RELA axis. These findings propose a new agent against EMs and support the development of ligustrazine-based treatment strategies for EMs.
Introduction
Endometriosis (EMs) is a debilitating gynecological disorder in which endometrial-like tissues grow outside the adjacent organs, including the bladder, ovaries, pelvic peritoneum, and colon (Li et al. Citation2021; Miller et al. Citation2021). Previous studies have shown that EMs is usually accompanied by dysmenorrhea, chronic pelvic pain, dysuria, dyschezia, and dysuria (Sansone et al. Citation2021; Veena et al. Citation2022). The statistics revealed that EMs is observed in 5–15% of reproductive-age women (Zhang et al. Citation2021) and affects approximately 165 million women in China (Shafrir et al. Citation2021; Wu et al. Citation2021). Although many efforts have been made to optimize the therapies for EMs, standard treatments, such as nonsteroidal anti-inflammatory drugs and pelvic surgery, do not benefit all patients (Shafrir et al. Citation2021). Thus, it is of great clinical significance to explore new therapeutic options for EMs.
Ligustrazine is an ingredient of Rhizoma Chuanxiong (Ligusticum chuanxiong Hort [Umbelliferae]) with therapeutic benefits (Jiang et al. Citation2021; Ma et al. Citation2021). The anti-inflammatory property of ligustrazine has been observed in various diseases. For instance, ligustrazine inhibited hepatic steatosis and oxidative stress, and suppresses the secretion of inflammatory cytokines in hepatic stellate cells (Zhang et al. Citation2016). Moreover, recent evidence has suggested that the combined use of ligustrazine ferulic acid, and tetrahydropalmatine attenuated epithelial-mesenchymal transformation during the development of EMs (Zhang et al. Citation2021). However, the mechanisms underlying the effects of ligustrazine on EMs remain unclear.
RELA, also known as p65, is a transcription factor that can bind to genes encoding pro-inflammatory cytokines, induces their transcription, and ultimately promotes the secretion of inflammatory cytokines (Khawaja et al. Citation2021). Signal transducer and activator of transcription 3 (STAT3) is a transcription factor and its activation has been found in EMs, as evidenced by an increased level of phosphorylated STAT3 (p-STAT3) (He et al. Citation2021). Furthermore, ligustrazine inhibited the activation of the STAT3 signaling pathway in mice with experimental autoimmune encephalomyelitis (Zhang et al. Citation2021). Insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1), a key RNA-binding protein that regulates the stability of mRNA, is known to be upregulated in EMs (Yi et al. Citation2010; Cruz-Zaragoza et al. Citation2021). Bioinformatics prediction from Starbase (http://starbase.sysu.edu.cn/index.php) (Zhang et al. Citation2022) indicated that IGF2BP1 could bind to the RELA mRNA. The above data led us to hypothesize that RELA, STAT3, and IGF2BP1 might be involved in ligustrazine-mediated progression of EMs.
In this study, we investigate the effects of ligustrazine on EMs and explore the potential involvement of RELA, STAT3, and IGF2BP1 in this process. The relationships among these factors were examined. Our findings suggest that ligustrazine attenuated inflammation in EMs via regulating the STAT3/IGF2BP1/RELA axis, which may provide new insights into the development of novel treatments for EMs.
Materials and methods
Tissue collection
Endometrial tissues were collected from patients with EMs or eutopic endometrium (EU), or normal subjects (n = 30 per group) in the Second Affiliated Hospital of Nanchang University between April 2020 and April 2021. Tissues were maintained at −80 °C. Patients who were diagnosed with EMs according to the common diagnostic guidelines were eligible for recruitment. The exclusion criteria were as follows: (1) Individuals who suffered from other diseases and are currently under treatment; (2) pregnant or lactating women; (3) patients who were allergic to probiotics or had used/were using antibiotics recently; and (4) alcoholics or smokers. This study (Clinical trial Register Number: 2022-35) was approved by the Ethics Committee of The Second Affiliated Hospital of Nanchang University and informed consent was obtained from each participant.
Cell culture and treatment
Human endometrial stromal cells (HESCs) were isolated from EMs, EU, or normal tissues as previously described (Li et al. Citation2021). Briefly, tissues were digested with 0.25% Type IA collagenase (Sigma) and strained through 250 and 38 μm stainless steel sieves, respectively, to remove undigested tissue and glandular cells. At least 95% of the isolated cells were positive for vimentin, but they were not stained for cytokeratin. Cells were contained in DMEM (Thermo Fisher Scientific) containing 1% penicillin, 10% fetal bovine serum, and 1% streptomycin (Sigma) in the condition of 37 °C and 5% CO2. To detect the effects of ligustrazine on EMs, HESCs were treated with 25, 50, 100, or 200 μM ligustrazine for 1, 3, 6, or 12 h.
Immunohistochemical (IHC) staining
Tissues were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) overnight, paraffin-embedded, and cut into 5 μm thick sections. After being deparaffinized and rehydrated, the sections were heated in sodium citrate buffer in a microwave for antigen retrieval, washed with PBS for 5 min (three times) at room temperature, incubated in 3% H2O2 at room temperature for 25 min, washed with PBS for 5 min (three times), and blocked and incubated in goat serum for 30 min. Then, the samples were stained with primary antibodies (Abcam), including anti-p-STAT3, anti-IGF2BP1, and anti-RELA (p65) overnight at 4 °C. Next, samples were incubated with an HRP-labeled secondary antibody for 30 min at 37 °C. Finally, freshly prepared diaminobenzidine was added for color development.
Cell transfection
HESCs (3 × 105 cells/well) were seeded in six-well plates. To silence STAT3 or IGF2BP1, cells were transfected with short hairpin RNA targeting STAT3 (STAT3 shRNA) or IGF2BP1 (IGF2BP1 shRNA), or negative control (shNC) for 24 h using Lipofectamine 2000 (Thermo Fisher Scientific). To overexpress STAT3 or IGF2BP1, cells were transfected with pcDNA3.1-STAT3 (Genepharma), pcDNA3.1-IGF2BP1 (Genepharma), or pcDNA3.1 (Genepharma) for 24 h using Lipofectamine 2000.
Western blotting
RIPA was used to isolate proteins from tissues or cells. The BCA kit (Beyotime) was used for protein quantification. Protein samples were separated by SDS-PAGE (10%) and then transferred to PVDF membranes (Bio-Rad). Then, the membranes were incubated with designated primary antibodies (Abcam), including anti-STAT3 (1:1000), anti-p-STAT3 (1:1000), anti-IGF2BP1 (1:1000), anti-RELA(p65) (1:1000), and anti-β-actin (internal control, 1:1000) overnight at 4 °C. After blocking, an anti-rabbit secondary antibody (1:5000; Abcam) was applied for 1 h. The Odyssey Imaging System was used to scan the membranes. The Odyssey software (v2.0, LI-COR Biosciences) was used for blot analysis.
Quantitative real-time PCR (RT-qPCR) detection
TRIzol (Invitrogen) was used to isolate total RNA from tissues. PrimeScript kit (Takara) and SYBR kit (Takara) were used for RT-qPCR. The primers were generated by GenePharma. The sequences of the primers were as follows:
STAT3 forward, 5′-GCTGCCCCATACCTGAAGAC-3′
STAT3 reverse, 5′-GTAGGCGCCTCAGTCGTATC-3′;
IGF2BP1 forward, 5′-GGCCTGAGAATGAGTG-3′
IGF2BP1 reverse, 5′-GAGGGGCAGACAGTGTTG -3′;
Tumor necrosis factor alpha (TNF-α) forward, 5′-CCAGAACTCCAGGCGGTGTC-3′
TNF-α reverse, 5′-GGCTACGGGCTTGTCACTCG-3′;
Interleukin 6 (IL-6) forward, 5′-TCCAGCCAGTTGCCTTCTTG-3′
IL-6 reverse, 5′-AGCCACTCCTTCTGTGACTC-3′;
Interleukin 1β (IL-1β) forward, 5′-ATGCCTCGTGCTGTCTGAC-3′
IL-1β reverse, 5′-TCCCGACCATTGCTGTTTCC-3′;
β-actin (internal control) forward, 5′-AAATCTGGCACCACACCTTC-3′
β-actin reverse, 5′-GGGGTGTTGAAGGTCTCAAA-3′.
The 2−ΔΔCt method was applied for quantification, and the data in the control group was set to 1.
Enzyme-linked immunosorbent assay (ELISA)
The levels of IL-1β, TNF-α, and IL-6 levels in tissue samples and HESC supernatants were detected by ELISA kits (Abcam) according to the manufacturer’s instructions.
Dual luciferase assay
The corresponding binding sequences of wild-type (WT) IGF2BP1 or mutant (Mut) IGF2BP1 were synthesized and then sub-cloned into the luciferase reporter plasmid pGL3 to obtain pGL3-WT-IGF2BP1 and pGL3-Mut-IGF2BP1 plasmids. The luciferase plasmids (WT or Mut) and STAT3 shRNA/shNC were transfected to HESCs using Lipofectamine 2000. After incubation for 48 h, the Dual-Luciferase System (Promega Corporation) was used to assess the luciferase activity. The Renilla luciferase activity served as the internal control.
RNA immunoprecipitation (RIP) assay
The correlation between IGF2BP1 and the RELA mRNA was assessed by RIP assay. Approximately 1 × 107 cells were harvested and lysed in 100% RIP lysis buffer with proteinase and RNase inhibitors. The RIP lysates were incubated with RIP buffer containing magnetic beads conjugated to anti-IGF2BP1 (Millipore) antibody or control IgG (Millipore). Meanwhile, IgG served as the negative control. The enrichment of IGF2BP1 and RELA was evaluated by RT-qPCR.
Actinomycin D assays
After 24 h of transfection, actinomycin D (5 µg/mL, Sigma) was added to the media of transfected HESCs. At 0, 1, 2, 3, or 4 h after incubation with actinomycin D, the mRNA level of RELA was assessed by RT-qPCR.
Chromatin immunoprecipitation (ChIP) assay
Cells were homogenized in immunoprecipitation lysis buffer (Beyotime) supplemented with protease inhibitor cocktail (Beyotime), phosphatase inhibitor cocktail (Beyotime), and EDTA (Beyotime). The lysates were centrifuged at 12,000 rpm for 15 min at 4 °C, and the supernatants were removed. Then, prepared lysates were precleared using 10 μL of Protein A + G agarose beads (cat no. 78610, Thermo Fisher Scientific) by incubation with rotation at 4 °C for 2 h. The antibodies of interest or control IgG were added to the precleared lysates, and 20 μL of Protein A + G agarose beads were then added to the mixture. The tubes were incubated with rotation at 4 °C overnight in each step. Anti-STAT3 antibody (Millipore) was used for ChIP. Immunoglobulin G (IgG) was used as the negative control. IGF2BP1 enrichment was assessed by RT-qPCR.
Statistical analysis
Three experiments were conducted in each group. The normality of the data was tested by the Kolmogorov–Smirnov test. All data were normally distributed and expressed as mean ± standard deviation. All statistical analyses were performed using GraphPad Prism 7 (GraphPad Software, Inc.). Paired Student’s t-test or unpaired Student’s t-test was applied to analyze the differences between the two groups. One-way ANOVA followed by Tukey’s test was for multi-group comparisons. p < 0.05 suggested statistical significance.
Results
STAT3, IGF2BP1, RELA, IL-6, TNF-α, and IL-1β were upregulated in EMs tissues
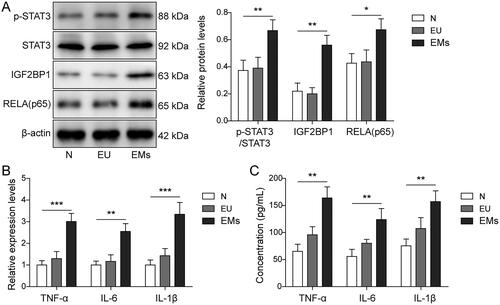
Firstly, the levels of p-STAT3, IGF2BP1, RELA, IL-6, TNF-α, and IL-1β in EMs were examined. The results showed that p-STAT3, IGF2BP1, and RELA were upregulated in EMs tissues in comparison with normal tissues (p < 0.05, and supplemental Figure S1). The levels of IL-6, TNF-α, and IL-1β were also notably upregulated in EMs (p < 0.01, ). Consistently, ELISA showed the concentrations of these pro-inflammatory cytokines in EMs tissues were significantly higher than those in EU and normal tissues (p < 0.01, ). Taken together, p-STAT3, IGF2BP1, RELA, TNF-α, IL-6, and IL-1β were upregulated in EMs tissues.
Figure 1. STAT3, IGF2BP1, RELA (p65), IL-6, TNF-α, and IL-1β were upregulated in EMs tissues. (A) The levels of p-STAT3, IGF2BP1, RELA (p65), and STAT3 in normal, EU, and EMs tissues were evaluated by Western blot. β-Actin was used for normalization. (B) The levels of IL-6, IL-1β, and TNF-α in normal, EU, and EMs tissues were detected by RT-qPCR. (C) The levels of IL-6, IL-1β, and TNF-α in normal, EU, and EMs tissues were determined by ELISA. *p < 0.05; **p < 0.01; **p < 0.001.
Ligustrazine significantly decreased the levels of STAT3, IGF2BP1, RELA, IL-6, TNF-α, and IL-1β in HESCs
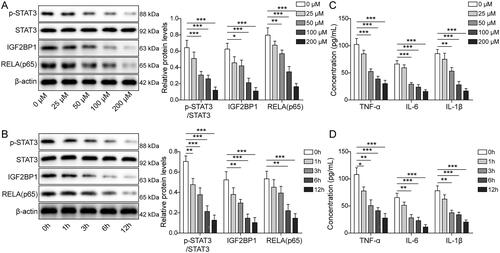
To investigate the role of ligustrazine in EMs, we treated HESCs with various concentrations of ligustrazine. As shown in , ligustrazine downregulated the expressions of p-STAT3, IGF2BP1, and RELA in HESCs (p < 0.05). Based on the above results, ligustrazine at 100 μM was used for subsequent experiments. We further found that ligustrazine inhibited the expression of p-STAT3, IGF2BP1, and RELA in HESCs in a time-dependent manner (p < 0.01, ). Moreover, ligustrazine time-dependently and decreased the levels of TNF-α, IL-6, and IL-1β in supernatants of HESCs (p < 0.01 and p < 0.05). These data suggest that ligustrazine significantly suppressed the expressions of p-STAT3, IGF2BP1, RELA, IL-6, TNF-α, and IL-1β in EMs.
Figure 2. Ligustrazine significantly inhibited the expression of STAT3, IGF2BP1, RELA (p65), IL-6, TNF-α, and IL-1β in HESCs. (A) HESCs were treated with 25, 50, 100, or 200 μM ligustrazine for 6 h. The levels of p-STAT3, STAT3, IGF2BP1, and RELA (p65) in HESCs were measured by Western blot. (B) HESCs were exposed to 100 μM ligustrazine for 1, 3, 6, or 12 h. The levels of p-STAT3, STAT3, IGF2BP1, and RELA (p65) in HESCs were detected by Western blot. (C) HESCs were treated with 25, 50, 100, or 200 μM ligustrazine for 6 h. The concentrations of IL-6, IL-1β, and TNF-α in HESC supernatants were determined by ELISA. (D) HESCs were exposed to 100 μM ligustrazine for 1, 3, 6, or 12 h. The concentrations of IL-6, IL-1β, and TNF-α in HESC supernatants were assessed by ELISA. *p < 0.05; **p < 0.01; **p < 0.001.
Ligustrazine inhibited RELA-mediated inflammation in HESCs via inhibiting the activation of STAT3
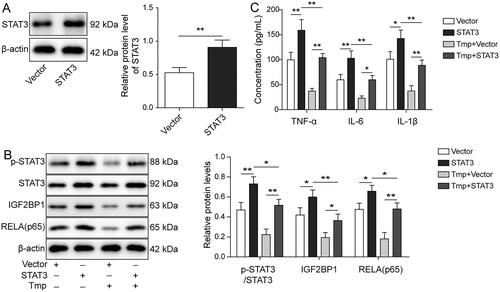
To investigate the mechanism by which ligustrazine regulated inflammatory responses in HESCs, HESCs overexpressing STAT3 were constructed. Western blot was then performed to evaluate transfection efficiency. As shown in , the expression of STAT3 in HESCs was significantly upregulated 1.72-fold by transfection with STAT3-overexpressed plasmids (p < 0.01). In addition, STAT3 overexpression markedly increased the expression of p-STAT3, IGF2BP1, and RELA in HESCs (p < 0.05), and it effectively reversed ligustrazine-induced downregulation of these proteins (p < 0.05, ). The decrease in the levels of TNF-α, IL-1β, and IL-6 levels in HESC supernatants by ligustrazine was also significantly restored by STAT3 overexpression (p < 0.05, ). Furthermore, the anti-inflammatory effect of ligustrazine was greatly diminished by overexpression of STAT3 (p < 0.01, ). The above results imply that ligustrazine inhibited RELA-mediated inflammation in HESCs via inhibiting the activation of STAT3.
Figure 3. Ligustrazine inhibited RELA (p65)-mediated inflammation in HESCs by inactivating STAT3. (A) HESCs were transfected with empty vector or pcDNA3.1-STAT3. The level of STAT3 in HESCs was measured by Western blot. (B) HESCs were transfected with empty vector or pcDNA3.1-STAT3, followed by treatment with or without 100 μM Ligustrazine for 6 h. The levels of p-STAT3, IGF2BP1, RELA (p65), and STAT3 in HESCs were assessed by Western blot. (C) The concentrations of IL-6, IL-1β, and TNF-α in HESC supernatants were measured by ELISA. *p < 0.05; **p < 0.01.
Ligustrazine alleviated RELA-mediated inflammation in HESCs by downregulating IGF2BP1
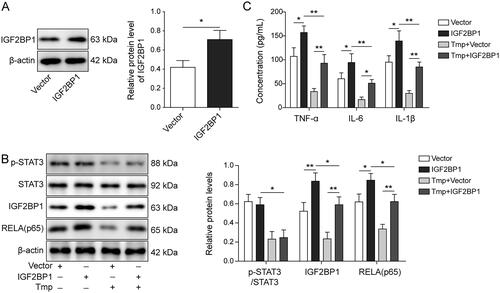
To explore the relationship between ligustrazine and IGF2BP1 in EMs, cells were transfected with pcDNA3.1-IGF2BP1. As shown in , the level of IGF2BP1 in HESCs was markedly increased 1.69-fold by the transfection with IGF2BP1-overexpressed plasmids. IGF2BP1 overexpression did not affect the level of p-STAT3, but increased the expression of RELA in HESCs (p < 0.05, ). However, this phenomenon was reversed by ligustrazine treatment (p < 0.01, ). The presence of ligustrazine also significantly abolished IGF2BP1 overexpression-induced inflammatory responses in HESCs (p < 0.01, ). Collectively, ligustrazine alleviated RELA-mediated inflammation in HESCs via downregulating IGF2BP1.
Figure 4. Ligustrazine alleviated RELA (p65)-mediated inflammation in HESCs by downregulating IGF2BP1. (A) HESCs were transfected with empty vector or pcDNA3.1-IGF2BP1. The level of IGF2BP1 in HESCs was measured by Western blot. (B) HESCs were transfected with empty vector or pcDNA3.1-IGF2BP1, followed by treatment with or without 100 μM ligustrazine for 6 h. The expression of p-STAT3, IGF2BP1, RELA (p65), and STAT3 in HESCs was detected by Western blot. (C) The concentrations of IL-6, IL-1β, and TNF-α in HESC supernatants were measured by ELISA. *p < 0.05; **p < 0.01.
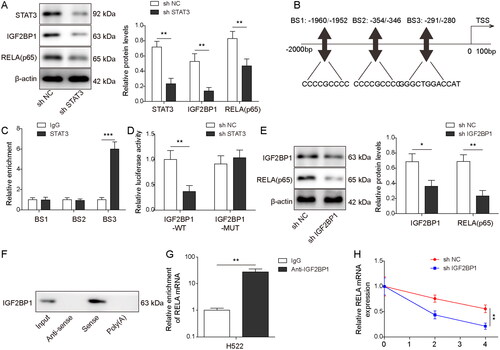
STAT3 bound to the promoter of the IGF2BP1 gene, and IGF2BP1 bound to the RELA mRNA
We further investigated the relationship among STAT3, IGF2BP1, and RELA. As illustrated in , transfection with STAT3 shRNA notably inhibited the expression of STAT3, IGF2BP1, and RELA in HESCs (p < 0.01). AnimalTFDB, a computational prediction tool, predicted that there might be three binding sites (BS) between STAT3 and IGF2BP1 (). ChIP assay then showed the enrichment of BS3 in the complexes precipitated with anti-STAT3 antibody (p < 0.001, ). The luciferase activity of WT-IGF2BP1 was notably decreased by STAT3 silencing (p < 0.01, ). Moreover, transfection with IGF2BP1 shRNA notably decreased the expressions of IGF2BP1 and RELA in HESCs (p < 0.05, ). In addition, IGF2BP1 could bind to the RELA mRNA (). Furthermore, IGF2BP1 silencing notably reduced the stability of the RELA mRNA (p < 0.01, ). Thus, it could be concluded that STAT3 could bind to the IGF2BP1 promoter, and IGF2BP1 could in turn bind to the RELA mRNA to promote RELA expression.
Figure 5. STAT3 bound to the promoter of IGF2BP1, and IGF2BP1 bound to the RELA mRNA. (A) HESCs were transfected with shNC or shSTAT3. The levels of STAT3, IGF2BP1, and RELA (p65) in HESCs were assessed by Western blot. (B) AnimalTFDB was used to predict the binding sites between IGF2BP1 and STAT3. (C) ChIP assay detected the enrichment of STAT3 on the IGF2BP1 promoter. (D) The luciferase activity of STAT3 on WT/Mut-IGF2BP1 was examined by dual-luciferase assay. (E) HESCs were transfected with shNC or shIGF2BP1. The levels of IGF2BP1 and RELA (p65) in HESCs were assessed by Western blot. (F, G) The enrichment of IGF2BP1 with the RELA mRNA was assessed by (F) RNA pull-down and (G) RIP assay. (H) The half-life of the RELA mRNA was detected by RT-qPCR. *p < 0.05; **p < 0.01.
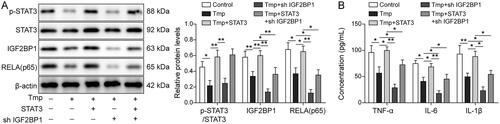
Ligustrazine attenuated inflammation during the progression of EMs via regulating the STAT3/IGF2BP1/RELA axis
Lastly, Western blot analysis was performed to ascertain the mechanism by which ligustrazine mediates inflammation in EMs. As illustrated in , ligustrazine-induced downregulation of p-STAT3, RELA, and IGF2BP1 was reversed by STAT3 overexpression (p < 0.05), while the effect of STAT3 on IGF2BP1 and RELA was reversed by IGF2BP1 silencing (p < 0.05). Meanwhile, the inhibitory effect of ligustrazine on IL-1β, IL-6, and TNF-α was significantly rescued by STAT3 overexpression (p < 0.05, ). However, IGF2BP1 downregulation restored this phenomenon (p < 0.05, ). To sum up, ligustrazine attenuated inflammation during the progression of EMs via regulating the STAT3/IGF2BP1/RELA axis.
Figure 6. Ligustrazine attenuated inflammation in EMs via regulating the STAT3/IGF2BP1/RELA axis. HESCs were subjected to the following treatment: 100 μM Ligustrazine, Ligustrazine + pcDNA3.1-STAT3, Ligustrazine + shIGF2BP1, or Ligustrazine + pcDNA3.1-STAT3 + shIGF2BP1. (A) The levels of STAT3, p-STAT3, IGF2BP1, and RELA (p65) in HESCs were measured by Western blot. (B) The concentrations of IL-6, IL-1β, and TNF-α in HESC supernatants were assessed by ELISA. *p < 0.05; **p < 0.01.
Discussion
Excessive secretion of pro-inflammatory cytokines, such as IL-1β, TNF-α, and IL-6, has been considered key contributors to the development of EMs (Bian et al. Citation2021; Kotlyar et al. Citation2021). In the present study, we showed that ligustrazine inhibited the production of TNF-α, IL-1β, and IL-6 in HESCs and therefore alleviated inflammation during the progression of EMs. Further analysis revealed, for the first time, that ligustrazine attenuated inflammation in EMs via regulating the STAT3/IGF2BP1/RELA axis. These data suggest that ligustrazine may act as a novel therapeutic agent for EMs.
STAT3 is a crucial member of the STAT3 signaling participates in diverse inflammatory responses and plays an indispensable role in the occurrence of various inflammatory diseases (Chowdhury et al. Citation2019; Yu et al. Citation2019). The involvement of STAT3 in the progression of EMs has also been reported. IL-37, an anti-inflammatory cytokine, inhibited the development of EMs in a mouse model by inhibiting STAT3 phosphorylation, while activation of STAT3 effectively reversed IL-37-induced dendritic cell maturation in EMs (Li et al. Citation2021). Besides, long non-coding RNA AFAP1-AS1 modulated the proliferation and apoptosis of endometrial stromal cells in EMs by activating the STAT3/transforming growth factor-β1/Smad axis (Huan et al. Citation2021). Consistent with previous literature, our data showed that ligustrazine treatment decreased the phosphorylation level of STAT3 in HESCs, suggesting that ligustrazine may act as an inhibitor of the STAT3 pathway in EMs.
The interaction between RELA and STAT3 serves as a key event in the pathogenesis of multiple inflammation-related diseases (Gao et al. Citation2021). In the present study, we found that ligustrazine reversed RELA-induced inflammation in HESCs by inhibiting the activation of STAT3. IGF2BP1 is an RNA binding protein that maintains mRNA stability (Ramesh-Kumar and Guil, Citation2022; Liu et al. Citation2022). Our data showed that IGF2BP1 reduced the stability of the RELA mRNA. Liu et al. (Citation2022) reported that silencing of IGF2BP1 restrained inflammatory responses in chronic inflammatory diseases, such as atherosclerosis. In addition, recent data showed that IGF2BP1 silencing inhibited p65 nuclear translocation and nuclear factor kappa B activation in lipopolysaccharide-induced macrophages (Xie et al. Citation2019). Then, we speculated that STAT3 might upregulate the expression of RELA by binding IGF2BP1. Further analysis of the relationships among STAT3, IGF2BP1, and RELA demonstrated that suggesting that STAT3 regulated the progression of EMs via regulating the IGF2BP1/RELA axis. Furthermore, the binding analyses indicated that STAT3 bound to the promoter of the IGF2BP1 gene.
The limitations of the present study should also be acknowledged. Firstly, our data were obtained from clinical tissue samples and cell culture experiments. Whether ligustrazine could inhibit inflammation in EMs should be validated in animal models. Secondly, we only examined the effects of ligustrazine on the STAT3/IGF2BP1/RELA axis, whether other inflammation-related signaling pathways are involved in ligustrazine-mediated progression of EMs warrants further investigation. Thirdly, the optimal dosage and treatment duration of ligustrazine for EMs need to be explored.
Conclusions
The present study shows that ligustrazine inhibits inflammatory responses in EMs by regulating the STAT3/IGF2BP1/RELA axis. These data not only highlighted the therapeutic potential of ligustrazine for EMs but also provided a basis for future development of ligustrazine-based treatment strategies for EMs.
Supplemental Material
Download TIFF Image (2.3 MB)Acknowledgements
We would like to give our sincere gratitude to the reviewers for their constructive comments.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analyzed during this study are included in this published article
Additional information
Funding
References
- Bian Y, Yuan L, Yang X, Weng L, Zhang Y, Bai H, Chen J. 2021. SMURF1-mediated ubiquitylation of SHP-1 promotes cell proliferation and invasion of endometrial stromal cells in endometriosis. Ann Transl Med. 9(5):362–362.
- Chowdhury I, Banerjee S, Driss A, Xu W, Mehrabi S, Nezhat C, Sidell N, Taylor RN, Thompson WE. 2019. Curcumin attenuates proangiogenic and proinflammatory factors in human eutopic endometrial stromal cells through the NF-κB signaling pathway. J Cell Physiol. 234(5):6298–6312.
- Cruz-Zaragoza LD, Dennerlein S, Linden A, Yousefi R, Lavdovskaia E, Aich A, Falk RR, Gomkale R, Schöndorf T, Bohnsack MT, et al. 2021. An in vitro system to silence mitochondrial gene expression. Cell. 184(23):5824–5837.e15.
- Gao M, Liu L, Zhang X, Li Z, Zhang M. 2021. Interleukin-6 reverses adriamycin resistance in nasal NK/T-cell lymphoma via downregulation of ABCC4 and inactivation of the JAK2/STAT3/NF-κB/P65 pathway. Environ Toxicol Pharmacol. 85:103639.
- He Y, Hung SW, Liang B, Zhang R, Gao Y, Chu CY, Zhang T, Xu H, Chung JPW, Wang CC. 2021. Receptor tyrosine kinase inhibitor sunitinib as novel immunotherapy to inhibit myeloid-derived suppressor cells for treatment of endometriosis. Front Immunol. 12:641206.
- Huan Q, Cheng SC, Du ZH, Ma HF, Li C. 2021. LncRNA AFAP1-AS1 regulates proliferation and apoptosis of endometriosis through activating STAT3/TGF-β/Smad signaling via miR-424-5p. J Obstet Gynaecol Res. 47(7):2394–2405.
- Jiang R, Xu J, Zhang Y, Zhu X, Liu J, Tan Y. 2021. Ligustrazine alleviate acute lung injury through suppressing pyroptosis and apoptosis of alveolar macrophages. Front Pharmacol. 12:680512.
- Khawaja H, Fazal N, Yaqub F, Ahmad MR, Hanif M, Yousaf MA, Latief N. 2021. Protective and proliferative effect of Aesculus indica extract on stressed human adipose stem cells via downregulation of NF-κB pathway. PLoS One. 16(10):e0258762.
- Kotlyar AM, Mamillapalli R, Flores VA, Taylor HS. 2021. Tofacitinib alters STAT3 signaling and leads to endometriosis lesion regression. Mol Hum Reprod. 27:gaab016.
- Li L, Liao Z, Ye M, Jiang J. 2021. Recombinant human IL-37 inhibited endometriosis development in a mouse model through increasing Th1/Th2 ratio by inducing the maturation of dendritic cells. Reprod Biol Endocrinol. 19(1):128.
- Li X, Xiong W, Long X, Dai X, Peng Y, Xu Y, Zhang Z, Zhang L, Liu Y. 2021. Inhibition of METTL3/m6A/miR126 promotes the migration and invasion of endometrial stromal cells in endometriosis†. Biol Reprod. 105(5):1221–1233.
- Liu HT, Zou YX, Zhu WJ, Sen L, Zhang GH, Ma RR, Guo XY, Gao P. 2022. LncRNA THAP7-AS1, transcriptionally activated by SP1 and post-transcriptionally stabilized by METTL3-mediated m6A modification, exerts oncogenic properties by improving CUL4B entry into the nucleus. Cell Death Differ. 29(3):627–641.
- Liu M, Tao G, Cao Y, Hu Y, Zhang Z. 2022. Silencing of IGF2BP1 restrains ox-LDL-induced lipid accumulation and inflammation by reducing RUNX1 expression and promoting autophagy in macrophages. J Biochem Mol Toxicol. 36(4):e22994.
- Ma X, Ruan Q, Ji X, Yang J, Peng H. 2021. Ligustrazine alleviates cyclophosphamide-induced hepatotoxicity via the inhibition of Txnip/Trx/NF-κB pathway. Life Sci. 274:119331.
- Miller JE, Lingegowda H, Symons LK, Bougie O, Young SL, Lessey BA, Koti M, Tayade C. 2021. IL-33 activates group 2 innate lymphoid cell expansion and modulates endometriosis. JCI Insight. 6(23):e149699.
- Ramesh-Kumar D, Guil S. 2022. The IGF2BP family of RNA binding proteins links epitranscriptomics to cancer. Semin Cancer Biol. 86(Pt 3):18–31.
- Sansone AM, Hisrich BV, Young RB, Abel WF, Bowens Z, Blair BB, Funkhouser AT, Schammel DP, Green LJ, Lessey BA, et al. 2021. Evaluation of BCL6 and SIRT1 as non-invasive diagnostic markers of endometriosis. Curr Issues Mol Biol. 43(3):1350–1360.
- Shafrir AL, Martel E, Missmer SA, Clauw DJ, Harte SE, As-Sanie S, Sieberg CB. 2021. Pelvic floor, abdominal and uterine tenderness in relation to pressure pain sensitivity among women with endometriosis and chronic pelvic pain. Eur J Obstet Gynecol Reprod Biol. 264:247–253.
- Veena KV, Siddamalla S, Deenadayal M, Shivaji S, Bhanoori M. 2022. DNMT1 and DNMT3B gene variants and their association with endometriosis in South Indian women. Mol Biol Rep. 49(1):321–329.
- Wu J, Fang X, Xia X. 2021. Identification of key genes and pathways associated with endometriosis by weighted gene co-expression network analysis. Int J Med Sci. 18(15):3425–3436.
- Xie J, Li Q, Zhu XH, Gao Y, Zhao WH. 2019. IGF2BP1 promotes LPS-induced NFκB activation and pro-inflammatory cytokines production in human macrophages and monocytes. Biochem Biophys Res Commun. 513(4):820–826.
- Yi YC, Wang SC, Chao CC, Su CL, Lee YL, Chen LY. 2010. Evaluation of serum autoantibody levels in the diagnosis of ovarian endometrioma. J Clin Lab Anal. 24(5):357–362.
- Yu H, Zhong Q, Xia Y, Li E, Wang S, Ren R. 2019. MicroRNA-2861 targets STAT3 and MMP2 to regulate the proliferation and apoptosis of ectopic endometrial cells in endometriosis. Pharmazie. 74:243–249.
- Zhang C, Zhang Y, Pan H, Tan Y, Wei Q, Dai X, Wei J, Chen Y. 2021. Combination of ferulic acid, ligustrazine and tetrahydropalmatine attenuates epithelial-mesenchymal transformation via Wnt/β-catenin pathway in endometriosis. Int J Biol Sci. 17(10):2449–2460.
- Zhang F, Jin H, Wu L, Shao J, Wu X, Lu Y, Zheng S. 2016. Ligustrazine disrupts lipopolysaccharide-activated NLRP3 inflammasome pathway associated with inhibition of Toll-like receptor 4 in hepatocytes. Biomed Pharmacother. 78:204–209.
- Zhang L, Lu X, Gong L, Cui L, Zhang H, Zhao W, Jiang P, Hou G, Hou Y. 2021. Tetramethylpyrazine protects blood-spinal cord barrier integrity by modulating microglia polarization through activation of STAT3/SOCS3 and inhibition of NF-кB signaling pathways in experimental autoimmune encephalomyelitis mice. Cell Mol Neurobiol. 41(4):717–731.
- Zhang M, Lu N, Li HJ, Guo XY, Lu L, Guo Y. 2022. Inhibition of lncRNA NEAT1 induces dysfunction of fibroblast-like synoviocytes in rheumatoid arthritis via miRNA-338-3p-mediated regulation of glutamine metabolism. J Orthop Surg Res. 17(1):401.
- Zhang Y, Li M, Li L, Xiao J, Chen Z. 2021. Randomized controlled study of the effects of DHEA on the outcome of IVF in endometriosis. Evid Based Complement Alternat Med. 2021:3569697.
