Abstract
Context
Daphnetin is a natural product with anti-inflammatory, antioxidant, and neuroprotective properties. Reports have found that it has a strong analgesic effect; however, its analgesic mechanism is unknown.
Objective
We explored the effect and mechanism of daphnetin on neuropathic pain (NP).
Materials and methods
The rat model of NP was established by ligation of the sciatic nerve. Male Sprague-Dawley rats were divided into six groups: Control, Model, Sham, morphine (0.375 mg/kg), and daphnetin (0.0625 and 0.025 mg/kg). Rats were intrathecally injected with drugs or normal saline once daily for three days. Hyperalgesia was evaluated by mechanical withdrawal threshold (MWT) and thermal withdrawal threshold (TWT). Protein levels were detected using ELISA, immunofluorescence, and western blotting.
Results
Compared to the Model group, daphnetin improved TWT (46.70 °C vs. 42.20 °C) and MWT (45.60 g vs. 23.60 g), reduced the expression of interleukin-1β (0.99 ng/g vs. 1.42 ng/g), interleukin-6 (0.90 ng/g vs. 1.52 ng/g), and tumor necrosis factor-α (0.93 ng/g vs. 1.52 ng/g) in the sciatic nerve. Daphnetin decreased the expression of toll-like receptor 4 (TLR4) (0.47-fold), phosphorylated inhibitor of NF-κB (p-IKBα) (0.29-fold), nuclear factor kappaB (NF-κB) (0.48-fold), glial fibrillary acidic protein (GFAP) (0.42-fold), CXC chemokine ligand type 1 (CXCL1) (0.84-fold), CXC chemokine receptor type 2 (CXCR2) (0.78-fold) in the spinal cord.
Discussion and conclusions
Daphnetin alleviates NP by inhibiting inflammation and astrocyte activation in the spinal cord, providing theoretical support for the extensive clinical treatment of NP.
Introduction
Pain is a sign of self-protection. Unlike physiological pain, however, neuropathic pain (NP) does not originate from the stimulation of distal nerve endings but is a secondary consequence of injury or disease of the nervous system (Kankowski et al. Citation2021). NP is often caused by the remodeling of nociceptors due to lesions in the peripheral or central nervous system, which triggers pain sensitization in the body (Li et al. Citation2016; Bouhassira Citation2019). At present, the main mechanism and research focus of NP is central sensitization originating from the spinal cord and brain (Ji et al. Citation2014). Currently, the clinical treatment of NP is based on tricyclic antidepressants and anticonvulsants. However, these drugs have limited potency with side effects that restrict their clinical application in the management of NP. Therefore, it is particularly important to discover novel drugs with stronger analgesic effects and fewer side effects for NP.
Most researchers have confirmed crosstalk between glial cells and neurons in NP; the role of glial cells in NP cannot be ignored (Inoue and Tsuda Citation2018). CXC chemokine ligand type 1 (CXCL1), also known as keratinocyte-derived chemokine (KC) or growth-related oncogene (GRO), is a member of the CXC family (Silva et al. Citation2017). CXCL1 acts as a ligand for CXC chemokine receptor type 2 (CXCR2), causing central sensitization and maintenance of NP (Yang et al. Citation2016). Nuclear factor kappa-B (NF-κB) is a transcription factor that transduces extracellular signals to affect gene expression (Xu et al. Citation2014). NF-κB participates in the regulation of tumor necrosis factor α (TNF-α) induced expression of CXCL1 in primary astrocytes (Lee et al. Citation2012). In addition, new evidence suggests that the activation of NF-κB is associated with the development of inflammatory pain, NP, and chronic pain (Khalid et al. Citation2018).
Coumarin and its derivatives are potential analgesic agents. For example, 7-hydroxycoumarin, edgeworin, edgeworoside A, and edgeworoside C have strong analgesic activities (Wang et al. Citation2020). In addition, a recent study showed that osthole and columbianadin (both having a coumarin scaffold) displayed strong antinociceptive activities in an NP model (Su et al. Citation2020). Daphnetin is a natural product isolated from Daphne giraldii Nitsche. (Thymelaeaceae). The structural formula of daphnetin is 7,8-dihydroxycoumarin (), which has many biological activities, such as neuroprotective, anti-inflammatory, antioxidant, and analgesic (Yu et al. Citation2014). Studies suggest that the anticoagulant effect of coumarins may be directly linked to the substitution of the acetamido and pyrazole rings (Abdelhafez et al. Citation2010; Kathuria et al. Citation2012). Although the parent structure of daphnetin is a coumarin structure, it does not have the above-mentioned substituent groups; therefore, its anticoagulant effect is relatively weak and has higher safety. A study found that daphnetin treatment of fibromyalgia in mice inhibited the upregulation of monoamine oxidase A (MOA) and the overexpression of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and thiobarbituric acid-responsive substance (TBAR) (Singh, Kaur, et al. Citation2021). Daphnetin can effectively inhibit neuronal oxidative stress and apoptosis and has a protective effect on neurons (Zhi et al. Citation2019). In animal experiments, daphnetin inhibited IL-17, IL-12 α, and interferon γ to treat experimental autoimmune encephalomyelitis (EAE) in mice (Wang et al. Citation2020). The analgesic mechanism of daphnetin is unclear; therefore, we aimed to verify whether daphnetin can interfere with the expression of chemokine CXCL1/CXCR2 by inhibiting the NF-κB pathway, reducing central sensitization, further exploring its analgesic mechanism.
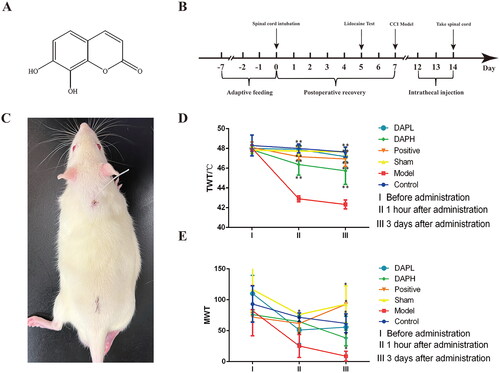
Figure 1. Daphnetin alleviates neuropathic pain in CCI rats. (A) The structural formula of daphnetin. (B) Experimental flowchart. (C) Subdural intubation in rats. The paralysis of the lower limbs of the rat in this figure is caused by the injection of lidocaine through the catheter, proving that the catheter is located in the L4–L5 position. (D) The change of TWT in rats (n = 12). (E) The change of MWT in CCI rats (n = 12). *p < .05, **p < .01 vs. Model group.
Materials and methods
Model establishment
Male Sprague-Dawley (SD) rats weighing 180–200 g (n = 72), animal license No. SCXK2020-0033 was provided by Beijing SBF Experimental Animal Technology Co., Ltd. They were placed in a suitable living environment maintained at a constant temperature and relative humidity. The rats were adapted to a 12 h dark/light cycle with access to feed and water ad libitum. The experiment was started after the rats were allowed to acclimate for one week. The animal experimental protocol of this study was reviewed and approved by the Medical and Experimental Animal Ethics Committee of the Beijing University of Chinese Medicine (No. BUCM4-202103302-1088).
After the rats were adaptively fed for seven days, a formal experiment was started. Rats anesthetized with 1.5% sodium pentobarbital (Beijing Chemical Reagent Company, China, 020402) (i.p.) were placed on the operating table. A 1.5 cm incision was made in the middle of the ileum. The muscles were separated, the coccygeal vertebrae were fully exposed, the intervertebral foramen was found on the right side of the first coccygeal vertebra, and the transparent cerebrospinal fluid flowed out after the puncture. A normal saline-filled EP-10 catheter (Smiths Medical, UK, 302021) was inserted horizontally forward at a distance of approximately 2 cm from the intervertebral foramen. The catheter was closed, and the muscle and skin were sutured and sterilized. The rats were further single-caged after surgery. Lidocaine (Shandong Hualu Pharmaceutical Co., Ltd., China, XB19H26) was injected through the catheter on the fifth postoperative day. Immediate paralysis of the rat’s lower limbs proved that the catheter was located at the L4–L5 segment (Storkson et al. Citation1996). The rats with successful spinal epidural intubation were randomly divided into five groups (n = 12): Model, Sham, Positive, high-dose daphnetin (DAPH), and low-dose daphnetin (DAPL), and 12 blank rats were selected as the control group.
On the seventh day after subdural intubation, the Model, DAPH, and DAPL groups were subjected to the establishment of the chronic constriction injury (CCI) model (Bennett and Xie Citation1988). After anesthesia, the rats were placed on the operating table, and a 2 cm incision was made after depilation of the left hind limb. After separating the muscle, the sciatic nerve was fully exposed and ligated with 4-0 suture four times with a ligation spacing of 1 mm. Disinfection of the wound after suturing the muscle and skin was carried out. The rats showed hindlimb weakness, limping, and licking behavior; tolerance to pain and temperature also decreased, proving that the model was successful.
Group and treatment
The positive group received an intrathecal injection of 0.375 mg/kg morphine (Qinghai Pharmaceutical Factory, China, 850807) once every 12 h for three consecutive days. The DAPH and DAPL groups were intrathecally injected with daphnetin standard (purchased from Shanghai Yuanye Bio-technology Co., Ltd., China, B21084, HPLC ≥ 98%) at doses of 0.0625 mg/kg and 0.025 mg/kg, respectively, once every 12 h, for three consecutive days. The Model and Sham groups were injected with the same amount of normal saline once every 12 h for three consecutive days (Su et al. Citation2020).
Behavioral assays
We used von Frey hair to measure the mechanical withdrawal threshold (MWT) of the rats (Dixon Citation1980). The rats were placed in special metal mesh cages, and the measurement was started after the rats stopped the behavior. The affected limbs of the rats were stimulated with von Frey hair of different specifications, and the relevant data were recorded. When the rat showed tremor or avoidance behavior, it was recorded as a positive reaction, and when there was no obvious behavioral change, it was recorded as a negative reaction. Five valid data were recorded from the first changed data, and the 50% positive reaction amount (ED50) was calculated.
The rats were placed in a cold/hot plate pain meter (PE34, IITC, USA) for 5 min before the start of the experiment. After the rats were acclimated to the environment and allowed to relax, the machine was switched on, and the temperature was programmed at a rate of 5 °C/min. Normally, when the rat detects the pain caused by elevated temperature, the hind limbs contract, and licking behavior is seen; the temperature of the instrument was suspended at this time, and the current temperature was recorded. Each rat was measured three times, with a break of 10 min, and the average value was considered as the thermal withdrawal threshold (TWT) (Wang et al. Citation2020).
Immunofluorescence staining
Three rats in each group were selected for paraffin sectioning of the L4–L5 spinal cord segment; four sections were prepared for every rat’s spinal cord. Rat spinal cord slices were incubated with primary antibodies from the following sources: rabbit antibodies (1:200, 80788 T, CST, USA), mouse antibodies GFAP (1:50, ab4648, Abcam, UK), rabbit antibodies NF-κB (1:200, 8242 T, CST, USA), mouse antibodies NeuN (1:100, 66836 − 1-lg, Proteintech, USA), rabbit antibodies CXCL1 (1:50, bs-10234R, Bioss, China), and rabbit antibodies CXCR2 (1:50, 61c0355, Affinity, USA) overnight at 4 °C. After washing with the primary antibodies, cells were incubated with Alexa Flour488 or Alexa Flour647. An image of the dorsal horn of the spinal cord on the affected side of the rat was obtained using a laser confocal microscope (Sp8, Leica, Germany) after sealing with DAPI. Images of the dorsal horn of the spinal cord were taken for analysis of fluorescence intensity using ImageJ software (1.8.0, NIH, USA).
Western blot
For each group, three rat L4–L5 spinal cord segments were selected and stored in liquid nitrogen; 600 mL of RIPA lysis solution (P0013C, Beyotime, China) was added for total protein extraction. After extracting proteins from rat spinal cord tissue, the protein concentration was adjusted to 1 μg/μL using the SDS-PAGE protein sample loading buffer (B1012, Applygen, China). Protein samples (10 μg) were separated by SDS-PAGE and transferred to PVDF membranes (R1DB92455, Millipore, Germany), which were then incubated with primary antibodies at 4 °C overnight. The primary antibodies used were toll-like receptor 4 (TLR4) (1:1000, ab13556, Abcam), IKBα (1:1000, ab12134, Abcam), p-IKBα (1:1000, ab133462, Abcam), and α-tubulin (1:5000, ab7291, Abcam). The electroporated membrane was washed with TBST, incubated with the secondary antibody for 1 h at room temperature, and developed. Quantitative analysis of the protein bands was performed using ImageJ software. All western blots represent data from three independent replicates.
ELISA
The sciatic nerve of rats was homogenized in PBS buffer, and the supernatant was collected to detect IL-1 β (rat IL-1 β ELISA Kit RGB & CHN lot:20220623. 60013 R), IL-6 (rat IL-6 ELISA Kit RGB & CHN lot:20220623. 60023 R), TNF-α (rat TNF-α ELISA Kit RGB & CHN lot:20220623. 60080 R). The L4-L5 spinal cord segment of rats was homogenized in PBS buffer, and the supernatant was collected to detect the contents of CXCL1 (rat CXCL1 ELISA Kit RGB & CHN lot:20220623. 60024 R) and CXCR2 (rat CXCR2 ELISA Kit RGB & CHN lot:20220623. 60671 R). Total protein content in the sciatic nerve and spinal cord was also detectd (TP Kit RGB & CHN lot:20210118).
Statistical method
SAS 8.2 software was used for the statistical analysis. All data are presented as mean ± standard deviation (S.D.). When the data in each group conformed to a normal distribution and had equal variance, a one-way analysis of variance was applied, and the LSD method was used to compare groups. Behavioral test data were analyzed using repeated measures ANOVA. A nonparametric test was used when the sample did not conform to a normal distribution. The confidence interval was set to 0.05. p < 0.05 was considered statistically significant, and p < 0.01 was considered significantly different.
Results
Daphnetin alleviates neuropathic pain (NP) in CCI rats
We maintained a 7-d recovery period after subdural intubation to ensure the healthy state of the rats before establishing the CCI model (). We determined that the EP-10 catheter was placed between the L4–L5 segment in rats through the lidocaine test, which was helpful for several accurate intrathecal injections in our subsequent experiments (). We measured rat TWT and MWT at three-time points: time point I, before modeling; time point II, 1 h after administration; and time point III, three consecutive days of administration. There were no significant differences in the TWT and MWT between the Sham and Control groups at these three-time points (p > 0.05), suggesting that intrathecal intubation did not lead to NP in rats. Behavioral indicators of the Model group were significantly different from those of the Sham group within time points II and III (p < 0.05), proving that the CCI model produced NP. These results showed that the rats in the Model group exhibited hyperalgesia to thermal and mechanical stimulations. The TWT and MWT of the positive, DAPH, and DAPL groups were significantly different from those of the Model group (p < 0.05) at time points II and III, indicating that the analgesic effect of daphnetin was equivalent to that of morphine ().
Daphnetin inhibits inflammatory factors release in the sciatic nerve and astrocytes activation in the spinal dorsal horn of CCI rats
Peripheral nerve injury can directly induce NP, and the underlying pro-inflammatory state of peripheral nerves often sustains pain development (Kuffler Citation2020). IL-1β is expressed in the dorsal root ganglia; its level increases after peripheral nerve extrusion and induces spontaneous neuronal electrical activity (Su et al. Citation2020). The high expression of IL-6 and TNF-α is also associated with NP (Stellwagen and Malenka Citation2006; Wei et al. Citation2019). We used an ELISA kit to detect the expression of IL-1β, IL-6, and TNF-α in the sciatic nerve of rats, and found that the expression of IL-1β, IL-6, and TNF-α in the Model group was significantly increased compared to those in the Sham group (p < 0.05). The expression of the above-mentioned inflammatory factors in the sciatic nerve of rats was significantly decreased after subdural injection of daphnetin for three consecutive days (compared with Model, p < 0.05). There was no significant difference in the expression of inflammatory factors between the Sham and Control groups (p > 0.05), proving that the subdural catheter did not induce the production of inflammatory factors in the sciatic nerve of the rats ().
Figure 2. Effects of daphnetin on the sciatic nerve and spinal cord astrocytes in CCI rats. (A–C) ELISA kits to detect the expression of IL-1β, IL-6, and TNF-α in sciatic nerve (n = 6). (D) Expression of GFAP in rat spinal cord in immunofluorescence staining (n = 3). (E) Immunofluorescence staining of rat spinal cord astrocyte marker GFAP (n = 3). *p < .05, **p < .01 vs. Model group.
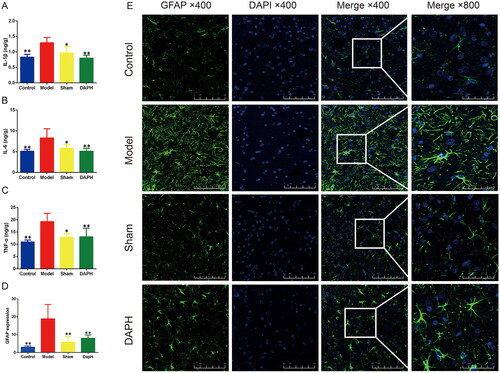
Astrocytes are activated by inflammation or peripheral nerve injury in the spinal cord and contribute to the induction of neuronal hyperactivity by releasing various molecules, leading to chronic pain (Tang et al. Citation2021). Rats were perfused and fixed, and the spinal cord was removed to obtain pathological sections. Spinal cord astrocyte activation marker GFAP was stained by immunofluorescence technique. The results showed that GFAP was highly expressed in the spinal cord of the Model group (compared with the Sham group, p < 0.05). This high expression was effectively inhibited after intrathecal injection of daphnetin (DAPH group compared with the Model group, p < 0.05). In addition, compared with the control group, GFAP in the spinal cord of the Sham group was not significantly increased (p > 0.05), indicating that the established administration method did not induce the activation of astrocytes in the spinal cord ().
Daphnetin inhibits the NF-κB expression in spinal cord astrocytes of CCI rats
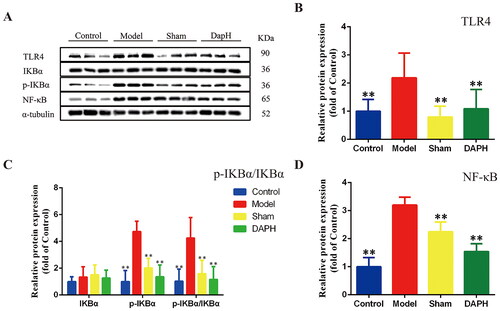
NF-κB, a ubiquitous transcription factor, is involved in the expression of genes critical for NP treatment (Ni et al. Citation2019). After spinal cord or peripheral nerve injury, NF-κB is abundantly expressed in astrocytes of the spinal cord (Miyoshi et al. Citation2008). Using western blot technology, we analyzed the protein expression of NF-κB in the spinal cord of rats and found that the protein expression of NF-κB in the spinal cord of the Model group was significantly upregulated (compared with the Sham group, p < 0.01). After the injection of daphnetin, compared with the Model group, the protein expression of NF-κB was significantly decreased (p < 0.01).
To explore how daphnetin interferes with the expression of NF-κB, we used western blot technology to explore the upstream and downstream related proteins of NF-κB. Toll-like receptor 4 (TLR4) is a pattern recognition receptor belonging to the toll-like receptor (TLR) family that contains an extracellular domain and an intracellular domain (Medzhitov et al. Citation1997). β-Arrestins, such as IKBα, can interact with TLR4 signaling molecules and mediate NF-κB activity (Zhang et al. Citation2020). We measured the protein expression of TLR4, IKBα, and p-IKBα by western blot technology and calculated the p-IKBα/IKBα protein ratio. Compared with the Sham group, the expression of TLR4 and p-IKBα and the ratio of p-IKBα/IKBα protein in the Model group were significantly increased (p < 0.01). After the injection of daphnetin, the expression of TLR4 and p-IKBα and the ratio of p-IKBα/IKBα protein were significantly downregulated (p < 0.01). The above experimental data proved that daphnetin regulates the protein expression of NF-κB in the spinal cord astrocytes of CCI rats by downregulating TLR4 and inhibiting the phosphorylation of IKBα ().
Figure 3. Effects of daphnetin on NF-κB pathway in the spinal cord of CCI rats. (A) Protein bands of TLR4, IKBα, p-IKBα, and NF-κB in the spinal cord (n = 3). (B–D) Normalized treatment of protein expression of TLR4, IKBα, p-IKBα, and NF-κB in the spinal cord (n = 3). *p < .05, **p < .01 vs. Model group.
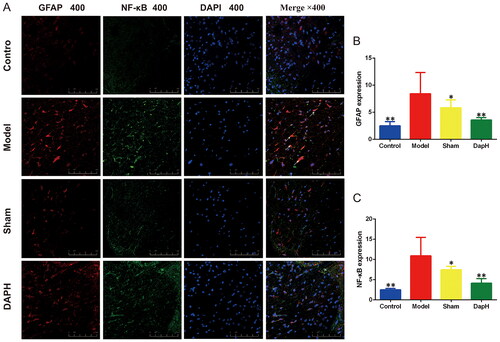
We performed double immunofluorescence staining for GFAP and NF-κB in the spinal cords of rats. ImageJ analysis revealed that the expression of NF-κB and GFAP in the spinal cord of the Model group was high (compared with the Sham group, p < 0.05), and NF-κB was expressed in the nucleus of astrocytes. After injection of daphnetin, the fluorescence intensities of NF-κB and GFAP were attenuated (compared to the Model group, p < 0.05), and only a part of NF-κB entered the nucleus of astrocytes. This trend corroborates the results of western blotting ().
Figure 4. Effects of daphnetin on NF-κB in the spinal cord astrocytes of CCI rats. (A) The expression of GFAP and NF-κB in the dorsal horn of the spinal cord in double immunofluorescence staining (n = 3). (B) Fluorescence intensity analysis of GFAP (n = 3). (C) Fluorescence intensity analysis of NF-κB (n = 3). *p < .05, **p < .01 vs. Model group.
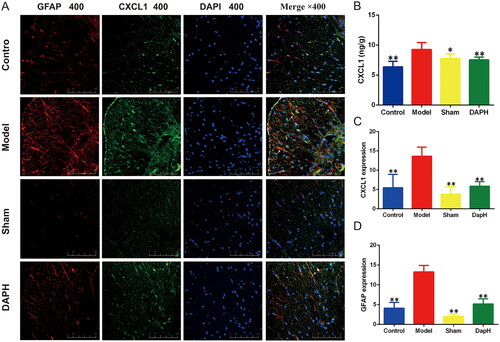
Daphnetin inhibits the CXCL1 expression in spinal cord astrocytes of CCI rats
Chemokine CXCL1, also known as KC or growth-associated oncogene (GRO), is a member of the CXC family and has been shown to play a key role in inducing and maintaining inflammation and NP through its preferred receptor CXCR2 (Silva et al. Citation2017). We detected the expression of CXCL1 in the rat spinal cord using an ELISA kit. The expression of CXCL1 in the Model group was significantly higher than that in the Sham group (p < 0.05). After intrathecal injection of daphnetin, the expression of CXCL1 in the DAPH group was lower than that in the Model group (p < 0.01). Using immunofluorescence double staining, we found that CXCL1 is expressed in astrocytes. Immunofluorescence analysis showed that the fluorescence intensities of GFAP and CXCL1 in the Model group increased in the dorsal horn of the spinal cord (compared with the Sham group, p < 0.01). The fluorescence intensity of CXCL1 in the DAPH group was significantly lower than that in the Model group (p < 0.01), which was consistent with the results of the ELISA ().
Figure 5. Effects of daphnetin on CXCL1 in the spinal cord of CCI rats. (A) Expression of GFAP and CXCL1 in the dorsal horn of the spinal cord in double immunofluorescence staining (n = 3). (B) The expression of CXCL1 in the spinal cord was detected using ELISA kit (n = 6). (C) Fluorescence intensity analysis of CXCL1 (n = 3). (D) Fluorescence intensity analysis of GFAP (n = 3). *p < .05, **p < .01 vs. Model group.
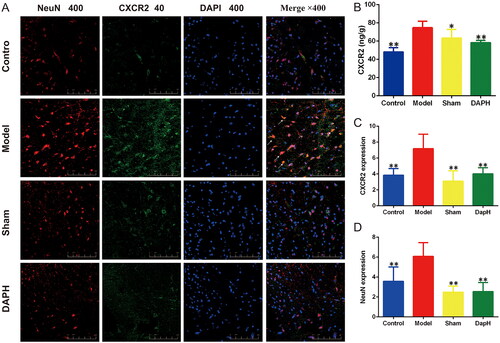
Daphnetin inhibits the expression of CXCR2 in spinal cord neurons of CCI rats
Neuronal CXCR2 expression in the spinal cord may be upregulated following peripheral nerve injury, contributing to neuroplasticity and nociceptor sensitization in NP (Cao et al. Citation2016). CXCL1 modulates excitatory synaptic transmission by increasing N-methyl-d-aspartate (NMDA) receptor-induced currents through CXCR2 in spinal neurons (Cao et al. Citation2014). We detected the expression of CXCR2 in the rat spinal cord using an ELISA kit. The expression of CXCR2 in the Model group was significantly higher than that in the Sham group (p < 0.05). After intrathecal injection of daphnetin, the expression of CXCR2 in the DAPH group was significantly lower than that in the Model group (p < 0.01). Immunofluorescence double staining showed that CXCR2 was expressed in neurons. Fluorescence intensity analysis revealed that the fluorescence intensities of NeuN and CXCR2 in the Model group were enhanced in the dorsal horn of the spinal cord (compared with the Sham group, p < 0.01). The fluorescence intensity of CXCR2 in the DAPH group was significantly lower than that in the Model group (p < 0.01), which was consistent with the ELISA results. In the Sham group, the fluorescence intensities of NeuN and CXCR2 were not significantly increased (compared to the Control group, p > 0.05) ().
Figure 6. Effects of daphnetin on CXCR2 in the spinal cord of CCI rats. (A) Expression of NeuN and CXCR2 in the dorsal horn of the spinal cord in double immunofluorescence staining (n = 3). (B) The expression of CXCR2 in the spinal cord was detected using ELISA kit (n = 6). (C) Fluorescence intensity analysis of CXCR2 (n = 3). (D) Fluorescence intensity analysis of NeuN (n = 3). *p < .05, **p < .01 vs. Model group.
Discussion
We investigated the analgesic effect of daphnetin on NP in CCI rats and explored the potential mechanism of action. After the sciatic nerve ligation, the rats showed hyperalgesia (mechanical and thermal hyperalgesia) and changes in biochemical parameters. These changes were ameliorated by injecting daphnetin into the subdural cavity. Daphnetin inhibited the upregulation of TLR4 and the phosphorylation of IKBα in the spinal cord of CCI rats, reduced the release of NF-κB, and indirectly inhibited the receptor activation of CXCR2 in neurons in the spinal dorsal horn to reduce the activation of astrocytes and the release of CXCL1.
Mechanical hyperalgesia and thermal hyperalgesia are core indicators for evaluating allodynia in NP (Khan et al. Citation2019). In our experiment, we evaluated pain in CCI rats 1 h after continuous intrathecal injection of daphnetin for three days. The results showed that the mechanical and thermal hyperalgesia of rats in the DAPH and DAPL groups were significantly improved after 1 h of a single administration, and the improvement lasted until the end of 3 d of administration. NP improvement in CCI rats by daphnetin reflects its protective effect on the spinal cord after peripheral nerve injury, which may be the key to daphnetin analgesia.
After peripheral nerve injury, innate immune cells release inflammatory factors such as IL-1β, IL-6, and TNF-α to act on the injury site and spinal cord, driving neuronal sensitization in the dorsal horn of the spinal cord (Zhao et al. Citation2017; Cowie et al. Citation2019). As shown in our data, the expression of IL-1β, IL-6, and TNF-α in the sciatic nerve significantly increased after ligation of the sciatic nerve in rats. Daphnetin, a member of the coumarin family, has various pharmacological effects, including neuroprotective and anti-inflammatory effects (Singh, Singh, et al. Citation2021). Therefore, after daphnetin administration, inflammatory factors in the sciatic nerve were effectively inhibited, showing a protective effect on peripheral nerves.
Chemokines, including CXC chemokines (α-chemokines), CC chemokines (β-chemokines), C chemokines (γ-chemokines), and CX3C chemokines (δ-chemokines) (Hughes and Nibbs Citation2018) are responsible for intercellular signaling. CXCL1 is upregulated in the spinal cord in NP states (Zhang et al. Citation2013). In this experiment, we observed upregulation of CXCL1 expression in the spinal cord of the Model group. Combined with immunofluorescence double staining, we further determined that CXCL1 is expressed in astrocytes in the spinal cord. We also found that astrocytes in the spinal cord of the Model group were massively activated and overexpressed GFAP. Spinal astrocytes have been shown to play an important role in the development and maintenance of NP (Ji et al. Citation2019). As the most abundant glial cell type in the central nervous system (CNS), astrocytes increase the activity of nociceptive neurons in the spinal cord by releasing various astrocyte mediators (Chiang et al. Citation2012). By comparing the data of the DAPH and Model groups, we found that daphnetin can inhibit the overexpression of CXCL1 in the spinal cord. In immunofluorescence double staining, we also found that the expression of CXCL1 in astrocytes of the DAPH group was lower, and that the expression of GFAP in astrocytes was attenuated. Therefore, daphnetin can attenuate the activation of astrocytes in the dorsal horn of the spinal cord and upregulation of CXCL1 in astrocytes, which are related to NP in CCI rats.
Multiple lines of evidence have implicated NF-κB in the development and maintenance of NP (Ni et al. Citation2019). Activation of NF-κB often occurs in tumors and mediates the production of CXCL1 in astrocytes (Xu et al. Citation2014). The results of the Model group support the maintenance of NP in CCI rats via NF-κB through the production of CXCL1 by spinal astrocytes. Western blotting results showed that daphnetin restricted the release of NF-κB in the spinal cord by inhibiting the expression of TLR4 and phosphorylation of IKBα. Immunofluorescence results showed that daphnetin inhibited NF-κB overexpression in the astrocytes of CCI rats. Inhibition of NF-κB can regulate the transcription of multiple inflammatory mediators, including chemokines and pro-inflammatory factors (IL-1β, IL-6, and TNF-α), thereby effectively improving pain behaviors (Zhou et al. Citation2018).
CXCR2 belongs to the CXCR family and is the primary receptor for CXC chemokines, which mediate angiogenesis and play a crucial role in cancer and various inflammatory diseases (Cheng et al. Citation2019). Peripheral nerve inflammation or nerve injury induces the expression of CXCL1 and CXCR2 in the distal dorsal root ganglion and spinal cord, which contributes to neuroplasticity and nociceptor sensitization in pathological pain (Marro et al. Citation2016). In the current study, the expression of CXCR2 in the spinal cord was upregulated in the Model group, indicating that the enhancement of CXCR2 in neurons is also involved in the regulation of pain in the supraspinal region. Daphnetin significantly reduced the level of CXCR2 in the spinal cord of CCI rats, and the quantitative analysis of immunofluorescence staining was consistent with the above results. Therefore, our study suggested that daphnetin-mediated attenuation of CXCL1/CXCR2 signaling may be a potential pathway for NP treatment in CCI rats.
Conclusion
Our data demonstrated that daphnetin could ameliorate NP by decreasing astrocyte activation through TLR4/NF-κB pathway and inhibiting the CXCL1/CXCR2 chemokine pair and attenuating neuronal sensitivity in the spinal dorsal horn. Our study revealed that daphnetin might be developed as a drug for the clinical treatment of NP.
Author contributions
Tianrui Zhang and Wulin Liang, the first author of this paper, were responsible for the theme and design of the experiment, the operation of animals, and the writing of the manuscript. Wenjing Ou assisted with the experiment and was responsible for recording the experimental data. Mingqian Zhang assisted with the experiment and was responsible for recording the experimental data. Shuang Cui was responsible for data analysis and image processing of the experiment. Shuofeng Zhang, the corresponding author of this article, was responsible for the revision and submission of this paper.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abdelhafez OM, Amin KM, Batran RZ, Maher TJ, Nada SA, Sethumadhavan S. 2010. Synthesis, anticoagulant and PIVKA-II induced by new 4-hydroxycoumarin derivatives. Bioorg Med Chem. 18(10):3371–3378.
- Bennett GJ, Xie YK. 1988. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 33(1):87–107.
- Bouhassira D. 2019. Neuropathic pain: definition, assessment and epidemiology. Rev Neurol. 175(1–2):16–25.
- Cao DL, Qian B, Zhang ZJ, Gao YJ, Wu XB. 2016. Chemokine receptor CXCR2 in dorsal root ganglion contributes to the maintenance of inflammatory pain. Brain Res Bull. 127:219–225.
- Cao DL, Zhang ZJ, Xie RG, Jiang BC, Ji RR, Gao YJ. 2014. Chemokine CXCL1 enhances inflammatory pain and increases NMDA receptor activity and COX-2 expression in spinal cord neurons via activation of CXCR2. Exp Neurol. 261:328–336.
- Cheng Y, Ma XL, Wei YQ, Wei XW. 2019. Potential roles and targeted therapy of the CXCLs/CXCR2 axis in cancer and inflammatory diseases. Biochim Biophys Acta Rev Cancer. 1871(2):289–312.
- Chiang CY, Sessle BJ, Dostrovsky JO. 2012. Role of astrocytes in pain. Neurochem Res. 37(11):2419–2431.
- Cowie AM, Dittel BN, Stucky CL. 2019. A novel sex-dependent target for the treatment of postoperative pain: the NLRP3 inflammasome. Front Neurol. 10(622):622.
- Dixon WJ. 1980. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 20:441–462.
- Hughes CE, Nibbs RJB. 2018. A guide to chemokines and their receptors. FEBS J. 285(16):2944–2971.
- Inoue K, Tsuda M. 2018. Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential. Nat Rev Neurosci. 19(3):138–152.
- Ji RR, Donnelly CR, Nedergaard M. 2019. Astrocytes in chronic pain and itch. Nat Rev Neurosci. 20(11):667–685.
- Ji RR, Xu ZZ, Gao YJ. 2014. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov. 13(7):533–548.
- Kankowski S, Grothe C, Haastert-Talini K. 2021. Neuropathic pain: spotlighting anatomy, experimental models, mechanisms, and therapeutic aspects. Eur J Neurosci. 54(2):4475–4496.
- Kathuria A, Priya N, Chand K, Singh P, Gupta A, Jalal S, Gupta S, Raj HG, Sharma SK. 2012. Substrate specificity of acetoxy derivatives of coumarins and quinolones towards Calreticulin mediated transacetylation: investigations on antiplatelet function. Bioorg Med Chem. 20(4):1624–1638.
- Khalid S, Ullah MZ, Khan AU, Afridi R, Rasheed H, Khan A, Ali H, Kim YS, Khan S. 2018. Antihyperalgesic properties of honokiol in inflammatory pain models by targeting of NF-kappaB and Nrf2 signaling. Front Pharmacol. 9:140.
- Khan A, Khan S, Kim YS. 2019. Insight into pain modulation: nociceptors sensitization and therapeutic targets. Curr Drug Targets. 20(7):775–788.
- Kuffler DP. 2020. Injury-induced effectors of neuropathic pain. Mol Neurobiol. 57(1):51–66.
- Lee YH, Kim SH, Kim Y, Lim Y, Ha K, Shin SY. 2012. Inhibitory effect of the antidepressant imipramine on NF-kappaB-dependent CXCL1 expression in TNFalpha-exposed astrocytes. Int Immunopharmacol. 12(4):547–555.
- Li XY, Wan Y, Tang SJ, Guan Y, Wei F, Ma D. 2016. Maladaptive plasticity and neuropathic pain. Neural Plast. 2016:4842159.
- Marro BS, Grist JJ, Lane TE. 2016. Inducible expression of CXCL1 within the central nervous system amplifies viral-induced demyelination. J Immunol. 196(4):1855–1864.
- Medzhitov R, Preston-Hurlburt P, Janeway CA Jr. 1997. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 388(6640):394–397.
- Miyoshi K, Obata K, Kondo T, Okamura H, Noguchi K. 2008. Interleukin-18-mediated microglia/astrocyte interaction in the spinal cord enhances neuropathic pain processing after nerve injury. J Neurosci. 28(48):12775–12787.
- Ni H, Wang Y, An K, Liu Q, Xu L, Zhu C, Deng H, He Q, Wang T, Xu M, et al. 2019. Crosstalk between NFkappaB-dependent astrocytic CXCL1 and neuron CXCR2 plays a role in descending pain facilitation. J Neuroinflammation. 16(1):1–16.
- Silva RL, Lopes AH, Guimaraes RM, Cunha TM. 2017. CXCL1/CXCR2 signaling in pathological pain: role in peripheral and central sensitization. Neurobiol Dis. 105:109–116.
- Singh L, Kaur A, Singh AP, Bhatti R. 2021. Daphnetin, a natural coumarin averts reserpine-induced fibromyalgia in mice: modulation of MAO-A. Exp Brain Res. 239(5):1451–1463.
- Singh L, Singh AP, Bhatti R. 2021. Mechanistic interplay of various mediators involved in mediating the neuroprotective effect of daphnetin. Pharmacol Rep. 73(5):1220–1229.
- Stellwagen D, Malenka RC. 2006. Synaptic scaling mediated by glial TNF-alpha. Nature. 440(7087):1054–1059.
- Storkson RV, Kjorsvik A, Tjolsen A, Hole K. 1996. Lumbar catheterization of the spinal subarachnoid space in the rat. J Neurosci Methods. 65(2):167–172.
- Su X, Wu B, Zhang W, Ji YH, Wang Q, Tan ZY. 2020. Inhibitory effects of columbianadin on nociceptive behaviors in a neuropathic pain model, and on voltage-gated calcium currents in dorsal root ganglion neurons in mice. Front Pharmacol. 10:1–14.
- Tang J, Bair M, Descalzi G. 2021. Reactive astrocytes: critical players in the development of chronic pain. Front Psychiatry. 12:682056.
- Wang D, Zhu B, Liu X, Han Q, Ge W, Zhang W, Lu Y, Wu Q, Shi L. 2020. Daphnetin ameliorates experimental autoimmune encephalomyelitis through regulating heme oxygenase-1. Neurochem Res. 45(4):872–881.
- Wang L, Yin C, Liu T, Abdul M, Zhou Y, Cao JL, Lu C. 2020. Pellino1 regulates neuropathic pain as well as microglial activation through the regulation of MAPK/NF-kappaB signaling in the spinal cord. J Neuroinflammation. 17(1):83.
- Wang LY, Xia H, Xia GY, Wei XH, Tian GH, Lin S. 2020. Research progress of natural non-alkaloids with analgesic activity. Zhongguo Zhong Yao Za Zhi. 45(24):5840–5865.
- Wei Z, Fei Y, Su W, Chen G. 2019. Emerging role of Schwann cells in neuropathic pain: receptors, glial mediators and myelination. Front Cell Neurosci. 13:116.
- Xu J, Zhu MD, Zhang X, Tian H, Zhang JH, Wu XB, Gao YJ. 2014. NFkappaB-mediated CXCL1 production in spinal cord astrocytes contributes to the maintenance of bone cancer pain in mice. J Neuroinflammation. 11(38):38.
- Yang LH, Xu GM, Wang Y. 2016. Up-regulation of CXCL1 and CXCR2 contributes to remifentanil-induced hypernociception via modulating spinal NMDA receptor expression and phosphorylation in rats. Neurosci Lett. 626:135–141.
- Yu WW, Lu Z, Zhang H, Kang YH, Mao Y, Wang HH, Ge WH, Shi LY. 2014. Anti-inflammatory and protective properties of daphnetin in endotoxin-induced lung injury. J Agric Food Chem. 62(51):12315–12325.
- Zhang P, Yang M, Chen C, Liu L, Wei X, Zeng S. 2020. Toll-like receptor 4 (TLR4)/opioid receptor pathway crosstalk and impact on opioid analgesia, immune function, and gastrointestinal motility. Front Immunol. 11:1455.
- Zhang ZJ, Cao DL, Zhang X, Ji RR, Gao YJ. 2013. Chemokine contribution to neuropathic pain: respective induction of CXCL1 and CXCR2 in spinal cord astrocytes and neurons. Pain. 154(10):2185–2197.
- Zhao H, Alam A, Chen Q, A Eusman M, Pal A, Eguchi S, Wu L, Ma D. 2017. The role of microglia in the pathobiology of neuropathic pain development: what do we know? Br J Anaesth. 118(4):504–516.
- Zhi J, Duan B, Pei J, Wu S, Wei J. 2019. Daphnetin protects hippocampal neurons from oxygen-glucose deprivation-induced injury. J Cell Biochem. 120(3):4132–4139.
- Zhou L, Hu Y, Li C, Yan Y, Ao L, Yu B, Fang W, Liu J, Li Y. 2018. levo-Corydalmine alleviates vincristine-induced neuropathic pain in mice by inhibiting an NF-kappa B-dependent CXCL1/CXCR2 signaling pathway. Neuropharmacology. 135:34–47.
