Abstract
Context
Zhibai Dihuang pill (ZD), a traditional Chinese medicine nourishes Yin and reduces internal heat, is believed to have therapeutic effects on urinary tract infections (UTIs).
Objective
To explore the effects and mechanism of modified ZD (MZD) on UTI induced by extended-spectrum β-lactamase (ESBLs) Escherichia coli.
Materials and methods
Thirty Sprague-Dawley rats were randomly divided into control, model (0.5 mL 1.5 × 108 CFU/mL ESBLs E. coli), MZD (20 g/kg MZD), LVFX (0.025 g/kg LVFX), and MZD + LVFX groups (20 g/kg MZD + 0.025 g/kg LVFX), n = 6. After 14 days of treatment, serum biochemical indicators, renal function indicators, bladder and renal histopathology, and urine bacterial counts in rats were determined. Additionally, the effects of MZD on ESBLs E. coli biofilm formation and related gene expression were analyzed.
Results
MZD significantly decreased the count of white blood cells (from 13.12 to 9.13), the proportion of neutrophils (from 43.53 to 23.18), C-reactive protein (from 13.21 to 9.71), serum creatinine (from 35.78 to 30.15), and urea nitrogen (from 12.56 to 10.15), relieved the inflammation and fibrosis of bladder and kidney tissues, and reduced the number of bacteria in urine (from 2174 to 559). In addition, MZD inhibited the formation of ESBLs E. coli biofilms (2.04-fold) and decreased the gene expressions of luxS, pfS and ompA (1.41–1.62-fold).
Discussion and conclusion
MZD treated ESBLs E. coli-induced UTI inhibited biofilm formation, providing a theoretical basis for the clinical application of MZD. Further study on the clinical effect of MZD may provide a novel therapy option for UTI.
Introduction
Urinary tract infections (UTIs) are infectious diseases caused by the abnormal reproduction of various pathogenic microorganisms in the urinary tract (Gupta et al. Citation2017). Complicated UTIs often lead to prolonged infection and eventually cause renal function damage and serious health hazards (Tandogdu and Wagenlehner Citation2016; Millner and Becknell Citation2019). Uropathogenic Escherichia coli (UPEC) is a typical bacterial UTI pathogen (Herrmann et al. Citation2002). Various virulence factors in the pathological group of UPEC provide more opportunities for bacterial survival (Behzadi et al. Citation2020). Currently, the main intervention measures for the clinical treatment of UTI are antibacterial drugs (Flores-Mireles et al. Citation2019). Approximately 60% of antibiotics are β-lactams, which have various chemical structures and are often used to treat different types of bacterial infectious diseases (Behzadi et al. Citation2020). The application of antimicrobials has led to β-lactamase production, resulting in drug-resistant bacterial strains (Issakhanian and Behzadi Citation2019). In a clinical study, 86% of ESBL-producing strains were isolated from UPEC, and there was neither a pattern of resistance nor ESBL production in UTI (Khonsari et al. Citation2021). The presence of extended-spectrum β-lactamase (ESBLs) E. coli makes the most common antimicrobial agent less effective in treating UTIs (Zowawi et al. Citation2015). Therefore, there is an urgent clinical need for alternative treatment options that target UTI pathogenesis.
Bacterial biofilms are a special survival mode for bacteria to adapt to the environment (Vuotto et al. Citation2017), which can resist host immunity and reduce antibiotic sensitivity (Pavithra and Doble Citation2008). When human body resistance is decreased, the remaining bacteria in the biofilm proliferate and release again, resulting in repeated attacks and prolonged infections (Tremblay et al. Citation2014). Studies have shown that biofilms are positively correlated with ESBL production and play a key role in UTIs (Shrestha et al. Citation2019). Destruction of this natural barrier of bacterial biofilm may be an effective way to overcome bacterial resistance and treat chronic clinical infections (Kostakioti et al. Citation2013).
An increasing number of studies have confirmed that traditional Chinese medicine (TCM) can effectively improve clinical symptoms and reduce the UTI recurrence rate (Yu et al. Citation2017; Li et al. Citation2019; Liu et al. Citation2019). TCM compounds have multi-directivity, multi-layer and multi-target characteristics, which make it difficult for bacteria to simultaneously produce multiple mutations against multiple antibacterial components (Zhang et al. Citation2013). Zhibai Dihuang pill (ZD) is a traditional compound TCM that nourishes Yin and reduces internal heat and is used to treat Yin-deficiency-heat (YDH) syndrome (Yi et al. Citation2020). Recently, ZD has been used to treat the accompanying symptoms of hyperthyroidism, nephrotic syndrome, UTI, and prostatitis (Liu et al. Citation2018). According to the literature, we adjusted ZD to obtain a compound TCM with a better curative effect against UTI. By observing the effects of the components of modified ZD (MZD) on the blood biochemical indices, histopathology, and urine bacterial content of the UTI rat model induced by ESBLs E. coli, and the effects of the medicated serum on the formation of ESBLs E. coli biofilms, the role and possible mechanism of MZD against ESBLs E. coli to treat UTI were explored.
Materials and methods
Preparation of MZD
The MZD used in this study was prepared by experts of the Affiliated Hospital of Traditional Chinese Medicine of Southwest Medical University, conformed with the compatibility principle, and was prepared uniformly by the pharmacy department of the hospital. Briefly, MZD is the fusion of Chinese classical prescriptions Zhibai Dihuang Pill and Bazheng Powder, including 15 g Phellodendron amurense Rupr. (Rutaceae), 10 g Anemarrhena asphodeloides Bunge (Liliaceae), 10 g Alpinia oxyphylla Miq. (Zingiberaceae), 12 g Cuscuta chinensis Lam. (Convolvulaceae), 15 g Plantago asiatica L. (Plantaginaceae), 12 g Polygonum aviculare L. (Polygonaceae), 12 g Dianthus superbus L. (Caryophyllaceae), 12 g Gardenia jasminoides J.Ellis (Rubiaceae), 20 g Astragalus mongholicus Bunge (Fabaceae), 15 g Poria cocos (Schw.) Wolf. (Polyporaceae), 12 g Atractylodes macrocephala Koidz. (Asteraceae), 10 g Scutellaria baicalensis Georgi (Lamiaceae) and 12 g Achyranthes bidentata Blume (Amaranthaceae). Water was added to the MZD mixture at a 10:1 ratio (water: plant material, v/w). After 30 min of soaking in water, the plant material was boiled twice for 1 h each and filtered. The filtrate was then concentrated to 3.33 g/mL using a rotary evaporator (RF02C, Shanghai Kangsheng Industrial Co., Ltd., China) and stored at 4 °C until use.
ESBLs E. coli culture
The ESBLs E. coli was provided by the Clinical Laboratory of the Affiliated Hospital of Traditional Chinese Medicine of Southwest Medical University. The cryopreserved ESBLs E. coli strain was regenerated by inoculation onto an agar plate and incubated in an incubator at 37 °C for 18 h. The regenerated bacterial strain was picked and inoculated into a lysogeny broth (LB) liquid medium and cultured at 37 °C at 150 rpm for 16–24 h (Hou et al. Citation2019), and the bacterial solution concentration was adjusted to 1.5 × 108 CFU/mL as seed liquid.
Animal experiments
Thirty 8-week-old specific-pathogen-free female Sprague-Dawley (SD) rats, weighing 200–220 g, were purchased from Chengdu Dossy Experimental Animals Co., Ltd. (license number: SCXK (Sichuan) 2020-030). Rats were kept at a room temperature of 22 °C, with a humidity of 50–60%, with good ventilation, under a 12 h light/dark cycle, and had free access to food and water. This study was performed in strict accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (Bayne Citation1996). All experimental protocols of this study were approved by the Animal Ethics Committee of Southwest Medical University (NO.:20221118-018; Luzhou, China), and all efforts were made to minimize animal suffering and reduce the number of animals used. The rats were randomly divided into five groups (n = 6): control group, model group, MZD group, LVFX group and MZD + LVFX group. After 24 h of water inhibition, rats were anesthetized by intraperitoneal injection of 0.8 mg/100 g 1% pentobarbital. The rats were supine, the ureteral intubation was inserted into the urethra, and the lower abdomen of the rats was pressed to empty the urine. Then 0.5 mL 1.5 × 108 CFU/mL ESBLs E. coli was slowly injected into the bladder with a 1 mL sterile syringe to establish a rat model of UTI (Johansson et al. Citation1987). The control group was injected with an equal volume of sterile normal saline solution. After the establishment of the model, each group was treated with corresponding drugs for 14 days (Xue et al. Citation2017). The MZD group was intragastrically administered 20 g/kg MZD, the LVFX group was intragastrically administered 0.025 g/kg LVFX, and the MZD + LVFX group was intragastrically administered 20 g/kg MZD and 0.025 g/kg/d LVFX, while the control and model groups were intragastrically administered the same dose of normal saline every day. After treatment, the rats were sacrificed by cervical dislocation, urine was collected for bacterial colony culture, and serum was collected for subsequent microbial experiments. Additionally, blood biochemical indexes were measured, and a histopathological examination of the kidney and bladder was performed.
Serum biochemical indicators assays
Blood was collected from the rats, and biochemical indices, such as white blood cells (WBC), the proportion of neutrophils (NEUT%), and renal function indices serum creatinine (Scr) and urea nitrogen (BUN) were determined a HITACHI 7600-020 automatic biochemistry analyzer (Shandong, China). Additionally, blood samples were centrifuged at 3000 rpm for 15 min to obtain serum. Serum C-reactive protein (CRP) expression was determined using enzyme-linked immunosorbent assay (ELISA). All operations were performed in strict accordance with the manufacturer’s instructions and were repeated at least thrice for each sample.
Histopathological staining
Bladder and kidney tissues were fixed in 4% paraformaldehyde for 24 h at room temperature, paraffin-embedded, and sectioned at 3 μm. Histopathological changes, inflammation, and fibrosis were observed by HE and Masson staining. Sections of bladder and kidney tissues were dewaxed with xylene and ethanol gradient and then stained with hematoxylin and eosin (HE) or Masson staining (Ozawa and Sakaue Citation2020). Histological analyses were performed using a light microscope (Nikon). Each tissue section was evaluated histologically, and images were taken at ×400 magnification.
Urine bacterial culture
The urine of different treatment rats was extracted from the bladder with sterile needles, and continuously diluted to 10−4, coated with 100 μL diluent on the plate, and cultured upside down in an incubator at 37 °C for 24 h, then the number of bacterial colonies was counted using light microscopy (Nikon).
Antibiotic resistance and MS-MZD sensitivity
Experimental antibiotics, including ampicillin (AMP, CAS: 7177-48-2), cefazolin (CFZ, CAS: 25953-19-9), cefotaxime (CTX, CAS: 64485-93-4), gentamicin (GEN, CAS: 1403-66-3), tetracycline (TE, CAS: 60-54-8), chloramphenicol (CAP, CAS: 56-75-7), and levofloxacin (LVFX, CAS: 100986-85-4), were all obtained from McLean Biochemical Technology Co., Ltd. (Shanghai, China). Stock solutions of experimental antibiotics were prepared at a concentration of 10.24 mg/mL, filtered, and sterilized at 0.22 µm. Medicated serum of MZD (MS-MZD) was obtained from previous animal experiments. In brief, abdominal aorta blood was collected 1 h after the last drug administration in rats and centrifuged at 3000 rpm for 15 min to separate the serum, which was filtered and sterilized a 0.22 µm before use. The minimal inhibitory concentration (MIC) of MS-MZD against ESBLs E. coli was determined using the broth microdilution method (Jorgensen and Ferraro Citation2009), which was recommended by the American Society for Clinical and Laboratory Standards (CLSI) for drug sensitivity testing. The MS-MZD dilution times were 1:1 to 1:2048, and the concentrations of antibiotics were 256–0.125 μg/mL, both of which were double-gradient dilutions.
Crystal violet dyeing
To determine the effect of MS-MZD on E. coli biofilm formation, five groups were randomly set up: control, negative serum control (SHRN), medicated serum of the MZD (MS-MZD, MIC50), levofloxacin (LVFX, MIC50), and MS-MZD + LVFX (MIC50). ESBLs E. coli was cultured overnight in LB liquid medium at 37 °C, and the concentration was adjusted to 1 × 109 CFU/mL. Then, 10 μL of ESBLs E. coli solution and 200 μL of media with different drugs were added to 96-well plates (Coffey and Anderson Citation2014). The culture solution was added to the control group, and each strain was made in three duplicate wells, and the culture was static at 37 °C. After 72 h of culture, 50 μL of 0.25 g/L crystal violet dye was added and stained at room temperature for 15 min. After rinsing with normal saline and air drying, 150 μL of 95% ethanol was added to each well and decolorized at room temperature for 10 min. The absorbance of each well was measured at 570 nm wavelength using a microplate reader (Bio-Rad Laboratories, Inc.).
Scanning electron microscopy (SEM)
Ultrastructural changes in the biofilm were observed using SEM (Leoney et al. Citation2020). Approximately 107 CFU/mL ESBLs E. coli solution was inoculated into the culture medium containing the different drugs. After incubation for 10 h, the supernatant was discarded after centrifugation at 5000 rpm for 5 min, 2.5% dialdehyde was added for fixation at room temperature for 48 h, and 1% osmic acid was added for 4 h, washed 10 times with PBS, dehydrated with an ethanol gradient, and replaced with acetone 10 times. The centrifugally concentrated liquid was dried using a CO2 critical point dryer (Emitech K850, Quorum, UK), and the dried bacterial powder was observed by SEM (FEI Quanta 200 F, Shanghai, China).
Real-time quantitative polymerase chain reaction (RT-PCR)
The levels of luxS, pfS and ompA in ESBLs E. coli were measured by RT-PCR (Sun et al. Citation2019). Briefly, the total RNA of ESBLs E. coli was obtained using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.), and cDNA was prepared using HiScript IIIRT SuperMix for qPCR (with gDNA wiper) (Vazyme Biotech Co., Ltd.). qPCR was performed using ChamQ™ SYBR qPCR Master Mix (Vazyme Biotech Co., Ltd.) with a CFX96™ RT-PCR detection system (Bio-Rad Laboratories, Inc.) at a final volume of 20 µL using the standard protocol as follows: 95 °C for 30 s, 40 cycles of 95 °C for 10 s and 60 °C for 30 s, 95 °C for 15 s, 60 °C for 60 s, and 95 °C for 15 s. Relative luxS, pfS, and ompA mRNA expression levels were determined using the comparative 2−ΔΔCt method with gapA as an internal reference gene. Primer sequences used in this study are listed in .
Table 1. The primer sequences used for real-time PCR assay.
Statistical analysis
All data were analyzed using SPSS software (version 22.0; IBM Corp.) and are shown as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used for comparison between the groups, Dunnett’s test was used for paired comparison (post hoc), and p < 0.05 was considered to indicate a statistically significant difference.
Results
Effects of MZD on UTI induced by ESBLs E. coli in rats
To investigate the effects of MZD on UTI in rats induced by ESBLs E. coli, the blood biochemical indices and renal function indices of rats were analyzed. Compared with the control group, WBC, NEUT%, CRP, Scr, and BUN of rats in the model group were significantly increased, showing an inflammatory response and renal function damage (p < 0.001, ). Rats in the MZD, LVFX, and MZD + LVFX groups exhibited significantly lower WBC, NEUT%, CRP, Scr, and BUN than those in the model group, suggesting that both MZD and LVFX can improve the inflammatory response and renal function damage in rats with UTI (p < 0.001, ).
Table 2. Blood biochemical and renal function indexes of rats.
The histopathological analysis of the bladder and kidney was performed, and as shown in , in the model group, there were vascular hyperplasia, dilation, and inflammatory cell infiltration in the bladder tissue lamina propria, the thickening and edema of the muscle and outer membrane were obvious, and blood vessels, lymphocytes, neutrophils, and macrophages were visible in the proliferative fibrous connective tissue. For kidney tissue, the local glomerular morphology and structure were fuzzy, the renal tubules were denatured and necrotic, the epithelial cell nuclei were flattened, lysed, or irregular, some lumens were slightly dilated, the surrounding interstitial fibrosis was more obvious, a large number of fibroblasts were hyperplastic, and lymphocyte infiltration was observed in the necrotic area. Inflammatory infiltration and necrosis of the bladder and kidney tissue were alleviated in the MZD, LVFX, and MZD + LVFX groups. The therapeutic effect of MZD combined with LVFX was significantly better than that of a single alone. There was no obvious degeneration, necrosis, or inflammatory cell infiltration in the bladder and kidney tissues of the MZD + LVFX group (). Additionally, as shown in , Masson staining was used to analyze bladder and kidney fibrosis. The percentage of fibrous tissue in the bladder and kidney in rats of the model group was significantly higher than that in the control group, fibrosis was decreased after MZD or LVFX treatment, and the curative effect of the MZD + LVFX group was better than that of the MZD or LVFX groups (p < 0.05, ).
Figure 1. Effects of MZD on tissue morphology of UTI induced by ESBLs E. coli in rats. (A) Representative images showing HE staining of bladder and kidney in rats. Magnification, ×400. (B) Representative images showing Masson staining of bladder and kidney in rats. Magnification, ×400. (C) Percentage of fibrotic tissue in the bladder and kidney of rats stained by Masson. Data were shown as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, compared with the control group; #p < 0.05, ##p < 0.01, ###p < 0.001, compared with the model group; &p < 0.05, &&p < 0.01, &&&p < 0.001, compared with the MZD + LVFX group. MZD: modified Zhibai Dihuang pill; LVFX: levofloxacin.
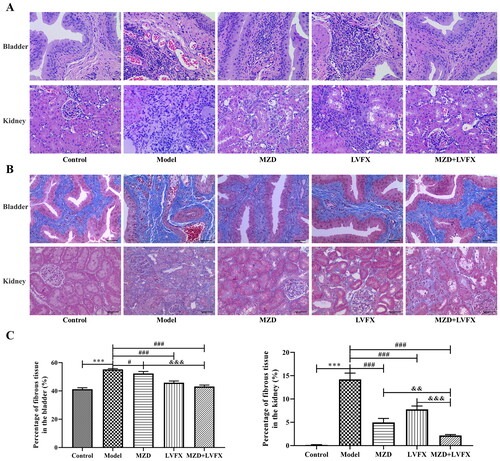
Figure 2. Urine bacterial culture of rats with UTI caused by ESBLs E. coli. (A) Representative plate showing the bacterial culture of urine. (B) The number of bacterial culture colonies in the urine of rats. Data are shown as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, compared with the control group; #p < 0.05, ##p < 0.01, ###p < 0.001, compared with the model group; &p < 0.05, &&p < 0.01, &&&p < 0.001, compared with the MZD + LVFX group. MZD: modified Zhibai Dihuang pill; LVFX: levofloxacin.
Urine bacterial culture of rats with UTI caused by ESBLs E. coli
Bladder urine from rats in each group was collected, cultured on agar plates, and counted. The results showed that there were no bacteria in the control group, and urine bacteria in the model group were significantly increased. MZD, LVFX, and MZD + LVFX eliminated urine bacteria in the model group (p < 0.05, ), and the effect of MZD + LVFX was significantly better than that of MZD or LVFX (p < 0.05, ).
Antibiotic resistance and MS-MZD sensitivity
As shown in , EDBLs E. coli showed different degrees of resistance to seven antibiotics. CFZ, CTX, GEN, CAP, and LVFX showed relatively low MICs of 1, 0.25, 16, 8, and 2 µg/mL, respectively, indicating good antibacterial effects. AMP and TE showed MIC levels of 256 and 128 µg/mL, respectively. The MIC of MS-MZD against ESBLs E. coli strains was a dilution ratio of 1:2 ().
Table 3. MIC (μg/mL or dilution ratio) of ESBLs E. coli.
Effects of MS-MZD on the formation of ESBLs E. coli biofilm
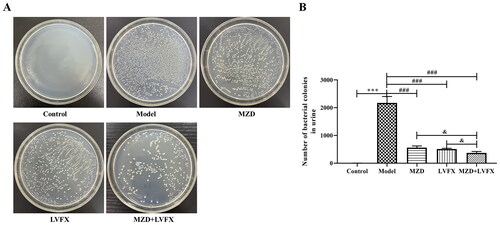
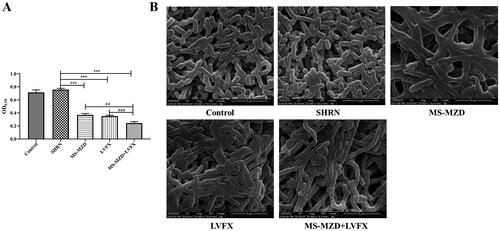
Biofilm formation further promotes the development and spread of bacteria, which are important causes of persistent bacterial infections. To further clarify the effects of MZD on ESBLs E. coli, we analyzed the biofilm-forming ability of ESBLs E. coli. As shown in , crystal violet staining showed that the biofilm formation ability in the SHRN group was the same as that in the control group (p > 0.05, ), indicating that serum did not affect biofilm formation. The biofilm formation ability of ESBLs E. coli in the MS-MZD, LVFX, and MS-MZD + LVFX groups was significantly lower than that in the model group, and the MS-MZD + LVFX group had the lowest value, which was lower than that in the MS-MZD or LVFX groups (p < 0.01, ). Additionally, the ultrastructure of ESBLs E. coli was observed by SEM, as shown in , ESBLs E. coli in the control and SHRN group showed a complete and smooth elongated structure. After different treatments, the cell lengths of the MS-MZD, LVFX, and MS-MZD + LVFX groups significantly increased, and all groups showed a certain bacteriostatic effect.
Figure 3. Comparison of the biofilm formation ability of ESBLs E. coli. (A) The biofilm formation was quantitatively determined by crystal violet staining. (B) Representative images showing biofilm formation observed by laser scanning electrolysis. Magnification, ×20,000. Data are shown as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, compared with the SHRN group; #p < 0.05, ##p < 0.01, ###p < 0.001, compared with the MS-MZD + LVFX group. SHRN: negative serum control group; MS-MZD: medicated serum of modified Zhibai Dihuang pill group; LVFX: levofloxacin group.
Effects of MS-MZD on the gene expression of ESBLs E. coli
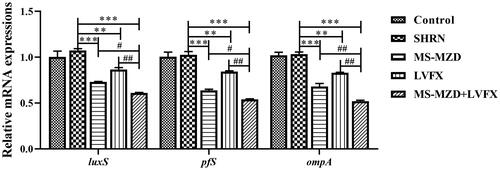
The luxS, pfS, and ompA expression in bacteria are closely associated with bacterial biofilm formation. In this study, the gene expression of luxS, pfS, and ompA in the SHRN group was not significantly different from that in the control group (p > 0.05, ), suggesting that the serum did not affect gene expression. MS-MZD and LVFX significantly reduced the gene expression of luxS, pfS, and ompA, and the difference was statistically significant compared to that of the SHRN group (p < 0.05, ). The MS-MZD + LVFX group further reduced the gene expression of luxS, pfS, and ompA compared with the MS-MZD or LVFX alone groups, and the effects were better than those of the single group (p < 0.05, ).
Figure 4. Effects of MS-MZD on the relative gene expression of luxS, pfS, and ompA in ESBLs E. coli. Data are shown as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, compared with the SHRN group; #p < 0.05, ##p < 0.01, ###p < 0.001, compared with the MS-MZD + LVFX group. SHRN: negative serum control group; MS-MZD: medicated serum of modified Zhibai Dihuang pill; LVFX: levofloxacin.
Discussion
UTIs are among the most common chronic and recurrent infectious diseases caused by various microbial pathogens (Choe et al. Citation2018). Studies have shown that 13 different microbial agents are involved in UTIs, including Escherichia coli, Streptococcus, Klebsiella, Staphylococcus, etc. (Behzadi and Behzadi Citation2008). Among these, the UPEC strain is the main strain causing different types of UTI and contains various virulence genes (Behzadi and Behzadi Citation2017; Hozzari et al. Citation2020). The presence of these virulence genes led to the rapid growth of ESBLs and metal-β-lactamase (MBL) of UPEC origin, but studies have shown that there is no significant correlation between the characteristics of ESBL production and bacterial antibiotic resistance (Khonsari et al. Citation2021). With the emergence of bacterial resistance, the use of antibiotics to treat complex UTIs caused by drug-resistant bacteria has become challenging (Waller et al. Citation2018). Due to the poor efficacy of antibiotic therapy strategies in treating UTIs associated with urinary pathogens, several new alternatives have been developed, including anti-adhesion therapy (Sarshar et al. Citation2020), anti-biofilm formation, etc. In modern medicine, TCM has a certain effect on the treatment of infectious diseases, and there are few reports of adverse reactions (Ma et al. Citation2019). In this study, ESBLs E. coli was used to construct a UTI rat model. After intragastric administration of MZD or LVFX in UTI rats, the renal function indices were significantly improved, pathological damage of the bladder and kidney was alleviated, and the number of bacteria in the urine was significantly inhibited. Additionally, MZD medicated serum significantly inhibited the formation of ESBLs E. coli biofilms and significantly decreased the luxS, pfS, and ompA gene expression in ESBLs E. coli, indicating that MZD may be an effective drug for UTI treatment.
UTI belongs to the category of drench syndrome in TCM, and TCM has great advantages in treating this disease due to its multi-target characteristics, integrity, and small toxicity and side effects (Liu et al. Citation2017). MZD, including Phellodendri chinensis cortex, Anemarrhenae rhizoma, Alpiniae oxyphyllae fructus, Cuscutae semen, Plantaginis semen, Polygoni avicularis herba, Dianthi herba, Gardeniae fructus, Astragali radixs, Poria, Atractylodis macrocephalae rhizoma, Scutellariae radix and Achyranthis bidentatae radix, were used in this study. Modern pharmaceutical research shows that Phellodendri chinensis cortex has an inhibitory effect on pathogenic bacteria such as dysentery Bacillus and Staphylococcus aureus (Sun et al. Citation2019), Anemarrhenae rhizoma has an inhibitory effect on E. coli, Proteus genus and other bacteria (Park et al. Citation2003), Dianthi herba has strong inhibitory activities against urogenital Chlamydia trachomatis (Li et al. Citation2000), Gardeniae fructus can inhibit Helicobacter pylori infection (Chang et al. Citation2017), Scutellariae radix protects mice from methicillin-resistant Staphylococcus aureus infection (Shi et al. Citation2020). Additionally, Cuscutae semen, Plantaginis semen, Polygoni avicularis herba, Astragali Radixs, Poria, Atractylodis macrocephalae rhizoma, and Achyranthis bidentatae radix are commonly used to tonify the kidney and can significantly improve renal function (Zhao et al. Citation2013; Zhang et al. Citation2014; Saremi et al. Citation2018; Wang et al. Citation2021; Zhao et al. Citation2021). The combination of these TCM accords with the idea of TCM syndrome differentiation treatment, which can not only treat the pathogen but also improve the symptoms and regulate the immunity of the body (Barrett et al. Citation2015). This study found that MZD had a good therapeutic effect on UTI caused by ESBLs E. coli, mainly manifested as improved renal function, alleviated inflammation and fibrosis of the bladder and kidney tissue, and significantly reduced the number of bacteria in the urine of rats, showing a strong antibacterial effect.
Gram-negative bacteria communicate with each other through the production and perception of diffused signaling molecules, a phenomenon known as quorum sensing (QS), in which bacterial biofilms play an important role in regulating many bacterial activities (Kai Citation2018). The structural characteristics of bacterial biofilms make them insensitive to antibiotics and the immune system, and biofilm formation can aggravate antibiotic resistance and make it difficult to treat bacterial infections (Chen and Wen Citation2011). Bacterial biofilm formation inhibition is considered a potential target for antibacterial drugs (Rabin et al. Citation2015). Studies have shown that Yunnan Baiyao can affect biofilm formation by Pseudomonas aeruginosa, inhibiting the virulence of bacteria (Zhao et al. Citation2013). Citrus flavonoids can inhibit biofilm formation and bacterial cell-cell signal transduction in E. coli (Vikram et al. Citation2010). Numerous studies have shown that TCM has antibacterial effects and can inhibit biofilm formation (Zhang et al. Citation2020). This study also found that MZD significantly inhibits the formation of ESBLs E. coli biofilms. Additionally, the formation of bacterial biofilms is regulated by various signaling pathways, and the QS system is one of the regulatory mechanisms closely associated with the pathogenicity of pathogens (Muhammad et al. Citation2020). In E. coli, QS inter-acoustic signaling molecules secreted by the bacteria are transported into the cells by luxS and then phosphorylated. Signaling molecules bind to the receptor protein luxS to regulate the formation and structure of biofilms (Williams Citation2002). Studies have shown that the inactivation of luxS and pfs significantly reduces the ability of E. coli to generate QS and induce biofilm formation (Wang et al. Citation2016). Moreover, ompA is a major regulatory gene for the formation of outer membrane proteins in E. coli, which regulates biofilm formation in E. coli and is closely related to the virulence of E. coli (Ma and Wood Citation2009). In this study, it was found that medicated serum of MZD significantly reduced the luxS, pfS, and ompA gene expressions, and showed an inhibitory effect on QS. The combined effect of MS-MZD and LVFX was better, suggesting that MZD might play a therapeutic role by inhibiting bacterial biofilm formation.
Conclusions
This study demonstrated that MZD could improve renal function, alleviate inflammation and fibrosis of bladder and kidney tissue, reduce the number of bacteria in urine, and show a good therapeutic effect on UTI induced by ESBLs E. coli. In addition, MZD medicated serum inhibited the formation of ESBLs E. coli biofilms and reduced the expression of luxS, pfS, and ompA, suggesting that MZD plays a therapeutic role in rats with UTI caused by ESBLs E. coli by inhibiting bacterial biofilm formation.
Author contributions
Kaifa Chen and Xin Liu conceived and designed the experiments, Kaifa Chen, Yongsheng Zhu and Hongwei Su performed the experiments, Kaifa Chen and Xin Liu analyzed the data, Hao Jiang contributed reagents/materials/analysis tools. Kaifa Chen and Xin Liu wrote the manuscript. All authors read and approved the final version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Barrett P, Flower A, Lo V. 2015. What’s past is prologue: Chinese medicine and the treatment of recurrent urinary tract infections. J Ethnopharmacol. 167:86–96.
- Bayne K. 1996. Revised guide for the care and use of laboratory animals available. American Physiological Society. Physiologist. 39:199, 208–111.
- Behzadi P, Behzadi E. 2008. The microbial agents of urinary tract infections at central laboratory of Dr. Shariati Hospital, Tehran, IRAN. Turkiye Klinikleri J Med Sci. 28:445–449.
- Behzadi P, García-Perdomo HA, Karpiński TM, Issakhanian L. 2020. Metallo-β-lactamases: a review. Mol Biol Rep. 47(8):6281–6294.
- Behzadi P. 2020. Classical chaperone-usher (CU) adhesive fimbriome: uropathogenic Escherichia coli (UPEC) and urinary tract infections (UTIs). Folia Microbiol (Praha). 65(1):45–65.
- Behzadi P, Behzadi E. 2017. Uropathogenic Escherichia coli: an ideal resource for DNA microarray probe designing. Bioinform Biomed Eng. 10209:12–19.
- Chang CH, Wu JB, Yang JS, Lai YJ, Su CH, Lu CC, Hsu YM. 2017. The suppressive effects of geniposide and genipin on Helicobacter pylori infections in vitro and in vivo. J Food Sci. 82(12):3021–3028.
- Chen L, Wen YM. 2011. The role of bacterial biofilm in persistent infections and control strategies. Int J Oral Sci. 3(2):66–73.
- Chen XP, Ali L, Wu LY, Liu C, Gang CX, Huang QF, Ruan JH, Bao SY, Rao YP, Yu D. 2018. Biofilm formation plays a role in the formation of multidrug-resistant Escherichia coli toward nutrients in microcosm experiments. Front Microbiol. 9:367.
- Choe HS, Lee SJ, Yang SS, Hamasuna R, Yamamoto S, Cho YH, Matsumoto T. 2018. Summary of the UAA-AAUS guidelines for urinary tract infections. Int J Urol. 25(3):175–185.
- Coffey BM, Anderson GG. 2014. Biofilm formation in the 96-well microtiter plate. Methods Mol Biol. 1149:631–641.
- Flores-Mireles A, Hreha TN, Hunstad DA. 2019. Pathophysiology, treatment, and prevention of catheter-associated urinary tract infection. Top Spinal Cord Inj Rehabil. 25(3):228–240.
- Gupta K, Grigoryan L, Trautner B. 2017. Urinary tract infection. Ann Intern Med. 167(7):Itc49–itc64.
- Herrmann V, Palma P, Géo MS, Lima RS. 2002. Urinary tract infections: pathogenesis and related conditions. Int Urogynecol J Pelvic Floor Dysfunct. 13(3):210–213.
- Hou Y, Yang M, Jiang H, Li D, Du Y. 2019. Effects of low-intensity and low-frequency ultrasound combined with tobramycin on biofilms of extended-spectrum beta-lactamases (ESBLs) Escherichia coli. FEMS Microbiol Lett. 366:026.
- Hozzari A, Behzadi P, Kerishchi Khiabani P, Sholeh M, Sabokroo N. 2020. Clinical cases, drug resistance, and virulence genes profiling in uropathogenic Escherichia coli. J Appl Genet. 61(2):265–273.
- Issakhanian L, Behzadi P. 2019. Antimicrobial agents and urinary tract infections. Curr Pharm Des. 25(12):1409–1423.
- Johansson SL, Anderström C, Schultz L, Larsson P. 1987. Enhancement of N-[4-(5-nitro-2-furyl)-2-thiazolyl]formamide-induced carcinogenesis by urinary tract infection in rats. Cancer Res. 47:559–562.
- Jorgensen JH, Ferraro MJ. 2009. Antimicrobial susceptibility testing: a review of general principles and contemporary practices. Clin Infect Dis. 49(11):1749–1755.
- Kai K. 2018. Bacterial quorum sensing in symbiotic and pathogenic relationships with hosts. Biosci Biotechnol Biochem. 82(3):363–371.
- Khonsari MS, Behzadi P, Foroohi F. 2021. The prevalence of type 3 fimbriae in uropathogenic Escherichia coli isolated from clinical urine samples. Meta Gene. 28:100881.
- Kostakioti M, Hadjifrangiskou M, Hultgren SJ. 2013. Bacterial biofilms: development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb Perspect Med. 3(4):a010306.
- Leoney A, Karthigeyan S, Asharaf AS, Felix AJW. 2020. Detection and categorization of biofilm-forming Staphylococcus aureus, Viridans streptococcus, Klebsiella pneumoniae, and Escherichia coli isolated from complete denture patients and visualization using scanning electron microscopy. J Int Soc Prev Community Dent. 10(5):627–633.
- Li JJ, Tu YY, Tong JZ, Wang PT. 2000. [Inhibitory activity of Dianthus superbus L. and 11 kinds of diuretic Traditional Chinese medicines for urogenital Chlamydia trachomatis in vitro]. Zhongguo Zhong Yao Za Zhi. 25:628–630. Chinese
- Li XQ, Zhu JY, Pan RR, Shen YL, Rahman K, Zhang CY, Zhang LJ, Luan X, Zhang H. 2019. Therapeutic effect of Dongbai-Tonglin-Fang, a Chinese herbal formula, on urinary tract infection in rat model. J Ethnopharmacol. 241:112028.
- Liu CM, Chen J, Yang S, Mao LG, Jiang TT, Tu HH, Chen ZL, Hu YT, Gan L, Li ZJ, et al. 2018. The Chinese herbal formula Zhibai Dihuang Granule treat Yin-deficiency-heat syndrome rats by regulating the immune responses. J Ethnopharmacol. 225:271–278.
- Liu SW, Guo J, Wu WK, Chen ZL, Zhang N. 2019. Treatment of uncomplicated recurrent urinary tract infection with Chinese medicine formula: a randomized controlled trial. Chin J Integr Med. 25(1):16–22.
- Liu SW, Xu XY, Xu J, Yuan JY, Wu WK, Zhang N, Chen ZL. 2017. Multi-drug resistant uropathogenic Escherichia coli and its treatment by Chinese medicine. Chin J Integr Med. 23(10):763–769.
- Ma Q, Wood TK. 2009. OmpA influences Escherichia coli biofilm formation by repressing cellulose production through the CpxRA two-component system. Environ Microbiol. 11(10):2735–2746.
- Ma Y, Chen M, Guo Y, Liu J, Chen W, Guan M, Wang Y, Zhao X, Wang X, Li H, et al. 2019. Prevention and treatment of infectious diseases by traditional Chinese medicine: a commentary. APMIS. 127(5):372–384.
- Millner R, Becknell B. 2019. Urinary tract infections. Pediatr Clin North Am. 66(1):1–13.
- Muhammad MH, Idris AL, Fan X, Guo Y, Yu Y, Jin X, Qiu J, Guan X, Huang T. 2020. Beyond risk: Bacterial biofilms and their regulating approaches. Front Microbiol. 11:928.
- Ozawa A, Sakaue M. 2020. New decolorization method produces more information from tissue sections stained with hematoxylin and eosin stain and Masson-trichrome stain. Ann Anat. 227:151431.
- Park HJ, Lee JY, Moon SS, Hwang BK. 2003. Isolation and anti-oomycete activity of nyasol from Anemarrhena asphodeloides rhizomes. Phytochemistry. 64(5):997–1001.
- Pavithra D, Doble M. 2008. Biofilm formation, bacterial adhesion and host response on polymeric implants–issues and prevention. Biomed Mater. 3(3):034003.
- Rabin N, Zheng Y, Opoku-Temeng C, Du Y, Bonsu E, Sintim HO. 2015. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med Chem. 7(4):493–512.
- Saremi J, Kargar Jahromi H, Pourahmadi M. 2018. Effect of Polygonum aviculare L. on nephrolithiasis induced by ethylene glycol and ammonium chloride in rats. Urol J. 15:79–82.
- Sarshar M, Behzadi P, Ambrosi C, Zagaglia C, Palamara AT, Scribano D. 2020. FimH and anti-adhesive therapeutics: A disarming strategy against uropathogens. Antibiotics (Basel). 9(7):397.
- Shi T, Li T, Jiang X, Jiang X, Zhang Q, Wang Y, Zhang Y, Wang L, Qin X, Zhang W, et al. 2020. Baicalin protects mice from infection with methicillin-resistant Staphylococcus aureus via alleviating inflammatory response. J Leukoc Biol. 108(6):1829–1839.
- Shrestha R, Khanal S, Poudel P, Khadayat K, Ghaju S, Bhandari A, Lekhak S, Pant ND, Sharma M, Marasini BP. 2019. Extended spectrum β-lactamase producing uropathogenic Escherichia coli and the correlation of biofilm with antibiotics resistance in Nepal. Ann Clin Microbiol Antimicrob. 18(1):42.
- Sun T, Li XD, Hong J, Liu C, Zhang XL, Zheng JP, Xu YJ, Ou ZY, Zheng JL, Yu DJ. 2019. Inhibitory effect of two traditional Chinese medicine monomers, berberine and matrine, on the quorum sensing system of antimicrobial-resistant Escherichia coli. Front Microbiol. 10:2584.
- Sun Y, Lenon GB, Yang AWH. 2019. Phellodendri cortex: a phytochemical, pharmacological, and pharmacokinetic review. Evid Based Complement Alternat Med. 2019:7621929.
- Tandogdu Z, Wagenlehner FM. 2016. Global epidemiology of urinary tract infections. Curr Opin Infect Dis. 29(1):73–79.
- Tremblay YD, Hathroubi S, Jacques M. 2014. [Bacterial biofilms: their importance in animal health and public health]. Can J Vet Res. 78:110–116.
- Vikram A, Jayaprakasha GK, Jesudhasan PR, Pillai SD, Patil BS. 2010. Suppression of bacterial cell-cell signalling, biofilm formation and type III secretion system by Citrus flavonoids. J Appl Microbiol. 109(2):515–527.
- Vuotto C, Longo F, Pascolini C, Donelli G, Balice MP, Libori MF, Tiracchia V, Salvia A, Varaldo PE. 2017. Biofilm formation and antibiotic resistance in Klebsiella pneumoniae urinary strains. J Appl Microbiol. 123(4):1003–1018.
- Waller TA, Pantin SAL, Yenior AL, Pujalte GGA. 2018. Urinary tract infection antibiotic resistance in the United States. Prim Care. 45(3):455–466.
- Wang J, Bao B, Meng F, Deng S, Dai H, Feng J, Li H, Wang B. 2021. To study the mechanism of Cuscuta chinensis Lam. and Lycium barbarum L. in the treatment of asthenospermia based on network pharmacology. J Ethnopharmacol. 270:113790.
- Wang X, Li S, Lu X, Hu P, Chen H, Li Z, Bu Z, Lang X, Wang X. 2016. Rapid method of luxS and pfs gene inactivation in enterotoxigenic Escherichia coli and the effect on biofilm formation. Mol Med Rep. 13(1):257–264.
- Williams P. 2002. Quorum sensing: an emerging target for antibacterial chemotherapy? Expert Opin Ther Targets. 6(3):257–274.
- Xue WY, Qi JC, Du L. 2017. Intervention effect and mechanism of curcumin in chronic urinary tract infection in rats. Asian Pac J Trop Med. 10(6):594–598.
- Yi WJ, Chen J, Li ZB, Jiang TT, Bi DQ, Liu CM, Yang S, Hu YT, Gan L, Tu HH, et al. 2020. Screening of potential biomarkers for Yin-deficiency-heat syndrome based on UHPLC-MS method and the mechanism of Zhibai Dihuang granule therapeutic effect. Anat Rec (Hoboken). 303(8):2095–2108.
- Yu GY, Tian ZJ, Sun Y, Yang HW, Han SJ, Miao RP, Fu XW, Xiong M, Wang YX, Tian JJ. 2017. [Review on advantages and evidence of treating and preventing urinary tract infection in traditional Chinese medicine]. Zhongguo Zhong Yao Za Zhi. 42:1439–1448. Chinese
- Zhang HW, Lin ZX, Xu C, Leung C, Chan LS. 2014. Astragalus (a traditional Chinese medicine) for treating chronic kidney disease. Cochrane Database Syst Rev. 22:Cd008369.
- Zhang L, Liang E, Cheng Y, Mahmood T, Ge F, Zhou K, Bao M, Lv L, Li L, Yi J, et al. 2020. Is combined medication with natural medicine a promising therapy for bacterial biofilm infection? Biomed Pharmacother. 128:110184.
- Zhang N, Huang L, Liu S, Wang Y, Luo Y, Jin X, Guo J, Ke Y, Chen J, Yuan X, et al. 2013. Traditional Chinese medicine: an alternative treatment option for refractory recurrent urinary tract infections. Clin Infect Dis. 56(9):1355.
- Zhao H, Xu J, Wang R, Tang W, Kong L, Wang W, Wang L, Zhang Y, Ma W. 2021. Plantaginis semen polysaccharides ameliorate renal damage through regulating NLRP3 inflammasome in gouty nephropathy rats. Food Funct. 12(6):2543–2553.
- Zhao YY, Feng YL, Bai X, Tan XJ, Lin RC, Mei Q. 2013. Ultra performance liquid chromatography-based metabonomic study of therapeutic effect of the surface layer of Poria cocos on adenine-induced chronic kidney disease provides new insight into anti-fibrosis mechanism. PLoS One. 8(3):e59617.
- Zhao ZG, Yan SS, Yu YM, Mi N, Zhang LX, Liu J, Li XL, Liu F, Xu JF, Yang WQ, et al. 2013. An aqueous extract of Yunnan Baiyao inhibits the quorum-sensing-related virulence of Pseudomonas aeruginosa. J Microbiol. 51(2):207–212.
- Zowawi HM, Harris PN, Roberts MJ, Tambyah PA, Schembri MA, Pezzani MD, Williamson DA, Paterson DL. 2015. The emerging threat of multidrug-resistant Gram-negative bacteria in urology. Nat Rev Urol. 12(10):570–584.
