?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Context
Danggui Buxue Decoction (DBD) is an effective complementary medicine in alleviating myelosuppression after chemotherapy (MAC). However, its mechanism of action is elusive.
Objective
To illustrate that regulating β-hydroxybutyric acid (β-OHB) metabolism and suppressing oxidative stress could be a potential mechanism of action for DBD in alleviating MAC.
Materials and methods
After HPLC quantification and dose testing (3, 6 and 10 g/kg, gavage) of DBD, Sprague-Dawley rats were divided into control, cyclophosphamide (CTX) (30 mg/kg CTX for 5 days, intraperitoneal administration) and CTX + DBD groups (6 g/kg DBD for 14 days, gavage). Blood cell counts, thigh bone histological examination, β-OHB levels, oxidative stress indices and HDAC1 activity were tested. The biological function of β-OHB was verified in vitro (hBMSC cells were incubated in culture mediums that contained 40 μM CTX and β-OHB in 0, 1, 2.5, 5, 10 mM) and in vivo (MAC rat model, 3 g/kg β-OHB for 14 days, gavage).
Results
Rats in the CTX + DBD group showed upregulated blood cell counts (118–243%), β-OHB levels (495 nmol/mL in blood, 122 nmol/mg in marrow supernatant) and downregulated HDAC1 activity (59%), and oxidative stress indices (60–85%). In vitro, 5 mM β-OHB improved hBMSC cell migration (123%) and proliferation (131%). In vivo, rats treated with 3 g/kg β-OHB showed upregulated blood cell counts (121–182%) and downregulated HDAC1 activity (64%) and oxidative stress indices (65–83%).
Discussion and conclusions
DBD, a traditional Chinese medicine, alleviates MAC by intervening in β-OHB metabolism and oxidative stress.
Introduction
Chemotherapy is one of the common methods widely used to treat malignant tumors by inhibiting or killing tumor cells. However, most chemotherapeutic drugs have low selectivity and may damage normal cells, tissues or organs at the same time, thereby inducing a variety of adverse reactions (Pich et al. Citation2019). Among them, myelosuppression after chemotherapy (MAC) is the most common side effect with the highest incidence. Nearly 80% of patients with malignant tumors have MAC, mainly manifested by the reduced number of platelets, erythrocytes, granulocytes and subsequent hematopoietic dysfunction (Qiu et al. Citation2021). MAC can also cause immune system dysfunction, leading to a severe infection or discontinued treatment (Hiam-Galvez et al. Citation2021). Currently, MAC is mainly treated by the infusion of hematopoietic growth factors, glucocorticoid hormone, etc. Although these methods have an immediate effect and good therapeutic action, they may also cause a series of iatrogenic diseases, such as severe bone pain, fever, interstitial pneumonia, cardiac failure and thrombus (He et al. Citation2021). Therefore, developing effective and safe methods to supplement or replace the above treatments is a topic of general clinical interest.
Traditional Chinese medicines (TCMs) are widely used as complementary medicines and have been shown to reduce toxicity and enhance the efficacy of chemotherapy (Yang et al. Citation2020). Related research has shown that TCM is a safe and effective adjuvant in the clinical prevention and treatment of MAC (Hou et al. Citation2017; Yang et al. Citation2021). Danggui Buxue Decoction (DBD) is a traditional recipe comprising Astragalus membranaceus (Fisch.) Bunge (Fabaceae) radix and Angelica sinensis (Oliv.) Diels (Apiaceae) radix at a fixed-ratio (5:1). As a classic formula for nourishing ‘Qi’ and enriching ‘Blood’, DBD has been confirmed to have a series of pharmacological effects, including promoting hematopoiesis, immunity regulation and anti-vasoconstriction (Guo et al. Citation2021; Liu, Chang et al. Citation2021). Clinically, DBD has a significant effect against chemotherapy-induced anemia, renal anemia, leukopenia and other hematopoietic dysfunction and, therefore, can be used for the prevention and treatment of MAC (Shi et al. Citation2020; Liu, Yang et al. Citation2021).
Modern pharmacological studies have revealed that in addition to the active components acting directly on the targets and signal pathways, TCM can also exert its efficacy indirectly by intervening in the endogenous metabolites (Yan et al. Citation2015). The small endogenous metabolites, such as amino acids, bile acids and lipids, work as functional ligands to influence environmental homeostasis, drug metabolism or disease progression (Wishart Citation2016). The disruption of metabolic homeostasis has been linked to biological mechanisms underlying several diseases (Wishart Citation2019).
Chemotherapy can induce serious oxidative stress injury (Rtibi et al. Citation2017), which has been shown to contribute to the pathophysiology of MAC by inducing hematopoietic stem cell (HSC) senescence (Wei and Frenette Citation2018). In a previous study, we demonstrated that the β-hydroxybutyric acid (β-OHB) level was directly correlated with the degree of MAC (Gao et al. Citation2019). As the major component of the ketone body (Møller Citation2020), β-OHB can serve as endogenous histone 1 deacetylase (HDAC1) inhibitor, which is associated with increased histone acetylation to initiate the expression of antioxidant transcription factor FoxO3a (Shimazu et al. Citation2013; Ji et al. Citation2022). FoxO3a can promote the expression of antioxidant proteins in response to oxidative stress, such as superoxide dismutase (SOD) (Samadi et al. Citation2021). Moreover, HDAC1 has been shown to be involved in hemocytogenesis (Rosborough et al. Citation2012; Adeshakin et al. Citation2020). Therefore, regulating the β-OHB/HDAC1 pathway and oxidative stress homeostasis in the hematopoietic microenvironment could be a potential effective method for the prevention and treatment of MAC.
In this study, we test the efficacy of DBD in alleviating myelosuppression and unravel its underlying mechanism using a cyclophosphamide (CTX)-induced MAC rat model. CTX, a traditional chemotherapeutic drug, induces serious myelosuppression (Zhang et al. Citation2022). We evaluated β-OHB levels, HDAC1 activity and related oxidative stress indices in the circulatory system and hematopoietic microenvironment. Furthermore, the biological function of β-OHB in suppressing oxidative stress and alleviating CTX-induced hematopoiesis dysfunction was verified both in vitro and in vivo. This study will provide insights into the mechanisms of DBD in alleviating MAC and help underpin the efficacy of DBD in preventing and treating several oxidative stress-related diseases.
Materials and methods
Chemicals and reagents
Cyclophosphamide (CTX, 97%, Lot No: L8C0S28) and β-hydroxybutyric acid (β-OHB, 95%, Lot No: LF70Q06) were purchased from Bailingwei (Beijing, China). Normal saline for injection and 4% paraformaldehyde were obtained from Rhawn (Shanghai, China). NP-40 lysis buffer, Tris-HCl and EDTA were purchased from Beyotime Biotechnology (Shanghai, China). hBMSC culture medium was purchased from iCell Bioscience (Shanghai, China). Herbal reference standards were obtained from Herbpurify Co., Ltd. (Chengdu, China), including: caffeic acid (98%, Lot No: K00311812016), ferulic acid (98%, Lot No: A00211812016), ononin (98%, Lot No: M01301904002), calycosin (98%, Lot No: M02111809026) and formononetin (98%, Lot No: C01801905020).
Preparation of DBD and chemical reagents
Dried decoction pieces of Astragalus membranaceus radix and Angelica sinensis radix were purchased in 2021 from Zhangzhongjing Pharmacy (Henan, China) and identified by Dr Zhang Nan; the specimens were stored at Xinxiang Medical University, Henan. DBD was prepared following the procedure described in a previous study (Liu, Chang et al. Citation2021). Briefly, 20 g Angelica sinensis radix and 100 g Astragalus membranaceus radix were weighed and immersed in 700 mL distilled water for 1 h, then decocted with boiling water for 1 h. Next, the decoction was filtered using gauze, and the residue was again decocted with 300 mL of boiling water for 1 h. The decoctions collected in both steps were combined and concentrated to 120 mL (1 g/mL) and stored at 4 °C until further use.
CTX for injection was prepared by dissolving CTX in normal saline to 10 mg/mL. The solution was sterile-filtered using 0.22 μm membrane filters and stored at 4 °C until further use.
Analysis methods for DBD fingerprint
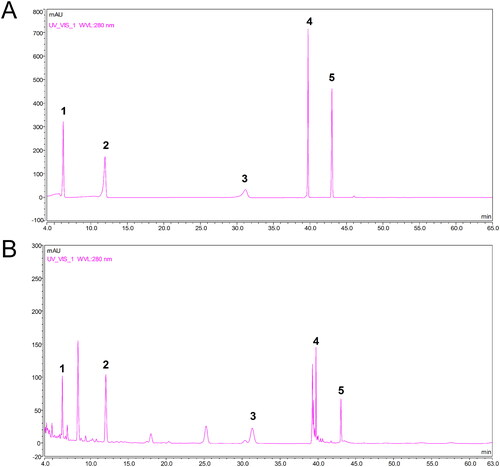
Deionized water (380 μL) was added to 20 μL DBD and vortex-mixed. After centrifugation (4 °C, 3500 g, 10 min), the supernatant was separated for DBD fingerprint analysis and the key active ingredients quantification (ThermoFisher Dionex Ultimate 3000, USA).
The analytical conditions were as follows: column (Ultimate XB-C18, 4.6 × 250 mm, 5 μm) temperature was set at 30 °C; a gradient elution with 1.00 mL/min flow rate (phase A: 0.1% formic acid, phase B: acetonitrile) was carried out with 85% A for 0–35 min, 50% A for 35–60 min, 20% A for 61–63 min then linearly brought back to 85% A and maintained for 2 min. UV detector was set at 280 nm.
Animal experiments and sample collection
All animal experiments were performed according to the Guide for the Care and Use of Laboratory Animals and approved by the Animal Ethics Committee of Xinxiang Medical University (approval number: XYLL-20210157). All the male Sprague-Dawley rats (SPF grade, 180 ± 10 g) were purchased from SiPeiFu Biotechnology Co., Ltd. (Beijing, China) and housed in a temperature-controlled environment under a 12-h dark/light cycle.
After one week of acclimatization to the raising condition, 30 male Sprague-Dawley rats were randomly divided into three groups: CTX + DBD (n = 12), CTX (n = 12) and control (C, n = 6). Rats in CTX + DBD and CTX groups were treated with CTX (30 mg/kg/day) by intraperitoneal injection (i.p.) for five consecutive days (Days 8–12) to establish the MAC model. Rats in CTX + DBD group were treated with DBD (6 g/kg/day) by intragastric administration (i.g.) for two weeks (Days 1–14). Rats in C group were treated with the same dose of distilled water (DW) and normal saline (NS).
Blood samples on Days 0, 7 and 14 were collected for blood cell counts and biochemical examination. Thigh bone samples were collected for histological examination and biochemical examination. All the samples were stored at −80 °C until analysis.
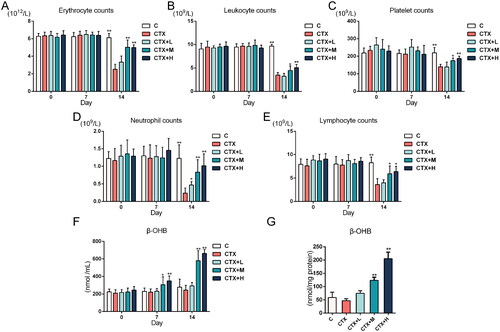
The administration dosage of DBD was confirmed as follows. Thirty male Sprague-Dawley rats were randomly divided into CTX group, control group and CTX + DBD (low, medium and high dosage) group. Rats in CTX + L, CTX + M and CTX + H group were treated with DBD by intragastric administration (i.g.) for two weeks (Days 1–14) at the dosage of 3, 6 and 10 g/kg/day, respectively. Rat blood cell counts were tested by automatic blood cell analyzer (Mindray BC-2800, Shenzhen, China). β-OHB in circulatory system and hematopoietic microenvironment were detected following the assay kits instruction. The results of DBD dosage experiment are shown in Discussion.
Biological sample preparation
All groups’ biological samples were treated with the same methods for preparation. After collection, the blood samples (containing anticoagulant) were divided into two parts: one was used for blood cell counts (including erythrocyte, leukocyte, platelet, neutrophil and lymphocyte), and the other was used to obtain plasma samples. Briefly, the blood samples were centrifuged (1000 g, 4 °C, 10 min), and the supernatant was separated to obtain plasma samples.
The right thigh bone samples of rats were collected, immediately immersed in 4% paraformaldehyde and used for histological examination. The left thigh bone was collected, weighed and triturated in NETN buffer (comprising 0.5% NP-40, 20 mM Tris-HCl, 1 mM EDTA) and used to assess the level of proteins in marrow samples.
Histological studies
After decalcifying, paraffin embedding and staining with hematoxylin & eosin (H&E), tissue sections of the right thigh samples were observed using confocal microscopy (Olympus FV1200, Japan).
Western blotting
The triturated bone samples were centrifuged (3500 g, 4 °C, 10 min), and the marrow supernatant and precipitate were separated and stored at −80 °C. Afterward, the marrow precipitate samples were washed using NETN buffer and resuspended in 100 μL 0.2 M HCl at 4 °C for 2 h. After centrifugation (3500 g, 4 °C, 10 min), 1 M Tris was added to the supernatant for neutralization, and histones were harvested. The protein level in each sample was measured using BCA Protein Assay kit (Beyotime Biotechnology, Shanghai, China). After adding loading buffer, histones samples were denatured in a boiling water bath for 10 min. The proteins were separated using SDS-PAGE and transferred onto PVDF membranes for immunoblotting. After blocking in 5% defatted milk for 30 min, PVDF membranes were incubated with anti-histone H3 (ab1791) and anti-acetylated histone H3K9 (ab32129) primary antibodies (Abcam, Shanghai, China) overnight at 4 °C. Subsequently, the PVDF membranes were washed and incubated with secondary antibodies at 25 °C for 30 min, and ECL solution (Beyotime Biotechnology, Shanghai, China) was added. The films were observed using ChemiDoc™ XRS + with Image Lab software (Bio-Rad, USA).
Biochemistry examination
Rat blood cell counts were tested by automatic blood cell analyzer (Mindray BC-2800, Shenzhen, China). Total antioxidant capacity (T-AOC), malondialdehyde (MDA) and superoxide dismutase (SOD) were detected by assay kits (Beyotime Biotechnology, Shanghai, China). 4-hydroxynonenal (4-HNE), advanced oxidation protein products (AOPP) and β-OHB were detected by ELISA kits (Jianglai Biological Technology Co., Ltd. Shanghai, China). HDAC1 and FoxO3a were detected by ELISA kits (Jining Industrial Co., Ltd. Shanghai, China). All biochemistry examinations were detected following the instructions.
Assessment of the biological function of β-OHB in vitro
Human bone marrow stromal cell (hBMSC) lines were purchased from iCell Bioscience (Shanghai, China). Cells were incubated in the hBMSC culture medium and cell culture dishes were incubated in constant temperature incubator (at 37 °C) containing 5% CO2.
Cell migration rate was evaluated using the wound healing test. Briefly, the cells were incubated in 24-well plates containing 40 μM CTX and β-OHB in 0, 1, 2.5, 5 and 10 mM. A wound was marked on the bottom of the plate, and the dimension of the wound was recorded under a microscope. After incubation in a serum-free medium for 24 h, the dimension of the wound was re-recorded, and the cell migration rate (%) was calculated as follows:
Cell proliferation rate was tested using Cell Counting Kit-8 (Beyotime Biotechnology, Shanghai, China). Briefly, the cells were seeded in 96-well plates containing 40 μM CTX and β-OHB in 0, 1, 2.5, 5 and 10 mM and incubated for 24 h. After incubation, 10 μL CCK-8 was added to each well, and the absorbance (OD) at 450 nm was measured using microplate reader (SpectraMax i3, USA).
Cell samples were collected from culture dishes. After cell counting, the culture medium was removed, the plates were washed using 4 °C phosphate-buffered solution and a quantitative cell lysis solution was added. Afterward, the solution was centrifuged (3500 g, 4 °C, 10 min), and the MDA and AOPP levels in the supernatant were estimated.
Assessment of the biological function of β-OHB in vivo
Thirty-eight male Sprague-Dawley rats were randomly divided into four groups: CTX + βOHB + DBD (n = 8), CTX-βOHB + DBD (n = 8), CTX + βOHB (n = 8), CTX (n = 8) and control (C, n = 6). Rats in CTX + βOHB + DBD, CTX-βOHB + DBD, CTX + βOHB and CTX groups were treated with CTX (30 mg/kg/day, i.p.) for five consecutive days (days 8–12) to establish MAC model. Rats in CTX + βOHB + DBD and CTX + βOHB groups were treated with β-OHB (3 g/kg/day, i.g.) for two weeks (Days 1–14). Rats in CTX + βOHB + DBD and CTX-βOHB + DBD groups were treated with DBD (6 g/kg/day, i.g.) for two weeks (Days 1–14). Rats in C group were treated with the same dose of distilled water (DW) and normal saline (NS). The administration dosage of β-OHB was decided based on previous related studies (Ji et al. Citation2022). The blood cell counts were tested using an automatic blood cell analyzer. The levels of MDA, SOD, AOPP, HDAC1, FoxO3a and β-OHB were detected following the assay kits’ instructions.
Statistical analysis
The experimental data were statistically analyzed using SPSS (version 25.0, USA) and GraphPad Prism (version 6.0, USA). All the results are shown as mean ± standard deviation (SD). Statistical significances between the two groups were obtained by two-tailed Student’s t-test and non-parametric analysis (Mann–Whitney U test). p < 0.05 was considered statistically significant. Pearson correlation analysis was used to evaluate the possible association between two continuous variables. The absolute value of Pearson correlation coefficient (P) greater than 0.7 represented highly -positive or -negative correlations (Mukaka Citation2012).
Results
Quality assurance of DBD
The HPLC analysis results of DBD are shown in . Five main active components in the DBD fingerprint were quantified using reference standards: caffeic acid (37.31 ± 5.33 μg/mL), ferulic acid (103.98 ± 8.50 μg/mL), ononin (83.06 ± 5.24 μg/mL), calycosin (47.25 ± 7.42 μg/mL) and formononetin (31.93 ± 6.32 μg/mL). Data are expressed as mean ± SD (n = 6).
Efficacy of DBD against CTX-induced MAC
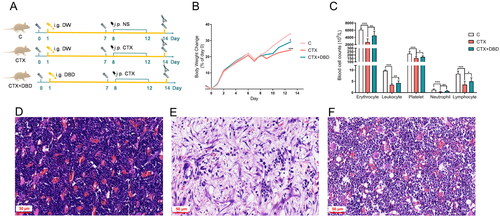
The process of sample collection is shown in . Compared with the C group, rats in the CTX group showed a decreased appetite, lower daily weight gain rate and lower blood cell counts (). These results indicated that the CTX group experienced more severe myelosuppression than the C group. Histological examination of thigh bone samples also revealed more severe damage, such as hematopoietic cell necrosis and inflammatory infiltration, in the CTX group than those in the C group (). However, rats treated with DBD showed milder symptoms with increased daily weight gain rate, elevated blood cell counts and hematopoietic cells and decreased inflammatory cells compared with those in the CTX group ()). The baseline blood cell counts (days 0 and 7) did not differ significantly. All these results confirmed the establishment of the CTX-induced MAC rat model and the potential efficacy of DBD to alleviate hematopoiesis dysfunction in the CTX-induced MAC rat model.
Figure 2. DBD alleviated hematopoiesis dysfunction in the CTX-induced MAC rat model. (A) Experimental protocols for MAC rat model establishment and sample collection. (B) Body weight changes compared with Day 0. (C) Blood cell counts on Day 14. Representative histological examination of thigh bone samples in C (D), CTX (E) and CTX + DBD (F) groups (scale bar, 50 μm). Data are expressed as mean ± SD. (C, control group) n = 6, (CTX, CTX-treated group) n = 12, (CTX + DBD, CTX and DBD-treated group) n = 12. *p < 0.05, **p < 0.01, ***p < 0.001.
β-OHB levels in the circulatory system and hematopoietic microenvironment
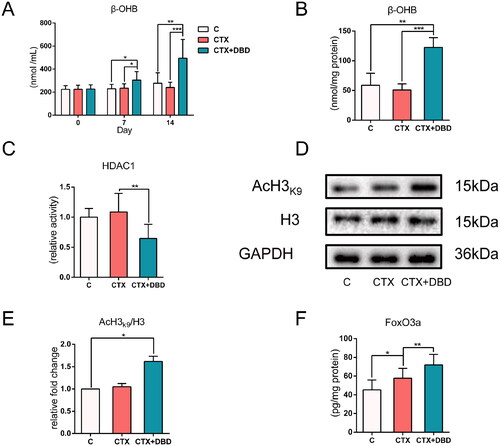
β-OHB in rat blood samples (Days 0, 7 and 14) and marrow supernatant samples (day 14) were analyzed to assess β-OHB metabolism in the circulatory system and hematopoietic microenvironment, respectively. Compared with the other two groups, β-OHB levels in the circulatory system of the rats in the CTX + DBD group differed significantly after oral administration of DBD for seven days; on Day 14, blood and marrow supernatant β-OHB levels were also significantly increased (). The results demonstrated that DBD could influence β-OHB metabolism.
Figure 3. β-OHB levels and HDAC1 activity. (A) β-OHB levels in blood samples on Days 0, 7 and 14. (B) β-OHB levels in marrow supernatant samples on Day 14. (C) HDAC1 relative activity in hematopoietic microenvironment on Day 14 (compared with C group). (D) Representative Western blot results of H3 and AcH3K9. (E) Acetylation ratio of AcH3K9 (data were normalized to C group). (F) FoxO3a expression levels in hematopoietic microenvironment on Day 14. Data are expressed as mean ± SD. (C, control group) n = 6, (CTX, CTX-treated group) n = 12, (CTX + DBD, CTX and DBD-treated group) n = 12. *p < 0.05, **p < 0.01, ***p < 0.001.
Changes in HDAC1 activity
To further explore the biological functions of β-OHB in the hematopoietic microenvironment, the relative activity of HDAC1 in the thigh bone marrow samples was detected (). Acetylated histone H3 lysine 9 (AcH3K9) has been used to measure histone acetylation levels and HDAC1 activity (Shimazu et al. Citation2013; Ji et al. Citation2022). AcH3k9 in the purified histone H3 was tested using Western blot (). HDAC1 can inhibit the expression of the antioxidant transcription factor FoxO3a. The level of FoxO3a can also represent HDAC1 activity to some extent (). On Day 14, HDAC1 relative activity, acetylation ratio of AcH3k9 and FoxO3a expression in the CTX + DBD group were significantly changed, suggesting that HDAC1 activity in hematopoietic microenvironment was inhibited after oral administration of DBD.
Oxidative stress indices in the circulatory system and hematopoietic microenvironment
T-AOC, SOD, MDA, 4-HNE and AOPP are the biomarkers for oxidative stress measurement (Marrocco et al. Citation2017; Sanchez-Rodriguez and Mendoza-Nunez Citation2019). These oxidative stress indices in rat blood (Days 0, 7 and 14) and thigh bone marrow samples (Day 14) were estimated to assess the oxidative stress levels in the circulatory system and hematopoietic microenvironment, respectively. The results revealed significant differences between the C, CTX and CTX + DBD groups on Day 14 in the circulatory system () and hematopoietic microenvironment (), which confirmed that MAC-induced oxidative stress damage could be alleviated by DBD.
Figure 4. Levels of oxidative stress indices in rat blood samples on Days 0, 7 and 14. (A) T-AOC. (B) SOD. (C) MDA. (D) 4-HNE. (E) AOPP. Data are expressed as mean ± SD. (C, control group) n = 6, (CTX, CTX-treated group) n = 12, (CTX + DBD, CTX and DBD-treated group) n = 12. *p < 0.05, **p < 0.01, ***p < 0.001.
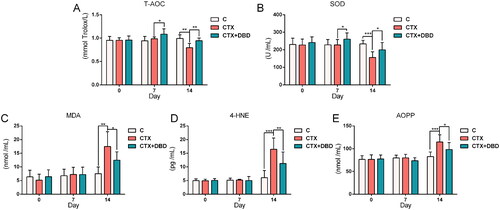
Figure 5. Levels of oxidative stress indices in rat thigh bone marrow samples on day 14. (A) T-AOC. (B) SOD. (C) MDA. (D) 4-HNE. (E) AOPP. Data are expressed as mean ± SD. (C, control group) n = 6, (CTX, CTX = treated group) n = 12, (CTX + DBD, CTX and DBD-treated group) n = 12. *p < 0.05, **p < 0.01, ***p < 0.001.
Biological function verification of β-OHB in vitro
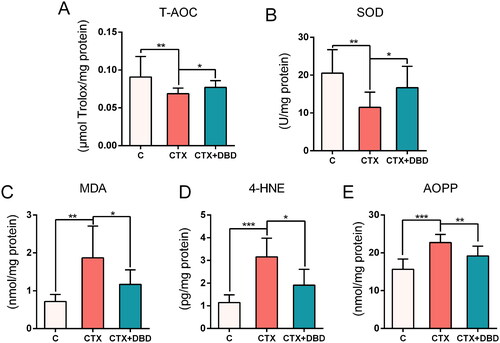
Pearson correlation results revealed that β-OHB levels had high negative correlations to HDAC1 activity (P = −0.779), oxidative stress (P = −0.852) and myelosuppression (P = −0.769). To verify that β-OHB can suppress hematopoietic microenvironment oxidative stress induced by CTX, we performed in vitro experiments using the hBMSC cell lines. After administration of CTX (40 μM) in the culture medium, cell migration () and proliferation () were suppressed owing to the drug toxicity. Meanwhile, the oxidative stress damage was significantly enhanced by CTX (). However, increasing the concentration of β-OHB in the culture medium improved the hBMSC cell migration and proliferation and suppressed the oxidative stress damage dose-dependently.
Figure 6. In vitro verification of the biological function of β-OHB. (A) hBMSC cell wound healing test (scale bar, 100 μm). (B) The relative migration rate of cells calculated by wound healing test, n = 5 per group. (C) The relative survival rate of cells, n = 18 per group. (D) MDA levels in hBMSC cell, n = 8 per group. (E) AOPP levels in hBMSC cell, n = 8 per group. Data are expressed as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001.
Biological function verification of β-OHB in vivo
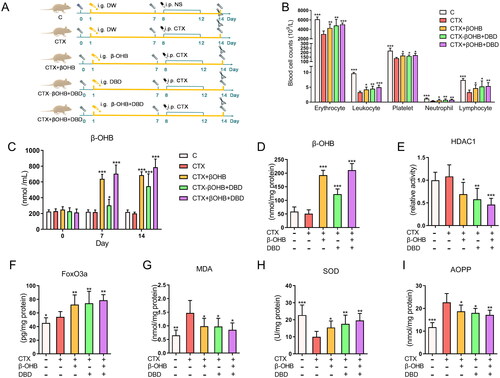
Another independent experiment in vivo was performed to further validate the biological function of β-OHB in suppressing oxidative stress and alleviating hematopoiesis dysfunction induced by CTX. β-OHB was administrated orally directly to simulate the effect of DBD in regulating β-OHB metabolism. The process of sample collection is shown in . Blood cell counts indicated that β-OHB could relieve hematopoiesis dysfunction effectively (). The baseline blood cell counts (Days 0 and 7) were also compared and did not show significant differences. The level of β-OHB in the blood of rats in the CTX + βOHB group increased nearly three times compared with that in rats in the C and CTX groups, after administration of β-OHB for seven days. On Day 14, β-OHB in blood and marrow supernatant samples of the CTX + βOHB group maintained a higher level () than that in the C and CTX groups. Meanwhile, compared with the CTX − βOHB + DBD group, HDAC1 relative activity, FoxO3a expression, MDA, SOD and AOPP levels in marrow supernatant samples on Day 14 reached similar levels in the CTX + βOHB group (). These findings indicated that β-OHB inhibited HDAC1 activity, suppressed oxidative stress and alleviated hematopoiesis dysfunction in the CTX-induced MAC rat model.
Figure 7. In vivo verification of the biological function of β-OHB. (A) Experimental protocols and sample collection strategy. (B) Blood cell counts on Day 14. (C) β-OHB levels in rat blood samples on Days 0, 7 and 14. (D–I) β-OHB levels, HDAC1 relative activity (compared with C group), FoxO3a expression, MDA levels, SOD activity and AOPP levels in hematopoietic microenvironment on Day 14. Data are expressed as mean ± SD. (C, control group) n = 6, (CTX, CTX treated group) n = 8, (CTX + βOHB, CTX and β-OHB-treated group) n = 8, (CTX − βOHB + DBD, CTX and DBD-treated group) n = 8, (CTX + βOHB + DBD, CTX, β-OHB and DBD-treated group) n = 8. Compared with the CTX group, *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
MAC, induced by chemotherapeutic drugs, is one of the most common adverse side effects that can lead to dysfunctional immune system (Hiam-Galvez et al. Citation2021). As a classic TCM formula, DBD is widely used as a complementary medicine to alleviate the side effects of MAC (Liu, Chang et al. Citation2021). In this study, we explored the efficacy of DBD against MAC and unraveled the underlying mechanism of action using an animal MAC model. Here, the MAC model was established by administering CTX to rats; subsequent experiments demonstrated the efficacy of DBD in alleviating the CTX-induced MAC in rats. Oxidative stress is a major etiological factor of MAC (Wei and Frenette Citation2018). Studies have demonstrated the efficacy of TCM mediated via the pharmacodynamics mechanisms by intervening endogenous metabolites (Yan et al. Citation2015). As an endogenous ketone metabolite, β-OHB inhibits HDAC1, promotes FoxO3a expression and suppresses oxidative stress (Shimazu et al. Citation2013). Our study demonstrated that the alleviating effects of DBD were mediated via the regulation of the β-OHB metabolism and oxidative stress homeostasis.
The hematopoietic microenvironment consists of bone marrow stromal cells, HSCs, cytokines, cell metabolites and extracellular matrix (Silva et al. Citation2021). The hematopoietic microenvironment is important for hematopoietic function recovery and HSCs regeneration (Liu et al. Citation2014). Therefore, it is necessary to consider the hematopoietic microenvironment as a critical research object, focusing on the dynamic changes in both the circulatory system and the hematopoietic microenvironment. To test this hypothesis, we developed a CTX-induced MAC rat model to evaluate the effect of DBD. CTX is a traditional chemotherapeutic drug and has been used to establish a MAC animal model (Zhang et al. Citation2022). In this study, rats were treated with CTX (i.p.) with 30 mg/kg/day for five consecutive days to establish the MAC model. With this method, rat blood cell counts kept dropping after CTX administration and fell to the lowest on Day 14. However, currently, no generally accepted method for clinical MAC treatment has been reported (Hou et al. Citation2017; Yang et al. Citation2021); therefore, we could not find an appropriate positive control drug for animal experiment model design. According to our results, ketogenic substances may be used as positive control drugs in follow-up studies.
Dosages of drugs can result in different pharmacological activities. In this study, preliminary experiments were conducted by administrating different dosages of DBD based on the human equivalent dose to a MAC rat model to confirm the oral dosage of DBD. The clinical dosage of DBD for humans is 1 g/kg, equivalent to 6.3 g/kg in rats (Guo et al. Citation2021). Therefore, we chose 6 g/kg as the medium dose, half of the clinical dosage (3 g/kg) as the low dose and a higher dosage (10 g/kg) as the high dose. The DBD oral dosage at 6 g/kg/day or 10 g/kg/day showed a significant effect on increased blood cell counts and β-OHB levels (). Based on these results, we selected 6 g/kg/day as the oral dosage of DBD in the present study.
Figure 8. Blood cell counts and β-OHB levels in DBD oral dosage experiments. (A) Erythrocyte counts, (B) Leukocyte counts, (C) Platelet counts, (D) Neutrophil counts, (E) Lymphocyte counts, and (F) β-OHB levels in blood samples on Days 0, 7 and 14. (G) β-OHB levels in marrow supernatant samples on Day 14. Data are expressed as mean ± SD. (C, control group) n = 6, (CTX, CTX-treated group) n = 6, (CTX + L, CTX and low dose DBD-treated group) n = 6, (CTX + M, CTX and medium dose DBD-treated group) n = 6, (CTX + H, CTX and high dose DBD-treated group) n = 6. Compared with the CTX group, *p < 0.05, ** p < 0.01.
β-OHB is the major component of the ketone body and can be oxidize to provide energy for organism. Besides, β-OHB also plays a role in immune regulation (Møller Citation2020). These functions of β-OHB are in accord with the theory of traditional Chinese medicine (nourishing ‘Qi’ and enriching ‘Blood’). After administration of DBD for two weeks, β-OHB levels in the blood and marrow supernatant samples revealed a significant increase, which proved that DBD could influence β-OHB metabolism. Moreover, on Day 7 (before CTX administration), the β-OHB level was significantly increased in the blood samples of the CTX + DBD group compared with that in other groups. Similar phenomena were also detected for some oxidative stress indices, which further suggest that the protective effects of DBD on MAC are associated with β-OHB metabolism and oxidative stress.
Histone deacetylation is one of the epigenetic modifications of histones and plays an important role in cell differentiation (Kim et al. Citation2020). Among histone deacetylases, HDAC1 can be inhibited by β-OHB, resulting in a higher level of FoxO3a (Ji et al. Citation2022). HDAC1 and FoxO3a can influence medullary hematopoiesis function and have high clinical application value (Ito and Suda Citation2014; Kim et al. Citation2020). Our results indicated that after taking DBD orally, HDAC1 activity was inhibited, and the FoxO3a level was increased, suggesting DBD has efficacy on histone deacetylation.
As progenitor cells, HSCs can differentiate into erythrocytes, platelets and immune cells in the hematologic system. The senescence of HSCs induced by chemotherapeutic drugs plays a crucial role in myelosuppression (Wei and Frenette Citation2018). A low level of oxidative stress in the hematopoietic microenvironment is conducive to the self-regenerative capability of HSCs. Conversely, a higher level of oxidative stress can inhibit regeneration and further induce senescence of HSCs (Shao et al. Citation2013). To evaluate oxidative stress levels, T-AOC, SOD, MDA, 4-HNE and AOPP in the circulatory system and hematopoietic microenvironment were tested. Of these indices, MDA and 4-HNE are the most investigated end product of lipid peroxidation (Sousa et al. Citation2017). 4-HNE is also a signal molecule for inflammation and indicates inflammatory responses associated with oxidative stress (Castro et al. Citation2017). AOPP, the oxidation product of proteins with chlorinated oxidants, is a biomarker of protein oxidative damage (Marrocco et al. Citation2017). AOPP also acts as a mediator of the inflammation process (Cristani et al. Citation2016). SOD is a major antioxidant enzyme that can scavenge free radicals and reduce oxidative stress levels (Serafini et al. Citation2006). T-AOC represents the total nonenzymatic antioxidant capacity. As these indices representing almost all types of oxidative stress biomarkers (Sanchez-Rodriguez and Mendoza-Nunez Citation2019) were evaluated in this study, the results could represent the effects of DBD on hematopoietic microenvironment oxidative stress levels from multiple aspects.
Pearson correlation is used to assess a possible association between two continuous variables (Muhaidat et al. Citation2022). In this study, Pearson correlations indicated that the pharmacodynamic effects of DBD could correlate with the biological function of β-OHB by inhibiting HDAC1 activity and resisting oxidative stress. However, we did not find a direct correlation between HDAC1 activity and FoxO3a level (P = −0.294). It has been reported that besides oxidative stress, inflammation also influences the expression of FoxO3a (Lin et al. Citation2004; Lee et al. Citation2013). The histological examination and biochemical indices evaluated in this study revealed the occurrence of inflammatory response together with myelosuppression. Based on these results, we speculated that FoxO3a level could be regulated by both HDAC1 and other inflammation-related pathways.
To further validate that the suppressing oxidative stress function of β-OHB plays a role in the mechanisms of DBD against MAC, we performed in vitro and in vivo verification experiments. In vitro experiments revealed that increasing the concentration of β-OHB in the culture medium could suppress CTX-induced oxidative stress damage in hBMSC cells. However, considering that blood cell counts are still the gold standard for MAC diagnosis and grading (Brigle et al. Citation2017), we chose in vivo rat model to further validate the biological function of β-OHB against MAC. The results demonstrated the HDAC1 activity inhibiting roles of β-OHB; it suppressed oxidative stress and alleviated hematopoiesis dysfunction in the MAC rat model. These results suggest that approaches to regulating β-OHB metabolism, such as the administration of DBD, could be applied to prevent and treat several oxidative stress-related diseases.
In our study, β-OHB was administrated directly to simulate the effect of DBD. However, compared with those in the CTX − βOHB + DBD group, rats in the CTX + βOHB group showed a higher β-OHB level, a similar effect on alleviating hematopoiesis dysfunction in the MAC rat model. The CTX + βOHB + DBD group showed the best regulation effect compared with other groups. These results revealed regulation of β-OHB metabolism could be one of the mechanisms of DBD in the prevention and treatment of MAC. Nevertheless, other underlying mechanisms could also be existed, which need further studies to gain better insights.
In summary, we demonstrate that the mechanism of DBD in alleviating CTX-induced myelosuppression is related to the regulation of β-OHB metabolism, HDAC1 activity and suppression of oxidative stress (). However, the complexities of TCM-effective substances and pharmacodynamic mechanisms still deserve further investigation. Our previous studies using systems pharmacology and molecular docking have shown that the active constituents of DBD, such as astragaloside and ferulic acid, could regulate β-OHB levels by influencing metabolic enzymes involved in β-OHB metabolism, such as β-hydroxybutyrate dehydrogenase; however, it still needs to be confirmed. Furthermore, according to TCM theory, DBD has two major drug effects (nourishing ‘Qi’ and enriching ‘Blood’). Therefore, further studies are required to explore the role of these two effects in the pharmacodynamic mechanisms of DBD. Our study also revealed that DBD could influence inflammation. β-OHB can inhibit inflammatory response by NLRP3 and NF-κB signal pathways (Youm et al. Citation2015; Wu et al. Citation2022). Therefore, investigating the relationship between β-OHB, inflammatory responses and MAC could be another approach to better understanding the precise mechanisms of DBD.
Conclusions
In this study, a CTX-induced MAC rat model was established to investigate the mechanisms of DBD in alleviating myelosuppression. Evaluation of β-OHB levels, HDAC1 activity and oxidative stress indices in the circulatory system and hematopoietic microenvironment revealed the alleviating effects of DBD against myelosuppression and in regulating β-OHB metabolism and oxidative stress homeostasis. Furthermore, we validated the biological function of β-OHB in alleviating CTX-induced hematopoiesis dysfunction and oxidative stress in vitro and in vivo. This study demonstrates that regulating β-OHB metabolism and suppressing oxidative stress can be the potential mechanisms of DBD to prevent and treat MAC. Our findings provide a new approach to understanding the pharmacodynamic mechanisms of DBD, suggesting DBD can be applied in the prevention and treatment of several oxidative stress-related diseases.
Author contributions
All authors made substantial contributions to the conception, study design, acquisition, analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to take responsibility and be accountable for the contents of the work.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adeshakin AO, Yan D, Zhang M, Wang L, Adeshakin FO, Liu W, Wan X. 2020. Blockade of myeloid-derived suppressor cell function by valproic acid enhanced anti-PD-L1 tumor immunotherapy. Biochem Biophys Res Commun. 522(3):604–611.
- Brigle K, Pierre A, Finley-Oliver E, Faiman B, Tariman J, Miceli T. 2017. Myelosuppression, bone disease, and acute renal failure: evidence-based recommendations for oncologic emergencies. Clin J Oncol Nurs. 21(5 Suppl):60–76.
- Castro JP, Jung T, Grune T, Siems W. 2017. 4-Hydroxynonenal (HNE) modified proteins in metabolic diseases. Free Radic Biol Med. 111:309–315.
- Cristani M, Speciale A, Saija A, Gangemi S, Minciullo P, Cimino F. 2016. Circulating advanced oxidation protein products as oxidative stress biomarkers and progression mediators in pathological conditions related to inflammation and immune dysregulation. Curr Med Chem. 23(34):3862–3882.
- Gao Y, Li W, Chen J, Wang X, Lv Y, Huang Y, Zhang Z, Xu F. 2019. Pharmacometabolomic prediction of individual differences of gastrointestinal toxicity complicating myelosuppression in rats induced by irinotecan. Acta Pharm Sin B. 9(1):157–166.
- Guo Y, Zhang Y, Hou Y, Guo P, Wang X, Zhang S, Yang P. 2021. Anticonstriction effect of MCA in rats by Danggui Buxue Decoction. Front Pharmacol. 12:749915.
- He M, Wang N, Zheng W, Cai X, Qi D, Zhang Y, Han C. 2021. Ameliorative effects of ginsenosides on myelosuppression induced by chemotherapy or radiotherapy. J Ethnopharmacol. 268:113581.
- Hiam-Galvez KJ, Allen BM, Spitzer MH. 2021. Systemic immunity in cancer. Nat Rev Cancer. 21(6):345–359.
- Hou B, Liu R, Qin Z, Luo D, Wang Q, Huang S. 2017. Oral Chinese herbal medicine as an adjuvant treatment for chemotherapy, or radiotherapy, induced myelosuppression: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2017:3432750.
- Ito K, Suda T. 2014. Metabolic requirements for the maintenance of self-renewing stem cells. Nat Rev Mol Cell Biol. 15(4):243–256.
- Ji L, He Q, Liu Y, Deng Y, Xie M, Luo K, Cai X, Zuo Y, Wu W, Li Q, et al. 2022. Ketone body beta-hydroxybutyrate prevents myocardial oxidative stress in septic cardiomyopathy. Oxid Med Cell Longev. 2022:2513837.
- Kim MY, Yan B, Huang S, Qiu Y. 2020. Regulating the regulators: the role of histone deacetylase 1 (HDAC1) in erythropoiesis. IJMS. 21(22):8460.
- Lee JC, Espeli M, Anderson CA, Linterman MA, Pocock JM, Williams NJ, Roberts R, Viatte S, Fu B, Peshu N, et al. 2013. Human SNP links differential outcomes in inflammatory and infectious disease to a FOXO3-regulated pathway. Cell. 155(1):57–69.
- Lin L, Hron JD, Peng SL. 2004. Regulation of NF-kappaB, th activation, and autoinflammation by the forkhead transcription factor foxo3a. Immunity. 21(2):203–213.
- Liu H, Yang H, Qin Z, Chen Y, Yu H, Li W, Zhu X, Cai J, Chen J, Zhang M. 2021. Exploration of the Danggui Buxue Decoction mechanism regulating the balance of ESR and AR in the TP53-AKT signaling pathway in the prevention and treatment of POF. Evid Based Complement Alternat Med. 2021:4862164.
- Liu M, Tan H, Zhang X, Liu Z, Cheng Y, Wang D, Wang F. 2014. Hematopoietic effects and mechanisms of Fufang Ejiao Jiang on radiotherapy and chemotherapy-induced myelosuppressed mice. J Ethnopharmacol. 152(3):575–584.
- Liu Y, Chang M, Hu Z, Xu X, Wu W, Ning M, Hang T, Song M. 2021. Danggui Buxue Decoction enhances the anticancer activity of gemcitabine and alleviates gemcitabine-induced myelosuppression. J Ethnopharmacol. 273:113965.
- Marrocco I, Altieri F, Peluso I. 2017. Measurement and clinical significance of biomarkers of oxidative stress in humans. Oxid Med Cell Longev. 2017:6501046.
- Møller N. 2020. Ketone body, 3-hydroxybutyrate: minor metabolite - major medical manifestations. J Clin Endocr Metab. 105(9):2884–2892.
- Muhaidat J, Albatayneh A, Abdallah R, Papamichael I, Chatziparaskeva G. 2022. Predicting COVID-19 future trends for different European countries using Pearson correlation. EuroMediterr J Environ Integr. 7(2):157–170.
- Mukaka M. 2012. Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 24(3):69–71.
- Pich O, Muinos F, Lolkema MP, Steeghs N, Gonzalez-Perez A, Lopez-Bigas N. 2019. The mutational footprints of cancer therapies. Nat Genet. 51(12):1732–1740.
- Qiu H, Cao S, Xu R. 2021. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun (Lond). 41(10):1037–1048.
- Rosborough BR, Castellaneta A, Natarajan S, Thomson AW, Turnquist HR. 2012. Histone deacetylase inhibition facilitates GM-CSF-mediated expansion of myeloid-derived suppressor cells in vitro and in vivo. J Leukoc Biol. 91(5):701–709.
- Rtibi K, Selmi S, Grami D, Sebai H, Amri M, Marzouki L. 2017. Irinotecan chemotherapy-induced intestinal oxidative stress: underlying causes of disturbed mucosal water and electrolyte transport. Pathophysiology. 24(4):275–279.
- Samadi M, Aziz SG, Naderi R. 2021. The effect of tropisetron on oxidative stress, SIRT1, FOXO3a, and claudin-1 in the renal tissue of STZ-induced diabetic rats. Cell Stress Chaperones. 26(1):217–227.
- Sanchez-Rodriguez MA, Mendoza-Nunez VM. 2019. Oxidative stress indexes for diagnosis of health or disease in humans. Oxid Med Cell Longev. 2019:1–32.
- Serafini M, Villano D, Spera G, Pellegrini N. 2006. Redox molecules and cancer prevention: the importance of understanding the role of the antioxidant network. Nutr Cancer. 56(2):232–240.
- Shao L, Wang Y, Chang J, Luo Y, Meng A, Zhou D. 2013. Hematopoietic stem cell senescence and cancer therapy-induced long-term bone marrow injury. Transl Cancer Res. 2:397–411.
- Shi X-Q, Zhu Z-H, Yue S-J, Tang Y-P, Chen Y-Y, Pu Z-J, Tao H-J, Zhou G-S, Yang Y, Guo M-J, et al. 2020. Integration of organ metabolomics and proteomics in exploring the blood enriching mechanism of Danggui Buxue Decoction in hemorrhagic anemia rats. J Ethnopharmacol. 261:113000.,.
- Shimazu T, Hirschey M, Newman J, He W, Shirakawa K, Le Moan N, Grueter C, Lim H, Saunders L, Stevens R, et al. 2013. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 339(6116):211–214.
- Silva WN, Costa AC, Picoli CC, Rocha BGS, Santos GSP, Costa PAC, Azimnasab-Sorkhabi P, Soltani-Asl M, da Silva RA, Amorim JH, et al. 2021. Hematopoietic stem cell stretches and moves in its bone marrow niche. Crit Rev Oncol Hematol. 163:103368.
- Sousa BC, Pitt AR, Spickett CM. 2017. Chemistry and analysis of HNE and other prominent carbonyl-containing lipid oxidation compounds. Free Radic Biol Med. 111:294–308.
- Wei Q, Frenette PS. 2018. Niches for hematopoietic stem cells and their progeny. Immunity. 48(4):632–648.
- Wishart DS. 2016. Emerging applications of metabolomics in drug discovery and precision medicine. Nat Rev Drug Discov. 15(7):473–484.
- Wishart DS. 2019. Metabolomics for investigating physiological and pathophysiological processes. Physiol Rev. 99(4):1819–1875.
- Wu Y, Teng Y, Zhang C, Pan Y, Zhang Q, Zhu X, Liu N, Su X, Lin J. 2022. The ketone body beta-hydroxybutyrate alleviates CoCrMo alloy particles induced osteolysis by regulating NLRP3 inflammasome and osteoclast differentiation. J Nanobiotechnology. 20(1):120.
- Yan S, Liu R, Jin H, Liu X, Ye J, Shan L, Zhang W. 2015. “Omics” in pharmaceutical research: overview, applications, challenges, and future perspectives. Chin J Nat Med. 13(1):3–21.
- Yang J, Zhu X, Yuan P, Liu J, Wang B, Wang G. 2020. Efficacy of traditional Chinese medicine combined with chemotherapy in patients with non-small cell lung cancer (NSCLC): a meta-analysis of randomized clinical trials. Support Care Cancer. 28(8):3571–3579.
- Yang S, Che H, Xiao L, Zhao B, Liu S. 2021. Traditional Chinese medicine on treating myelosuppression after chemotherapy. Medicine. 100(4):e24307.
- Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D'Agostino D, Planavsky N, Lupfer C, Kanneganti TD, et al. 2015. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 21(3):263–269.
- Zhang H, Zhang L, Yang C, Zhang Y, Li J, Zhang X, Chen J, Huang B, Zhao D, Li X, et al. 2022. Prevention effect of protopanaxadiol-type saponins saponins and protopanaxatriol-type saponins on myelosuppression mice induced by cyclophosphamide. Front Pharmacol. 13:845034.