Abstract
Context
Chinese medicinal herbs (CMH) have been considered a potentially efficacious approach for patients with breast cancer that experience adverse effects from endocrine treatment.
Objective
To investigate the impact of CMH on endocrine therapy-induced side effects in patients with hormone receptor-positive (HR+) breast cancer.
Methods
Ten databases (e.g., PubMed, Web of Science, Cochrane Library, China National Knowledge Information Database and other databases) were searched up to 20 May 2022. The search terms included Chinese herb, breast cancer, endocrine therapy, clinical trial and their mesh terms. The study selection and data extraction were performed by two independent reviewers. The risk of bias was evaluated using the Cochrane risk of bias method.
Results
A total of 31 studies with 2288 patients were included. There were significant improvements in bone mineral density (BMD) [lumbar BMD (MD 0.08, 95% CI 0.07 to 0.09, p < 0.00001) and femoral neck BMD (MD 0.08, 95% CI 0.07 to 0.10, p < 0.00001)] and bone gal protein (BGP) (MD 0.24, 95% CI 0.17 to 0.31, p < 0.00001), with a significant reduction in triglycerides (MD −0.53, 95% CI −1.00 to −0.07, p < 0.05) and no effect on estradiol levels (MD 0.90, 95% CI −0.31 to 2.12, p = 0.15).
Conclusions
CMH combined with complementary therapy can moderately reduce endocrine therapy-induced side effects, including bone loss and dyslipidemia in patients with HR + breast cancer, revealing the potential role of CMH in treating (HR+) breast cancer. More high-quality RCTs are warranted to further validate the effectiveness and safety of CMH.
Keywords:
Introduction
Breast cancer is the primary cause of cancer-related deaths among females. According to the Global Cancer Statistics 2020, breast cancer now exceeds lung malignancy as the most widely identified cancer in females, with an anticipated 2.3 million new cases (11.7%) and 0.69 million new deaths (6.9%) (Sung et al. Citation2021). In the United States, over 250,000 breast cancers are diagnosed annually (Siegel et al. Citation2020), and nearly 80% of breast cancers are hormone receptor-positive (HR+) (DeSantis et al. Citation2019). Most of these patients are dependent on endocrine therapy.
Endocrine therapies, such as aromatase inhibitors and tamoxifen, have been used as first-line therapy for patients with HR+ breast cancer (Johnston et al. Citation2009; Aggelis and Johnston Citation2019). Continuing tamoxifen for 10 years rather than 5 years has been reported to lead to further reductions in recurrence and mortality rates, especially after 10 years (Davies et al. Citation2013). Although endocrine therapy has been shown to benefit survival in patients with breast cancer, long-term adherence to endocrine therapy remains a major concern, partly due to the side effects of treatment, such as dyslipidemia, hot flashes, vaginal dryness, and musculoskeletal symptoms, which affect one’s quality of life, social function, and adherence to treatment (Condorelli and Vaz-Luis Citation2018; Lee et al. Citation2020). Consequently, identifying new therapeutic methods to mitigate the adverse effects of endocrine therapy is critical.
Traditional Chinese medicine (TCM) is commonly used in breast cancer therapy, and its use dates back more than 2000 years (Sun Citation2014). Indeed, 86.4% of patients with breast cancer in China are treated with TCM, such as Chinese medicinal herbs (CMH) (Cui et al. Citation2004; Chen et al. Citation2008). It is worth noting that the percentage of Western nations using TCM is growing, with 9–69% of patients in these counties using TCM (Ernst Citation2000; Harris and Rees Citation2000). According to study statistics, approximately 65% in Australia and 33.1% in Taiwan of patients with cancer use one form or another of complementary and alternative therapies (CAT), with up to 36% of CAT users opting for TCM (Cooke et al. Citation2012; Porter et al. Citation2017). Survivors of breast cancer constitute the largest user population. CMH is the most common TCM treatment (Molassiotis et al. Citation2005; Boon et al. Citation2007; Cooke et al. Citation2012; Porter et al. Citation2017; Kuo et al. Citation2018). In addition, 25–47% of patients with cancer in North America use herbal remedies to support their therapy (Yin et al. Citation2013). TCM usage, particularly CMH, is believed to reduce the toxic side effects of cancer treatment, such as relieving hot flashes and bone loss induced by endocrine therapy (Li et al. Citation2016; Huang et al. Citation2017), improving immune function (Wong et al. Citation2005), increasing general well-being and quality of life (Porter et al. Citation2017), reducing metastasis and recurrence, and prolonging life expectancy (Jiang et al. Citation2021).
CMH has been suggested as a potentially useful treatment for patients with breast cancer undergoing endocrine therapy. Consequently, the current study sought to perform a meta-analysis and comprehensive systematic review to assess the efficacy of CMH as an adjunctive therapy to minimize the negative impacts of endocrine therapy in breast cancer treatment.
Methods
Design
Detailed findings were presented using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. The PROSPERO registration number for this systematic review is CRD42021262536.
Search strategy
The main electronic databases, including PubMed, Embase, Cochrane Library, Web of Science, clinicaltrials.gov, Ovid, EBSCO, China National Knowledge Information Database (CNKI), Wang Fang, and Chinese Science and Technology Database (CSTJ), were searched using the following search keywords: (Chinese herb OR Chinese herbal medicine OR herb medicine OR Chinese herb therapy OR herbal remedy OR herb therapy) AND (breast neoplasms OR breast cancer OR breast lesions) AND (endocrine therapy) AND (clinical trial OR randomized controlled trial OR randomized controlled trial), with minor modifications based on the specifics of each database search. Only original research publications with a publication date before 20 May 2022 were sourced, and only articles in English and Chinese were considered.
PICOS
Types of studies
Randomized clinical trials (RCTs) were included if they were of patients with breast cancer receiving endocrine therapy.
Participants
All participants were females with HR+ (progesterone receptor and/or estrogen receptor-positive) breast cancer, regardless of cancer stage, age, or race. Patients who did not receive Chinese herbal treatment were excluded from the study.
Interventions and control
CMH (single or compound) was used as the intervention. There were no limitations to the dosage form, dosage, or course of treatment. CMH can be administered before, during, or after endocrine therapy, whether injected or administered orally. This review included studies on CMH versus placebo, CMH in combination with Western drugs versus Western drugs only, or CMH alone versus Western drugs to reduce the side effects of endocrine therapy.
Outcomes
Outcome data were classified as (1) cancer treatment-induced bone loss, including bone mineral density (BMD), beta cross-laps (β-CTX), bone gal protein (BGP), and bone-specific alkaline phosphatase (BALP). (2) Menopausal symptoms, including luteinizing hormone (LH), follicle-stimulating hormone (FSH), Kupperman score, estradiol (E2), and endometrial thickening. (3) Dyslipidemia, including total high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglyceride (TG), and cholesterol (TC) levels.
Exclusion criteria
Studies were excluded if they did not include a mean value or standard deviation (SD), if the full text was unavailable, or if neither could be obtained despite repeated attempts to contact the authors. In addition, studies with inadequate methodological quality (Jadad score <3) were excluded.
Study selection
Two reviewers separately examined the titles and abstracts of all studies. Based on the specified inclusion criteria, the entire text matching the inclusion criteria (according to the screening of titles and abstracts) was collected, followed by the retrieval of possibly related references. A consensus was established, or a third investigator was consulted as necessary.
Data extraction
The following data were extracted: general information (publication year, first author’s name, and title of study), participant characteristics (sex, age, disease stage, and sample size), intervention characteristics (description of the intervention and control groups, dosage of Western medicine and CMH, treatment duration and frequency), outcome measures (mean, SD, significance level, and units of measurement), and Jadad scale.
Methodological quality assessment
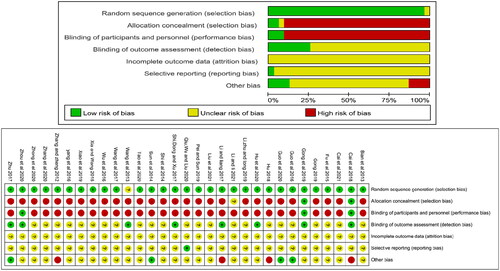
The risk of bias for the included studies was assessed based on the Cochrane Handbook for Systematic Reviews evaluation criteria in the following six domains: performance bias (blinding of personnel and participants), selection bias (concealment of the random sequence generation and allocation), attrition bias (incomplete outcome data), detection bias (blinding of outcome evaluation), reporting bias (reporting selectively), and other biases. Each type of bias was categorized as high, low, or unclear. For the generation of random sequencing, low risk means that the researcher has specified how the random sequence was generated, such as random numbers generated by a computer or randomized digital table. In contrast, there is a high risk if random sequence generation contains no random component. For allocation concealment, a low risk refers to the inability of the subject or investigator to predict the assignment, such as using a central randomization system or an ordered, opaque envelope. For performance bias, low risk conditions were considered if the outcome was not affected by a lack of blinding of participants. Likewise, there is a minimal risk of detection bias when the evaluators are blinded or blinding is not implemented but does not affect the results. Low risk conditions were judged when no missing data or missing data did not affect the outcome or the correct way to count the lost data. Selective bias: low risk means that there is an available study protocol, or there is no study protocol, and all expected outcomes have been reported, whereas a high risk means that significant results are not reported or incomplete. Two authors independently determined whether each area had low, high, or unclear bias. When there was disagreement between the two reviewers, a third reviewer was consulted to help reach a decision.
Statistical analyses
The Cochrane Collaboration Review Manager Software (RevMan 5.3.0) was used for the data analysis. For all included studies, all outcome data were presented as the mean ± SD, and the rate of adverse reactions was reported as dichotomous data. To evaluate the heterogeneity, I2 values were determined. If I2 was ≤25%, we used a fixed-effects model; otherwise, if I2 was > 25%, we adopted a random-effects model. In this study, breast cancer subgroups were determined based on TCM syndrome differentiation principles, which primarily involve two factors: strengthening the body (+), Bu Shen, or a combination of strengthening and removing pathological products (±), Bu Shen shu gan. Funnel plots were used to assess the possibility of publication bias.
Results
Characteristics of included studies
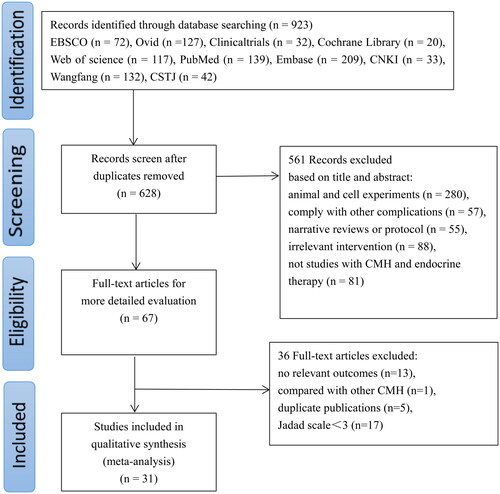
Database searches identified 923 studies, of which 628 records remained after removing duplicates. Furthermore, 561 articles were excluded after their titles and abstracts were reviewed because they involved animal experiments, combined other complications, were narrative reviews or protocols and were not clinical studies related to CMH and endocrine therapy. After reading the full texts of 67 papers, 13 studies with no relevant outcomes, one study that compared different CMH, five duplicate studies, and 17 trials with a Jadad score (less than three points) indicating poor methodological quality were excluded. Therefore, 31 studies (Zhang and Zheng Citation2012; Bian et al. Citation2013; Wang et al. Citation2013; Shi et al. Citation2014; Sun et al. Citation2014; Fu et al. Citation2015; Xia and Wang Citation2015; Guo et al. Citation2016; Wu et al. Citation2016; Yang et al. Citation2016; Li and Liang Citation2017; Shi et al. Citation2017; Wang et al. Citation2017; Zhu Citation2017; Cai et al. Citation2018; Hu Citation2018; Gong Citation2019; Gong et al. Citation2019; Li et al. Citation2019; Pei and Sun Citation2019; Xiao et al. Citation2019; Guo et al. Citation2020; Hu et al. Citation2020; Qiu et al. Citation2020; Tao et al. Citation2020; Zhang et al. Citation2020; Zhong et al. Citation2020; Zhou et al. Citation2020; Cai et al. Citation2021; Li and Li Citation2021; Liu et al. Citation2021) were included in the meta-analysis. shows a flowchart of the article search procedure.
Figure 1. Flowchart of the article-searching procedure. CSTJ: Chinese Science and Technology Database; CNKI: China National Knowledge Information database, CMH: Chinese medicinal herbs.
The included studies involved 2288 patients, including 1117 patients in the control group and 1130 patients in the CMH group, with 41 patient dropouts. All included patients were pathologically diagnosed with HR(+) breast cancer. displays the baseline characteristics of the included studies. And displays Chinese herbal components of the included studies.
Table 1. Characteristics of the included studies.
Table 2. Chinese herbal components of the included studies.
Risk of bias within studies
As shown in , all 31 studies reported randomization; however, one study did not report the randomization method (Wang et al. Citation2013). Two studies (Cai et al. Citation2018; Gong et al. Citation2019) reported allocation concealment, two studies (Cai et al. Citation2018; Gong et al. Citation2019) reported double-blinding, and one (Zhou et al. Citation2020) reported blinding using placebos as controls. The performance bias of these three studies was assessed as low risk. The detection bias of eight studies (Wang et al. Citation2013; Li and Liang Citation2017; Shi et al. Citation2017; Zhu Citation2017; Cai et al. Citation2018; Gong et al. Citation2019; Hu et al. Citation2020; Zhou et al. Citation2020) was assessed as low risk for using objective measures, while the remaining 23 studies were judged as unclear. Regarding reporting bias, one study (Qiu et al. Citation2020) reported negative and positive results and was judged low risk, while the rest were considered unclear. The attrition bias of all studies was unclear.
Endocrine therapy-induced bone loss
Bone mineral density (BMD)
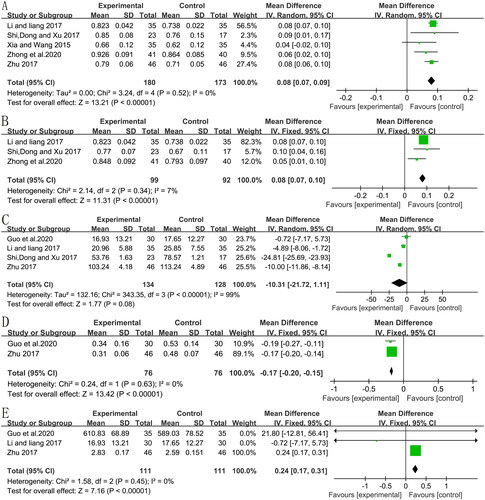
Five RCTs (Xia and Wang Citation2015; Li and Liang Citation2017; Shi et al. Citation2017; Zhu Citation2017; Zhong et al. Citation2020) analyzed the BMD level (five included the lumbar BMD, and three included the femoral neck BMD) and showed a significant pooled mean improvement in the BMD of the lumbar spine (, MD:0.08, 95% CI:0.07–0.09, p < 0.00001, I2 = 0%) and femoral neck (, MD:0.08, 95% CI:0.07–0.10, p < 0.00001, I2 = 7%).
Bone-specific alkaline phosphatase (BALP)
Four RCTs (Li and Liang Citation2017; Shi et al. Citation2017; Zhu Citation2017; Guo et al. Citation2020) including 262 patients (134 in CMH groups and 128 in control groups) showed that CMH decreased the BALP level (MD: −10.31, 95% CI: −21.72 to 1.11, p = 0.08, I2 = 99%) compared to the control group (). The sensitivity analysis showed that the heterogeneity remained high even after removing the studies one by one.
Beta cross-laps (B-CTX)
In two studies (Zhu Citation2017; Guo et al. Citation2020) that compared CMH to a control group, the amount of B-CTX was significantly reduced by CMH (, MD = −0.17; 95% CI: −0.20 to −0.15, p<0.00001, I2 = 0%).
Bone gal protein (BGP)
Three studies (Li and Liang Citation2017; Zhu Citation2017; Guo et al. Citation2020) involving 222 subjects suggested that CMH resulted in significant improvements in BGP compared to the control group (, MD = 0.24; 95% CI: 0.17–0.31, p < 0.00001, I2 = 0%).
Menopausal symptoms
Estradiol (E2)
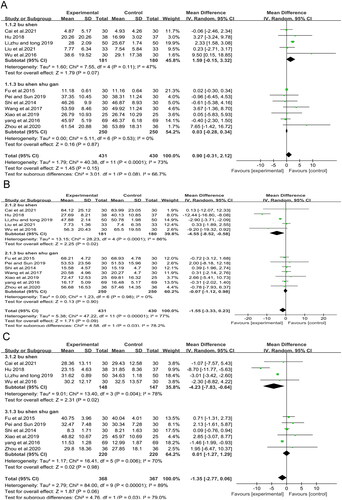
The level of E2 was also assessed in 12 studies (Shi et al. Citation2014; Fu et al. Citation2015; Wu et al. Citation2016; Yang et al. Citation2016; Wang et al. Citation2017; Hu Citation2018; Li et al. Citation2019; Pei and Sun Citation2019; Xiao et al. Citation2019; Zhou et al. Citation2020; Cai et al. Citation2021; Liu et al. Citation2021) but CMH had no significant effect (MD = 0.90; 95% CI: −0.31 to 2.12, p = 0.15, I2 = 73%). Subgroup analysis also showed no significant effect in either the Bu Shen or Bu Shen shu gan subgroups (, MD = 1.59; 95% CI: −0.15 to 3.32, p = 0.07, I2 = 47%, and MD = 0.03; 95% CI: −0.28 to 0.34, p = 0.87, I2 = 0%, respectively).
Follicle-stimulating hormone (FSH)
Regarding the FSH level, the reduction of FSH in the CMH group was not significantly different from the control group (MD = −1.55; 95% CI: −3.33 to 0.23, p = 0.09, I2 = 77%) in 12 studies (Shi et al. Citation2014; Fu et al. Citation2015; Wu et al. Citation2016; Yang et al. Citation2016; Wang et al. Citation2017; Hu Citation2018; Li et al. Citation2019; Pei and Sun Citation2019; Xiao et al. Citation2019; Zhou et al. Citation2020; Cai et al. Citation2021; Liu et al. Citation2021) that included 861 patients (). Subgroup analysis of Bu Shen shu gan also showed no significant difference (MD = −0.07; 95% CI: −1.12 to 0.98, p = 0.90, I2 = 0%), whereas subgroup analysis of Bu Shen showed a significant decrease in FSH (, MD = −4.55; 95% CI: −8.52 to −0.58, p = 0.02, I2 = 86%).
Luteinizing hormone (LH)
For the LH level, ten studies (Shi et al. Citation2014; Fu et al. Citation2015; Wu et al. Citation2016; Yang et al. Citation2016; Hu Citation2018; Li et al. Citation2019; Pei and Sun Citation2019; Xiao et al. Citation2019; Zhou et al. Citation2020; Cai et al. Citation2021) involving 735 patients showed no statistically significant difference between the CMH and control groups (MD = −1.35; 95% CI: −2.77 to 0.06, p = 0.06, I2 = 89%), whereas subgroup analysis of Bu Shen revealed a significant reduction with Bu Shen CMH (MD = −4.23; 95% CI: −7.83, −0.64, p = 0.02, I2 = 78%) (). Because all subgroups showed high heterogeneity, other factors influenced the heterogeneity.
Endometrial thickening
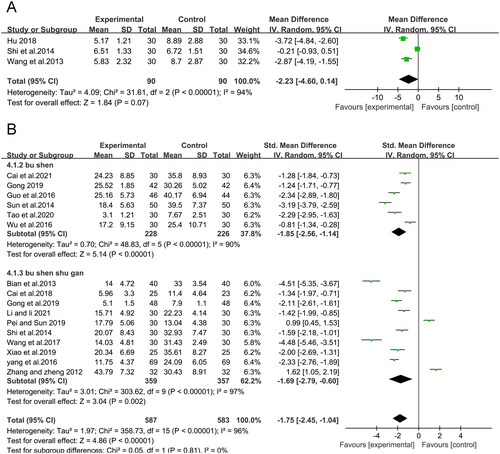
Three RCTs (Wang et al. Citation2013; Shi et al. Citation2014; Hu Citation2018) analyzed endometrial thickening in 180 participants, showing that CMH treatment significantly reduced endometrial thickening (MD = −2.23, 95% CI: −4.60 to 0.14, p = 0.07) with substantive heterogeneity (I2 = 94%) (). The pooled outcome showed low heterogeneity (I2 = 0%, p < 0.00001) with an MD of −3.37 (95% CI −4.22 to −2.51) following sensitivity analysis in which the study of Shi et al. (Citation2014) was excluded.
Kupperman score
shows that the CMH-treated group had significantly lower Kupperman scores than the control group (SMD = −1.75; 95% CI: −2.45 to −1.04, p<0.00001, I2 = 96%) in 16 studies of 1170 patients. All studies adopted the amended Kupperman to calculate the Kupperman score, with 13 studies (Zhang and Zheng Citation2012; Bian et al. Citation2013; Shi et al. Citation2014; Sun et al. Citation2014; Guo et al. Citation2016; Yang et al. Citation2016; Wang et al. Citation2017; Cai et al. Citation2018; Gong Citation2019; Gong et al. Citation2019; Pei and Sun Citation2019; Xiao et al. Citation2019; Li and Li Citation2021) defining the amended Kupperman according to the same criteria (symptom severity multiplied by symptom index, 0–63 points), while the other three (Wu et al. Citation2016; Tao et al. Citation2020; Cai et al. Citation2021) did not specify the grading methods. However, the meta-analysis showed the same results after removing these articles. The subgroup analysis showed that the Kupperman score was also significantly reduced in each subgroup. Notably, each subgroup also showed high heterogeneity, indicating that other factors affected the heterogeneity.
Dyslipidemia
Total cholesterol (TC)
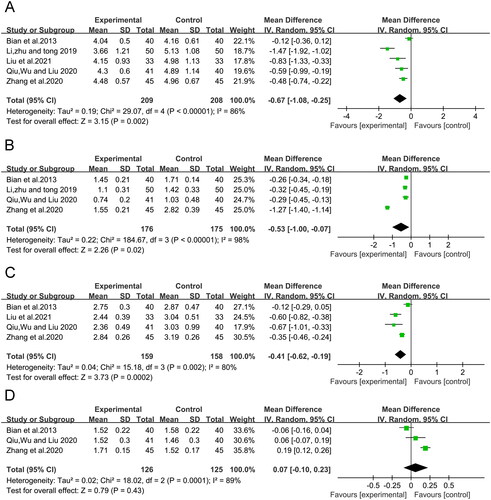
Five RCTs (Bian et al. Citation2013; Li et al. Citation2019; Qiu et al. Citation2020; Zhang et al. Citation2020; Liu et al. Citation2021), including 417 participants, showed a significant reduction in TC (MD = −0.67, 95% CI: −1.08 to −0.25, p = 0.002) in the CMH-treated group compared to the control group. However, the heterogeneity among the studies was I2 = 86% and remained unchanged after the sensitivity analysis ().
Triglycerides (TG)
The pooled results showed that CMH treatment significantly reduced TG compared to the control group (MD = −0.53, 95% CI: −1.00 to −0.07, p < 0.00001). However, a sensitivity analysis was conducted due to the substantial heterogeneity among the studies (I2 = 98%) (). The heterogeneity was significantly reduced (I2 = 0%) when the study by Zhang et al. (Citation2020) was removed.
Low-density lipoprotein (LDL)
Four RCTs (Bian et al. Citation2013; Qiu et al. Citation2020; Zhang et al. Citation2020; Liu et al. Citation2021) with 317 participants showed a significant reduction in LDL (MD = −0.41, 95% CI: −0.62 to −0.19, p = 0.0002) in the CMH-treated group compared to the control group. Again, the heterogeneity among the studies was I2 = 80% and remained unchanged following sensitivity analysis, in which those studies were excluded ().
High-density lipoprotein (HDL)
The meta-analysis also showed no statistically significant difference between the CMH and control groups in improving HDL levels (MD = 0.07, 95% CI: −0.10 to 0.23, p = 0.43, I2 = 89%) ().
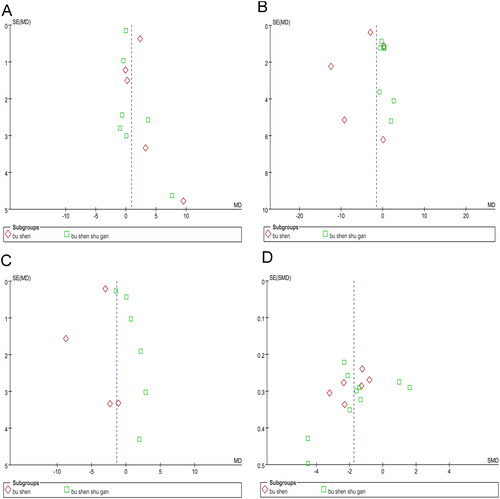
Publication bias
The asymmetric funnel plots in indicate a publication bias for E2, FSH, and LH. The funnel plot in shows an almost symmetrical distribution, indicating no publication bias in the Kupperman score. For the other outcomes, funnel plot analysis was not conducted because of the insufficient number of studies included.
Discussion
CMH has been used clinically to relieve adverse effects experienced by patients with breast cancer. Multiple systematic reviews and meta-analyses have evaluated the efficacy of CMH in patients with breast cancer to prevent chemotherapy-related adverse effects (Zhang et al. Citation2007; Kim et al. Citation2015; Leggett et al. Citation2015; Zhu et al. Citation2016; Zhu Citation2017; Li et al. Citation2020). However, few studies have assessed the effectiveness of CMH treatment on side effects associated with endocrine therapy in HR(+) breast cancer. This study aimed to perform a comprehensive meta-analysis and systematic review to investigate whether CMH can reduce endocrine therapy-induced adverse effects in patients with HR(+) breast cancer.
This meta-analysis revealed a significant alleviation of bone loss (measured by BMD and BGP) and dyslipidemia (measured by TG). However, due to unexplained high heterogeneity and/or the limited number of included studies, accurate conclusions cannot be drawn regarding the effect of CMH on B-CTX, TC, and LDL in patients with HR(+) breast cancer.
Regarding the evaluation of menopausal symptoms, the levels of E2, FSH, LH, endometrial thickening, and Kupperman score were presented as continuous data. Due to the significant heterogeneity, subgroup analyses were conducted based on TCM breast cancer therapy principles, which primarily include two aspects: strengthening the body (Bu Shen) or a combination of strengthening and removing pathological products (Bu Shen shu gan).
Bu Shen CMH is believed to regulate hormones such as E2 and FSH (Cao et al. Citation2021), which may impair the efficacy of endocrine suppressive therapy in patients with HR(+) breast cancer. However, this study showed that increased CMH treatment did not affect E2 levels. Subgroup analysis showed that E2 levels were stable, with no significant decrease in E2 levels in either Bu Shen and Bu Shen shu gan CMH subgroups. The heterogeneity was low among subgroups, which suggested that different principles of CMH treatment may be the main factor leading to the heterogeneity of E2 levels; however, Bu Shen shu gan CMH did not affect the FSH levels, while Bu Shen CMH did. However, it is worth noting that the subgroup of Bu Shen also showed high heterogeneity, indicating that other factors were affecting the heterogeneity. Although the meta-analysis revealed significant differences in the LH level, endometrial thickening, and Kupperman score, an accurate conclusion cannot be drawn due to high heterogeneity and/or the limited number of included studies.
Limitations
This study has several limitations. First, the overall quality of the included studies was poor, as most trials neglected to specify the allocation concealment method and the blindness of participants and assessors, resulting in an uncertain or high risk of performance and selection bias, which may compromise the applicability of the results. In addition, most studies did not follow up with participants, resulting in unclear long-term effects of CMH on endocrine therapy-induced side effects in patients with HR(+) breast cancer. Second, all research on CMH was conducted in China and published in Chinese, which makes it difficult for other countries to recognize the effect of CMH. Further studies on the impact of CMH published in English are needed. Third, although a subgroup analysis was conducted based on TCM treatment theory, there was still high heterogeneity in the FSH, LH, and Kupperman scores. The main reason for the heterogeneity among the studies was the diversity and complex description of CMH interventions. Therefore, it was not possible to compare the dosage and duration of CMH.
Conclusions
As an adjuvant treatment for patients with HR(+) breast cancer, CMH can alleviate bone loss and decrease TG levels. However, more well-designed, large-sample, multicenter randomized controlled studies are needed to investigate the impact of CMH on reducing endocrine therapy-induced side effects, including bone loss, menopausal symptoms, and dyslipidemia in patients with HR + breast cancer.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aggelis V, Johnston SRD. 2019. Advances in endocrine-based therapies for estrogen receptor-positive metastatic breast cancer. Drugs. 79(17):1849–1866.
- Bian WH, Li L, Zhang XQ, Yang J, Ying Y, Le YZ, Chen L, Cao DY. 2013. Shugan Tiaoyinyang Fang in ameliorating symptoms caused by tamoxifen after breast cancer surgery and improving the quality of life:aRCT. J Nanjing Univ Trad Chin Med. 29:529–531. Chinese.
- Boon HS, Olatunde F, Zick SM. 2007. Trends in complementary/alternative medicine use by breast cancer survivors: comparing survey data from 1998 and 2005. BMC Womens Health. 7:4.
- Cai JY, Gan JW, Yue SB, Zhuo CL, Chen QT, Zhang GL, Jin Y. 2021. Effect of Fuzheng Xiaoliu decoction on quality of life, inflammatory factors and sex hormones in postmenopausal breast cancer endocrine therapy patients. Shenzhen J Integr Tradit Chin West Med. 31:71–74. Chinese.
- Cai LL, Guo Q, Cao WL, Wu Y, Guo ZN, Xu Y. 2018. Effect of Chaiguilongmu decoction in improving adverse reactions of endocrine therapy for breast cancer: a randomized double-blind controlled study. China Pharm. 27:16–19. Chinese.
- Cao Y, Chen Y, Wang P, Lu J, Han X, She J. 2021. Network pharmacology and experimental validation to explore the molecular mechanisms of Bushen Huoxue for the treatment of premature ovarian insufficiency. Bioengineered. 12(2):10345–10362.
- Chen Z, Gu K, Zheng Y, Zheng W, Lu W, Shu XO. 2008. The use of complementary and alternative medicine among Chinese women with breast cancer. J Altern Complement Med. 14(8):1049–1055.
- Condorelli R, Vaz-Luis I. 2018. Managing side effects in adjuvant endocrine therapy for breast cancer. Expert Rev Anticancer Ther. 18(11):1101–1112.
- Cooke M, Mitchell M, Tiralongo E, Murfield J. 2012. Complementary and alternative medicine and critical care nurses: a survey of knowledge and practices in Australia. Aust Crit Care. 25(4):213–223.
- Cui Y, Shu XO, Gao YT, Wen WQ, Ruan ZX, Jin F, Zheng W. 2004. Use of complementary and alternative medicine by Chinese women with breast cancer. Breast Cancer Res Treat. 85(3):263–270.
- Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, Abraham M, Medeiros Alencar VH, Badran A, Bonfill X, et al. 2013. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 381(9869):805–816.
- DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, Jemal A, Siegel RL. 2019. Breast cancer statistics, 2019. CA Cancer J Clin. 69(6):438–451.
- Ernst E. 2000. Prevalence of use of complementary/alternative medicine: a systematic review. Bull World Health Organ. 78:252–257.
- Fu Y, Yun Q, Zhu XM. 2015. Influences of Heixiaoyao San combined with Shensiwei on clinical measures and quality of life in breast cancer patients with menopausal-like symptoms induced by tamoxifen. Oncol Progr. 13:541–544. Chinese.
- Gong J. 2019. 42 cases of perimenopausal syndrome after endocrine therapy of breast cancer treated with Guipi decoction. Chiang-hsi Chung I Yao. 50:38–40. Chinese.
- Gong LJ, Lv ZY, Ding XW, Shi J. 2019. Clinical study on Shugan Liangxue decoction in improving perimenopausal syndrome in the patients with breast cancer using postoperative medical castration. China Mod Doc. 57:127–130. Chinese.
- Guo L, Wu DL, Du XF. 2016. Effect of Modified Xiangbei Yangrong Tang based on differentiation in treating metastatic breast cancer treated. Chin J Exp Tradit Med Formulae. 22:160–164. Chinese.
- Guo Q, Yao C, Guo YF, Wang C. 2020. Effects of Guilu Sanhuang Decoction combined with calcium on bone metabolism in patients with breast cancer treated by endocrine therapy. Chin J Inf Tradit Chin Med. 27:41–45. Chinese.
- Harris P, Rees R. 2000. The prevalence of complementary and alternative medicine use among the general population: a systematic review of the literature. Complement Ther Med. 8(2):88–96.
- Hu WD. 2018. Clinical Effect of Ruqing Tang combined with western medicine therapy for breast cancer of qi-yin deficiency type. New J Tradit Chin Med. 50:154–156. Chinese.
- Hu MX, Zhu CX, Xu QQ, Sun KF. 2020. Effect of Chaishao Jieyu decoction combined with Pingxiao capsule on the patients with luminal breast cancer after operation. J Hainan Med Univ. 26:1640–1644. Chinese.
- Huang XH, Liang RH, Su L, Guo W, Wang CJ. 2017. Mechanism of Bushen Jianpi decoction in preventing and treating osteoporosis caused by aromatase inhibitors in breast cancer treatment. Cancer Biomark. 18(2):183–190.
- Jiang H, Li M, Du K, Ma C, Cheng Y, Wang S, Nie X, Fu C, He Y. 2021. Traditional Chinese Medicine for adjuvant treatment of breast cancer: Taohong Siwu decoction. Chin Med. 16(1):129.
- Johnston S, Pippen J Jr, Pivot X, Lichinitser M, Sadeghi S, Dieras V, Gomez HL, Romieu G, Manikhas A, Kennedy MJ, et al. 2009. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol. 27(33):5538–5546.
- Kim W, Lee WB, Lee JW, Min BI, Baek SK, Lee HS, Cho SH. 2015. Traditional herbal medicine as adjunctive therapy for breast cancer: A systematic review. Complement Ther Med. 23(4):626–632.
- Kuo YT, Chang TT, Muo CH, Wu MY, Sun MF, Yeh CC, Yen HR. 2018. Use of complementary Traditional Chinese Medicines by adult cancer patients in Taiwan: a nationwide population-based study. Integr Cancer Ther. 17(2):531–541.
- Lee YK, Lee EG, Kim HY, Lee Y, Lee SM, Suh DC, Yoo JI, Lee S. 2020. Osteoporotic fractures of the spine, hip, and other locations after adjuvant endocrine therapy with aromatase inhibitors in breast cancer patients: a meta-analysis. J Korean Med Sci. 35(46):e403.
- Leggett S, Koczwara B, Miller M. 2015. The impact of complementary and alternative medicines on cancer symptoms, treatment side effects, quality of life, and survival in women with breast cancer–a systematic review. Nutr Cancer. 67(3):373–391.
- Li CS, Li ZY. 2021. Clinical observation of Chaihu plus modified Longgu Muli decoction on perimenopausal syndrome of estrogen receptor-positive breast cancer caused by tamoxifen. Hebei J Tradit Chin Med. 43:1620–1623. Chinese.
- Li JJ, Liang LC. 2017. Clinical observation of aromatase inhibitor-associated osteopenia treated by Bushen therapy. J Shanxi Coll Tradit Chin Med. 18:27–29. Chinese.
- Li S, So TH, Tang G, Tan HY, Wang N, Ng BFL, Chan CKW, Yu EC, Feng Y. 2020. Chinese herbal medicine for reducing chemotherapy-associated side-effects in breast cancer patients: a systematic review and meta-analysis. Front Oncol. 10:599073.
- Li Y, Zhu X, Bensussan A, Li P, Moylan E, Delaney G, McPherson L. 2016. Herbal medicine for hot flushes induced by endocrine therapy in women with breast cancer: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2016:1327251.
- Li XR, Zhu JQ, Tong ZY. 2019. Effect of Rehmanniae decoction combined with auricular acupuncture on cognitive ability, hormone level, blood lipid and vitamin D content of patients with postmenopausal breast cancer. Chin Arch Tradit Chin Med. 37:246–249. Chinese.
- Liu H, Zhou L, Zhang QQ, Ling J, Zhang R, Liu LF. 2021. Influence of strengthening endocrine therapy of Jiawei Erxian decoction joint ovarian function suppression on quality of life, Traditional Chinese Medicine syndromes, blood lipids and sex hormones of breast cancer patient in pre-menopausal hormones with receptor-positive. Hebei J Tradit Chin Med. 43:283–287. Chinese.
- Molassiotis A, Fernández-Ortega P, Pud D, Ozden G, Scott JA, Panteli V, Margulies A, Browall M, Magri M, Selvekerova S, et al. 2005. Use of complementary and alternative medicine in cancer patients: a European survey. Ann Oncol. 16(4):655–663.
- Pei JW, Sun TZ. 2019. Clinical study of Danzhi Xiaoyao powder combined with Erxian decoction in the treatment of class II climacteric syndrome after endocrine therapy for breast cancer. Acta Chin Med. 34:1973–1976. Chinese.
- Porter D, Cochrane S, Zhu X. 2017. Current usage of Traditional Chinese Medicine for breast cancer-a narrative approach to the experiences of women with breast cancer in Australia-a pilot study. Medicines (Basel. 4(2):20.
- Qiu JS, Wu YY, Liu C. 2020. Clinical study of Baohe pill in treatment of dyslipidemia in endocrine therapy of breast cancer. Res Integr Tradit Chin West Med. 12:303–307. Chinese.
- Shi H, Dong J, Xu YB. 2017. Longniu Bugu decoction for prevention and treatment of bone loss caused by endocrine therapy of premenopausal breast cancer. Chin J Endocr Surg. 11:380–383. Chinese.
- Shi YF, Wei QL, Zhou LN. 2014. Yiyuan Shengjing decoction treat similar climacteric syndrome of breast cancer after treated with Tamoxifen. J Zhejiang Chin Med Univ. 38:1172–1182. Chinese.
- Siegel RL, Miller KD, Jemal A. 2020. Cancer statistics, 2020. CA Cancer J Clin. 70(1):7–30.
- Sun Y. 2014. The role of Chinese medicine in clinical oncology. Chin J Integr Med. 20(1):3–10.
- Sun XF, Liu H, He YN, Liu CJ, Cui SD. 2014. Fuzheng Xiaoai decoction 50 cases of syndrome endocrine treatment of breast cancer after operation. Chin J Exp Tradit Med Formulae. 20:192–195. Chinese.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. 2021. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71(3):209–249.
- Tao ZH, Yu XW, Tong X, Fang MH, Jin YJ, Xu ZG, Su Q. 2020. Clinical observation on the treatment of perimenopausal like syndrome with breast cancer endocrine therapy by Yishen Kang’ai Fang. Smart Healthcare. 6:91–94. Chinese.
- Wang JC, Sun CH, Liu HM, Yan WH. 2013. Effects of the Tiaohe decoction on premenopausal breast cancer PR(+) ER(+) HER-2(-) endometrium. Clin J Chin Med. 5:15–16. Chinese.
- Wang WS, You JL, Xue Q. 2017. Efficacy observation of Yishen-chenqian formula treatment Tamoxifen side effects of endocrine stage of breast cancer. Shanxi J Tradit Chin Med. 33:23–25. Chinese.
- Wong CK, Bao YX, Wong EL, Leung PC, Fung KP, Lam CW. 2005. Immunomodulatory activities of Yunzhi and Danshen in post-treatment breast cancer patients. Am J Chin Med. 33(3):381–395.
- Wu JP, Feng N, Li Y, Bei LM, Li XL, Liu Y, Shi Y, Gao BC, Yang BF. 2016. Experimental research on the effect of Ruqing decoction on the levels of the hormone for patients with breast cancer of Qi and Yin deficiency syndrome. Inf Tradit Chin Med. 33:53–56.
- Xia YP, Wang H. 2015. The clinical observation of the treatment of osteoporosis with Jintiange capsules in postmenopausal breast cancer patients after surgery. Chin J Osteoporos. 21:812–816. Chinese.
- Xiao H, Xi KJ, Fang NQ. 2019. Modified Zhibai Dihuang decoction alleviating the adverse reactions of letrozole in endocrine therapy for breast cancer. Contemporary Med. 25:9–12. Chinese.
- Yang HF, Luo H, Yang OO, Hu ZJ, He JL, Xu HB. 2016. Effects of Erzhi pills and Guizhi decoction for the reproductive hormone, modified Kupperman score and ki67 expression of premenopausal Luminal type breast cancer patients with tidal fever after treatment. China Med Her. 13:175–178. Chinese.
- Yin SY, Wei WC, Jian FY, Yang NS. 2013. Therapeutic applications of herbal medicines for cancer patients. Evid Based Complement Alternat Med. 2013:302426.
- Zhang M, Liu X, Li J, He L, Tripathy D. 2007. Chinese medicinal herbs to treat the side-effects of chemotherapy in breast cancer patients. Cochrane Database Syst Rev. 2007(2):CD004921.
- Zhang XP, Wang C, Wang QW, Cao SH, Shao XY, Liang S, Feng M, Wang HH, Yao C. 2020. Study on the effect of Sanhuang decoction on chronic stress and blood viscosity of breast cancer patients. Chin J Surg Oncol. 12:534–538. Chinese.
- Zhang CL, Zheng YL. 2012. Clinical study of Dan Zhi Xiao Yao San and Er Zhi Wan Jia Jian in treating the hormone therapy of breast cancer the clinical observation of after menopause syndrome. China J Chin Med. 27:6–8. Chinese.
- Zhong SS, Wan H, Li R, Mao JL, Qu WC, Lu YY, Zhang C. 2020. Clinical effect of spleen-tonifying and kidney-nourishing prescription in the prevention and treatment of aromatase inhibitor-induced arthralgia. J Anhui Univ Chin Med. 39:20–24. Chinese.
- Zhou J, Zhao F, Zhan XF. 2020. Clinical observation of similar climacteric syndrome due to breast cancer with endocrine therapy treated by Prosperous Ankun soup. Xinjiang J Tradit Chin Pharm. 38:6–9. Chinese.
- Zhu QL. 2017. Xianlinggubao capsules combined with calcium agents in treatment of osteoporosis due to hormonal therapy after breast cancer operation: effect on bone metabolism. Chin J Gen Surg. 26:670–674. Chinese.
- Zhu L, Li L, Li Y, Wang J, Wang Q. 2016. Chinese herbal medicine as an adjunctive therapy for breast cancer: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2016:9469276.