Abstract
Context
Hawthorn leaves are a kind of widely used medicinal plant in China. The major ingredient, hawthorn leaves flavonoids (HLF), have cardiotonic, cardioprotective, and vascular protective effects.
Objective
The study evaluated the protective role of HLF in cardiac remodelling and the underlying mechanisms under simulated microgravity by hindlimb unloading rats.
Materials and methods
Adult male Sprague-Dawley rats were divided into control, HLF, HU (hindlimb unloading) and HU + HLF groups (n = 8). After HU and daily intragastric administration at the dose of 100 mg/kg/d for 8 weeks, cardiac function and structure were evaluated by biochemical indices and histopathology. We identified the main active compounds and mechanisms involved in the cardioprotective effects of HLF via bioinformatics and molecular docking analysis, and relative signalling pathway activity was verified by Western blot.
Results
HLF treatment could reverse the HU-induced decline in LV-EF (HU, 55.13% ± 0.98% vs. HU + HLF, 71.16% ± 5.08%), LV-FS (HU, 29.44% ± 0.67% vs. HU + HLF, 41.62% ± 4.34%) and LV mass (HU, 667.99 ± 65.69 mg vs. HU + HLF, 840.02 ± 73.00 mg). Furthermore, HLF treatment significantly increased NPRA expression by 135.39%, PKG by 51.27%, decreased PDE5A by 20.03%, NFATc1 by 41.68% and Rcan1.4 by 54.22%.
Conclusions
HLF plays a protective effect on HU-induced cardiac remodelling by enhancing NPRA-cGMP-PKG pathway and suppressing the calcineurin-NFAT pathway, which provides a theoretical basis for use in clinical therapies.
Introduction
Humans live in the earth’s environment under 1 G of gravity, and our organ systems have adapted to work in this environment. Under the long-term conditions of space or simulated microgravity, microgravity induces changes in cardiac adaptation, which reduces the workload of the heart and causes the loss of cardiac mass. Microgravity causes a chronic decline in metabolic and oxygen uptake, which reduces the demand for cardiac output, leading to cardiac atrophy and decreased cardiac function (Dorfman et al. Citation2007; Zhong et al. Citation2016). Importantly, it has been reported that the heart may lead to atrophy with a proportion of 8–10% after 10 days of spaceflight (Tuday and Berkowitz Citation2007). Previous studies have shown that cardiac performance is altered under these conditions, including increased incidence of cardiac arrhythmias and decreased left ventricular stroke volume (Platts et al. Citation2009). Furthermore, cardiac dysfunction and cardiac remodelling induced by simulated microgravity have been observed in rats, mice, and rhesus monkeys (Sun et al. Citation2019; Zhong et al. Citation2021).
A variety of countermeasures have been used to protect the heart during space travel, including exercise training; however, there is no evidence that cardiac mass and function are restored by the countermeasures. In recent years, traditional Chinese medicine (TCM) has become an important strategy in the prevention and treatment of cardiac diseases. Its curative effects are remarkable, and its toxicity and side effects are low (Lin et al. Citation2019). The characteristics of TCM include its multi-channel and multi-target action, and it has unique advantages over other measures in preventing and treating cardiac diseases (Ren et al. Citation2020).
Hawthorn (Crataegus spp. [Rosaceae]) leaves have been used as a medicinal plant for a long period of time in China. Hawthorn leaves flavonoids (HLF) are the major active ingredient in hawthorn leaves, they include various flavonoids, such as vitexin-4″-O-glucoside, vitexin-2″-O-rhamnoside, quercetin, hyperoside, and vitexin (Ma et al. Citation2007; Zhu et al. Citation2015). The flavonoid extracts of hawthorn leaves are widely used to treat cardiovascular diseases (Koch and Malek Citation2011). Pharmacological studies have proven that these hawthorn leaves flavonoids extracts to possess cardiotonic, cardioprotective and vascular protective effects (Koch and Malek Citation2011). Therefore, these hawthorn leaves' flavonoids may be employed in the prophylactic and therapeutic treatment of myocardial damage and cardiac dysfunction. Treatment with flavonoids, such as rutin, hyperoside, and quercetin attenuates atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP), which ameliorates myocardial damage (Yan et al. Citation2013; Imam et al. Citation2017; Wang et al. Citation2018). However, the mechanism of HLF in reducing cardiac remodelling induced by simulated microgravity is unclear. In this research, we studied the effects and underlying mechanisms of HLF in simulated microgravity-induced cardiac remodelling in rats.
Material and methods
Animals
All animal experiments were performed in accordance with the ARRIVE guidelines and the Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Xi’an International Medical Center Hospital (Lot: 2022050). Adult male Sprague-Dawley rats (180 ± 20 g) were purchased from the Laboratory Animal Center of Xi’an Jiaotong University of P.R. China. The rats were housed under standard laboratory conditions (12 h light/dark cycle and temperature of 24 ± 1 °C and free access to a standard diet and distilled water.
HLF
HLF (purity 80%) was purchased from Herb-key (NO: ZZSZ-025, Shaanxi, China). The main compounds of HLF were obtained as described previously (Zhu et al. Citation2015), analyzed by Agilent 1200-6460 system and identified by MassBank (https://massbank.eu/MassBank/Search). The 1.25 mg powdered HLF were dissolved in 100 mL distilled water and stored at 4 °C.
Model and treatment
After 1 week of acclimation, the rats were randomly divided into four study groups (n = 8): Ctrl (control group), HLF, HU (hindlimb unloading), and HU + HLF. There was no statistical difference in the average body weight of the rats between the groups. Rats in the HU and HU + HLF groups maintained HU for 8 weeks by continuous suspension by the tail at 30° continuous (Morey-Holton and Globus, Citation2002). Animals were housed in individual cages and allowed to move their forelimbs freely. The rats in the HU + HLF group were given HLF during the tail suspension. The HLF and HU + HLF groups received HLF (100 mg/kg/d) via gavage, and the Ctrl and HU groups received distilled water. Previous studies reported that oral treatment with hydroalcoholic extracts from hawthorn leaves improved cardiac function and prevented myocardial infarction at an optimal dose of 100 mg/kg/d (Veveris et al. Citation2004), and 100 mg/kg/d hawthorn leaves extract prevented L-NAME-induced hypertension (Koçyildiz et al. Citation2006). Accordingly, we used a similar HLF dose in this study. The body weight of the rats was monitored weekly.
Cardiac function and structure
Cardiac function and structure after 8 weeks of tail suspension were evaluated via two-dimensional echocardiography (vevo2100, Visualsonics, USA). Under light inhalational anesthesia with isoflurane, the fur was removed from the collar of the rats to the middle of the chest, and the rats were placed in supine position. The interventricular septum (IVS), left ventricular internal diastolic (LVID), left ventricular posterior wall (LVPW), left ventricular ejection fractions (LV-EF), left ventricular fraction shortening (LV-FS), and left ventricular mass (LV mass) were measured.
Blood pressure monitoring
A non-invasive blood pressure (BP) monitor (CODA-HT4, Kent scientific corporation, USA) was used to measure the systolic, diastolic, and mean BP indirectly by the tail artery in conscious rats. The BP measurement was performed three times in a row and the average value was taken.
Sample collection and preparation
All rats were euthanized after 8 weeks of HU, and heart samples were isolated immediately after the rats were sacrificed. The heart weight of rats was assessed, and the heart weight index (HW/BW) was calculated by dividing heart weight by body weight. All samples were snap-frozen in liquid nitrogen and stored at −80 °C until analysis.
CK-MB, cTnT and cGMP
Rats were anaesthetized with sodium pentobarbital (30 mg/kg) by intraperitoneal injection. Blood samples were separated from the common carotid artery, centrifuged at 5000 rpm for 10 min, and the serum was collected. Serum levels of creatine kinase-MB (CK-MB) (no. F15213, Westang, Shanghai, China), cardiac troponin T (cTnT) (no. F15267, Westang, Shanghai, China), and cyclic guanosine 3′, 5′-monophosphate (cGMP) (68AT-cGMP-S100, RayBiotech, USA) were measured using a corresponding enzyme-linked immunosorbent assay (ELISA) kit.
Hematoxylin and eosin staining
Rat myocardial tissues were washed with pre-cooled physiological saline solution and fixed with paraformaldehyde for 48 h at room temperature. The left ventricular apex was embedded into paraffin conventionally, and paraffin blocks were cut into 4 μm thick sections. Tissue slices were stained with hematoxylin and eosin (HE) and myocardial morphology was assessed via a light microscope (DM1000-3000, Leica, Germany). CaseViewer was used to measure and quantify cell size.
Bioinformatics and molecular docking analysis
To analyze the potential molecular mechanism of HLF, the major constituents of HLF were obtained, as described previously (Zhu et al. Citation2015), identified by Agilent 1200-6460 system and distinguished by MassBank (https://massbank.eu/MassBank/Search). The gene symbols of cardiac remodeling were obtained from the GeneCards Human Database Gene Search (https://www.genecards.org/), with a prediction confidence score cutoff of 3.0. These selected gene symbols were enriched via DAVID Functional Annotation Bioinformatics Microarray Analysis (https://david.ncifcrf.gov/). The KEGG pathways of selected symbols were saved. Next, docking studies between relative proteins, such as natriuretic peptide receptor A (NPRA), phosphodiesterase 5 A (PDE5A), cGMP-dependent protein kinase (PKG) and nuclear factor of activated T-cells (NFATc1), and the major constituents of HLF were performed using Discovery Studio software (Discovery Studio Client 2019, China). The structures of relative proteins were obtained from the Protein Data Bank (http://www.pdb.org). The structures of flavonoids were obtained from Pubchem (https://pubchem.ncbi.nlm.nih.gov/) and ZINC (https://zinc.docking.org/). After removing water molecules, the structures of the proteins were optimized, and the binding site were obtained according to the receptor cavity of each protein. The docking between proteins and flavonoids were going finally.
Western blot
Left ventricular tissue was lysed in RIPA lysis buffer (50 mM Tris pH 7.4, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS) on ice for 15 min. Then, the suspension was centrifuged at 12000 g for 20 min at 4 °C. Protein concentration was determined using a BCA kit (7780S, Cell Signalling Technology, MA, USA) and adjusted with loading buffer (P0015A, Beyotime, Shanghai, China). The same quantity of protein sample (10 μg) was separated by 8-12% SDS-polyacrylamide gel electrophoresis and blotted onto a polyvinylidene fluoride membranes (Immobilon P; Millipore, Bedford, MA, USA). The membranes were blocked in 5% non-fat milk at room temperature for 2 h and incubated with primary antibodies against NPRA (1:1000 dilution, ab14536, Abcam, Cambridge, UK), PKG (1:1000 dilution, #3248, Cell Signalling Technology, MA, USA), PDE5A (1:1000 dilution, ab259945, Abcam, Cambridge, UK), NFATc1 (1:500 dilution, sc-7294, Santa Cruz, CA, USA), Rcan1.4 (1:500 dilution, sc-377507, Santa Cruz, CA, USA), and α-tubulin (1:5000 dilution, 11224-1-AP, proteintech, USA) overnight at 4 °C. Then horseradish peroxidase (HRP)-conjugated secondary antibodies were incubated with the membranes at 37 °C for 1 h. Detection of antibody binding was followed by chemiluminescence detection (ECL-001, Zhuangzhibio, Shaanxi, China). The expression of GAPDH and α-tubulin were used to calculate the relative amounts of protein expression as an internal standard.
Immunohistochemistry
The myocardial tissue slices were baked at 65 °C for 30 min, then dehydrated in xylene and rehydrated in stepped alcohol. Endogenous peroxidase was blocked with 300 mL/L H2O2 in methanol for 20 min. Antibodies of NPRA (1:200 dilution, ab14536, Abcam, Cambridge, UK) and Rcan1.4 (1:500 dilution, sc-377507, Santa Cruz, CA, USA) were incubated overnight with tissue sections at 4 °C. Next, the corresponding secondary antibodies were added and retained for 1 h at room temperature. After DAB color was developed with chromogen for 10 min and rinsed in running tap water for 5 min, slices were counterstained with hematoxylin.
Statistical analysis
Statistical analysis was performed using SPSS software (version 13.0, SPSS, Inc., Chicago, IL, USA) and GraphPad Software (Prism 6). A total of eight samples were included in each group. Student’s t-test was used to obtain P-values. p < 0.05 indicated a statistically significant difference.
Results
HLF increased body and heart weights of HU rats
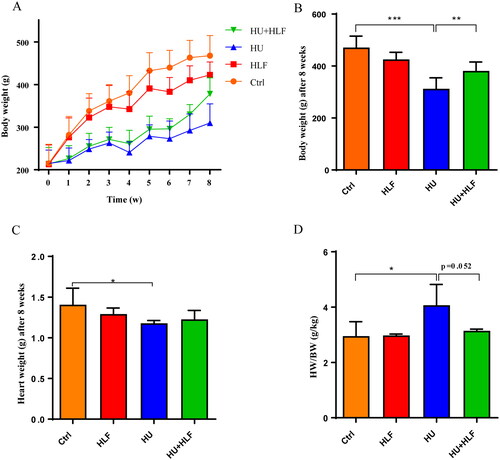
The body weights of HU rats were reduced significantly (p<0.01) compared with ctrl rats after 2 weeks of HU. After 4 weeks of HLF treatment, a significant increase in body weight was observed compared with HU (). After 8 weeks of HU, heart weight was decreased in the study groups compared with ctrl group, but there was no significant change in the HLF treatment group (). However, after 8 weeks of HU, the heart weight index of HU group was increased compared with ctrl group ().
Figure 1. Effects of HU and HLF treatment on body weight and heart weight index (HW/BW). HLF ameliorated the fall in body weight induced by HU during 8 weeks (A). HLF improved the reduction in body weight after 8 weeks HU (B). HU led to a decrease in heart weight (C). HLF attenuated the increase in HWI after 8 weeks HU (D). Values are means ± SD, n = 8, *p < 0.05, **p < 0.01, ***p < 0.001.
HU induced progressive cardiac dysfunction in adult rats, while HLF treatment reduced cardiac damage accompanied by restoring blood pressure
Obvious changes in cardiac structure and function were detected in the rats after 8 weeks of HU (). LV-EF was comparable between the groups; it was significantly lower in the HU group than in the control group at 8 weeks. In contrast, there was a greater increase in LV-EF in the HU + HLF group than in the HU group (). A significant decrease in LV-FS was observed in HU group compared with the control group; however, the decrease in LV-FS due to HU was attenuated by HLF treatment (). Furthermore, a significant decrease in LV mass after 8 weeks of HU was observed compared with the control group; however, HLF treatment significantly increased LV mass compared with the HU group (). The data indicate that HU-induced cardiac dysfunction, while HLF treatment reduced HU-induced cardiac dysfunction in adult rats.
Figure 2. Changes of HU and HLF treatment in cardiac structure left ventricular function and blood pressure. (A) Representative echocardiography of four groups. (B) Left ventricular ejection fraction (LV-EF). (C) Left ventricular fractional shortening (LV-FS). (D) Left ventricular mass (LV Mass). (E) Systolic blood pressure. (F) Diastolic blood pressure. (G) Mean arterial blood pressure. Values are means ± SD, n = 8, *p < 0.05, **p < 0.01, ***p < 0.001.
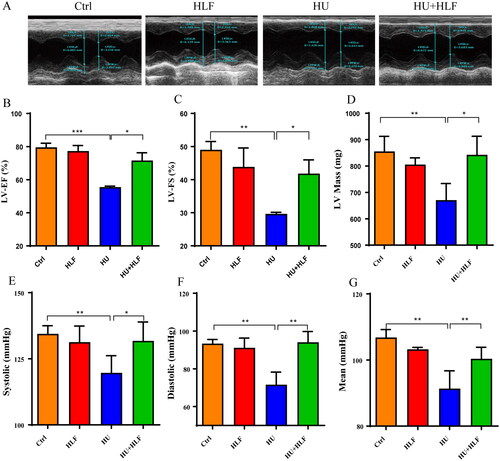
To assess whether HU with or without HLF treatment induced hemodynamic changes and thereby affected cardiac function, we measured the systolic, diastolic and mean BP. Compared With the control rats, HU induced a significant decrease in mean arterial BP, diastolic BP and systolic BP. However, HLF treatment significantly attenuated low BP ().
HLF decreased the levels of myocardial biomarkers, meanwhile, protected against HU-induced cardiac remodeling
To evaluate myocardial injury, serum CK-MB and cTnT levels were determined (). Compared with the control group, the HU group had significantly increased serum CK-MB, and cTnT levels. A significant decrease in myocardial biomarker levels was associated with HLF treatment compared with HU. Overall, these data suggest that HLF treatment attenuates myocardial injury in HU rats.
Figure 3. Concentration analyses of myocardial injury biomarkers and cardiomyocyte morphology analyses following 8 weeks HU or HLF treatment. HLF alleviated the increase in CK-MB (A) and cTnT (B) after 8 weeks HU. (C) Cardiomyocyte morphology (40×; bar = 20 μm). (D) The width of the cardiomyocyte. Values are means ± SD, n = 8, **p < 0.01, ***p < 0.001.
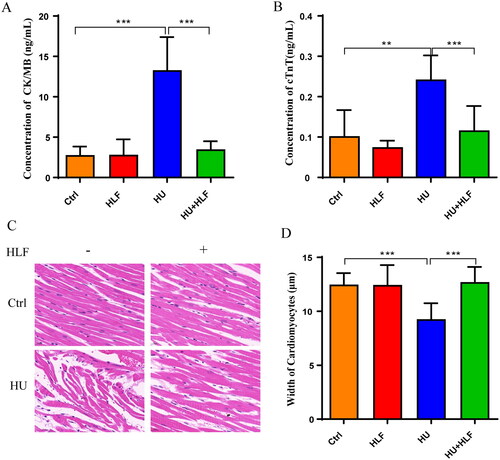
To confirm the effect of HU with or without HLF treatment on cardiac remodeling, HE staining was used to investigate cardiomyocyte morphology. As shown in , cardiomyocytes had disorderly arrangement, but the disordered arrangement of cardiomyocytes was ameliorated with HLF treatment. The cell size of cardiomyocytes decreased after HU; however, HLF treatment opposed the effects of HU ().
Bioinformatics and molecular docking prediction
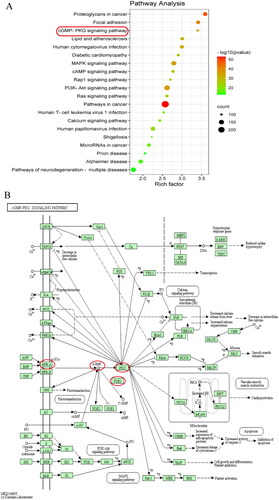
To analyze the potential molecular mechanism, gene symbols of cardiac remodelling were enriched. The homo sapiens of these selected symbols were mapped to many KEGG pathways (). Many signalling pathways related to the cardiovascular system were predicted. The cGMP-PKG signalling pathway () was one of the closely related pathways. In previous reports, the NPRA-cGMP-PKG signalling pathway was shown to inhibit the calcineurin-NFAT pathway in cardiomyocytes (Tokudome et al. Citation2005), and the calcineurin-NFAT pathway was shown to play an important role in cardiac dysfunction. Therefore, the docking between the major proteins of the NPRA-cGMP-PKG and calcineurin-NFAT signalling pathways and the major flavonoids was going.
Figure 4. Signalling pathway prediction based on bioinformatics. (A) Results of the major 20 signalling pathways enrichment analysis. (B) The cGMP-PKG signalling pathway was downloaded from KEGG PATHWAY Database (https://www.kegg.jp/kegg/pathway.html).
Five major flavonoid compounds of HLF were unambiguously identified as vitexin-4″-O-glucoside, vitexin-2″-O-rhamnoside, vitexin, rutin, and hyperoside (). The accuracy of docking between target proteins NPRA, PDE5A, PKG, and NFATc1 (PDB ID code: 1jdn, 3bjc, 4r4l and 5sve, respectively) and the five major HLF compounds was assessed. Lower binding energy was associated with better interaction between the receptor and ligand. Molecular docking of the compounds with PKG and NFATc1 exhibited strong interaction between the compounds with proteins (). With PKG, vitexin and hyperoside exhibited H-bonding interactions, carbon-hydrogen bonds and van der Waals to combine (). With NFATc1, vitexin-4″-O-glucoside, vitexin-2″-O-rhamnoside, vitexin, and hyperoside exhibited H-bonding interaction, pi-Alkyl and van der Waals to combine (). The results successfully predicted the binding ability of major components of HLF and target proteins. Therefore, PKG and NFATc1 were demonstrated to be targets of HLF.
Figure 5. The major five compounds of HLF are vitexin-2″-O-rhamnoside (A), vitexin-4″-O-glucoside (B), vitexin (C), rutin (D) and hyperoside (E).
Figure 6. View of the molecular docking simulation between proteins and major compounds of HLF. (A) The binding site of PKG (PDB ID code: 4r4l) (B) Binding site of NFATc1 (PDB ID code: 5sve) (C)-(H) 2D and 3D view of the interactions between proteins and major compounds of HLF. (C) PKG and vitexin. (D) PKG and hyperoside. (E) NFATc1 and vitexin-4″-O-glucoside. (F) NFATc1 and vitexin-2″-O-rhamnoside. (G) NFATc1 and vitexin. (H) NFATc1 and hyperoside.
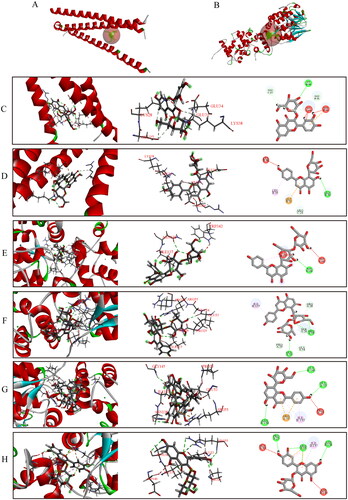
Table 1. The binding energy (kcal/mol) between compounds and target proteins.
HLF regulated HU-Induced progressive cardiac dysfunction via NPRA-cGMP-PKG signalling
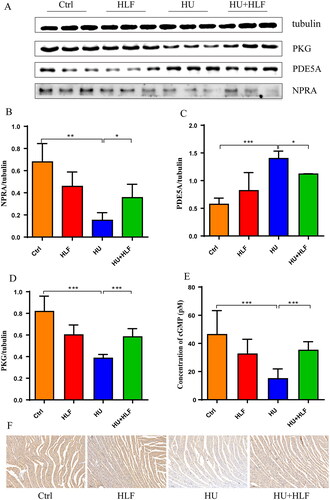
To gain more insight into the effect of HLF treatment on the signalling pathways involved in HU-induced cardiac dysfunction, we examined the levels of cGMP and the protein levels of NPRA, PKG, and PDE5A in myocardial tissue in the four groups. As shown in , quantification analysis revealed that PKG and PDE5A levels were increased while NPRA level decreased in rats after 8 weeks of HU; however, there was obvious amelioration in HU rates treated for 8 weeks with HLF. After 8 weeks of HU, a significant decrease in cGMP was observed compared with the ctrl; however, cGMP was increased following HLF treatment (). In parallel, immunohistochemistry demonstrated that cardiac myocytes in the HU group were exhibited weak staining for NPRA, the samples were weakly positive for NPRA (). In contrast, HLF-treated samples exhibited enhanced positivity for NPRA. These results indicate that HLF regulates HU-induced progressive cardiac dysfunction via NPRA-cGMP-PKG signalling.
Figure 7. HLF treatment activates NPRA-cGMP-PKG signalling pathway in rats after simulated microgravity. (A) Representative Western blots for NPRA, PKG and PDE5A in left ventricular tissue from rats after 8 weeks of HU. (B-D) Quantification analysis of relative protein in A. (E) Concentration analyses of cGMP. (F) Immunohistochemistry for NPRA in myocardial tissue slices after 8 weeks of HU (10×; bar =100 μm). Values are means ± SD, n = 8, *p < 0.05, **p < 0.01, ***p < 0.001.
HLF inhibited activation of calcineurin-NFAT signalling in cardiac myocytes
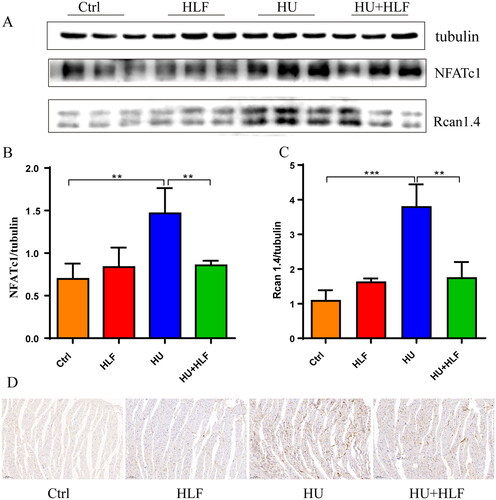
In previous reports, NPRA-cGMP-PKG signalling was shown to inhibit the calcineurin-NFAT pathway in cardiac myocytes (Tokudome et al. Citation2005). We detected levels of calcineurin-NFAT pathway-related proteins Rcan1.4 and NFATc1 in myocardial tissue from the four groups. In general, the protein levels of Rcan1.4 and NFATc1 in myocardial tissues of HU rats were higher than that in ctrl rats, while expression was decreased in the HLF treatment group (). Furthermore, immunohistochemistry revealed that Rcan1.4 was abundantly expressed in the HU group, with decreased intensity in the HLF treatment group (). These results suggest that HLF inhibit the activation of calcineurin-NFAT signalling in cardiac myocytes.
Figure 8. HLF treatment inhibits the Calcineurin-NFAT signalling pathway in rats after simulated microgravity. (A) Representative Western blots for NFATc1 and Rcan1.4 in left ventricular tissue from rats after 8 weeks of HU. (B-C) Quantification analysis of relative protein in A. (D) Immunohistochemistry for Rcan1.4 in myocardial tissue slices after 8 weeks of HU (10×; bar = 100 μm). Values are means ± SD, n = 8, *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
Evidence of cardiac remodelling induced by simulated microgravity has been well documented. TCM has become important in the prevention and treatment of various diseases, and the flavonoids in TCM are widely used in the prevention and treatment of cardiac diseases. This is the first study to demonstrate the regulatory effect of plant flavonoids on simulated microgravity-induced cardiac remodelling. The results indicate that HLF treatment decreases levels of myocardial biomarkers, ameliorates cardiac dysfunction and orthostatic hypotension, and corrects the disordered arrangement of cardiomyocytes to protect against simulated microgravity-induced cardiac remodelling. The underlying mechanisms of the protective effects of HLF in simulated microgravity-induced cardiac remodelling correspond with the role of HLF in activating the NPRA-cGMP-PKG signalling pathway and inhibiting the calcineurin-NFAT signalling pathway, as shown in .
Figure 9. Mechanism of HLF protects from cardiac remodelling induced by simulated microgravity. Red represents the effect of simulated microgravity, green represents the therapeutic effect of HLF, and black represents proven effects. Arrows indicate activation, T-shapes indicate inhibition. The underlying mechanisms of HLF protective effects in simulated microgravity-induced cardiac remodeling are corresponding with the role of HLF in activating NPRA-cGMP-PKG signalling pathways and inhibiting calcineurin-NFAT signalling pathways.
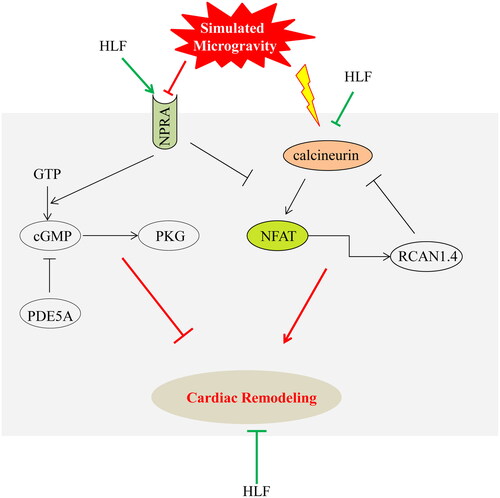
Studies on the pharmacological effects of hawthorn leaves have shown that they have anti-ischemia/reperfusion injury, anti-arrhythmia, hypolipidemic, vasodilatory, anti-inflammatory, hypotensive and cardioprotective effects (Sadek et al. Citation2018; Shatoor et al. Citation2019; Bujor et al. Citation2020). YiXinTongRuanJiaoNang, of which the main constituent is HLF, is widely used to treat angina pectoris and coronary heart disease in China. The major constituents of HLF such as vitexin-4″-O-glucoside, vitexin-2″-O-rhamnoside, vitexin, rutin, and hyperoside have been used to prevent and treat cardiovascular diseases (Ma et al. Citation2007). Among these constituents, vitexin-4″-O-glucoside and vitexin-2″-O-rhamnoside inhibit anoxia/reoxygenation injury in the heart (Li et al. Citation2008), vitexin attenuates myocardial ischemia/reperfusion injury by regulating mitochondrial dysfunction (Xue et al. Citation2020), hyperoside improves cardiac function and ameliorates myocardial hypertrophy and fibrinogen deposition by suppressing the NLRP1 inflammation pathway after myocardial infarction (Yang et al. Citation2021), and rutin exhibits cardioprotective effects by enhancing antioxidant enzyme activity, inhibiting fibrosis-related genes, and opposing the anti-inflammatory effect induced by LPS (Xianchu et al. Citation2018). Considering the pharmacological activity and clinical application of HLF, the cardioprotective effect of HLF was investigated and evaluated in a model of cardiac remodelling in rats exposed to simulated microgravity. As shown in the , cardiac atrophy with a proportion of 16.38% was observed in HU rats, but the loss of body weight with a proportion of 33.81% was observed in HU rats. It leads to the increase of HW/BW, but there was no significant change in the HLF treatment group. Moreover, CK-MB and cTnt are usually elevated only for a short term in circulation and rapidly declined afterwards. The study suggests the degree of myocardial injury was mild, leading to compensatory elevation of CK-MB and cTnT in circulation. Our results revealed that HU of rats led to cardiac atrophy, as evidenced by decreased relative cell size, and HLF treatment effectively opposed this situation.
Under physiological conditions, cGMP acts as an important secondary messenger that can be catalyzed by the transmembrane GC-coupled natriuretic peptide receptors (NPRs), NPRA and NPRB. The NPRs interact with natriuretic peptide, causing the activation of the intracellular GC domain and synthesis of cGMP. Furthermore, cGMP mediates various biological functions through cGMP-dependent protein kinases (PKG, also known as cGK).
There are a number of important factors that regulate cardiac function induced by various stimuli, including ANP and BNP (Lemaitre et al. Citation2022). Research has shown that circulating ANP concentrations continuously increase when astronauts experience weightlessness (Frings-Meuthen et al. Citation2020). Similarly, the expression of ANP was increased in the myocardium of rats after simulated microgravity for 4 weeks (Zhang et al. Citation2013). The specific receptors NPRA and NPRB, which mediate the physiological actions of natriuretic peptides, exhibit guanylyl cyclase activity in their domains (Silberbach and Roberts Citation2001). However, ANP has a much higher affinity for NPRA receptor than NPRB, so NPRA receptor is the physiological receptor (Hashimoto et al. Citation1994). The increased guanylyl cyclase activity due to the binding of natriuretic peptides to NPRA receptors, results in increased cGMP concentrations (Silberbach and Roberts Citation2001). Interestingly, decreased plasma levels of cGMP were reported in astronauts on long-term spaceflights (Rössler et al. Citation2001). Furthermore, reduced mRNA expression of GC-A (NPRA) was observed in highly metastatic melanoma cells under simulated microgravity (Ivanova et al. Citation2018). These findings demonstrate that the NPRA-cGMP-PKG pathway may be inhibited in space or simulated microgravity. In our study, NPRA-cGMP-PKG signalling in the myocardium was suppressed; HLF regulated simulated microgravity-induced progressive cardiac dysfunction via NPRA-cGMP-PKG signalling, which indicated activation of the NPRA-cGMP-PKG signalling pathway by HLF in HU rats.
Calcineurin is a calcium-dependent phosphatase. Sustained stimulation of calcineurin activity in the heart can cause pathological cardiac hypertrophy, which progresses rapidly to dilated hypertrophy and even heart failure (Molkentin et al. Citation1998). The activity of calcineurin is both inhibited and facilitated by association with the regulator of calcineurin1 (RCAN1, Dscr1 or Mcip1) (Vega et al. Citation2003). RCAN1, located on chromosome 21, is highly expressed in the brain, heart, and skeletal muscle (Fuentes et al. Citation2000). Three main RCAN1 isoforms have been detected as Rcan1.1, Rcan1.2 and Rcan1.4 (Genescà et al. Citation2003). Rcan1.4 has been identified as an endogenous regulator of phosphatase calcineurin which is associated with the activation of nuclear factor of activated T cells (NFAT) (Hogan et al. Citation2003; Soleimanpour et al. Citation2010). Topically secreted natriuretic peptides protect the heart from excessive cardiac remodeling by activating cardiac GCA by inhibiting the calcineurin-NFAT pathway (Tokudome et al. Citation2005). Moreover, expression of activated PKG prevents cardiomyocytes from hypertrophy due to calcineurin activation (Vega et al. Citation2003). This suggests that PKG acts on calcineurin in the hypertrophic signalling cascade. According to the literature, we speculated that calcineurin-NFAT signalling may be altered in HU rats. Employing molecular docking, we found that strong interaction of the compounds with NFATc1. In addition, we observed a significant increase in calcineurin-NFAT signalling after 8 weeks of HU, while HLF treatment significantly inhibited calcineurin-NFAT signalling in HU rats. These findings indicate that the protective effects of HLF in simulated microgravity-induced cardiac remodelling are associated with inhibition of the calcineurin-NFAT signalling pathway.
We will conduct further studies with RNA/DNA interference and specific inhibitors in rats to confirm the importance of these two signalling pathways and their involvement in regulating HLF in simulated microgravity-induced cardiac remodeling. Furthermore, HLF contains multiple active components, and it is worth exploring which component plays a major role in the treatment of simulated microgravity-induced cardiac remodeling. Therefore, we will continue to search for the major components of HLF that prevent and treat simulated microgravity-induced cardiac remodelling to increase the body of evidence for HLF in preventing and treating simulated microgravity-induced cardiac remodelling.
Conclusion
HLF has an amelioration effect on simulated microgravity-induced cardiac remodelling in rats, leading to decreased serum levels of CK-MB and cTnT, increased body weight, heart weight and BP, and amelioration of cardiac dysfunction. In addition, we discovered that HLF is involved in the regulation of simulated microgravity-induced cardiac remodelling through NPRA-cGMP-PKG signalling and the calcineurin-NFAT signalling pathway. Therefore, HLF have the potential to be a preventive measure for cardiac remodeling induced by simulated microgravity.
Author contribution
Tian Liu and Yuqi Ma analyzed and wrote the manuscript. Hui Zhao revised the manuscript. Xi Shen and Yan Niu treated data curation and visualization. Pengli Wang, Yuehuan Hu and Bing Yan interpreted data and prepared figures. Mingxia Zhang analyzed data. Jun Yu conceived and designed research. All authors have participated sufficiently in the study and approved the final version.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bujor A, Miron A, Luca S, Skalicka-Wozniak K, Silion M, Trifan A, Girard C, Demougeot C, Totoson P. 2020. Vasorelaxant effects of Crataegus pentagyna: links with arginase inhibition and phenolic profile. J Ethnopharmacol. 252:112559.
- Dorfman T, Levine B, Tillery T, Peshock R, Hastings J, Schneider S, Macias B, Biolo G, Hargens A. 2007. Cardiac atrophy in women following bed rest. J Appl Physiol. 103(1):8–16.
- Frings-Meuthen P, Luchitskaya E, Jordan J, Tank J, Lichtinghagen R, Smith S, Heer M. 2020. Natriuretic peptide resetting in astronauts. Circulation. 141(19):1593–1595.
- Fuentes J, Genescà L, Kingsbury T, Cunningham K, Pérez-Riba M, Estivill X, de la Luna S. 2000. DSCR1, overexpressed in Down syndrome, is an inhibitor of calcineurin-mediated signalling pathways. Hum Mol Genet. 9(11):1681–1690.
- Genescà L, Aubareda A, Fuentes J, Estivill X, De La Luna S, Pérez-Riba M. 2003. Phosphorylation of calcipressin 1 increases its ability to inhibit calcineurin and decreases calcipressin half-life. Biochem J. 374(Pt 2):567–575.
- Hashimoto Y, Ozaki J, Yasuhara M, Hori R, Suga S, Itoh H, Nakao K, Inui K. 1994. Functional evidence for an apical ANP receptor in LLC-PK1 kidney epithelial cells. Eur J Pharmacol. 268(3):443–445.
- Hogan PG, Chen L, Nardone J, Rao A. 2003. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 17(18):2205–2232.
- Imam F, Al-Harbi N, Al-Harbia M, Korashy H, Ansari M, Sayed-Ahmed M, Nagi M, Iqbal M, Khalid Anwer M, Kazmi I, et al. 2017. Rutin attenuates carfilzomib-induced cardiotoxicity through inhibition of NF-κB, hypertrophic gene expression and oxidative stress. Cardiovasc Toxicol. 17(1):58–66.
- Ivanova K, Eiermann P, Tsiockas W, Hemmersbach R, Gerzer R. 2018. Differential regulation of cGMP signaling in human melanoma cells at altered gravity: simulated microgravity down-regulates cancer-related gene expression and motility. Microgravity Sci Technol. 30(4):457–467.
- Koçyildiz Z, Birman H, Olgaç V, Akgün-Dar K, Melikoğlu G, Meriçli A. 2006. Crataegus tanacetifolia leaf extract prevents L-NAME-induced hypertension in rats: a morphological study. Phytother Res. 20(1):66–70.
- Koch E, Malek F. 2011. Standardized extracts from hawthorn leaves and flowers in the treatment of cardiovascular disorders–preclinical and clinical studies. Planta Med. 77(11):1123–1128.
- Lemaitre M, Jannin A, Chevalier B, Vantyghem M. 2022. The heart, an endocrine gland: natriuretic peptides. Ann Endocrinol. 83(1):59–62.
- Li P, Fu J, Li X. 2008. Effect of haw leaf extract and its preparation on polymorphonuclear leucocyte adhesion during HUVEC anoxia/reoxygenation injury. Chin J Integr Tradit West Med. 28:716–720.
- Lin PY, Chu CH, Chang FY, Huang YW, Tsai HJ, Yao TC. 2019. Trends and prescription patterns of traditional Chinese medicine use among subjects with allergic diseases: a nationwide population-based study. World Allergy Organ J. 12(2):100001.
- Ma G, Jiang X, Chen Z, Ren J, Li C, Liu T. 2007. Simultaneous determination of vitexin-4″-O-glucoside and vitexin-2″-O-rhamnoside from Hawthorn leaves flavonoids in rat plasma by HPLC method and its application to pharmacokinetic studies. J Pharm Biomed Anal. 44(1):243–249.
- Molkentin J, Lu J, Antos C, Markham B, Richardson J, Robbins J, Grant S, Olson E. 1998. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 93(2):215–228.
- Morey-Holton E, Globus R. 2002. Hindlimb unloading rodent model: technical aspects. J Appl Physiol (1985). 92(4):1367–1377.
- Platts S, Martin D, Stenger M, Perez S, Ribeiro L, Summers R, Meck J. 2009. Cardiovascular adaptations to long-duration head-down bed rest. Aviat Space Environ Med. 80(5 Suppl):A29–36.
- Rössler A, Noskov V, László Z, Polyakow V, Hinghofer-Szalkay H. 2001. Permanent depression of plasma cGMP during long-term space flight. Physiol Res. 50(1):83–90.
- Ren Y, Huo M, Ma J, Feng W, Qiao Y, Zhang Y. 2020. Study on components efficacy of Salviae miltiorrhizae Radix et Rhizoma based on systematic traditional Chinese medicine. Chin J Chinese Mater Med. 45:3251–3258.
- Sadek M, Mousa S, El-Masry S, Demain A. 2018. Influence of Hawthorn (Crataegus oxyacantha) leaves extract administration on myocardial infarction induced by isoproterenol in rats. CA. 7(1):1–12.
- Shatoor AS, Al Humayed S, Alkhateeb MA, Shatoor KA, Aldera H, Alassiri M, Shati AA. 2019. Crataegus aronia protects and reverses vascular inflammation in a high fat diet rat model by an antioxidant mechanism and modulating serum levels of oxidized low-density lipoprotein. Pharm Biol. 57:38–48.
- Silberbach M, Roberts C. 2001. Natriuretic peptide signalling: molecular and cellular pathways to growth regulation. Cell Signal. 13(4):221–231.
- Soleimanpour SA, Crutchlow MF, Ferrari AM, Raum JC, Groff DN, Rankin MM, Liu C, De León DD, Naji A, Kushner JA, et al. 2010. Calcineurin signalling regulates human islet {beta}-cell survival. J Biol Chem. 285(51):40050–40059.
- Sun H, Ling S, Zhao D, Li Y, Zhong G, Guo M, Li Y, Yang L, Du J, Zhou Y, et al. 2019. Panax quinquefolium saponin attenuates cardiac remodeling induced by simulated microgravity. Phytomedicine. 56:83–93.
- Tokudome T, Horio T, Kishimoto I, Soeki T, Mori K, Kawano Y, Kohno M, Garbers D, Nakao K, Kangawa K. 2005. Calcineurin-nuclear factor of activated T cells pathway-dependent cardiac remodeling in mice deficient in guanylyl cyclase A, a receptor for atrial and brain natriuretic peptides. Circulation. 111(23):3095–3104.
- Tuday E, Berkowitz D. 2007. Microgravity and cardiac atrophy: no sex discrimination. J Appl Physiol. 103(1):1–2.
- Vega RB, Bassel-Duby R, Olson EN. 2003. Control of cardiac growth and function by calcineurin signalling. J Biol Chem. 278(39):36981–36984.
- Veveris M, Koch E, Chatterjee S. 2004. Crataegus special extract WS 1442 improves cardiac function and reduces infarct size in a rat model of prolonged coronary ischemia and reperfusion. Life Sci. 74(15):1945–1955.
- Wang X, Liu Y, Xiao L, Li L, Zhao X, Yang L, Chen N, Gao L, Zhang J. 2018. Hyperoside protects against pressure overload-induced cardiac remodeling via the AKT signalling pathway. Cell Physiol Biochem. 51(2):827–841.
- Xianchu L, Lan Z, Ming L, Yanzhi M. 2018. Protective effects of rutin on lipopolysaccharide-induced heart injury in mice. J Toxicol Sci. 43(5):329–337.
- Xue W, Wang X, Tang H, Sun F, Zhu H, Huang D, Dong L. 2020. Vitexin attenuates myocardial ischemia/reperfusion injury in rats by regulating mitochondrial dysfunction induced by mitochondrial dynamics imbalance. Biomed Pharmacother. 124:109849.
- Yan L, Zhang J, Wang B, Lv Y, Jiang H, Liu G, Qiao Y, Ren M, Guo X. 2013. Quercetin inhibits left ventricular hypertrophy in spontaneously hypertensive rats and inhibits angiotensin II-induced H9C2 cells hypertrophy by enhancing PPAR-γ expression and suppressing AP-1 activity. PLoS One. 8(9):e72548.
- Yang Y, Li J, Rao T, Fang Z, Zhang J. 2021. The role and mechanism of hyperoside against myocardial infarction in mice by regulating autophagy via NLRP1 inflammation pathway. J Ethnopharmacol. 276:114187.
- Zhang W, Lu Y, Yang H, Xu P, Chang H, Yu Z. 2013. Four-week simulated weightlessness increases the expression of atrial natriuretic peptide in the myocardium. Acta Physiol Sin. 65:143–148.
- Zhong G, Li Y, Li H, Sun W, Cao D, Li J, Zhao D, Song J, Jin X, Song H, et al. 2016. Simulated microgravity and recovery-induced remodeling of the left and right ventricle. Front Physiol. 7:274.
- Zhong G, Zhao D, Li J, Liu Z, Pan J, Yuan X, Xing W, Zhao Y, Ling S, Li Y. 2021. WWP1 deficiency alleviates cardiac remodeling induced by simulated microgravity. Front Cell Dev Biol. 9:739944.
- Zhu S, Yan H, Niu K, Zhang S. 2015. Simultaneous determination of seven components from hawthorn leaves flavonoids in rat plasma by LC-MS/MS. J Chromatogr Sci. 53(6):909–914.
