Abstract
Context
Previous studies have highlighted significant therapeutic effects of Qiqilian (QQL) capsule on hypertension in spontaneously hypertensive rats (SHRs); however, its underlying molecular mechanism remains unclear.
Obejective
We investigated the potential mechanism by which QQL improves hypertension-induced vascular endothelial dysfunction (VED).
Materials and methods
In vivo, SHRs were divided into four groups (20 per group) and were administered gradient doses of QQL (0, 0.3, 0.6, and 1.2 g/kg) for 8 weeks, while Wistar Kyoto rats were used as normal control. The vascular injury extent, IL-1β and IL-18 levels, NLRP3, ASC and caspase-1 contents were examined. In vitro, the effects of QQL-medicated serum on angiotensin II (AngII)-induced inflammatory and autophagy in human umbilical vein endothelial cells (HUVECs) were assessed.
Result
Compared with the SHR group, QQL significantly decreased thickness (125.50 to 105.45 μm) and collagen density (8.61 to 3.20%) of arterial vessels, and reduced serum IL-1β (96.25 to 46.13 pg/mL) and IL-18 (345.01 to 162.63 pg/mL) levels. The NLRP3 and ACS expression in arterial vessels were downregulated (0.21- and 0.16-fold, respectively) in the QQL-HD group compared with the SHR group. In vitro, QQL treatment restored NLRP3 and ASC expression, which was downregulated approximately 2-fold compared with that of AngII-induced HUVECs. Furthermore, QQL decreased LC3II and increased p62 contents (p < 0.05), indicating a reduction in autophagosome accumulation. These effects were inhibited by the autophagy agonist rapamycin and enhanced by the autophagy inhibitor chloroquine.
Conclusion
QQL effectively attenuated endothelial injury and inflammation by inhibiting AngII-induced excessive autophagy, which serves as a potential therapeutic strategy for hypertension.
Introduction
Hypertension is a prevalent cardiovascular disease. Globally, the number of patients with hypertension aged 30–79 has doubled to 1.28 billion since 1990 (NCD-RisC Citation2021). In patients with hypertension, the continuous increase in arterial pressure damages the blood vessels and impairs the function of many target organs, including the heart, kidneys, and brain, eventually increasing the risk of diseases such as cardiac hypertrophy, cerebral hemorrhage, and atherosclerosis. High blood pressure causes a longer disease course and substantial harm, making it a leading cause of premature death worldwide. The vascular endothelium is the largest endocrine and paracrine organ with crucial and diverse physiological functions. The dysfunction of vascular endothelium owing to organic damage may result in the onset and progression of hypertension. Furthermore, hypertension can cause endothelial damage, thus creating a vicious cycle. Vascular remodelling refers to the adaptive functional and structural alterations that occur in blood vessels in response to the changes in the internal and external environment (Baumbach and Heistad Citation1989). Vascular remodeling-related disease is regulated by the renin–angiotensin system, inflammatory response, and redox regulation (Whiteford et al. Citation2016). As a chronic inflammatory condition, the onset and progression of hypertension are also associated with immune hyperactivation and the release of inflammatory mediators in vivo (Vanhoutte et al. Citation2009).
NLRP3 inflammasomes comprise NLRP3, ASC, and caspase-1 precursor complexes (Zhou et al. Citation2011). During vascular endothelial injury, NLRP3 inflammasomes are activated, leading to increased inflammation. Inflammasomes can be activated by pattern recognition receptors, including PAMPs and DAMPs. Activated NLRP3 receptors bind to the ASC proteins and recruit the precursor caspase-1 to form the NLRP3 inflammatory complex. Subsequently, precursor caspase-1 is activated and sheared to caspase-1, further activating IL-1β and IL-18 precursors and promoting the massive release of inflammatory factors IL-1β and IL-18, ultimately leading to an inflammatory response. Additionally, inflammasomes regulate caspase-1-dependent programmed pyroptosis that results in cell death under pathological conditions of inflammation and stress (Liu et al. Citation2018; Zhou et al. Citation2018). The reactive oxygen species (ROS) pathway is a common pathway for NLRP3 inflammasome activation, and most activators induce ROS generation and activate NLRP3 downstream (Liu et al. Citation2018). Consequently, cellular ROS levels can indirectly characterize NLRP3 inflammasome activation.
Traditional Chinese medicine (TCM) has a long history of successfully treating hypertension and related complications such as vascular dysfunction (Hao et al. Citation2015). Many TCM herbal compounds have been effectively used in the intervention of cardiovascular diseases, and significant progress has been made in understanding their action mechanisms (Hao et al. Citation2017, Xue et al. Citation2021). Several herbal active ingredients, including astragaloside IV, ellagic acid, curcumin, salidroside, quercetin, resveratrol, purple sweet potato pigment, and honokiol, possess anti-inflammatory and antioxidant effects. Astragaloside IV can decrease inflammasome accumulation and inflammatory factor levels while inhibiting the Notch signaling pathway by blocking the NLRP3/Calpain-1 pathway, thereby alleviating MCT-induced PAH (Sun et al. Citation2021). Ellagic acid inhibits the NLRP3 inflammasome pathway via antioxidant activity and alleviates MCT-induced PAH rats (Tang et al. Citation2015). Curcumin inhibits hypertension-induced VSMC phenotypic transformation and abnormal proliferation by blocking NLRP3 inflammasomes (Sun et al. Citation2017). Furthermore, salidroside alleviates atherosclerosis-induced endothelial senescence through reduces IL-1β-induced vascular inflammation by suppressing NLRP3 inflammasomes (Hu et al. Citation2020), which plays a similar role as quercetin in alleviating vascular inflammation (Li et al. Citation2021). Resveratrol (Bal et al. Citation2022), purple sweet potato pigment (Wang et al. Citation2019, Sun et al. Citation2019), and honokiol (Huang et al. Citation2020) inhibit endothelial and myocardial senescence by blocking NLRP3 inflammasomes. Moreover, these herbal ingredients have the potential to alleviate cardiovascular senescence by modulating NLRP3 inflammasomes and exert anti-ageing activity and cardiovascular system protection through this mechanism. Therefore, they may be considered potential drugs for exerting anti-hypertension and anti-cardiovascular ageing effects by targeting NLRP3 inflammasomes.
Our preliminary findings indicate that Qiqilian (QQL) capsule can effectively lower blood pressure while also protecting the endothelial function of hypertensive blood vessels in patients with hypertension (Yue et al. Citation2010, Qin et al. Citation2013). Animal experiments have revealed that QQL can protect against vascular endothelial injury by decreasing the expression of inflammatory factor high-sensitivity C-reactive protein, mediating malondialdehyde and superoxide dismutase activity (Zhang and Yue Citation2012). However, the molecular mechanism by which QQL improves endothelial function remains unclear. Thus, in this study, we investigated the protective effects and potential mechanisms of QQL in hypertension-induced vascular endothelial dysfunction (VED).
Materials and methods
Animals and treatment
A 0.2 g/mL QQL suspension was formulated from the QQL capsules prepared at the Ethnomedicine Research and Development Base of Rui Kang Hospital, Guangxi University of Chinese Medicine. Animals used in this study were obtained from Beijing Vital River Laboratory Animal Technology Company (Beijing, China), and all animal experiments were conducted at the Animal Experiment Center of the Guangxi University of Chinese Medicine. All protocols and experiments involving animals were conducted following the Affidavit of Approval of Animal Ethicals and Welfare and approved by the Guangxi University Chinese Medicine Institutional Animal Ethical and Welfare Committee (Approval No. DW20210722-003).
The sample size of animal experiments was based on our previous study (Luo et al. Citation2015) on the efficacy of QQL on spontaneously hypertensive rats (SHRs) (Supplementary materials: Table S1). Here, 20 male Wistar Kyoto (WKY) rats [SPF level, certificate: SCXK(Jing)2016-0006] and 80 sex-matched 8-weeks-old SHRs [SPF level, certificate: SCXK(Jing)2016-0006] were acclimatized in the laboratory for 1 week prior to gavage treatment. All experimental rats were housed in plastic cages in SPF animal room at 22 ± 2 °C with a 12 h light/dark cycle. The SHRs were randomly categorized into three groups, each containing 20 individuals: QQL-LD group (receiving 0.3 g/kg QQL), QQL-MD group (receiving 0.6 g/kg QQL), and QQL-HD group (receiving 1.2 g/kg QQL). These doses were equivalent to 0.5-, 1-, and 2-fold doses used in clinical applications, respectively. Furthermore, one group of SHRs was gavaged with an equal amount of saline (blank group), and the WKY group was also gavaged with an equal amount of saline (control group). The remaining WKY rats were used for obtaining QQL-medicated serum. Gavage was performed once a day for 8 weeks. Samples were obtained after the rats fasted for 24 h (). Following the sacrifice of the rats under deep anaesthesia, aortic tissues were quickly extracted to obtain blood for serum samples, which were stored at −80 °C. Arterial tissue samples were fixed in 4% paraformaldehyde for tissue staining, immunohistochemistry, and immunofluorescence. Finally, the extracted arterial tissues were fixed in pre-cooled 2.5% glutaraldehyde for examination under an electron microscope.
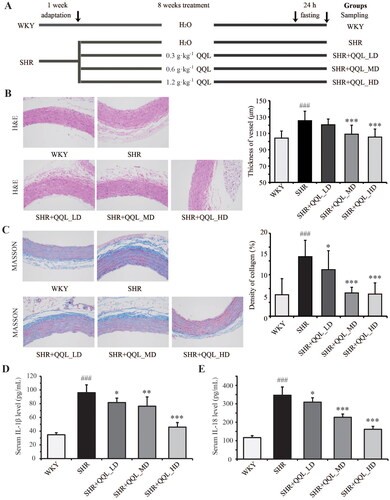
Figure 1. QQL attenuates arterial damage and inflammation of SHR. (A) The workflow of the animal treatment by QQL. (B) Images of H&E staining (200×) of arterial roots and thickness of arterial vessels (n = 8). (C) Images of MASSON staining (200×) and collagen density of arterial vessels (n = 8). (D) The serum level of IL-1β (n = 8). E) The serum level of IL-18 (n = 8). ### indicates a significant difference (p < 0.001) in SHR group compared with WKY group. * indicates a significant difference (p < 0.05), ** indicates a significant difference (p < 0.01), *** indicates a significant difference (p < 0.001) in QQL treatment groups compared with SHR group.
QQL-medicated serum preparation
The QQL-medicated serum was prepared with a minor modification of the method described by Fei et al. (Citation2019). For the preparation of drug-medicated serum, an additional 30 male 8-week-old WKY rats [SPF level, certificate: SCXK(Jing)2016-0006] were divided into two groups and treated with QQL (1.2 g/kg) and an equal amount of water twice a day for one week, respectively. One hour after the last administration, blood was collected from the abdominal aorta, centrifuged for 10 min at 4 °C, filtered through 0.22 μm microporous filter membrane and stored in −80 °C. Before the treatment of human umbilical vein endothelial cells (HUVECs), the QQL-medicated serum and drug-free serum were thawed and incubated for 30 min at −56 °C.
Drug concentration assessment using CCK-8 assay
The primary HUVECs and a complete medium (ECM basal medium, 10% FBS, 1% ECGS and 1% P/S) for enlarged cultivation of endothelial cells were purchased from Zhong Qiao Xin Zhou Biotechnology Company (Shanghai, China). To determine the optimal drug concentration, HUVECs were incubated with a concentration gradient of angiotensin II (AngII; 0, 10−5, 10−6, 10−7 and 10−8 M). Similarly, for the treatment of QQL-medicated serum, a gradient proportion (2.5, 5, 7.5 and 10%) of QQL-medicated serum along with AngII (10−6 M) was applied to HUVECs. After 24 h of treatment, HUVEC viability was determined using CCK-8 Cell Proliferation and Cytotoxicity Assay Kit (Solarbio, Beijing, China) as previously described (Zhao et al. Citation2022).
Experiment grouping and treatment of HUVECs
To investigate the effect of QQL on AngII-induced inflammatory damage, the HUVECs were categorized into seven treatment groups (each with three replicates):
Control group: HUVECs cultivated without drug treatment.
AngII group: AngII-treated HUVECs cultivated with drug-free serum.
AngII + QQL group: AngII-treated HUVECs cultivated with QQL-medicated serum (7.5%).
AngII + RAPA group: AngII-treated HUVECs cultivated with drug-free serum, along with rapamycin (RAPA) treatment (20 nM).
AngII + CQ group: AngII-treated HUVECs cultivated with drug-free serum, along with chloroquine (CQ) treatment (10 μM).
AngII + QQL + RAPA group: AngII-treated HUVECs cultivated with QQL-medicated serum (7.5%), along with RAPA treatment (20 nM).
AngII + QQL + CQ group: AngII-treated HUVECs cultivated with QQL-medicated serum (7.5%), along with CQ treatment (10 μM).
The treatment groups with drug-containing serum were administered to supplement the control serum to 10% of the medium serum ratio. After 24 h of treatment, cells from each group were digested using trypsin-EDTA enzyme solution, dispersed and mixed, centrifuged, washed for cell precipitation, and collected for further analysis. All the cell experiments were set with three replicates.
Enzyme-linked immunosorbent assay
The levels of inflammatory factors IL-1β and IL-18 were measured in the serum of rats (eight replicates) and supernatant of cell cultures (three replicates) using ELISA kits (Jiangsu Enzyme Immune Industrial Co., Ltd., Jiangsu, China) according to the manufacturer’s instructions.
Determination of ROS content
HUVECs were collected by centrifugation after 24 h of drug treatment. Following the manufacturer’s instructions and the protocol description before (Zhao et al. Citation2021), the cellular ROS content was assessed using the Reactive Oxygen Species Assay Kit (Solarbio, Beijing, China) and quantified using flow cytometry by measuring the fluorescent signal intensity of DCF, which is the product of the DCFH-DA probe oxidized by ROS.
Histopathological staining
Aortic tissues originating from six rats were fixed with 4% paraformaldehyde, embedded in paraffin, and longitudinally sliced into 4 µm sections. The sections were then dewaxed and stained with hematoxylin and eosin (H&E). Furthermore, Masson staining was performed to identify collagen fibres in the aorta. The strained tissues were examined using optical microscopy (Nikon Eclipse E100), and the images were captured using an imaging system (Nikon DS-U3). The thickness of the vessel and density of collagen was calculated using ImageJ software.
Immunohistochemistry analysis
Five sections of each group were subjected to dewaxing and rehydration using gradient alcohol. Antigen retrieval was performed, followed by treatment with 3% H2O2 to inhibit the activity of endogenous peroxidase, serum-based blocking, incubation with primary antibodies (NLRP3, ab263899; ASC, ab175449; Abcam, USA), and incubation with HRP-labeled secondary antibodies. DAB staining was used to restain nuclei with hematoxylin. The stained sections were observed under an optical microscope system (Olympus BX53), followed by image capture. The quantitative analysis of protein expression levels was performed by ImageJ software.
Immunofluorescence staining
For each group, five sections were subjected to the standard immunofluorescence protocol, including dewaxing and rehydration, antigen retrieval, blocking with serum, and incubation with primary and fluorescent secondary antibodies. After nuclear re-staining, DAPI was observed under a fluorescent microscope (Olympus BX53) following the sealing of the slice, and images were then captured. Staining images were relatively quantified using ImageJ software.
RT–PCR
Arterial tissues from nine individuals were pooled into three replicates randomly. Total RNA was extracted from the arterial tissues following the Animal Tissue Total RNA Extraction Kit instructions and reverse-transcribed into first-strand cDNA using Novozymes HiScript II Q RT SuperMix for qPCR (+gDNA wiper) to remove gDNA. Subsequently, the target genes were amplified using ChemQ Universal SYBR qPCR Master Mix and the quantitative fluorescence signal was detected using the AnalytikJena qTOWERE2.2 Fluorescence PCR instrument. The 2-ΔΔCt method was used to evaluate gene expression, which was normalized against internal reference gene GAPDH. The primer sequences used in this study are listed in .
Table 1. List of primers used for real-time PCR.
Western blotting
Arterial tissues from nine individuals were pooled into three replicates randomly. Following liquid nitrogen treatment, tissues were ground into powder, and mixed with RIPA protein lysate containing 1% cocktail, sonicated for 1–2 min to extract the total protein and centrifuged at 1000 rpm for 10 min (4 °C) to collect the protein supernatant. The BCA protein quantification kit was used to determine the protein concentration. Equal amounts of proteins were subjected to SDS–PAGE electrophoresis, transferred to PVDF membranes, blocked with 5% skim milk powder, and incubated with primary antibodies (NLRP3, ab263899; ASC, ab175449; caspase-1, ab286125; LC3, ab192890; Beclin-1, ab207612; p62, ab109012; Abcam, USA) and HRP-labeled secondary antibodies (Goat Anti-Mouse IgG(H + L), SA00001-1; Goat Anti-Rabbit IgG(H + L), SA00001-2; Proteintech, China). GAPDH (60004-1-Ig, Proteintech, China) served as an internal reference. Finally, the membranes were incubated in an ECL chromogenic solution, and the signal was detected using the Amersham Imager 680 imaging system (GE Healthcare, Chicago, USA). The relative quantitative analysis of protein bands was evaluated using ImageJ software.
Transmission electron microscopy
The cellular ultrastructural alterations and a number of autolysosomes were assessed using TEM. Treated HUVECs in each group were fixed with 2.5% glutaraldehyde and post-fixed with 1% osmic acid for 2 h. They were then rinsed three-times with PBS for 15 min each gradient was dehydrated with different concentrations of ethanol (50%, 70%, 80%, 90%, 95%, and 100%) for 15 min each, and embedded in epoxy resin. After ultrathin sectioning (60-80 nm), the samples were double-stained with uranium lead (2% uranyl acetate saturated aqueous solution and lead citrate, each for 15 min). Finally, the sections were imaged using a transmission electron microscope (Hitachi, HT7700-SS, Tokyo, Japan).
Statistical analysis
Data were presented as mean ± SD (standard deviation). Statistical analysis was performed using SPSS 26 for Windows System. The Shapiro-Wilk test was used to assess the normal distribution of the variables. The statistical analyses of multiple comparison were performed using one-way ANOVA and the post hoc least significant difference (LSD) method to observe the significant differences between sample groups. p < 0.05 was considered statistically significant.
Results
QQL ameliorates arterial vascular injury in SHRs
The experimental animals were kept according to the protocol depicted in . No significant differences were observed in the body weight in each group before and after treatment, indicating that QQL did not significantly affect the growth of SHRs. In SHRs, H&E staining revealed thickened and hyperplastic artery blood vessels, which were ameliorated by QQL therapy. The amelioration effect was more prominent at higher QQL doses (SHR, 125.50 ± 11.66 μm; QQL-LD, 120.55 ± 7.05 μm; QQL-MD, 109.01 ± 11.00 μm; QQL-HD, 105.45 ± 9.82 μm) (). Furthermore, Masson staining confirmed arterial vessel hyperplasia and significant collagen deposition in SHRs, which were significantly improved by QQL treatment (SHR, 8.61 ± 2.37%; QQL-LD, 6.74 ± 2.73%; QQL-MD, 3.36 ± 0.82%; QQL-HD, 3.20 ± 1.62%) (). Inflammatory factors were assessed in the serum of each group using ELISA. The findings showed that the serum IL-1β and IL-18 levels were significantly increased in the SHR group (90.25 ± 11.25 and 345.01 ± 45.73 pg/mL, respectively) compared with the WKY group (34.75 ± 3.20 and 116.59 ± 13.83 pg/mL, respectively) and were restored in the high-dose QQL treatment groups (46.13 ± 6.97 and 162.63 ± 20.56 pg/mL, respectively) ().
QQL reduces NLRP3 and ASC expression in the arterial vasculature of SHRs
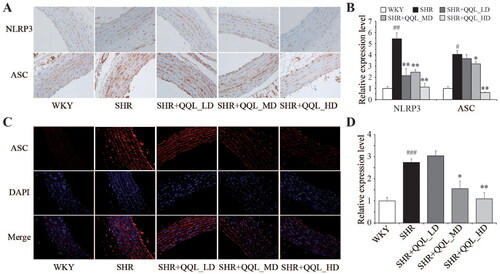
The expression of NLRP3 and ASC proteins in each group was identified using immunohistochemistry to determine the protective effect of QQL on arterial vessels. The findings suggested that the expression of NLRP3 and ASC proteins in the arterial vessels of SHRs was significantly higher than that in the WKY group, which was restored considerably by QQL treatment, particularly in the high-dose QQL treatment groups. The percentage determination of the staining area shows that the relative expression levels of NLRP3 and ASC decreased to 0.21- and 0.16-fold, respectively, in the high-dose QQL treatment group compared with those in the SHR group (). Further immunofluorescence determination of ASC protein expression in arterial vessels yielded results consistent with that of immunohistochemistry ().
Figure 2. QQL ameliorates the inflammasome of arterial in SHR. (A) The positive expression of NLRP3 and ASC in arterial was observed by immunohistochemistry analysis. (B) Relative quantitative analysis of NLRP3- and ASC-positive cells in arterial tissue (n = 5). (C) The positive expression of ASC in arterial observed by immunofluorescence analysis. (D) Relative quantitative analysis of ASC-positive cells in arterial tissue (n = 5). # indicates significant difference (p < 0.05), ## indicates significant difference (p < 0.01), ###indicates significant difference (p < 0.001) in SHR group compared with WKY group. *indicates a significant difference (p < 0.05), ** indicates significant difference (p < 0.01) in QQL treatment groups compared with SHR group.
QQL decreases the expression of inflammatory molecules in SHRs
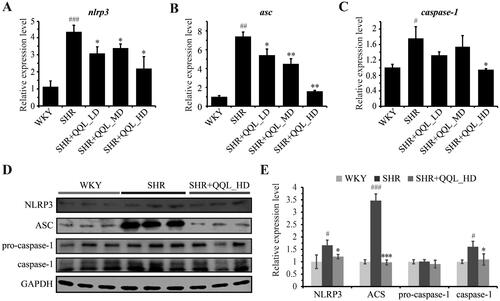
Fluorescent quantitative PCR was used to evaluate the gene expression of inflammatory pathways and their downstream molecules in arterial vessels, and the results were consistent with immunohistochemical and immunofluorescence assays at the tissue level. The expression of NLRP3 and ASC was significantly higher in arterial vessels of SHRs than in the WKY group but significantly lower in the QQL high-dose group (). Furthermore, the caspase-1 gene expression exhibited the same trend (). Moreover, western blotting results highlighted that QQL significantly reduced the high expression of NLRP3, ASC, caspase-1 proteins in SHRs (). These findings indicate that QQL significantly alleviates inflammation in arterial vessels of SHRs.
Figure 3. QQL reduces the expression of inflammatory-related genes and proteins in SHR (n = 3). (A) mRNA expression level of NLRP3. (B) the mRNA expression level of ASC. (C) mRNA expression level of caspase-1. (D) Protein expression levels of NLRP3, ASC, caspase-1 and cleaved-caspase-1. (E) Relative quantification of the expression levels of NLRP3, ASC, caspase-1 and cleaved-caspase-1. # indicates significant difference (P value < 0.05), ## indicates significant difference (p < 0.01), ### indicates significant difference (p < 0.001) of SHR group compared with WKY group. * indicates a significant difference (p < 0.05), ** indicates a significant difference (p < 0.01), *** indicates a significant difference (p < 0.001) of QQL treatment groups compared with SHR group.
Assessment of cell viability of HUVECs under AngII and QQL treatment
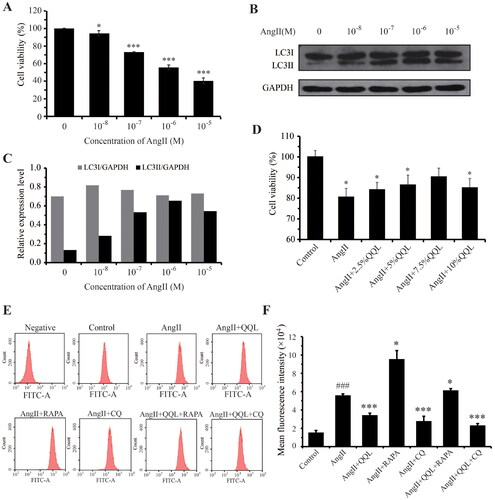
We evaluated the optimal in vitro concentration of AngII in HUVECs and the optimal treatment concentration of QQL-medicated serum. Furthermore, 10−6 M was found to be an approximate semi-lethal concentration of AngII (). AngII significantly increased the LC3II protein expression (). Treatment with 7.5% QQL-medicated serum with 10−6 M AngII significantly enhanced the proliferation rate of HUVECs ().
Figure 4. QQL ameliorates ROS generation in AngII-induced HUVECs. (A) Cell viability under different concentrations of AngII measured by CCK-8. (B) Protein levels of LC3 determined by western blot assay. (C) Relative expression levels of LC3I/GAPDH and LC3II/GAPDH were quantified by densitometric analysis. (D) Cell viability under the different percentages of QQL-medicated serum measured by CCK-8 (n = 3). (E) ROS accumulation in HUVECs was detected by flow cytometry with the DCFH-DA probe (n = 3). (F) Mean fluorescence intensity was statistically analyzed in each group. ### indicates a significant difference (p < 0.001) in AngII group compared with the control group. * indicates a significant difference (p < 0.05), *** indicates a significant difference (p < 0.001) in treatment groups compared with the control group in (A) and (D), and in treatment groups compared with AngII group in (F).
QQL eliminates AngII-induced ROS accumulation in HUVECs
The cellular ROS levels were measured in the control, AngII, AngII + QQL, AngII + RAPA, AngII + CQ, AngII + QQL + RAPA, and AngII + QQL + CQ groups to determine whether QQL ameliorated the cellular dysfunction of AngII-induced HUVECs through antioxidant effects. The findings revealed that AngII treatment significantly increased the intracellular ROS accumulation in HUVECs. QQL, CQ, and QQL + CQ treatment significantly reduced the ROS levels, with a decrease of 0.61-, 0.46-, and 0.41-fold, respectively, compared with that in the AngII group. However, ROS levels did not decrease in the AngII + QQL + RAPA treatment group ().
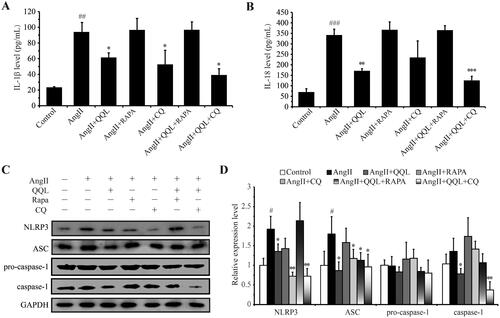
QQL alleviates inflammation in AngII-induced HUVECs
ELISA was performed on IL-1β and IL-18 in the cell supernatant of each group of samples to evaluate the effect of QQL on the cellular inflammation level in HUVECs. The findings revealed that AngII significantly increased the supernatant IL-1β and IL-18 levels of HUVECs (91.67 ± 17.14 and 332.21 ± 34.63 pg/mL, respectively), whereas QQL treatment significantly decreased their levels = (59.13 ± 8.83 and 170.44 ± 7.28 pg/mL, respectively). Furthermore, IL-1β and IL-18 levels were higher in the QQL + RAPA treatment group, and QQL + CQ treatment (39.50 ± 11.62 and 126.05 ± 24.75 pg/mL, respectively) also had a significant effect (). Western blot analysis of key markers of cellular inflammasomes showed a significant increase in the levels of NLRP3, ASC, and cleaved-Caspase 1 in the AngII treatment group, which were reversed by QQL treatment. Furthermore, QQL + CQ effectively reduced inflammatory complex protein levels, whereas QQL + RAPA did not have a significant effect ().
Figure 5. QQL reduces the inflammation in AngII-induced HUVECs. (A) The cell supernatant level of IL-1β (n = 3). (B) The cell supernatant level of IL-18 (n = 3). (C) Protein expression levels of NLRP3, ASC, caspase-1 and cleaved-caspase-1. (D) Relative quantification of the expression levels of NLRP3, ASC, caspase-1 and cleaved-caspase-1 (n = 3). # indicates significant difference (P value < 0.05), ## indicates significant difference (P value < 0.01), ### indicates significant difference (P value < 0.001) in AngII group compared with control group. * indicates a significant difference (P value < 0.05), ** indicates a significant difference (P value < 0.01), *** indicates a significant difference (P value < 0.001) in QQL treatment groups compared with AngII group.
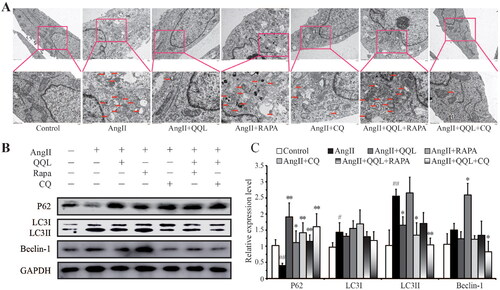
QQL inhibits excessive autophagy in AngII-induced HUVECs via the autophagic pathway
A TEM ultrastructural study was conducted on cells from each sample group to determine the influence of QQL on the autophagy level of HUVECs. The results revealed that AngII-induced HUVECs had a large number of cytolysosomes or autophagic lysosomes and abnormal mitochondrial structure. However, QQL treatment significantly inhibited the production of cytolysosomes or autophagic lysosomes. The QQL + RAPA treatment had no significant effect, whereas the CQ and QQL + CQ treatments also had a considerable effect (). Furthermore, western blot analysis of autophagy-related key markers revealed a substantial increase in LC3II and Beclin-1 expression levels in the AngII treatment group, as well as suppression of p62/SQSTM1 expression, which was reversed by QQL treatment. In addition, these changes induced by QQL could be inhibited by the combined treatment of RAPA and enhanced by the combined treatment of CQ ().
Figure 6. QQL suppressed autophagy in AngII-induced HUVECs. (A) Ultrastructure by TEM under different treatments of HUVEC. Red arrow indicates the location of autophagosomes. (B) Protein expression levels of p62, LC3I/II and Beclin-1. (C) Relative quantification of the expression levels of p62, LC3I/II and Beclin-1 (n = 3). # indicates significant difference (P value < 0.05), ## indicates significant difference (P value < 0.01) of SHR group compared with WKY group. * indicates significant difference (P value < 0.05), ** indicates significant difference (P value < 0.01) of QQL treatment group compared with SHR group.
Discussion
Endothelial dysfunction is a common feature of cardiovascular disease, characterized by oxidative stress and inflammatory response. Our previous study demonstrated that QQL can alleviate vascular injury in animals via its antioxidant effects and by reducing ET and AngII vasoconstrictor secretion. The present study showed that QQL ameliorated the proliferation and inflammation of aortic vessels in SHRs at the pathological, physiological, and molecular levels. Furthermore, the QQL was shown to repair AngII-induced cellular inflammation, viability inhibition, and ROS stress in HUVECs at the cellular level. Further analysis of autophagy-related indicators validated that QQL can inhibit AngII-induced excessive autophagy in HUVECs.
AngII production is linked to endothelial dysfunction caused by hypertension, making it the most common method for establishing hypertension cell models in vitro (Dimmeler et al. Citation1997, Mirabito Colafella and Danser Citation2017). Therefore, we examined the role of QQL in AngII-induced cellular inflammation in HUVECs. ROS is a crucial intracellular signalling regulator whose overproduction-induced oxidative stress has been associated with AngII-induced tissue damage and cellular dysfunction, including endothelial dysfunction (Dharmashankar and Widlansky Citation2010, Silva et al. Citation2012, Bian et al. Citation2017). Our findings showed that QQL can reverse AngII-induced ROS levels in HUVECs and significantly downregulate AngII-induced overproduction of pro-inflammatory factors IL-1β and IL-18 in the supernatant of HUVECs. Moreover, at the molecular level, QQL substantially decreased the protein expression levels of NLRP3, ASC, and pro-caspase in HUVECs induced by AngII treatment. These findings suggest that QQL enhances the antioxidant properties of HUVECs, reduces ROS levels, and eliminates AngII-induced cellular inflammation and endothelial dysfunction by blocking the assembly and activation of the NLRP3/ASC/pro-caspase inflammatory complex.
Autophagy is a crucial cellular process that degrades organelles, intracellular proteins, and other macromolecules and recycles degradation products. Autophagy can occur in both physiological and pathological states (Kim and Lee Citation2014). The involvement of autophagy in cell survival and death depends on its degree. Mild autophagy protects cells, whereas excessive autophagy over-digests cellular components and induces cell death (Denton et al. Citation2015). Autophagy activation is crucial in AngII-induced HUVEC functional impairment (Liu et al. Citation2021). Impaired autophagy is linked to the accumulation of ROS and the activation of inflammasomes (Deretic et al. Citation2013, Li et al. Citation2015). RAPA regulates cellular autophagy, glucose metabolism, and protein-nucleic acid synthesis homeostasis by inhibiting the mTOR pathway. Moreover, it inhibits vascular oxidative stress and AMPK activation and reverses endothelial dysfunction in senescent vessels. Furthermore, RAPA can inhibit NLRP3 inflammasomes and stimulate cellular autophagy, making it a potential drug for treating cardiovascular senescence by regulating NLRP3 inflammasomes. The mechanism by which autophagy and TLR inhibitor CQ ameliorate AngII-induced aortic remodelling in mice may be associated with the inhibition of excessive activation of autophagy (Tian et al. Citation2018). Additionally, CQ and hydroxychloroquine have ameliorative effects in experimental pulmonary hypertension models, with inhibition of endothelial cell autophagy and lysosomal degradation pathways being the primary action mechanisms (Long et al. Citation2013). In our study, the submicroscopic structure of cellular autophagy in the control, AngII, AngII + QQL, AngII + RAPA, AngII + CQ, AngII + QQL + RAPA, and AngII + QQL + CQ groups were examined to investigate whether QQL regulates inflammation and injury in HUVECs through autophagy. The findings suggest that AngII-induced excessive autophagy could be ameliorated by QQL treatment. Furthermore, it achieved minimal when it was co-treated with CQ. However, co-treatment of QQL with AngII + RAPA exhibited a synergistic effect. Hence, the alleviation of AngII-induced inflammation in HUVECs by QQL is most likely mediated by the inhibition of excessive autophagy. These results were further validated by the expression levels of autophagy marker proteins in each group of HUVECs.
Conclusions
QQL effectively suppress inflammatory reaction and reduce oxidative stress in SHRs and AngII-induced HUVECs by regulating NLRP3-ASC pathway and the inhibitor of ROS accumulation. Furthermore, these roles of QQL in the treatment of hypertension might contribute by inhibiting AngII-induced excessive autophagy in endothelial cells. These findings suggested that QQL can serve as a potential therapeutic strategy for hypertension patients in the clinic.
Authors’ contributions
Yuan Luo and Guihua Yue conceived the experimental design and edited the manuscript; Yun Ye and Zhenyuan Tan participated in the experimental study; Xiaocong Ma contributed the statistical analysis and results visualization. All authors have read and approved the final manuscript.
Supplemental Material
Download MS Word (16.3 KB)Acknowledgements
We thank Jinlin Cheng (Nanning Current Science Biotechnology Co., Ltd.) for editing this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analyzed during this study are included in this published article and its additional files.
Additional information
Funding
References
- Bal NB, Bostanci A, Sadi G, Donmez MO, Uludag MO, Demirel-Yilmaz E. 2022. Resveratrol and regular exercise may attenuate hypertension-induced cardiac dysfunction through modulation of cellular stress responses. Life Sci. 296:120424.
- Baumbach GL, Heistad DD. 1989. Remodeling of cerebral arterioles in chronic hypertension. Hypertension. 13(6 Pt 2):968–972.
- Bian F, Cui J, Zheng T, Jin S. 2017. Reactive oxygen species mediate angiotensin II-induced transcytosis of low-density lipoprotein across endothelial cells. Int J Mol Med. 39(3):629–635.
- Denton D, Xu T, Kumar S. 2015. Autophagy as a pro-death pathway. Immunol Cell Biol. 93(1):35–42.
- Deretic V, Saitoh T, Akira S. 2013. Autophagy in infection, inflammation and immunity. Nat Rev Immunol. 13(10):722–737.
- Dharmashankar K, Widlansky ME. 2010. Vascular endothelial function and hypertension: insights and directions. Curr Hypertens Rep. 12(6):448–455.
- Dimmeler S, Rippmann V, Weiland U, Haendeler J, Zeiher AM. 1997. Angiotensin II induces apoptosis of human endothelial cells. Protective effect of nitric oxide. Circ Res. 81(6):970–976.
- Fei YX, Zhao B, Yin QY, Qiu YY, Ren GH, Wang BW, Wang YF, Fang WR, Li YM. 2019. Ma Xing Shi Gan decoction attenuates PM2.5 induced lung injury via inhibiting HMGB1/TLR4/NFkappaB signal pathway in rat. Front Pharmacol. 10:1361.
- Hao P, Jiang F, Cheng J, Ma L, Zhang Y, Zhao Y. 2017. Traditional Chinese medicine for cardiovascular disease: evidence and potential mechanisms. J Am Coll Cardiol. 69(24):2952–2966.
- Hao PP, Jiang F, Chen YG, Yang J, Zhang K, Zhang MX, Zhang C, Zhao YX, Zhang Y. 2015. Traditional Chinese medication for cardiovascular disease. Nat Rev Cardiol. 12(6):318.
- Hu R, Wang MQ, Ni SH, Wang M, Liu LY, You HY, Wu XH, Wang YJ, Lu L, Wei LB. 2020. Salidroside ameliorates endothelial inflammation and oxidative stress by regulating the AMPK/NF-kappaB/NLRP3 signaling pathway in AGEs-induced HUVECs. Eur J Pharmacol. 867:172797.
- Huang PP, Fu J, Liu LH, Wu KF, Liu HX, Qi BM, Liu Y, Qi BL. 2020. Honokiol antagonizes doxorubicin-induced cardiomyocyte senescence by inhibiting TXNIPmediated NLRP3 inflammasome activation. Int J Mol Med. 45(1):186–194.
- Kim KH, Lee MS. 2014. Autophagy–a key player in cellular and body metabolism. Nat Rev Endocrinol. 10(6):322–337.
- Li H, Xiao L, He H, Zeng H, Liu J, Jiang C, Mei G, Yu J, Chen H, Yao P, et al. 2021. Quercetin attenuates atherosclerotic inflammation by inhibiting galectin-3-NLRP3 signaling pathway. Mol Nutr Food Res. 65(15):e2000746.
- Li L, Tan J, Miao Y, Lei P, Zhang Q. 2015. ROS and autophagy: interactions and molecular regulatory mechanisms. Cell Mol Neurobiol. 35(5):615–621.
- Liu D, Sun WP, Chen JW, Jiang Y, Xue R, Wang LH, Murao K, Zhang GX. 2021. Autophagy contributes to angiotensin II induced dysfunction of HUVECs. Clin Exp Hypertens. 43(5):462–473.
- Liu D, Zeng X, Li X, Mehta JL, Wang X. 2018. Role of NLRP3 inflammasome in the pathogenesis of cardiovascular diseases. Basic Res Cardiol. 113(1):5.
- Liu Q, Zhang D, Hu D, Zhou X, Zhou Y. 2018. The role of mitochondria in NLRP3 inflammasome activation. Mol Immunol. 103:115–124.
- Long L, Yang X, Southwood M, Lu J, Marciniak SJ, Dunmore BJ, Morrell NW. 2013. Chloroquine prevents progression of experimental pulmonary hypertension via inhibition of autophagy and lysosomal bone morphogenetic protein type II receptor degradation. Circ Res. 112(8):1159–1170.
- Luo Y, Yue GH, Yun YE, Lei FU. 2015. Protective effect of qiqilian capsule against left ventricular hypertrophy of spontaneously hypertensive rats. Chin J Exper Trad Med Form. 21:116–120.
- Mirabito Colafella KM, Danser AHJ. 2017. Recent advances in angiotensin research. Hypertension. 69(6):994–999.
- NCD Risk Factor Collaboration (NCD-RisC). 2021. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 398(10304):957–980.
- Qin Y, Zhu Z, Zhang A, Yue G. 2013. Effect of Qiqilian capsule on life quality of hypertension patients. Jiangsu J Trad Chin Med. 45:25–27.
- Silva BR, Pernomian L, Bendhack LM. 2012. Contribution of oxidative stress to endothelial dysfunction in hypertension. Front Physiol. 3:441.
- Sun C, Diao Q, Lu J, Zhang Z, Wu D, Wang X, Xie J, Zheng G, Shan Q, Fan S, et al. 2019. Purple sweet potato color attenuated NLRP3 inflammasome by inducing autophagy to delay endothelial senescence. J Cell Physiol. 234(5):5926–5939.
- Sun HJ, Ren XS, Xiong XQ, Chen YZ, Zhao MX, Wang JJ, Zhou YB, Han Y, Chen Q, Li YH, et al. 2017. NLRP3 inflammasome activation contributes to VSMC phenotypic transformation and proliferation in hypertension. Cell Death Dis. 8(10):e3074.
- Sun Y, Lu M, Sun T, Wang H. 2021. Astragaloside IV attenuates inflammatory response mediated by NLRP-3/calpain-1 is involved in the development of pulmonary hypertension. J Cell Mol Med. 25(1):586–590.
- Tang B, Chen GX, Liang MY, Yao JP, Wu ZK. 2015. Ellagic acid prevents monocrotaline-induced pulmonary artery hypertension via inhibiting NLRP3 inflammasome activation in rats. Int J Cardiol. 180:134–141.
- Tian X, Zhao X, Liu D, Zhang Y, Qu L, Wu P, He L. 2018. Effect of chloroquine in angiotensin II induced vascular remodeling in mice. J Clin Pathol Res. 38:1595–1600.
- Vanhoutte PM, Shimokawa H, Tang EH, Feletou M. 2009. Endothelial dysfunction and vascular disease. Acta Physiol. 196(2):193–222.
- Wang R, Liu L, Liu H, Wu K, Liu Y, Bai L, Wang Q, Qi B, Qi B, Zhang L. 2019. Reduced NRF2 expression suppresses endothelial progenitor cell function and induces senescence during aging. Aging (Albany NY). 11(17):7021–7035.
- Whiteford JR, D, Rossi G, Woodfin A. 2016. Mutually supportive mechanisms of inflammation and vascular remodeling. Int Rev Cell Mol Biol. 326:201–278.
- Xue Z, Li Y, Zhou M, Liu Z, Fan G, Wang X, Zhu Y, Yang J. 2021. Traditional herbal medicine discovery for the treatment and prevention of pulmonary arterial hypertension. Front Pharmacol. 12:720873.
- Yue G, Mo L, Luo Y. 2010. Effect of Yiqihuoxuejiedu decoction on inflammatory factor and von Willebrand factor of spontaneously hypertensive pressure rats. J Emerg Tradit Chin Med. 19:1365–1367.
- Zhang Z, Yue G. 2012. Protective effects of Yiqi Huoxue Jiedu recipe on the function of blood vessel endothelium in spontaneous hypertension rats. China Pharmacy. 23:200–202.
- Zhao N, Yang FE, Zhao CY, Lv SW, Wang J, Liu JM, Wang S. 2021. Construction of pH-dependent nanozymes with oxygen vacancies as the high-efficient reactive oxygen species scavenger for oral-administrated anti-inflammatory therapy. Adv Healthc Mater. 10(23):e2101618.
- Zhao ZQ, Zhang BL, Chu HQ, Liang L, Chen BZ, Zheng H, Guo XD. 2022. A high-dosage microneedle for programmable lidocaine delivery and enhanced local long-lasting analgesia. Biomater Adv. 133:112620.
- Zhou R, Yazdi AS, Menu P, Tschopp J. 2011. A role for mitochondria in NLRP3 inflammasome activation. Nature. 469(7329):221–225.
- Zhou W, Chen C, Chen Z, Liu L, Jiang J, Wu Z, Zhao M, Chen Y. 2018. NLRP3: a novel mediator in cardiovascular disease. J Immunol Res. 2018:5702103.
