Abstract
Context
The genus Glaucium Mill., one of the important Papaveraceae family plants, is rich in isoquinoline alkaloids and distributed worldwide.
Objective
Isolation and identification of bioactive alkaloids from Glaucium grandiflorum Boiss. & Huet. subsp. refractum (Nabelek) Mory var. torquatum (Cullen) Mory and G. corniculatum (L.) Rudolph var. corniculatum (Aslan Citation2012), and investigation of their antioxidant and anticholinesterase activities.
Materials and methods
The aerial parts of each plant were dried, powdered, and percolated with methanol, then each extract was fractionated between 50% aqueous acetic acid and petroleum. Their aqueous acidic layer was adjusted to pH 7–8 with NH4OH and extracted with chloroform, the extract was subjected to CC separation and isolation. Structures of the isolated alkaloids were elucidated by 1D and 2D-NMR and mass spectral analyses. The alkaloid extracts and their pure alkaloids were tested for anti-cholinesterase (AChE and BuChE) and antioxidant (ABTS, CUPRAC, β-carotene linoleic acid tests) activities in vitro.
Results
Methanol extracts of Glaucium grandiflorum subsp. refractum var. torquatum and G. corniculatum var. corniculatum afforded a novel compound glauciumoline and seven known isoquinoline alkaloids three of which have an aporphine-type and the other five have a protopine-type skeleton. Among them, trans-protopinium (7) and cis-protopinium (8) were isolated from a Glaucium species for the first time. Tertiary amine extracts (TAEs) of both plants showed very strong acetylcholinesterase inhibitory activity. The TAE of the plants also showed strong antioxidant activity while the isolated alkaloids showed no meaningful activity in the anticholinesterase and antioxidant tests.
Discussion and conclusions
Glaucium species are considered promising therapeutic agents in the treatment of Alzheimer’s disease.
Introduction
Glaucium Mill. (Papaveraceae) is one of the most diverse genera, distributed worldwide. The genus includes about 28 species (Kadereit Citation1993; Mingli and Grey-Wilson Citation2008; Aykurt et al. Citation2017; Akaberi et al. Citation2021) native to southern Europe and Mediterranean region, and central and south-western Asia. Most of the Glaucium species mainly grown in Iran (17 species) (Mory Citation1979), and Turkey (12 taxa) (Aslan Citation2012; Yıldırımlı Citation2012; Yıldız et al. Citation2015; Aykurt et al. Citation2017).
In the Flora of Turkey, the genus Glaucium is represented by 12 taxa, 7 of which are endemic. Glaucium grandiflorum Boiss.& Huet. has two varieties, var. grandiflorum Boiss.& Huet. and var. torquatum Cullen, the latter is an endemic species. Glaucium corniculatum (L.) Rud. is one of the biodiverse species with three subspecies; subsp. phoeniceum (Crantz) Holmboe, subsp. refractum (Nábelek) Cullen, subsp. tricolor (Besser) Holmboe and seven varieties; var. caricum (Stapf) Kuntze, var. corniculatum (L.) Rudolph, var. flavum (Crantz) Kuntze, var. fulvum (Sm.) Kuntze, var. grandiflorum (Boiss. & A.Huet) Kuntze, var. leiocarpum (Boiss.) Kuntze, var. pilosum Kuntze (Güner et al. Citation2012).
The genus is well known for its various pharmacological and biological activities including antimicrobial, anti-inflammatory, antitussive, antioxidant, hypoglycemic, bronchodilator and cytotoxic activities (Arafa et al. Citation2016). Glaucium species have been used as laxative, sedative, hypnotic, antidiabetic purposes and in the treatment of dermatitis (Zargari Citation1990; Shafiee and Morteza-Semnani Citation1998). In another study, the antibacterial activity of the methanol and chloroform extracts of the aerial parts of three Glaucium species; G. grandiflorum, G. oxylobum and G. paucilobum has been tested using the disk diffusion method and exhibited concentration-dependent activity against a series bacteria (Morteza-Semnani et al. Citation2005).
In folk medicine, fruits of Glaucium grandiflorum have been used for purification of blood and in the treatment of ophthalmic diseases (Baytop Citation1994). G. flavum Crantz., is one of the most studied Glaucium species which shows antitussive, antioxidant (Spasova et al. Citation2008), hypoglycemic (Cabo et al. Citation1988) and hypotensive effects (Orallo et al. Citation1995).
Turkish Glaucium species were first investigated by the Gozler group for isolation and structure elucidation of their alkaloids (Coşar et al. Citation1981; Gozler Citation1982). However, their biological activities were mostly investigated by other researchers later on (Sari Citation2001; Ozsoy et al. Citation2018).
Aerial parts of the Glaucium species are very rich in alkaloids, particularly aporphine-type isoquinoline alkaloids. Aporphinoids were also isolated from roots of Algerian Glaucium flavum which showed anti-tumour effects against human cancer cells (Bournine et al. Citation2013).
Glaucine has been used commonly as an antitussive and antihypertensive agent for a long time (Dimant and Bardashevskaia Citation1974). Glaucine was also widely known for antitussive, bronchodilatory and anti-inflammatory properties (Chiu et al. Citation2012), and its drugs found in eastearn European market, especially in Bulgaria as cough suppressant (Meyer et al. Citation2013) Glaucine act also as weak dopamine D1 and D2 receptor antagonist (Meyer et al. Citation2013). The antitussive effect of glaucine is comparable to codeine without signs of opiate withdrawal after long intake (Zhang et al. Citation2007).
In one study, glaucine was found to be a significant cytotoxic agent against cervical cancer HeLa cells (Hoet et al. Citation2004). In another study, a series of 18 aporphinoids, including glaucine, have been investigated in vitro against human poliovirus and found to be significantly active (Chen et al. Citation2013). Aporphine alkaloids can further inhibit calcium ion channels. They caused relaxation of the thoracic aorta of rats by suppressing the Ca2+ influx through both voltage- and receptor-operated calcium channels (Ivorra et al. Citation1993; Eltze et al. Citation2002).
Glaucine was found to be the major alkaloid of Glaucium species, especially in the species G. flavum (Arafa et al. Citation2016). However, in this study, glaucine was not obtained from the extracts of the endemic species G. grandiflorum subsp. refractum var. torquatum (Aslan Citation2012), while G. corniculatum var. corniculatum () afforded glaucine with a low yield (0.94% = 45 mg/4.75 g tertiary amine extract [TAE]) (Kusman Citation2018). A earlier study, carried out by Gozler (Citation1982) on the alkaloid extracts of former species, afforded glaucine at high yield (70%), which was collected from Ankara, the capital city of Turkey. This result should be attributed to the collection of the plant from a different location and a different season as well as other factors (Bogdanov et al. Citation2015).
Figure 1. Images of some Glaucium species. A: Glaucium grandiflorum subsp. refractum var. torquatum (Aslan Citation2012); B: Glaucium corniculatum var. corniculatum.

In this study, the extracts and isolated alkaloids from the two Glaucium species were investigated phytochemically with their bioactivity. In the literature, only a few anticholinesterase and antioxidant activity studies have been performed with alkaloid extracts of Glaucium species rather than their isolated alkaloids (Akaberi et al. Citation2021). However, the selected two Glaucium species have not been investigated for their anticholinesterase and antioxidant activities heretofore. Therefore, investigation of both species, particularly endemic species G. grandiflorum subsp. refractum var. torquatum (Aslan Citation2012), for the mentioned activities, would be worthwhile.
Materials and methods
General experimental procedure
1H- and 13C-NMR spectra were recorded on a Varian ID-6508 at 600 MHz for proton and 150 MHz for carbon with tetramethylsilane (TMS) as an internal standard. Some NMR spectra, particularly 2D NMR, were run with a Bruker Avance 500 MHz in the Drug Application and Research Center (DARC), Bezmialem Vakif University, Istanbul, Turkey. Chemical shifts values are given in ppm (δ scale), coupling constants(J) in Hz.
Optical rotations were measured on Rudolph Research Analytical, Autopol V Plus with AutofillTM Automatic Polarimeter. Measurements were taken at a temperature of 25 °C and a wavelength of 589 nm. Compounds concentrations were set to 0.267 g/100 mL.
Spots and bands were detected with a UV Camag spectrometer (254 and 366 nm). Mass spectral measurements were taken in Zivak® Tandem Gold Triple quadrupole (Istanbul, Turkey) mass spectrometer. HRMS spectra were run on Thermo ORBITRAP Q-EXACTIVE in Drug Application and Research Center (DARC). Column chromatography was conducted with silica gel 60 (0.063–0.200 mm) (Merck No: 1.07734). Thin-layer chromatography (TLC) was performed on silica gel 60 F254 plates (Merck No: 1.05554).
Methanol, chloroform, cyclohexane, diethylamine were purchased from Merck.
Plant material
Glaucium grandiflorum Boiss. & Huet. subsp. refractum var. torquatum (Aslan Citation2012) Cullen was collected at the flowering stage from Van (Eastern of Turkey, 38° 5.72″ N, 43° 2.75″ E) in May, 2016 and identified by Prof. Dr. Şükran Kültür (the plant specimen is preserved in the ISTE Herb.; ISTE 110.338), and G. corniculatum (L.) Rud. var. corniculatum was collected at the flowering stage from Konya (Center of Turkey, 38° 01.125″ N, 32° 30.033″ E) in June, 2016, and identified by Prof. Dr. Osman Tugay (the plant specimen is preserved in the KNYA Herb.; KNYA 30.113).
Extraction, fractionation and isolation
Air-dried and powdered aerial parts of Glaucium grandiflorum subsp. refractum var. torquatum (Aslan Citation2012) (1008.9 g) were percolated with methanol and the dried methanol extracts was obtained as 26.00 g (yield 2.6%). It was then fractionated between 50% aqueous acetic acid and petroleum ether. The aqueous acidic layer was adjusted to pH 7–8 with NH4OH (conc.) and extracted with CHCI3 (×6), collected chloroform layers were dried over Na2SO4, and the solvents were evaporated. The crude chloroform extract (1.2 g) was chromatographed on a column of silica gel. Dried chloroform extract elution was started with CHCl3 100%, and continued addition of MeOH as gradient [CHCl3:MeOH (9:1, 8:2, 7:3, 5:5)] successively, and then finally MeOH (100%) to collect 39 fractions. The obtained eluates were further purified using preparative TLC on silica gel with cyclohexane:diethylamine (8:2) or chloroform:cyclohexane:diethylamine (7:3:1). All compounds were identified by 1H- and 13C NMR techniques and Mass spectrometric analyses.
Air-dried and powdered aerial parts of G. corniculatum var. corniculatum (534.50 g) were percolated with methanol and then evaporated. Dried methanol extract was obtained as 132.00 g (yield 25%). The same extraction procedure used for the Glaucium corniculatum var. corniculatum was also applied for this species. The crude chloroform extract (4.75 g) of G. corniculatum var. corniculatum was chromatographed on a silica gel column. The column elution was started with CHCl3, and gradients CHCl3:MeOH mixtures (9:1, 8:2, 7:3, 5:5), and then only MeOH solvent, successively, and 62 fractions were collected. The similar fractions were combined and were further purified using preparative TLC Si-gel plates which developed cyclohexane:diethylamine (8:2) or chloroform:cyclohexane:diethylamine (7:3:1) solvents system.
Antioxidant activity
Antioxidant activities of the extracts and pure compounds were investigated for β-carotene-linoleic acid (Miller Citation1971), ABTS cation radical decolorization (Re et al. Citation1999) and cupric reducing antioxidant capacity (CUPRAC) (Apak et al. Citation2004) methods. The antioxidant activity of the extracts and compounds were compared with the standards of BHT, BHA and α-tocopherol.
β-Carotene lipid peroxidation inhibition method
Lipid peroxidation inhibition test method is also known as total antioxidant activity. This method is based on measuring the inhibition of conjugated diene hydroperoxides resulting from linoleic acid oxidation and the principle of lightening β-carotene (Miller Citation1971).
ABTS cation radical scavenging activity method
In this test assay, green/blue coloured ABTS radical is formed by oxidation of ABTS with persulphate. The method is based on the principle that the ABTS radical decolouration at 600–750 nm wavelength by reduction. The free radical scavenging activity of antioxidant substances is calculated as equivalent to Trolox and the results are given as the ‘TEAC value’ (Re et al. Citation1999).
Cupric-reducing antioxidant capacity method (CUPRAC)
According to this antioxidant method, neocuproin (2,9-dimethyl-1,10-phenanthroline) and Cu (II) are placed in the same well. The analysis was performed using Cu (I), which is formed by the reduction of Cu (II) by the antioxidant substance, giving a maximum absorbance at a wavelength of 450 nm by formation of complex with neocuproin, calculated as equivalent to Trolox, and the result is given as the ‘TEAC value’ (Apak et al. Citation2004).
Anticholinesterase activity
Acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitory activities of the extracts and isolated compounds were measured by slightly modifying the spectrophotometric method developed by Ellman et al. (Citation1961). Galantamine was used as a reference compound.
Statistical analysis
All bioactivity data were obtained in triplicate and the presented statistical data were analysed using Graphpad Prism version 9.00 (GraphPad Software, La Jolla CA).
Results
In this study, two known aporphine alkaloids, corydine (1), and isocorydine (2), and two protopine-type alkaloids; one known as protopine (5), another one the new alkaloid glauciumoline (3), were isolated (; ) from the aerial parts of the G. grandiflorum subsp. refractum var. torquatum (Aslan Citation2012), collected from Van, a city located in the Eastern-Anatolia. The other Glaucium species, G. corniculatum var. corniculatum (aerial parts), collected from Konya, afforded five alkaloids. These are a known aporhine alkaloid glaucine (4) and three known protopine type alkaloids; N-methyl canadine (6), trans-protopinium (7) and cis-protopinium (8), in addition to the new protopine-type alkaloid, glauciumoline (3) (; ).
Figure 2. Structures of the compounds isolated from G. grandiflorum subsp. refractum var. torquatum (Aslan Citation2012) and G. corniculatum var. corniculatum.
Table 1. Amounts of the isolated alkaloids from Glaucium species.
Their structures were elucidated based on spectral analyses, particularly by 1D- and 2D 1H- and 13C-NMR experiments and mass spectroscopic measurements. Although three known aporphine alkaloids (glaucine, corydine and isocorydine) and two known protopine alkaloids (5 and 6) were previously isolated from different Glaucium species, compound 3 was isolated from nature for the first time, and isolation of compounds 7 and 8 was isolated for the first time from a Glaucium species, in this study.
Compound 3 was isolated as a yellow amorphous solid, and its possible alkaloid structure was proven by the observation of a positive test result after spraying Dragendorff’s reagent.
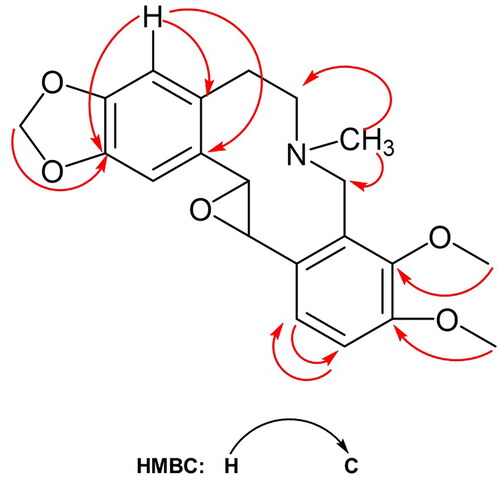
The 1H NMR spectrum of compound 3 () exhibited a characteristic two proton singlet at δ 5.95, and the appearance of a carbon chemical shift (δ 102.92) in the 13C NMR spectrum attributed to the presence of a dioxo methylene group in the skeleton. It should be positioned either on ring A or ring D. In the 1H NMR spectrum, four aromatic proton signals resonated at δ 6.98, 6.96, 6.94 and 6.68. Among them, two proton signals as singlets at δ 6.98 and 6.68 which could be attributed to H-1 and H-4, respectively. Their correlated carbon signals (C-1 and C-4) observed at δ 107.30 and 109.03, respectively, based on HSQC experiments. The resonance of a proton singlet at δ 6.68 (H-4) showed two-bond and three-bond away correlations with a carbon signal at 134.07 ppm corresponding to C-4a and C-14a in the HMBC spectrum which verified the location of dioxo methylene group on ring A (between C-2 and C-3). The 13C NMR spectrum () by an APT experiment showed the presence of 21 carbon signals consisting of a methylene dioxo moiety, two methoxy groups, three methylene pairs, 2 aliphatic and 4 aromatic methines, eight aromatic quaternary carbons, and a N-methyl signal in the molecule.
Table 2. 1H and 13C NMR data of protopine alkaloids glauciumoline (3), protopine (5), N-methyl canadine (6), trans-protopinium (7) and cis-protopinium (8).
The two distinct methoxy signals, resonated at δ 3.83 and 3.78, should be located at C-9 and C-10. This pattern is properly assigned by their carbon signals correlations in the HSQC and HMBC () experiments. The other two aromatic proton signals appeared as two doublets which have an AB spin system coupled to each other with a characteristic ortho J coupling value (7.8 Hz) which should be attached to C-9 and C-10 protons on ring D. The two methoxy protons and their corresponding carbons were determined based on HSQC experiments, and by HMBC experiments observing two-bond and three-bond away correlations () from H to C atoms.
The presence of an epoxy group in the structure of compound 3 was observed by two proton signals resonated at δ 3.32 brs (H-13) and 3.92 m (C-14) which coupled each other. Their vicinity followed by 1D and 2D NMR experiments. Their carbon signals resonated at δ 48.92 (C-13) and at δ 59.66 (C-14) which were correlated with their proton signals by the HSQC experiment. In the 1H NMR spectrum, three methylene pairs were observed; C-5 methylene pair with proton signals at δ 3.13 and 2.94 as multiplets corresponding to a C signal at δ 29.34; and for C-6 methylene pair with at δ 3.20 and 2.77 protons as multiplets corresponding to a C signal at δ 55.09; and for C-8 assignment with at δ 4.08 and 3.98 protons as multiplets corresponding to a C signal at δ 51.79 based on the 13C-NMR, HSQC, and HMBC experiments. N-Methyl protons resonated at δ 2.23 and C signal at δ 39.65 and showed three-bond away correlations with both C-6 and C-8 in the HMBC.
In fact, the structure of compound 3 resembled a protopine-type alkaloid based on above NMR data with a ten-member ring formed by B and C rings, which looks like allocryptopine (Kubala et al. Citation2011). But, instead of an oxo group, an epoxy group is present between C-13 and C-14, followed by two methine signals and their carbon signals mentioned above. The HRMS spectrum showed a peak at m/z 370.16330 [M + H]+ corresponding to the formula C21H24NO5 (theoretical mass: 370.16490) with 10.5 relative double bound (RDB) equivalent. Considering the complete assignment of protons and carbons through the aid of the 1D- and 2D NMR spectra and high-resolution mass spectral (HRMS) analysis results, the structure of the new compound 3 was elucidated, and the trivial name was given as glauciumoline.
Antioxidant activities of each extract and their alkaloids were investigated by three test (ABTS, CUPRAC and lipid peroxidation inhibition) methods.
As antioxidant activity test assays, the extracts showed moderate activity in the ABTS method. In the CUPRAC antioxidant capacity test, G. grandiflorum subsp. refractum var. torquatum (Aslan Citation2012) extract showed strong activity while G. corniculatum var. corniculatum extract showed moderate activity at the same concentration (50 μg/mL) ().
Figure 4. Trolox Equivalent Antioxidant Capacities of the extracts calculated with respect to the ABTS and CUPRAC methods. G. grandiflorum subsp. refractum var. torquatum (Aslan Citation2012) tertiary amine extract: GG TAE; G. corniculatum var. corniculatum tertiary amine extract: GC TAE; G. grandiflorum subsp. refractum var. torquatum (Aslan Citation2012) tertiary amine extract: GG TAE; G. grandiflorum subsp. refractum var. torquatum (Aslan Citation2012) quaternary amine extract: GG QAE. Test results for each extract shown with superscript capital letters (A–C) indicate significant differences (p<0.05) according to the Fisher test.
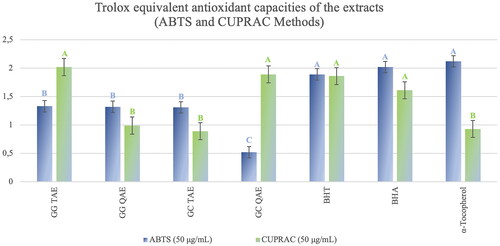
According to the ABTS activity results carried out at a concentration of 50 μg/mL, tertiary and quaternary amine extracts of G. grandiflorum subsp. refractum var. torquatum (Aslan Citation2012) showed strong antioxidant activity (1.33 ± 0.12 and 1.32 ± 0.24, respectively) compared to the standards () as well as the TAE of G. corniculatum var. corniculatum (1.31 ± 0.54), but quaternary amine extract of G. corniculatum var. corniculatum showed a moderate activity ().
According to the CUPRAC activity test results, TAE of G. grandiflorum subsp. refractum var. torquatum (Aslan Citation2012) showed very strong antioxidant activity (2.02 ± 0.01 mol/g) at a concentration of 50 μg/mL, even higher than standard compounds (BHT, BHA, and α-tocopherol) as well as quaternary amine extract of G. corniculatum var. corniculatum. Therefore, the extracts showed high CUPRAC capacity except for G. corniculatum var. corniculatum TAE ().
Considering β-carotene lipid peroxidation inhibition activity test results of the extracts obtained from Glaucium species, it was seen that TAEs of both plants showed high activity compared with the standards (). Quaternary amine extracts also showed fairly high β-carotene-linoleic acid test results.
Figure 5. β-Carotene lipid peroxidation inhibition results of the Glaucium extracts. G. grandiflorum var. torquatum tertiary amine extract: GG TAE; G. grandiflorum subsp. refractumvar. torquatum (Aslan Citation2012) quaternary amine extract: GG QAE; G. corniculatum var. corniculatum tertiary amine extract: GC TAE; G. corniculatum subsp. refractum var. corniculatum (Aslan Citation2012) quaternary amine extract: GC QAE; Test results for each extract shown with superscript capital letters (A–D) indicate significant differences (p<0.05) according to the Fisher test.
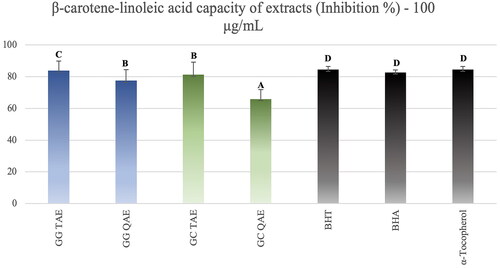
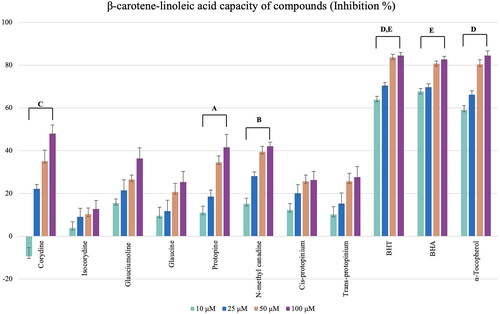
However, none of the alkaloids showed activity in the ABTS and CUPRAC methods which were tested at 50 μg/mL. The isolated alkaloids tested for lipid peroxidation inhibitory activity by the β-carotene-linoleic acid test assay, corydine (1) showed relatively good activity with 48.02% inhibition at 100 μM, and N-methyl canadine (6) and protopine (5) followed ().
Figure 6. Lipid peroxidation inhibition results of the alkaloids from the Glaucium species. BHT, BHA, and α -Tocopherol were used as positive standards). Test results for each compound shown with superscript capital letters (A–E) indicate significant differences (p<0.001) according to the Fisher test.
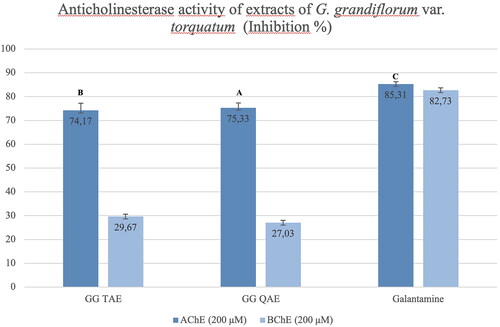
The anticholinesterase activity of tertiary and quaternary amine extracts of G. grandiflorum subsp. refractum var. torquatum (Aslan Citation2012) were tested, and both extracts showed high inhibition (74.17% and 75.33% at 200 μM, respectively) on AChE enzyme compared with the galantamine. In contrast, the tertiary and quaternary amine extracts showed weak inhibition against the BChE enzyme (29.67% and 27.03% at 200 μM, respectively) ().
Figure 7. Anticholinesterase activity results of the G. grandiflorum subsp. refractum var. torquatum (Aslan Citation2012) extracts. GG TAE: Tertiary amine extract; GG QAE: Quaternary amine extract. Test results for each extract shown with superscript capital letters (A–E) indicate significant differences (p<0.05) according to the Fisher test.
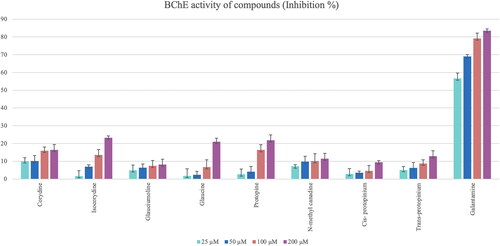
The anticholinesterase activity test results of the G. corniculatum var. corniculatum extract were examined and the TAE showed high inhibition against AChE and BChE enzymes which is equivalent to that of galantamine (81.88% and 83.13% at 200 μM, respectively). Quaternary amine extract of G. corniculatum var. corniculatum also showed high inhibition on the AChE enzyme compared with the galantamine (77.83% inhibition at 200 μM) (). However, the isolated alkaloids showed almost no anticholinesterase activity, except for cis-protopinium ().
Figure 8. Anticholinesterase inhibition activity results of the G. corniculatum var. corniculatum extracts. GC TAE: Tertiary amine extract; GC QAE: Quaternary amine extract. Test results for each extract shown with superscript capital letters (A–E) indicate significant differences (p<0.01) according to the Fisher test.
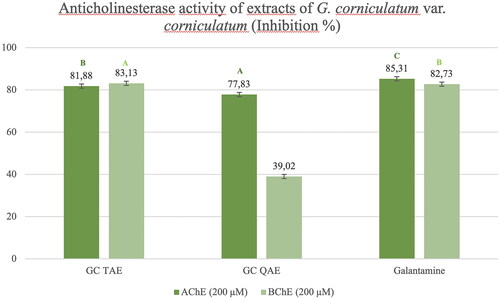
Figure 9. Acetylcholinesterase (AChE) inhibition activity results of isolated alkaloids from the Glaucium species. Statistical analysis was not performed because the test substances were not active.
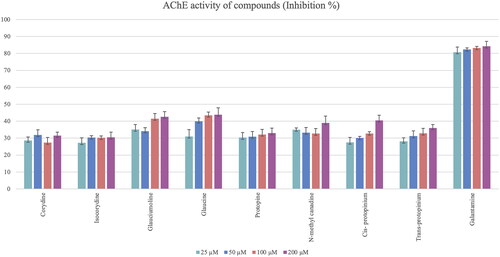
Glaucine and the new compound glauciumoline showed the highest AChE inhibitory activity among all tested alkaloids () while BChE inhibition activity results of the isolated alkaloids were found to be very low. Isocorydine, protopine and glaucine showed only weak inhibition on BChE enzyme at a concentration of 200 μM ().
Figure 10. Butyrylcholinesterase (BChE) inhibitory activity results of the isolated alkaloids from the Glaucium species. Statistical analysis was not performed because the test substances were not active.
Compound 1: 1H and 13C NMR data in . ESI-MS (–) m/z (%) = 341 [M]+ (calcd for C20H23NO4): 342 [M + H]+, 340 [M-H]+, 326 [M-CH3]+, 323 [M-H2O]+, 310 [M-OCH3]+, 298 [M-C2H5N]+, 279 [(M-OCH3)-OCH3]+.
Table 3. 1H and 13C NMR data of aporphine alkaloids corydine (1), isocorydine (2) and glaucine (4).
Compound 2: 1H and 13C NMR data in . ESI-MS (–) m/z (%) 341 [M]+ (calcd for C20H23NO4): 342 [M + H]+, 340 [M-H]+, 326 [M-CH3]+, 323 [M-H2O]+, 310 [M-OCH3]+, 298 [M-C2H5N]+, 283 [298-CH3]+.
Compound 3: [α]24,3D: −2,250. 1H and 13C NMR data are in . HRMS spectrometer (ORBITRAP Q-EXACTIVE), ESI (+) m/z 370.16330 [M + H]+ C21H24NO5 (RDB equiv. value: 10.5), (theoretical mass: 370.16490), 279.15790 (C16H23O4), 163.03813 (C9H7O3).
Compound 4: 1H and 13C NMR data in . ESI-MS (-) m/z (%) = 355 [M]+ (calcd for C21H25NO4): 355 [M]+, 356 [M + H]+, 354 [M-H]+, 340 [M-CH3]+, 338 [M-H2O]+, 324 [M-OCH3]+, 297 [(M-OCH3)-CO]+.
Compound 5: 1H and 13C NMR data in . ESI-MS (–) m/z (%) =353 [M]+ (calcd for C20H19NO5): 353 [M]+, 354 [M + H]+, 323 [M-OCH2]+, 206, 148.
Compound 6: 1H and 13C NMR data in . ESI-MS (–) m/z (%) = 354 [M]+ (calcd for C21H24NO4): 354 [M]+, 355 [M + H]+, 339 [M-CH3]+, 336 [M-H2O]+.
Compound 7: 1H and 13C NMR data in . ESI-MS (–) m/z (%) = 355 [M]+ (calcd for C20H21NO5): 355 [M]+, 356 [M + H]+, 332, 320, 188.
Compound 8: 1H and 13C NMR data in . ESI-MS (–) m/z (%) = 355 [M]+ (calcd for C20H21NO5): 355 [M]+, 356 [M + H]+, 332, 320, 188.
Discussion
The aerial parts of Glaucium species contain a large amount of isoquinoline alkaloids, most of which have an aporphine ring or a protopine ring. Although Glaucium species were found to be rich in an aporphine alkaloid glaucine (Israilov et al. Citation1979; Daskalova et al. Citation1988), in this study, glaucine was found only in the TAE of G. corniculatum var. corniculatum, and not at high amount (∼1%) in comparison with G. flavum species which is one of the most analysed Glaucium species and its major alkaloid glaucine which has an aporphine ring, investigated for many bioactivities, and glaucine, was found to be as an antitussive agent, and its drugs were commonly used by people (Meyer et al. Citation2013). However, most of the extracts of the Glaucium species were investigated rather than their isolated alkaloids. Glaucine and other aporphine alkaloids were studied for cytotoxic/anticancer, anti-inflammatory, antinociceptive, antiplasmodial and anti-AChE activities. A number of protopine alkaloids were investigated for anti-inflammatory, cardiovasoprotective, antithrombotic, neuroprotective, antimicrobial and antispasmodic activities (Akaberi et al. Citation2021).
Recent studies also indicate that Glaucium species may be used for the treatment of neurodegenerative diseases, such as Alzheimer’s and/or Parkinson’s disease, due to their bioactive alkaloids (Tuzimski et al. Citation2022). Some studies with the G. grandiflorum subsp. refractum var. grandiflorum extracts exhibited high antioxidant, anti-AChE , anti-inflammatory and DNA-damaging properties (Aslan Citation2012; Orhan et al. Citation2004; Ozsoy et al. Citation2018).
In our study, the extracts of both Glaucium species (G. corniculatum var. corniculatum and G. grandiflorum subsp. refractum var. torquatum (Aslan Citation2012)) also showed high antioxidant activity ( and ) as well as high anti-AChE activity ( and ). Therefore, a correlation was observed between these two activities.
In the treatment of Alzheimer’s disease, AChE inhibition is the most effective way for patients because AChE inhibitory property has been accepted as an important marker for detecting active substances in neurodegenerative diseases (Anand et al. Citation2014). In previous studies, it has been proven that when exposing various cell types to oxidative stress, AChE expression increases in both gene and protein levels (Kocanci et al. Citation2022). In this study, glaucine and glauciumoline showed significant AChE inhibitory activity () while the other isolated alkaloids showed weak or no activity.
Conclusions
Oxidative stress is a significant property in the mechanism of neurodegenerative diseases. Therefore, the interest in medicinal plants, particularly their alkaloids (such as galantamine), has increased in finding more active and safe new agents in the treatment of neurodegenerative disorders/diseases. In one of the recent studies, alkaloid extracts of G. corniculatum showed suppressed oxidative stress-induced neuronal apoptosis (Dolanbay et al. Citation2021). In another recent review article, neuroprotective and anti-cholinesterase activities, besides some other activities of Glaucium species, were reported (Akaberi et al. Citation2021).
In this study, we obtained a new alkaloid (3), trivially named glauciumoline, having a protopine-type isoquinoline structure, and its novelty comes from the formation of a 10-member ring by B and C rings with an epoxy group between C-13 and C-14. Although most of the isolated alkaloids have not shown even weak anticholinesterase activity results, glaucimoline and glaucine exhibited relatively stronger AChE inhibitory activity results.
Obtained activity results of the extracts and isolated compounds from two Glaucium species have been compared with the previous studies on Glaucium species. The two extracts (TAE and QAE) prepared from both Glaucium species showed higher anticholinesterase activity than the pure alkaloids ( and ). The tertiary and quaternary amine extracts of both species shown strong AChE activity (13.83 ± 0.02–14.87 ± 0.03) which are comparable with the reference compound galantamine. However, only TAE of G. corniculatum var. corniculatum showed very strong butrylcholinesterase activity.
Both species extracts also exhibited high antioxidant capacity in general. In particular, they showed high lipid peroxidation inhibitory activity by a β-carotene-linoleic acid test method () as well as high CUPRAC and ABTS antioxidant capacity (). However, isolated alkaloids have not shown CUPRAC and ABTS activity. In contrast, they showed good lipid peroxidation inhibitory activity ().
As a result, both Glaucium extracts showed higher antioxidant and anticholinesterase activity than their pure isolated alkaloids, indicating that TAEs of both Glaucium species probably contain other secondary metabolites besides alkaloids. Thus, the strong activity of the extracts might be observed due to the total synergistic effect of all of these compounds.
Acknowledgements
This study is a part of one of the authors Tuba KUŞMAN’s doctorate thesis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Akaberi T, Shourgashti K, Emami SA, Akaberi M. 2021. Phytochemistry and pharmacology of alkaloids from Glaucium spp. Phytochemistry. 191:112923.
- Anand R, Gill KD, Mahdi AA. 2014. Therapeutics of Alzheimer’s disease: past, present and future. Neuropharmacol. 76:27–50.
- Apak R, Güçlü K, Özyürek M, Karademir SE. 2004. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J Agric Food Chem. 52(26):7970–7981.
- Arafa AM, Mohamed MES, Eldahmy SI. 2016. The aerial parts of yellow horn poppy (Glaucium flavum Cr.) growing in Egypt: ısoquinoline alkaloids and biological activities. J Pharm Sci Res. 8:323–332.
- Aslan S. 2012. Glaucium Mill. In: Guner A, Aslan S, Ekim T, Vural M, Babaç MT, editors. List of plants of Turkey (vascular plants). İstanbul: İstanbul Nezahat Gökyiğit Botanical Garden Publications; p. 663–664.
- Aykurt C, Yıldız K, Özçandır A, Mungan F, Deniz İG. 2017. Glaucium alakirensis (Papaveraceae), a new species from Southern Anatolia, Turkey. Phytotaxa. 295(3):255–262.
- Baytop T. 1994. Dictionary of plant names in Turkish (Ankara). Ankara, Turkey: Turkish Language Association Publications.
- Bogdanov MG, Keremedchieva R, Svinyarov I. 2015. Ionic liquid-supported solid–liquid extraction of bioactive alkaloids. III. Ionic liquid regeneration and glaucine recovery from ionic liquid-aqueous crude extract of Glaucium flavum Cr. (Papaveraceae). Sep. Purif Technol. 155:13–19.
- Bournine L, Bensalem S, Peixoto P, Gonzalez A, Maiza-Benabdesselam F, Bedjou F, Wauters J-N, Tits M, Frédérich M, Castronovo V, et al. 2013. Revealing the anti-tumoral effect of Algerian Glaucium flavum roots against human cancer cells. Phytomedicine. 20(13):1211–1218.
- Cabo J, Cabo P, Jimenez J, Zarzuelo A. 1988. Glaucium flavum Crantz. Part v: hypoglycemic activity of the aqueous extract. Phytother Res. 2(4):198–200.
- Chen J, Gao K, Liu T, Zhao H, Wang J, Wu H, Liu B, Wang W. 2013. Aporphine alkaloids: a kind of alkaloids’ extract source, chemical constitution and pharmacological actions in different botany: a review. Asian J Chem. 25(18):10015–10027.
- Chiu CC, Chou HL, Wu PF, Chen HL, Wang HM, Chen CY. 2012. Bio-functional constituents from the stems of Liriodendron tulipifera. Molecules. 17(4):4357–4372.
- Coşar G, Bilgehan H, Gözler T. 1981. The antibacterial effects of some alkaloids isolated from Glaucium flavum Crantz. Mikrobiyol Bul. 15(2):105–109.
- Daskalova E, Iskrenova E, Kiryakov HG, Evstatieva L. 1988. Minor alkaloids of Glaucium flavum. Phytochemistry. 27(3):953–955.
- Dimant MI, Bardashevskaia SP. 1974. Glaucine treatment of hypertensive disease. Vrachebnoe delo. 12:24–26.
- Dolanbay SN, Kocanci FG, Aslim B. 2021. Neuroprotective effects of allocryptopine-rich alkaloid extracts against oxidative stress-induced neuronal damage. Biomed Pharmacother. 140:111690.
- Ellman GL, Courtney KD, Andres V, Feather-Stone RM. 1961. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 7:88–95.
- Eltze M, Grebe T, Michel MC, Czyborra P, Ullrich B. 2002. Affinity profile at α1-and α2-adrenoceptor subtypes and in vitro cardiovascular actions of (+)-boldine. Eur J Pharmacol. 443(1–3):151–168.
- Gozler T. 1982. Alkaloids of Turkish Glaucium Species. 1. Alkaloids of Glaucium grandiflorum var. torquatum. Planta Med. 46(11):179–180.
- Güner A, Aslan S, Ekim T, Vural M, Babaç MT. editors. 2012. Türkiye Bitkileri Listesi (Damarlı Bitkiler), Nezahat Gökyiğit Botanik Bahçesi ve Flora Araş Der Yayını. Istanbul.
- Hoet S, Stevigny C, Block S, Opperdoes F, Colson P, Baldeyrou B, Lansiaux A, Bailly C, Quetin-Leclercq J. 2004. Alkaloids from Cassytha filiformis and related aporphines: antitrypanosomal activity, cytotoxicity, and interaction with DNA and topoisomerases. Planta Med. 70(5):407–413.
- Israilov I, Karimova S, Yunusov M. 1979. Glaucium alkaloids. Chem Nat Compd. 15(2):103–113.
- Ivorra MD, Martinez F, Serrano A, D'ocon P. 1993. Different mechanism of relaxation induced by aporphine alkaloids in rat uterus. J Pharm Pharmacol. 45(5):439–443.
- Kadereit JW. 1993. Papaveraceae. In: Kubitzki K, Rohwer JG, Bittrich V, editors. Flowering plants dicotyledons. The families and Genera of vascular plants. Vol. 2. Berlin, Heidelberg, Germany: Springer; p. 494–506.
- Kocanci FG, Hamamcioglu B, Aslim B. 2022. The relationship between neuroprotective activity and antigenotoxic and acetylcholinesterase inhibitory effects of Glaucium corniculatum extracts. Braz J Pharm Sci. 58:1–14.
- Kubala M, Vacek J, Popa I, Janovska M, Kosina P, Ulrichova J, Travnicek Z, Šimánek V. 2011. The fluorescence properties and NMR analysis of protopine and allocryptopine. J Lumin. 131(7):1340–1345.
- Kuşman T. 2018. Isolation of bioactive glaucine and other alkaloids from some Glaucium species grown in Turkey and quantification with high performance liquid chromatography [Doctoral dissertation (Ph. D. dissertation)]. İstanbul: Istanbul University.
- Meyer GM, Meyer MR, Wink CS, Zapp J, Maurer HH. 2013. Studies on the in vivo contribution of human cytochrome P450s to the hepatic metabolism of glaucine, a new drug of abuse. Biochem Pharmacol. 86(10):1497–1506.
- Miller HA. 1971. Simplified method for the evaluation of antioxidants. J Am Oil Chem Soc. 48(2):91–91.
- Mingli Z, Grey-Wilson C. 2008. Glaucium Miller. Flora Chin. 7:282–283.
- Morteza-Semnani K, Saeedi M, Mahdavi MR. 2005. Antibacterial studies on extracts of three species of Glaucium. from Iran. Pharm Biol. 43(3):234–236.
- Mory B. 1979. Beitrage Zur Kenntnis der Sippenstruktur der Gattung Glaucium Miller (Papaveraceae). Feddes Repert. 89(9–10):499–544.
- Orallo F, Alzueta AF, Campos-Toimil M, Calleja JM. 1995. Study of the in vivo and in vitro cardiovascular effects of (+)-glaucine and N-carbethoxysecoglaucine in rats. Br J Pharmacol. 114(7):1419–1427.
- Orhan I, Sener B, Choudhary MI, Khalid A. 2004. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of some Turkish medicinal plants. J Ethnopharmacol. 91(1):57–60.
- Ozsoy N, Yilmaz-Ozden T, Aksoy-Sagirli P, Şahin H, Sarı A. 2018. Antioxidant, anti-acetylcholinesterase, anti-inflammatory and DNA protection activities of Glaucium grandiflorum var. grandiflorum. Iran J Pharm Res. 17(2):677–684.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 26(9–10):1231–1237.
- Sari A. 2001. The constituents of the aerial parts of Glaucium grandiflorum var. grandiflorum. Acta Pharm. Turc. 43:89–92.
- Shafiee A, Morteza-Semnani K. 1998. Crabbine and other alkaloids from the aerial parts of Glaucium paucilobum. Planta Med. 64(7):680–680.
- Spasova M, Philipov S, Nikolaeva-Glomb L, Galabov AS, Milkova T. 2008. Cinnamoyl-and hydroxycinnamoyl amides of glaucine and their antioxidative and antiviral activities. Bioorg Med Chem. 16(15):7457–7461.
- Tuzimski T, Petruczynik A, Szultka-Młyńska M, Sugajski M, Buszewski B. 2022. Isoquinoline alkaloid contents in Macleaya cordata extracts and their acetylcholinesterase and butyrylcholinesterase inhibition. Molecules. 27(11):3606.
- Yıldırımlı Ş. 2012. Turkey’s plant diversity paradise for Gypsum: Kepen, Sivrihisar, Eskişehir, 13 new members Türkiye. Herb J Syst Bot. 19:34–38.
- Yıldız K, Mungan F, Batır MB, Kılıç M, Kuh M. 2015. Revision on Glaucium Mill. in Turkey, Celal Bayar University coordination of scientific research projects. Project Number 2013–2018. p. 77.
- Zargari A. 1990. Medicinal plants. Vol. 1. 5th ed. Tehran: Tehran University Publications; p. 154–157.
- Zhang A, Zhang Y, Branfman R, Baldessarini RJ, Neumeyer JL. 2007. Advances in development of dopaminergic aporphinoids. J Med Chem. 50(2):171–181.