Abstract
Context
Periplaneta americana L. (Blattariae) is used as a treatment for ulcerative colitis (UC) in Chinese traditional medicine.
Objective
To evaluate the antioxidative activity of P. americana whole body ethanol extract (PAE) on UC mice and whether glycine and proline could be used for quality control and identification of active PAE components.
Materials and methods
NCM460 cells were pre-incubated in PAE, AA-L, AA-M, and AA-H (low, high and medium doses of proline and glycine), then treated with recombinant human TNF-α. The glutathione (GSH), malondialdehyde (MDA), superoxide dismutase (SOD) and reactive oxygen (ROS) levels were determined. UC mice were fed with water containing 2.5% dextran sulfate sodium (w/v) after pre-treatment with different doses of PAE once a day for 7 days. ELISA was used to detect the concentrations of inflammation-related factors. Colon tissues of mice were used to detect the activity of myeloperoxidase (MPO), GSH, MDA, and SOD. Histological changes were observed using H&E staining. The expression of target proteins was determined by western blotting.
Results
In vivo, PAE treatment reduced the DAI score more than in the model group, restoring the weight and colonic length. It also reduced the severity of colitis, and inflammatory and oxidative stress intensity. Additionally, western blotting showed that the Nrf2 pathway was activated by PAE. In vitro PAE significantly alleviated TNF-α-induced cell damage and oxidative stress, which is relevant to the activation of the Nrf2 pathway.
Conclusions
PAE may relieve oxidative stress through the Nrf2 signaling pathway, and proline and glycine may be used as active components of its antioxidative stress activity.
Introduction
Ulcerative colitis (UC) is an inflammatory bowel disease (IBD), mainly in the colon and rectum, and is closely related to the immune system (Xue et al. Citation2018). UC has a complex etiology, and several contributory factors may influence its disease activity, including lifestyle habits and immune dysfunction in the colon (Adams and Bornemann Citation2013). The incidence and occurrence of UC have increased globally in recent years (Ungaro et al. Citation2017). Its pathogenesis is complex, making clinical treatment difficult. Some drugs such as mesalazine, glucocorticoids, methotrexate, tofacitinib, and biologics may relieve UC, but their use is greatly restricted by side effects including diarrhea, vomiting, and hypokalemia (Curro et al. Citation2017; Honap et al. Citation2020). Meanwhile, traditional Chinese medicines (TCM) (Ali et al. Citation2012) show good efficacy and safety with respect to UC and have important developmental value. Furthermore, despite the general understanding of the various causes of UC, available therapies are currently limited and ineffective. At present, there are no permanent drugs for the complete cure of UC but anti-inflammatory agents such as 5-aminosalicylic acid (ASA), sulfasalazine, corticosteroids, immunosuppressive agents, and monoclonal antibodies are known treatment options.
Periplaneta americana L. (Blattariae) was first recorded in “Shen Nong Ben Cao Jing” in China more than 2,000 years ago. Its extracts have been widely used in traditional Chinese medicine due to its functions in activating blood circulation and inducing diuresis (Ma et al. Citation2018). It is widely used in TCM clinics to treat various ulcers (Zeng et al. Citation2019). Moreover, drugs derived from P. americana such as the Kangfuxin Solution (Z430209995, Sichuan Kelun Pharmaceutical Co., Ltd.), Ganlong capsule (Z20050113, Kunming Sainuo Pharmaceutical Co., Ltd.), and Xinmailong Injection (Z20060443, Yunnan Tengyao Pharmaceutical Co., Ltd.) are now extensively used to treat chronic heart failure and gastrointestinal ulcers and have been approved by the China Food and Drug Administration (CFDA), having achieved beneficial curative effects in clinical research. Oxidative stress plays an important role in UC pathogenesis. The occurrence of intestinal inflammation could promote the overproduction of reactive oxygen species (ROS) by immune cells, and the overexpression of ROS can damage intestinal epithelial cells, further aggravating intestinal inflammation (Piechota-Polanczyk and Fichna Citation2014). Zeng et al. (Citation2019) revealed that P. americana whole-body ethanol extract (PAE) had a protective effect in a UC model by inhibiting oxidative stress. However, the mechanism by which PAE acts on antioxidative stress has not been well described.
The components of animal drugs are complex, making it very difficult to separate and identify active components. Previous studies (Li Citation2017; Zhu et al. Citation2018; Liao et al. Citation2022) demonstrated that polypeptides and amino acids are the main active substances of PAE. However, the active components of PAE in UC treatment have not been fully identified. Glycine (Wang et al. Citation2014) and proline (Li et al. Citation2007) can alleviate IBD by regulating inflammation and oxidative stress. Thus, we speculate that glycine and proline may be active components of PAE for UC treatment.
This study investigated the effects of PAE on dextran sodium sulfate (DSS)-induced UC mouse models and TNF-α-induced NCM460 cells. We integrated the possible mechanisms of the extract’s antioxidative effect in these models and investigated whether glycine and proline may be used for quality control and identification of active components in PAE.
Materials and methods
Animals specimen
Female C57BL/6 mice (6–8 weeks old, 18–20 g) were obtained from Chengdu Dossy Experimental Animals Co., Ltd. (Chengdu, China). Throughout the experiments, the mice were kept in a 12 h dark/light cycle at a room temperature of 23 ± 2 °C and a relative humidity of 55 ± 5%, with free access to a standard diet and pure water. The experiments followed the rules of the Animal Ethics Committee of Dali University, which approved all experiments to control and supervise animal specimen (protocol number 2020-0918).
Materials
Minimum essential medium (MEM) and phosphate buffer were purchased from Corning Inc. (NY, USA). Fetal bovine serum (FBS) and trypsin EDTA (0.25%) were bought from Gibco Life Technologies (NY, USA). Proline (CAS: 344-25-2), alanine (CAS: 56-41-7), leucine (CAS: 61-90-5), glycine (CAS: 56-40-6), valine (CAS: 7004-03-7), glycine (CAS: 56-40-6), tryptophan (CAS: 73-22-3), tyrosine (CAS: 60-18-4), threonine (CAS: 72-19-5), isoleucine (CAS: 73-32-5), and phenylalanine (CAS: 3617-44-5) were purchased from Shanghai Adamas Reagent Co., Ltd. (Shanghai, China). Dextran sodium sulfate (DSS) (MW: 40000 Da) (CAS: 9011-18-1) was obtained from Shanghai Bide Pharmaceutical Technology Co., Ltd. (Shanghai, China), and tolbutamide (CAS: 64-77-7) and mesalazine (5-ASA) (CAS: 89-57-6) were purchased from Shanghai Aladdin Bio-Chem Technology Co., Ltd. (Shanghai, China). Kits for TNF-α, IL-6, IL-10, and IL-17 were purchased from R&D Systems, Inc. (CA, USA). Kits for inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and myeloperoxidase (MPO) were purchased from Nanjing Jiancheng Bioengineering Institute (Jiangsu, China). Cell Counting Kit-8 (CCK8) assays were obtained from Dojindo Molecular Technologies (Shanghai, China). Kits for bovine serum albumin (BSA), glutathione (GSH), malondialdehyde (MDA), and superoxide dismutase (SOD) were purchased from Shanghai Beyotime Biotechnology (Shanghai, China). Primary rabbit monoclonal antibodies against Nrf2 and β-tubulin were purchased from Cell Signaling Technology (Danvers, MA, USA). Antibodies against HO-1, NQO-1, GCLM, and GPX2 were purchased from Abcam Inc. (Cambridge, UK). CoraLite488-conjugated AffiniPure goat anti-mouse or anti-rabbit IgG (H + L) antibodies were obtained from Yeason Group, Inc. (Shanghai, China). Recombinant human TNF-α was purchased from Absin Bioscience Inc. (Shanghai, China).
PAE extraction
The whole dried body of P. americana was provided by Dali University and identified by Professor Zizhong Yang. The voucher specimen was stored at the College of Pharmacy, Dali University. P. americana (100 g) was degreased with petroleum ether, dried in an oven at 60 °C, and crushed to obtain P. americana crude powder. The powder was then screened through a 20 µm mesh and extracted twice with 1000 mL of 70% ethanol, heated at 70 °C for 2 h, and finally collected by freeze-drying after filtration.
LC-MS/MS analysis
Sample treatment
Stock solutions of amino acids and tolbutamide as the internal standard (IS) were dissolved at 2 mg/mL in water containing 1% formic acid. The stock solution was mixed and diluted with water to form a working solution (100, 500, 1000, 5000, 10000, 25000, and 50000 ng/mL for alanine and glycine; 10, 50, 100, 500, 1000, 2500, and 5000 ng/mL for leucine, isoleucine, phenylalanine, proline, threonine, tryptophan, tyrosine, and valine; 100 ng/mL for tolbutamide). Working solutions for PAE were dissolved in water at 100 μg/mL and filtered through a 0.2-μm nylon 66 filter head.
Separate stock solutions with calibration standards were used to prepare quality controls (QCs) at three concentration levels: high (QC-H), medium (QC-M), and low (QC-L). The QC-H was 4000 ng/mL for alanine and glycine, and 800 ng/mL for the remaining analytes. The QC-M was 25,000 ng/mL for alanine and glycine, and 500 ng/mL for the remaining analytes. The QC-L was 10,000 ng/mL for alanine and glycine, and 200 ng/mL for the remaining analytes.
Methods
An ACQUITY HPLC system (Waters, Manchester, USA) and an API 4000 triple quadrupole mass spectrometer (AB Sciex, Foster City, USA) were used. The chromatographic separation was performed on an X-bridge Amide column (150 × 4.6 mm, 3 mm) (Waters, Milford, USA), and optimal chromatographic separation was achieved using a gradient of solvent A (10 mM ammonium formicate in Milli-Q water containing 0.15% formic acid) as shown in . The parameters for multiple reaction monitoring (MRM) are listed in .
Table 1. High performance liquid phase conditions.
Table 2. Acquisition parameters in MRM mode.
Accuracy and repeatability
Accuracy and repeatability were determined using four replicate samples of the standard stock solution and PAE samples. Accuracy and repeatability were evaluated using the relative standard deviation (RSD). An RSD of less than 15% was considered acceptable.
Recovery
The recoveries of all analytes were measured using three QC concentration levels, with five replicates. The peak areas were determined using the above chromatographic conditions, and the spiked recoveries were calculated. Recovery (%) = ([measured amount–sample content]/amount added × 100%) and consistency and reproducibility were evaluated using the RSD. An RSD of less than 15% was considered acceptable.
Alleviation of DSS-induced UC symptoms in mice by PAE
In this study, the mice were randomly divided into six groups (n = 10) with different treatments for 21 days: normal control (NC), model, mesalazine (5-ASA, 200 mg/kg, gavage), low-dose PAE (PAE-L, 80 mg/kg, gavage), medium-dose PAE (PAE-M, 160 mg/kg, gavage), and high-dose PAE (PAE-H, 320 mg/kg, gavage). Mice in the model and NC groups were administered saline. On the seventh day, the mice in the NC group were fed with sterile water, while the others were fed with water containing 2.5% DSS (w/v).
Throughout the experiment, the body weight of the mice was recorded daily, and the disease activity index (DAI) was measured according to previously described criteria (Hamamoto et al. Citation1999). All mice were sacrificed by CO2 inhalation 14 d after starting DSS intake. Blood was collected and centrifuged at 10,000 rpm for 10 min at 4 °C, and the supernatants were collected for further enzyme-linked immunosorbent assay (ELISA) detection of IL-6, IL-17, IL-10, TNF-α, iNOS, and COX-2. Moreover, colon samples were collected, and cut in two, and one of the sections was fixed in 4% paraformaldehyde, embedded in paraffin, and then cut into 4-μm thick sections. The colons were stained with hematoxylin and eosin (H&E) according to standard procedures. The other section was homogenized in 0.1 M phosphate buffer (pH 7.4), centrifuged at 10,000 rpm for 10 min, and the supernatant was collected. ELISA kits were used to measure the levels of oxidative stress-related cytokines, including MPO, GSH, MDA, and SOD.
Determining the antioxidative stress effect of PAE in vitro
Measurement of intracellular ROS
First, 5 × 103 NCM460 cells were incubated and cultured in a 96-well plate for 12 h. Next, the medium was carefully cleaned, and the cells were washed twice with PBS. The cells were then incubated with 100 μL FBS-free medium containing PAE-L (40 μg), PAE-M (80 μg), PAE-H (160 μg), AA-L (0.32 μg proline and 0.44 μg glycine), AA-M (0.64 μg proline and 0.88 μg glycine), and AA-H (1.28 μg proline and 1.76 μg glycine) for 2 h, then treated with 50 ng/mL recombinant human TNF-α for 6 h. Finally, the cells were washed twice with PBS, and cell proliferation was assessed using the CCK-8 assay following the manufacturer’s instructions. Intracellular ROS were determined using the ROS Assay Kit (Beyotime, Shanghai, China). All experiments were performed three times.
Measurement of intracellular SOD, GSH, and MDA
First, 5 × 103 NCM460 cells were incubated and cultured in a 12-well plate for 12 h. Next, the medium was carefully cleaned, and the cells were washed twice with PBS. The cells were then pre-incubated in 1 mL of FBS-free medium containing PAE (400 μg) and AA-L (3 μg proline and 4 μg glycine), AA-M (6 μg proline and 8 μg glycine), and AA-H (12 μg proline and 16 μg glycine) for 2 h, and treated with 50 ng/mL recombinant human TNF-α for 6 h. At the end of the treatment, the cells were lysed using a solicitor. The lysates were centrifuged at 10,000 rpm for 10 min at 4 °C, and the supernatants were collected and used for immediate determination of SOD, GSH, and MDA levels.
Cell lines
NCM460 human epithelial cells (Shanghai Cell Resource Center, Shanghai, China) were grown in MEM containing 10% FBS and 1% penicillin-streptomycin (Corning Inc., NY, USA), and cultured at 37 °C with 5% CO2.
Western blotting
The total protein of the cells and colon was extracted using radioimmunoprecipitation assay buffer containing 1% protein phosphatase inhibitor. The proteins were then separated using 10% SDS-PAGE and transferred onto nitrocellulose filter membranes. After blocking for 2 h at room temperature with 1 × TBST containing 5% non-fat milk, the membranes were incubated overnight at 4 °C with HO-1 (1:1000), GPX2 (1:1000), GCLM (1:1000), β-tubulin (1:1000), NQO1 (1:1000), Nrf2 (1:1000), and Histone h3 (1:1000) primary antibodies. The membranes were then incubated with secondary antibodies (1:2000) for 2 h. Next, the membranes were washed, and the proteins visualized using enhanced chemiluminescence (Yeason, Shanghai, China). Amer Sham Imager 600 chemiluminometric (GE Healthcare Bio-Sciences AB, Sweden) was used for signal acquisition and imaging.
Statistical analysis
The data are expressed as mean ± standard error of the mean (SEM) using the GraphPad Prism software 8.0. The values of diverse groups were evaluated using one-way analysis of variance (ANOVA); p-values < 0.05 were considered statistically significant.
Results
LC-MS/MS analysis
Specificity
Typical MRM chromatograms of blank substrate, alanine, glycine, tryptophan, threonine, tyrosine, isoleucine, valine, proline, phenylalanine and tolbutamide working solutions and PAE samples are shown in . The reactions of the ten analytes were significantly different. Thus, blank substrate and working solutions of different concentrations were used [1000 ng/mL for leucine, isoleucine, phenylalanine, proline, threonine, tryptophan, tyrosine, valine, and internal standard (IS) tolbutamide; 100 ng/mL for tolbutamide; 50,000 ng/mL for alanine and glycine] for better comparison with the PAE samples. In the blank substrate, there were no amino acid peaks at the respective retention times. Additionally, leucine and isoleucine were well separated from the analytes, and there was no interference with quantification.
Figure 1. Representative MRM chromatograms of (a) blank matrix, (b) blank matrix containing alanine (50000 ng/mL), glycine (50000 ng/mL), leucine (1000 ng/mL), isoleucine (1000 ng/mL), phenylalanine (1000 ng/mL), proline (1000 ng/mL), threonine (1000 ng/mL), tryptophan (1000 ng/mL), tyrosine (1000 ng/mL), valine (1000 ng/mL) as well as tolbutamide (IS, 100 ng/mL) and (c) PAE samples. (A) alanine, (B) glycine, (C) leucine and isoleucine, (D) phenylalanine, (E) proline, (F) threonine, (G) tryptophan, (H) tyrosine, (I) valine, (J) tolbutamide.
Linearity and sensitivity
The linear ranges were 5000–250,000 ng/mL for alanine and glycine, 100–5000 ng/mL for leucine, isoleucine, phenylalanine, proline, threonine, tryptophan, tyrosine, and valine, and the S/N values for all standard samples were more than 10. The linear equations were as follows: alanine: y = 0.0032x + 2.99e-017 (r = 0.9998), glycine: y = 0.000157x + 2.96e-018 (r = 0.9994), leucine: y = 0.0366x + 1.33e-015 (r = 0.9995), isoleucine: y = 0.0256x + 4.99e-016 (r = 0.9997), phenylalanine: y = 0.0453x + 1.85e-015 (r = 0.9993), proline: y = 0.00336x + −4.77e-018 (r = 0.9999), threonine: y = 0.0158x + 2.83e-016 (r = 0.9996), tryptophan: y = 0.017x + (–1.7e-016) (r = 0.9998), tyrosine: y = 0.00109x + 1.69e-017 (r = 0.9994), valine: y = 0.0336x + 1.08e-015 (r = 0.9995). The correlation coefficients (r) were all greater than 0.99, indicating a good adaptation of the calibration curve. The results of the standard curve calculation showed that the amounts of the ten compounds, i.e., alanine, glycine, tryptophan, threonine, tyrosine, valine, proline, phenylalanine, leucine, and isoleucine in PAE, were 15.7, 11, 0.779, 3.03, 3.38, 6.03, 8.09, 2.99, 7.57, and 3.64 mg/g, respectively.
Accuracy and repeatability
As shown in , the RSD ranged from 1.8–4.2% for the ten analytes. All results were within acceptable limits, indicating that the instrument could accurately quantify amino acids. The repeatability was evaluated as shown in , and the RSD ranged 2.2–5.3% for the ten analytes. All results met the criteria reported in previous studies (Prinsen et al. Citation2016), indicating that the method was reproducible and could precisely determine amino acids.
Table 3. Precision and Accuracy for Each Analyte.
Table 4. Repeatability for Each Analyte.
Recovery
As shown in , the amino acid recoveries were assessed using three QC samples. The RSD of all samples was less than 10% at different concentration levels, indicating that the detection of amino acids was not significantly influenced by the substrate. Furthermore, the recoveries of all amino acids were consistent and reproducible.
Table 5. Recovery for Each Analyte.
PAE alleviates UC symptoms induced by DSS in mice
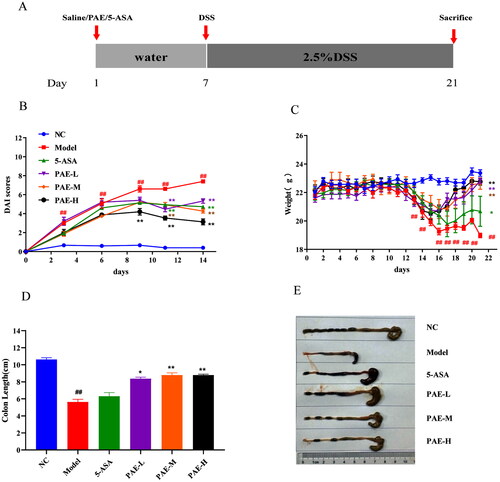
The DAI score, an essential clinical manifestation of UC, is defined as an important indicator in evaluating colonic injury (Samman et al. Citation2018). The body weight changes of the mice throughout the experiment clearly reflected their physiological status. As the experiment progressed, mice in the model group showed wasting, bloody stools, and diarrhea, and the DAI scores (6.93 ± 0.2) were elevated relative to those of the NC group (0.4 ± 0.13). In this study, mice in the DSS group showed greater body weight loss (18.98 ± 0.16) than those in the NC group (23.07 ± 0.22). However, PAE (160 and 320 mg/kg) attenuated body weight loss (22.18 ± 0.39, 23.07 ± 0.19) during the progression of colitis in mice (). In the model mice, the DAI score significantly increased in the presence of DSS but dramatically decreased with PAE treatment (L, 5.33 ± 0.23; M, 4.27 ± 0.21; H, 3.13 ± 0.31) (). Additionally, we observed that the colonic shortening induced by DSS (5.75 ± 0.43) was restored after PAE treatment (L, 8.4 ± 0.26; M, 8.61 ± 0.35; H, 8.79 ± 0.16) ().
Figure 2. Role of PAE pre-treatment on the of DSS-induced UC mice. (A) Administration time. (B) DAI scores of mice in various groups. (C) Changes in body weight of mice during the experiment. (D) Photographs of mouse colons. (E) Colon length of mice. Error bars represent the mean ± SEMs (n = 10/group). ##p < 0.01, #p < 0.05 compared to the NC group; **p < 0.01, *p < 0.05 compared to the model group. PAE: P. americana extract; 5-ASA: Mesalazine, 200 mg/kg; PAE-L: 80 mg/kg, PAE-M: 160 mg/kg, PAE-H: 320 mg/kg.
PAE improves the colonic histopathological status in UC mice
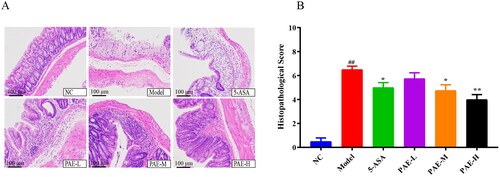
Colonic histological changes were illustrated in the sections stained with H&E. As shown in , unlike the mice in the NC group, those in the model group showed colonic tissue damage, including severe epithelial damage, mucosal inflammatory cell infiltration, and crypt deformation, which are consistent with the results of previous studies (Chen et al. Citation2015). However, treatment with PAE (160 and 320 mg/kg) and 5-ASA significantly reduced (p < 0.05 or p < 0.01) histological inflammation and protected the crypt structure of the colon (p < 0.05 or p < 0.01). Our results showed that treatment with PAE may exhibit beneficial effects in attenuating DSS-induced colitis.
Figure 3. Histologic examination of colonic tissues. (A) HE representative images of different groups (200× magnification). (B) Histopathological Score. Error bars represent the mean ± SEMs (n = 4/group). ##p < 0.01, #p < 0.05 compared to the NC group; **p < 0.01, *p < 0.05 compared to the model group. PAE: P. americana extract; 5-ASA: Mesalazine, 200 mg/kg; PAE-L: 80 mg/kg, PAE-M: 160 mg/kg, PAE-H: 320 mg/kg.
PAE decreases the levels of inflammatory cytokines in the serum of UC mice
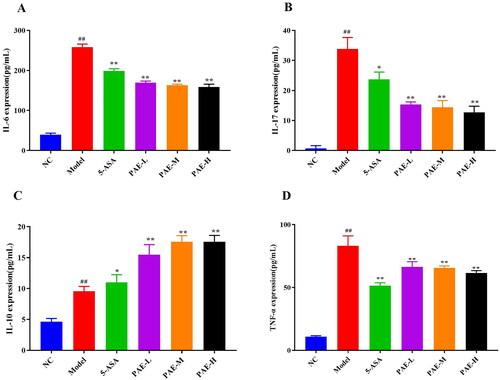
As shown in , the levels of TNF-α, IL-17, and IL-6 in the model group were more markedly increased (p < 0.01) than in the NC group. However, PAE reduced the increase in TNF-α, IL-17, and IL-6 levels which could lead to the development of inflamed mucosa and superficial ulcers in UC mice (Neurath Citation2014). Additionally, all dosages abnormally reduced the expressions of IL-6, TNF-α, and IL-17, and upregulated the expression of the IL-10 anti-inflammatory factor. These results confirmed that the inflammation-reducing effect of PAE may be connected to regulation of the DSS-induced expression of serum-associated inflammatory factors in mice with UC.
Figure 4. PAE regulates related inflammatory factors in mice. IL-6, IL-17, IL-10, and TNF-α were measured by ELISA. Error bars represent the mean ± SEMs (n = 6/group). ##p < 0.01, #p < 0.05 compared to the NC group; **p < 0.01, *p < 0.05 compared to the model group. PAE: P. americana extract; 5-ASA: Mesalazine, 200 mg/kg; PAE-L: 80 mg/kg, PAE-M: 160 mg/kg, PAE-H: 320 mg/kg.
Effect of PAE on iNOS and COX-2 in the serum of UC mice
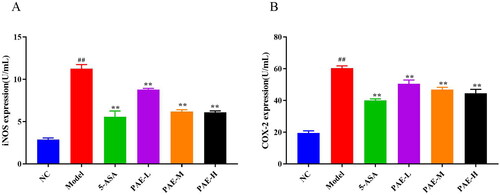
As shown in , relative to the NC group, the levels of serum iNOS and COX-2 were higher in DSS-induced colitis mice (p < 0.01). Therefore, treatment with 5-ASA and PAE markedly inhibited iNOS and COX-2 expression (p < 0.01).
Figure 5. PAE reduced iNOS and COX-2 expression levels in mice serum. iNOS and COX-2 in the serum of UC mice were measured by ELISA. Error bars represent the mean ± SEMs (n = 6/group). ##p < 0.01, #p < 0.05 compared to the NC group; **p < 0.01, *p < 0.05 compared to the model group. PAE: P. americana extract; 5-ASA: Mesalazine, 200 mg/kg; PAE-L: 80 mg/kg, PAE-M: 160 mg/kg, PAE-H: 320 mg/kg.
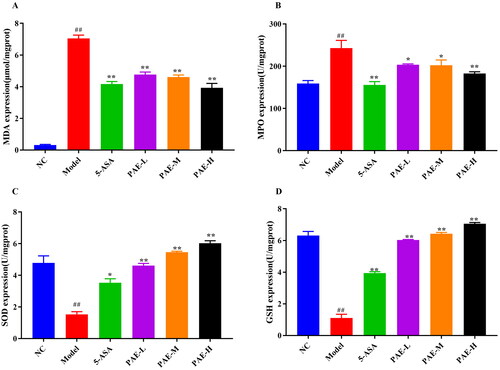
Antioxidative stress effects of PAE in UC mice
As demonstrated in , the MPO activity and MDA content in the colonic homogenates of mice in the model group were upregulated, while the expression levels of SOD and GSH were markedly downregulated relative to the NC group (p < 0.01). This demonstrated that the model group had a higher oxidative stress level. However, when UC mice were treated with PAE or 5-ASA, the GSH content and SOD activity were increased (p < 0.05, p < 0.01), and the activity levels of MPO and MDA were decreased (p < 0.05). These results indicated that PAE has a protective effect against UC through its antioxidative function.
Figure 6. Antioxidative Stress Effect of PAE in UC mice. Levels of MDA (A), MPO (B), SOD (C), and GSH activity (D). Error bars represent the mean ± SEMs (n = 6/group). ##p < 0.01, #p < 0.05 compared to the NC group; **p < 0.01, *p < 0.05 compared to the model group. PAE: P. americana extract; 5-ASA: Mesalazine, 200 mg/kg; PAE-L: 80 mg/kg, PAE-M: 160 mg/kg, PAE-H: 320 mg/kg.
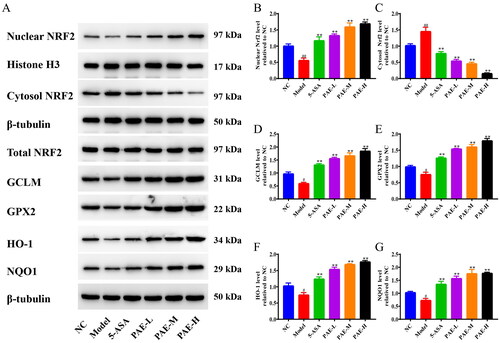
PAE exerts antioxidative stress effects by activating the Nrf2 signaling pathway
As shown in , relative to the NC group, the expression of nuclear Nrf2 protein was decreased and cytoplasmic Nrf2 protein expression was markedly upregulated in the model group (p < 0.05, p < 0.01). This was reversed after PAE intervention, indicating that PAE had a favorable effect on promoting the translocation of Nrf2. Additionally, relative to the NC group, the model group showed lower activity levels of GPX2, GCLM, HO-1, and NQO1 (p < 0.05). However, the protein expression levels of GPX2, GCLM, HO-1, and NQO1 were significantly higher in the PAE and 5-ASA groups (p < 0.01).
Figure 7. PAE activated the Nrf2 signaling pathway in DSS-induced UC colons. (A) Expression bands of nuclear Nrf2, cytoplasmic Nrf2, total Nrf2, GCLM, GPX2, HO-1, and NQO1 by western blotting. Expression levels of nuclear Nrf2 (B), cytosolic Nrf2 (C), GCLM (D), GPX2 (E), HO-1 (F), and NQO1 (G). Error bars represent the mean ± SEMs (n = 3/group). ##p < 0.01, #p < 0.05 compared to the NC group; **p < 0.01, *p < 0.05 compared to the model group. PAE: P. americana extract; 5-ASA: Mesalazine, 200 mg/kg; PAE-L: 80 mg/kg, PAE-M: 160 mg/kg, PAE-H: 320 mg/kg.
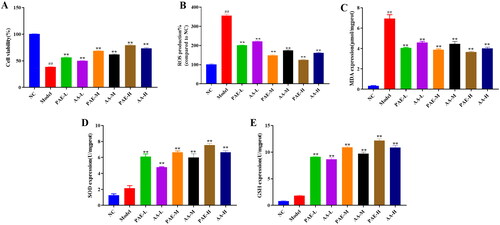
Antioxidative effect of PAE and AA on TNF-α-induced NCM460 cells
As demonstrated in , the model group had lower cell vitality (38.2%), higher ROS production (356.1%) and MDA (6.94 ± 0.67) content (p < 0.01) than the NC group, suggesting that the model group had a higher degree of oxidative stress, which may be related to cell damage induced by TNF-α. Additionally, different AA and PAE doses significantly enhanced the GSH (AA-L, 8.67 ± 0.47; AA-M, 9.71 ± 0.5; AA-H, 10.88 ± 0.66) (PAE-L, 9.12 ± 0.19; PAE-M, 10.92 ± 0.34; PAE-H, 12.2 ± 0.51) and SOD (AA-L, 4.79 ± 0.1; AA-M, 5.99 ± 0.81; AA-H, 6.66 ± 0.31) (PAE-L, 6.1 ± 0.6; PAE-M, 6.64 ± 0.32; PAE-H, 7.56 ± 0.35) activity (p < 0.05), and noticeably reduced ROS production (AA-L, 221.8%; AA-M, 171.8%; AA-H, 161.5%) (PAE-L, 201.1%; PAE-M, 147.9%; PAE-H, 125.5%) and MDA (AA-L, 4.58 ± 0.2; AA-M, 4.46 ± 0.41; AA-H, 4 ± 0.23) (PAE-L, 4.05 ± 0.11; PAE-M, 3.88 ± 0.16; PAE-H, 3.66 ± 0.12) content (p < 0.05). These results indicate that the antioxidative effect of PAE on TNF-α-induced NCM460 cells may be derived from their glycine and proline contents.
Figure 8. Antioxidative effect of PAE on TNF-α-induced NCM460 cells. Cell viability (%) (A), Levels of ROS production (B), MDA (C), SOD (D) and GSH (E). Error bars represent the mean ± SEMs (n = 3/group). ##p < 0.01, #p < 0.05 compared to NC group; **p < 0.01, *p < 0.05 compared to model group. PAE: P. americana extract, PAE-L: 400 μg/mL, PAE-M: 800 μg/mL, PAE-H: 1600 μg/mL; AA-L (3 μg/mL proline and 4 μg/mL glycine), AA-M (6 μg/mL proline and 8 μg/mL glycine), AA-H (12 μg/mL proline and 16 μg/mL glycine).
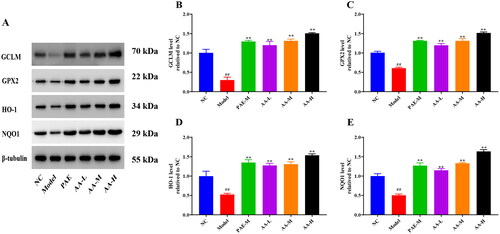
Effects of PAE and AA on the Nrf2 signaling pathway in TNF-α-induced NCM460 cells
As shown in , the model group had lower levels of GPX2, GCLM, HO-1, and NQO1 (p < 0.01) than the NC group. However, these levels were significantly increased after treatment with PAE and AA (p < 0.01). GPX2, GCLM, HO-1, and NQO1 are downstream proteins of Nrf2. These results indicated that the antioxidative stress effect of PAE on TNF-α-induced NCM460 cells may occur through the activation of Nrf2, which may be related to the glycine and proline content.
Figure 9. PAE activated the Nrf2 signaling pathway in TNF-α-induced NCM460 cells. (A) Representative expression of GCLM, GPX2, HO-1, and NQO1. Relative expression of GCLM (B), GPX2 (C), HO-1 (D), and NQO1 (E). Error bars represent the mean ± SEMs (n = 3/group). ##p < 0.01, #p < 0.05 relative to NC group; **p < 0.01, *p < 0.05 relative to model group. PAE: P. americana extract, PAE-L: 400 μg/mL, PAE-M: 800 μg/mL, PAE-H: 1600 μg/mL; AA-L (3 μg/mL proline and 4 μg/mL glycine), AA-M (6 μg/mL proline and 8 μg/mL glycine), AA-H (12 μg/mL proline and 16 μg/mL glycine).
Discussion
In this study, we investigated the antioxidative effect of PAE on DSS-induced mice and attempted to clarify its molecular mechanisms and active components. First, the quality of PAE was evaluated using LC-MS/MS, providing the basis for studying its pharmacodynamics and pharmacological mechanisms. A UC model was experimentally induced by the DSS method. It showed symptoms like those observed during induction in patients with UC, including shortened colon length, diarrhea, weight loss, and reduced activity, which are consistent with previous studies (Eichele and Kharbanda Citation2017). We demonstrated that PAE may improve the pathological symptoms of colitis, including shortened colon length, diarrhea, and weight loss, and reduce the DAI score—a reliable indicator of disease severity (Huang et al. Citation2017). Additionally, H&E staining showed that PAE reduced inflammatory cell infiltration and epithelial cell destruction caused by the recruitment of immune cells and activation of chemokines at the inflammatory site of the colon in UC mice. These results collectively indicate that PAE markedly relieved colonic inflammation and exerted a protective effect against DSS-induced UC in mice.
UC is a recurrent inflammatory disease characterized by an imbalance in cytokine secretion. An imbalance of the intestinal mucosal immune system exists in the presence of anti-inflammatory (e.g., IL-10, IL-4) and pro-inflammatory factors (e.g., TNF-α, IL-6, IL-17) of UC pathogenesis. Thus, beneficial modulation of inflammatory factors may provide a viable therapeutic strategy for UC treatment. In this study, PAE regulated cytokine parameters in the serum of mice with DSS-induced colitis, via decreases in TNF-α, IL-6, IL-17, and increases in IL-10.
Oxidative stress is a state of imbalance between oxidation and antioxidation. Inflammatory stimulation can result in oxidative stress, which in turn damages colon tissue and aggravates UC symptoms (Kruidenier et al. Citation2003; D'Inca et al. Citation2004). COX-2 and iNOS, widely acknowledged as inducible enzymes, are rarely secreted under normal conditions but are secreted by fibroblasts and monocyte macrophages in response to in vivo stimulation. Furthermore, overexpression of iNOS and COX-2 could contribute to the development of oxidative damage. In this study, iNOS and COX-2 expression levels were markedly elevated in the model group; however, this effect was reversed by PAE intervention. SOD, MDA, GSH, and MPO are common indicators in evaluating the level of oxidative stress. SOD, MDA, and GSH belong to the phase II control detoxifying enzymes of Nrf2. MPO is a peroxidase produced by activated neutrophils. In this study, intervention with PAE evidently decreased the activity of MDA and MPO and enhanced the levels of SOD and GSH. These results indicate that PAE may alleviate DSS-induced UC in mice by enhancing the antioxidant system and inhibiting lipid peroxidation.
Based on this relationship between UC and oxidative stress, we further explored the molecular mechanism by which PAE regulates oxidative stress in UC mice. Nrf2 is a key regulator in the expression of antioxidant proteins and detoxification enzymes in the cellular oxidative stress system (Zhang et al. Citation2014). Under normal conditions, Nrf2 binds to Kelch-like ECH-associated protein 1 (Keap1) in the cytoplasm. When oxidative stress occurs, the Keap cysteine residues are modified, altering the conformation and leading to the release of Nrf2 and its entry into the nucleus. Here, it regulates antioxidative proteins and phase II detoxification enzymes (e.g., GPX2, NQO1, HO-1, and GCLM) through interaction with the antioxidant response element ARE (Moura et al. Citation2015). Through these molecular mechanisms, Nrf2 can reduce cellular oxidative stress and exert protective effects. In this study, cytoplasmic Nrf2 protein expression was markedly decreased in the PAE and 5-ASA intervention groups relative to that in the model group, and the level of nuclear Nrf2 increased in a dose-dependent manner. This suggests that PAE may activate the Nrf2 pathway by promoting Nrf2 nuclear translocation. When the Nrf2 pathway is activated, the expression of Nrf2-regulated downstream proteins, including GCLM, NQO1, HO-1, and GCLM, is also significantly increased (Surh et al. Citation2008). Additionally, the expressions of the Nrf2-regulated downstream proteins GCLM, GPX2, HO-1, and NQO1 were significantly increased after PAE intervention. Therefore, PAE is expected to restore redox balance by activating the Nrf2 signaling pathway, thus inhibiting oxidative stress and inflammatory response, and preventing the aggravation of colitis injury.
The pathogenesis of UC is complex, and treatment is relatively difficult. TCM has shown to be effective, economical, and highly safe in the treatment of UC, due to its multiple active ingredients and targets. Additionally, as an insect, P. americana is rich in amino acids; however, their role in the pharmacological activity of PAE remains unknown. He et al. (Citation2018) reported that amino acids may play an important role in intestinal growth and maintaining mucosal integrity and barrier function. Previous studies have also found that glycine (Petrat et al. Citation2012; Wang et al. Citation2014) and proline (Li et al. Citation2007) can alleviate IBD; the mechanism is associated with the reduction of inflammation, antioxidative stress, and regulation of inflammation-related factors. In PAE, the glycine and proline content are relatively high. We developed a reproducible and stable amino acid method of analysis, and prepared a mixture of glycine and proline in the same proportions as those present in in vitro PAE, including PAE-L and AA-L, PAE-M and AA-M, and PAE-H and AA-H. In this study, treatment with PAE and AA effectively inhibited cell damage and oxidative stress in TNF-α-induced NCM460 cells. Additionally, PAE inhibited oxidative stress better than AA at the same amino acid concentration. These results show that the antioxidative stress effect of PAE is related to the levels of glycine and proline, but there may be other substances that have antioxidative stress effects as well. Furthermore, we found that AA significantly upregulated the GCLM, GPX2, HO-1, and NQO1 levels, which are regulated by Nrf2; thus, the antioxidative effect of PAE by modulating the Nrf2 pathway may be related to the glycine and proline levels. TCM is characterized by the combined therapeutic action of multiple active ingredients. A single active ingredient usually cannot explain the mechanism of action of TCM. Therefore, we speculate that the active ingredients of PAE that exert an antioxidative effect may be combined with other substances in addition to amino acids.
Conclusions
We show that PAE can alleviate intestinal tissue injury and suppress inflammatory response and oxidative stress in a UC model by activating the Nrf2 signaling pathway and promoting Nrf2 nuclear translocation. Moreover, we confirmed that the active components of PAE against oxidative stress are proline and glycine. We also speculate that PAE may contain other active substances against oxidative stress in addition to amino acids.
Acknowledgements
Jianzhong Wu thanks Dali University and Shanghai Synergy Pharmaceutical Sciences Co., Ltd. for their support in completing the thesis through this work.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Adams SM, Bornemann PH. 2013. Ulcerative colitis. Am Fam Physician. 87(10):699–705.
- Ali T, Shakir F, Morton J. 2012. Curcumin and inflammatory bowel disease: biological mechanisms and clinical implication. Digestion. 85(4):249–255.
- Chen G, Yang Y, Liu M, Teng Z, Ye J, Xu Y, Cai X, Cheng X, Yang J, Hu C, et al. 2015. Banxia xiexin decoction protects against dextran sulfate sodium-induced chronic ulcerative colitis in mice. J Ethnopharmacol. 166:149–156.
- Curro D, Pugliese D, Armuzzi A. 2017. Frontiers in drug research and development for inflammatory bowel disease. Front Pharmacol. 8:400.
- D'Inca R, Cardin R, Benazzato L, Angriman I, Martines D, Sturniolo GC. 2004. Oxidative DNA damage in the mucosa of ulcerative colitis increases with disease duration and dysplasia. Inflamm Bowel Dis. 10(1):23–27.
- Eichele DD., Kharbanda KK. 2017. Dextran sodium sulfate colitis murine model: an indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J Gastroenterol. 23:6016–6029.
- Hamamoto N, Maemura K, Hirata I, Murano M, Sasaki S, Katsu K. 1999. Inhibition of dextran sulphate sodium (DSS)-induced colitis in mice by intracolonically administered antibodies against adhesion molecules [endothelial leucocyte adhesion molecule-1 (ELAM-1) or intercellular adhesion molecule-1 (ICAM-1.)]. Clin Exp Immunol. 117(3):462–468.
- He F, Wu C, Li P, Li N, Zhang D, Zhu Q, Ren W, Peng Y. 2018. Functions and signaling pathways of amino acids in intestinal inflammation. Biomed Res Int. 2018:9171905.
- Honap S, Chee D, Chapman TP, Patel M, Kent AJ, Ray S, Sharma E, Kennedy J, Cripps S, Walsh A, et al. 2020. Real-world effectiveness of tofacitinib for moderate to severe ulcerative colitis: a multicentre UK experience. J Crohns Colitis. 14(10):1385–1393.
- Huang YF, Zhou JT, Qu C, Dou YX, Huang QH, Lin ZX, Xian YF., Xie YL., Lai XP., et al. 2017. Anti-inflammatory effects of Brucea javanica oil emulsion by suppressing NF-kappaB activation on dextran sulfate sodium-induced ulcerative colitis in mice. J Ethnopharmacol. 198:389–398.
- Kruidenier L, Kuiper I, Van Duijn W, Mieremet-Ooms MA, van Hogezand RA, Lamers CB, Verspaget HW. 2003. Imbalanced secondary mucosal antioxidant response in inflammatory bowel disease. J Pathol. 201(1):17–27.
- Li P, Yin YL, Li D, Kim SW, Wu G. 2007. Amino acids and immune function. Br J Nutr. 98(2):237–252.
- Li N, Li GZ, Wang JC, Shen LG, Yong SJ. 2017. Chemical components and biological activities in Periplaneta america. Prog in Mod Biomed. 17:7.
- Liao Q, Pang L, Li JJ, Zhang C, Li JX, Zhang X, Mao T, Wu DT, Ma XY, Geng FN, et al. 2022. Characterization and diabetic wound healing benefits of protein-polysaccharide complexes isolated from an animal ethno-medicine Periplaneta americana L. Int J Biol Macromol. 195:466–474.
- Ma X, Hu Y, Li X, Zheng X, Wang Y, Zhang J, Fu C, Geng F. 2018. Periplaneta americana ameliorates dextran sulfate sodium-induced ulcerative colitis in rats by Keap1/Nrf-2 activation, intestinal barrier function, and gut microbiota regulation. Front Pharmacol. 9:944.
- Moura FA, de Andrade KQ, Dos Santos JCF, Araujo ORP, Goulart MOF. 2015. Antioxidant therapy for treatment of inflammatory bowel disease: does it work? Redox Biol. 6:617–639.
- Neurath MF. 2014. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 14(5):329–342.
- Petrat F, Boengler K, Schulz R, de Groot H. 2012. Glycine, a simple physiological compound protecting by yet puzzling mechanism(s) against ischaemia-reperfusion injury: current knowledge. Br J Pharmacol. 165(7):2059–2072.
- Piechota-Polanczyk A, Fichna J. 2014. Review article: the role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn Schmiedebergs Arch Pharmacol. 387(7):605–620.
- Prinsen H, Schiebergen-Bronkhorst BGM, Roeleveld MW, Jans JJM, de Sain-van der Velden MGM, Visser G, van Hasselt PM, Verhoeven-Duif NM. 2016. Rapid quantification of underivatized amino acids in plasma by hydrophilic interaction liquid chromatography (HILIC) coupled with tandem mass-spectrometry. J Inherit Metab Dis. 39(5):651–660.
- Samman FS, Elaidy SM, Essawy SS, Hassan MS. 2018. New insights on the modulatory roles of metformin or alpha-lipoic acid versus their combination in dextran sulfate sodium-induced chronic colitis in rats. Pharmacol Rep. 70(3):488–496.
- Surh YJ, Kundu JK, Na HK. 2008. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 74(13):1526–1539.
- Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel J-F. 2017. Ulcerative colitis. Lancet. 389(10080):1756–1770.
- Wang W, Wu Z, Lin G, Hu S, Wang B, Dai Z, Wu G. 2014. Glycine stimulates protein synthesis and inhibits oxidative stress in pig small intestinal epithelial cells. J Nutr. 144(10):1540–1548.
- Xue M, Shi L, Wang W, Chen S, Wang L. 2018. An overview of molecular profiles in ulcerative colitis-related cancer. Inflamm Bowel Dis. 24(9):1883–1894.
- Zeng C, Liao Q, Hu Y, Shen Y, Geng F, Chen L. 2019. The role of Periplaneta americana (Blattodea: Blattidae) in modern versus traditional Chinese medicine. J Med Entomol. 56(6):1522–1526.
- Zhang T, Kimura Y, Jiang S, Harada K, Yamashita Y, Ashida H. 2014. Luteolin modulates expression of drug-metabolizing enzymes through the AhR and Nrf2 pathways in hepatic cells. Arch Biochem Biophys. 557:36–46.
- Zhu JJ, Yao S, Guo X, Yue BS, Ma XY, Li J. 2018. Bioactivity-guided screening of wound-healing active constituents from American cockroach (Periplaneta americana). Molecules. 23(1):101.



