Abstract
Context
Qingyi granules can be used to effectively treat patients with severe acute pancreatitis (SAP).
Objective
To elucidate the role of gut microbiota-mediated metabolism in the therapeutic effects of Qingyi granules.
Materials and methods
Sprague–Dawley rats were grouped into the sham operation, SAP model, Qingyi granule intervention (Q, 1.8 g/kg) and emodin intervention (E, 50 mg/kg) groups and observed for 24 h. H&E staining and ELISA were used for histopathological analysis and serum enzyme and cytokine assays. 16S rDNA sequencing and UHPLC-HRMS were used for gut microbiota analysis and untargeted metabolomics.
Results
In SAP rats, Qingyi granules decreased the pancreatic pathological score (Q, 7.4 ± 1.14; SAP, 11.6 ± 1.14, p < 0.01); serum amylase (Q, 121.2 ± 6.7; SAP, 144.3 ± 8.86, p < 0.05), lipase (Q, 566 ± 20.34; SAP, 656.7 ± 29.32, p < 0.01), and diamineoxidase (Q, 492.8 ± 26.08; SAP, 566.1 ± 26.83, p < 0.05) activities; and IL-1β (Q, 29.48 ± 0.88; SAP, 36.17 ± 1.88, p < 0.01), IL-6 (Q, 112.2 ± 3.57; SAP, 128.9 ± 9.09, p < 0.05) and TNF-α (Q, 215.3 ± 8.67; SAP, 266.4 ± 28.03, p < 0.05) levels. SAP induced Helicobacter and Lactobacillus overgrowth and suppressed Romboutsia and Allobaculum growth and caused aberrations in bacterial metabolites, which were partly reversed by Qingyi granules.
Discussion and Conclusions
Qingyi granules can modulate the gut microbiota and metabolic abnormalities to ameliorate SAP. Multi-omics approaches allow systematic study of the pharmacological mechanisms of compound prescriptions for critical illnesses.
Introduction
Severe acute pancreatitis (SAP) is a critical illness that originates in the pancreas and rapidly spreads throughout the body, and its incidence has risen with the increasing incidence of acute pancreatitis (AP) in the UK, Sweden, Denmark, the Netherlands, Norway, and Japan; SAP has a mortality rate of 20–40% (Lankisch et al. Citation2015; Boxhoorn et al. Citation2020). Infection is a predominant factor underlying the high mortality; in particular, enterogenic infection is the main causative agent, as evidenced by the discovery of bacteria in the blood stream of SAP patients (Werge et al. Citation2016; Li et al. Citation2018). In fact, some researchers have suggested that the gut microbiota affects the progression and severity of AP (Zheng et al. Citation2019; Zhu et al. Citation2019). SAP induces intestinal damage, followed by changes in intestinal microecology, which in turn exacerbates intestinal barrier disruption and leads to enterogenic infection (Capurso et al. Citation2012). The pathogenesis of SAP is driven by microbiota-derived metabolites, as shown in recent studies, and a healthy microbiota composition can lead to the release of favorable metabolites such as nicotinamide mononucleotide and short-chain fatty acids to relieve AP (Wang et al. Citation2022; Liu et al. Citation2023). To date, the gut microbiota and the metabolites these microbes produce in SAP patients are largely unknown and need to be explored for the development of new therapeutic strategies for SAP management.
Qingyi granules, prepared by modifying the Qingyi decoction used in traditional Chinese medicine (TCM), include Bupleurum chinense DC. (Apiaceae), Gardenia jasminoides J.Ellis (Rubiaceae), Scutellaria baicalensis Georgi (Lamiaceae), Paeonia lactiflora Pall. (Paeoniaceae), Corydalis yanhusuo (Y.H.Chou & Chun C.Hsu) W.T.Wang ex Z.Y.Su & C.Y.Wu (Papaveraceae), Saussurea costus (Falc.) Lipsch. (Compositae), Rheum officinale Baill. (Polygonaceae) and Natrii Sulfas, and they exhibit effects in dispersing stagnation, relieving pain, bowel purgation and circulation invigoration in SAP treatment (Chen W et al. Citation2015; Guo and Guo Citation2019). As a compound formula shown to have clinical efficacy, Qingyi decoction is being widely investigated, and many of its pharmacological mechanisms have been revealed. In an SAP rat model, Qingyi decoction decreased the level of secretory phospholipase A2 in the intestine, maintained intestinal barrier integrity, and prevented bacterial endotoxin-activated NF-κB signaling to restrain the spread of inflammation (Zhang et al. Citation2015; Su et al. Citation2019). In addition, pancreatic acinar cell injury in acute biliary pancreatitis was found to be ameliorated by Qingyi decoction via effects on the Gpbar1-NF-κB-p-RIP signaling pathway (Zhang et al. Citation2019). Proteomic studies have shown that Qingyi granules regulated 9 proteins related to inflammatory cell migration and infiltration to protect against SAP-induced lung injury (Sun et al. Citation2019). These studies have provided a wealth of insights into the therapeutic mechanisms of Qingyi decoction at the molecular level, particularly into protein-mediated signal transduction. Notably, the digestive tract is the first site for drug reaction, and the blood circulation serves mainly as the vehicle for drug delivery and signal feedback. Therefore, a systematic evaluation combining intestinal microbiota composition and serum metabolite profiling can complement studies to further clarify the extensive pharmacological activities of Qingyi granules.
Emodin is the main effective ingredient of Rheum officinale, the primary drug in Qingyi granules. Studies have shown that emodin can induce circulating neutrophil apoptosis through the Ca2+-calpain1-caspase12-caspase-3 signaling pathway to protect SAP rats from excessive inflammation (Wang et al. Citation2016), In addition, emodin suppresses the P2X7/NLRP3 signaling pathway and reactive oxygen species generation to reduce the production of proinflammatory factors (Xia et al. Citation2019; Zhang et al. Citation2019). Moreover, sodium taurocholate-induced pancreatic acinar cell damage could be attenuated by emodin via miR-30a-5p/HTRA1 (Xiang et al. Citation2017). Considering that the crude drug emodin is poorly absorbed, intestinal interplay was predicted to contribute to its distal effects. Here, emodin was tested in a parallel group to study whether there were similarities between the compound formula in Qingyi granules and monomeric emodin in SAP treatment. We integrated microbiome research and metabolomics to investigate SAP-associated gut dysbiosis and serum metabolite alterations, aiming to reveal the potential enterogenic repair mechanism of Qingyi granules and emodin.
Materials and methods
Animals and pharmaceuticals
Sixty-four male Sprague–Dawley rats weighing 180–220 g were provided by the specific-pathogen free animal center of Dalian Medical University and kept in a clean animal laboratory with ad libitum access to food and water. All animal protocols were approved by the Committee on the Ethics of Animal Experiments of Dalian Medical University (No. AEE19001). Qingyi granules were provided by the Pharmaceutical Department of the First Affiliated Hospital of Dalian Medical University. The herbs in Qingyi granules include Bupleurum chinense, Gardenia jasminoides, Scutellaria baicalensis, Paeonia lactiflora, Corydalis yanhusuo, Saussurea costus, Rheum officinale, and Natrii Sulfas. Each of the voucher specimens was kept in the Clinical Laboratory of Integrative Medicine at the First Affiliated Hospital of Dalian Medical University. The formula preparation, chemical profiling and quality control have been reported by our team previously (Wei et al. Citation2021). Emodin (C15H10O5, MW: 270.24, CAS: 518-82-1), an anthraquinone derivative that is also named 2-methyl-4,5,7-trihydroxyanthraquinone and has a light yellow to orange appearance, was obtained from Solarbio Life Sciences Co. (IE0070), China.
SAP model establishment and drug intervention
After adaptive feeding for one week, rats were randomly grouped as follows: sham operation (SO, n = 12), SAP model (SAP, n = 20), Qingyi granule intervention (Q, n = 16) and emodin intervention (E, n = 16). Rats in the SO group received an approximately 1 cm incision at the medioventral line with a slight abdominal flap. Rats in the SAP, Q and E groups were subjected to SAP model establishment as previously described (Jiao et al. Citation2022). Two hours after modeling, rats in the Q group received gavage with Qingyi granules at a dosage of 0.18 g/mL and 1 mL/100 g body weight as described in our previous work (Jiao et al. Citation2022) once and then again 12 h later; rats in the E group was treated with emodin twice, as in the Q group, at a dosage of 50 mg/kg using a modified protocol based on previous studies (Xiang et al. Citation2017; Hu et al. Citation2022); rats in the SO and SAP groups received an equivalent amount of water instead. The administered dosage of emodin was approximately 5- to 10-times the amount of emodin contained in the Qingyi granules based on a rough test.
Sample collection
Twenty-four hours after model establishment, all animals were sacrificed via acute blood loss, using a 5 mL syringe to draw blood samples from the abdominal aorta of rat under anesthesia. Blood samples were centrifuged to collect serum for enzyme, inflammatory factor and metabolite analyses. Feces in the distal ileum were collected directly from the rat intestinal lumen under sterile conditions, immediately placed into sterilized tubes and frozen in liquid nitrogen. All fecal samples, except that from 1 mouse in the E group that died close to the experimental end point, were obtained from surviving mice, and thus, the number of stool samples for microbiota analysis was n = 12 in the SO group, n = 12 in the SAP group, n = 13 in the Q group and n = 13 in the E group. Pancreatic and intestinal tissues were isolated and processed for histopathological examination.
Histopathological examination, plasma enzyme assay and cytokine assay
Pancreatic and ileal tissues fixed in paraformaldehyde were dehydrated, embedded in paraffin and cut into 4 μm slices, which were stained with hematoxylin and eosin and observed under a light microscope. For pancreatic histopathological scoring, five fields of each of the 6 samples in each experimental group were evaluated according to the extent of pancreatic edema, inflammation, vacuolization and necrosis under a high-power field (Rongione et al. Citation1997). Plasma enzymes and cytokines were assessed by enzyme linked immunosorbent assay.
16S rDNA sequencing and bioinformatics analysis
The stool bacterial DNA of rats was extracted with a DNA Stool Mini Kit (Qiagen, Germany). After determining the concentration and purity by a simpliNano, we diluted the DNA to 1 ng/μL and amplified the V3–V4 region of bacterial 16S rDNA by using specific primers with barcodes. Next, we performed PCR and established sequence libraries to load on the IonS5TMXL platform to obtain raw reads (Jiao et al. Citation2022). QIIME quality filters were used to screen the raw reads and obtain clean reads. Next, we used Pick_de_novo_otus.py to prepare operational taxonomic unit (OTU) tables. Sequences with ≥ 97% similarity were assigned to the same OTUs. For each OTU, we picked the representative sequences and exploited the Ribosomal Database Project classifier to annotate taxonomic information. Nonmetric multidimensional scaling (NMDS) of the core OTUs was employed to reveal the structural differences in the gut microbiota among the different groups, and the Shannon–Wiener index was calculated to observe the community diversity among the four groups. Finally, we used linear discriminant analysis with effect size (LEfSe) to identify the differentially abundant species between the SO and SAP groups and visualized them with a cladogram.
Untargeted metabolomics analysis through ultrahigh-performance liquid chromatography coupled to high-resolution mass spectrometry (UHPLC-HRMS)
Serum samples were thawed, and 400 μL of a methanol-acetonitrile (1:1) (Thermo Fisher Scientific, USA) mixed solution was added to 100 μL of serum. After vortexing and centrifugation, 200 μL of the supernatant was retained and dried by a centrifugal concentrator, and the dried extracts were cryopreserved before analysis. For chromatographic separation, sample extracts were redissolved in 130 μL of 25% methanol. Untargeted metabolomics analysis was conducted on an UltiMate 3000 UHPLC-HRMS (Ji et al. Citation2021).
Statistical analysis
Statistical analysis was conducted with SPSS 23.0. The values are reported as the mean ± SD or mean ± SEM for quantitative variables and percentage for categorical variables. One-way ANOVA was performed to investigate quantitative data alterations among four groups; Fisher’s exact test was used for percentage comparison among four groups. Statistical significance was set as a p value < 0.05. GraphPad Prism 7.0 was utilized for diagramming. To observe the correlations between the four main differentially abundant bacteria at the genus level and serum metabolites, Spearman’s correlation coefficient was established by R software.
Results
Qingyi granules and emodin attenuated pancreatic inflammation in SAP rats
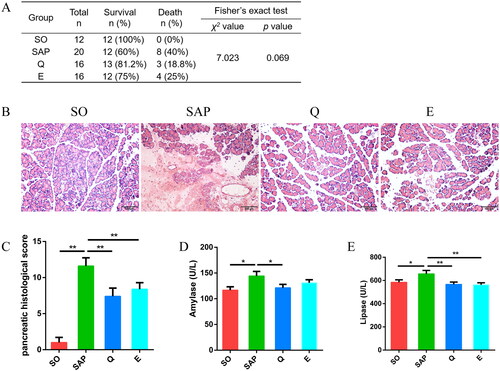
Both Qingyi granules and emodin intervention showed a decreasing trend for SAP-associated mortality and symptoms in rats. The mortality rate decreased from 40% in the SAP group to 18.8% in the Q group and 25% in the E group despite a p value of 0.069 by Fisher’s exact test (). Histopathological analysis showed mild pancreatic acinar cell edema and reduced infiltration of inflammatory cells after intervention with Qingyi granules and emodin (). Correspondingly, rats in the Q and E groups had lower pancreatic pathological scores than those in the SAP group (SO: 1 ± 0.71; SAP: 11.6 ± 1.14; Q: 7.4 ± 1.14; E: 8.4 ± 0.89, p < 0.01, ). In addition, rats in the SAP group had higher levels of serum amylase and lipase than those in the SO group (amylase, SO: 116.8 ± 6.51 U/L; SAP: 144.3 ± 8.86 U/L, p < 0.05; lipase, SO: 584.2 ± 21.72 U/L; SAP: 656.7 ± 29.32 U/L, p < 0.05; ), while Qingyi granule treatment significantly decreased the levels of serum amylase and lipase (amylase, Q: 121.2 ± 6.7 U/L, p < 0.05; lipase, Q: 566 ± 20.34 U/L, p < 0.01; ), and emodin significantly decreased the level of lipase (E: 562.9 ± 17.32 U/L, p < 0.01, ). These data indicated that both Qingyi granule and emodin treatment improved the pancreatic inflammation of SAP rats.
Figure 1. Both Qingyi granules and emodin attenuated pancreatic injuries and serum enzymatic indices in SAP rats. (A) Mortality of rats among the four groups. (B) Histological observation of the pancreas (HE, ×200). (C) Pancreatic histological scoring among the four groups. (D, E) Amylase and lipase levels in the serum among the four groups. SO, sham operation group; SAP, severe acute pancreatitis; Q, Qingyi granules; E, emodin. *p < 0.05; **p < 0.01.
Qingyi granules and emodin inhibited the systemic inflammatory response of SAP rats
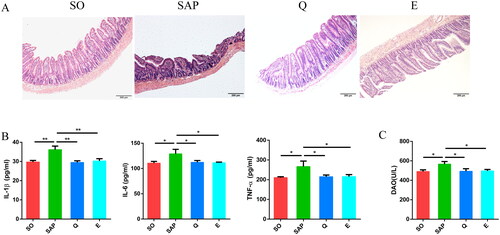
SAP rats displayed extensive intestinal injury, while rats in the Q and E groups exhibited improved intestinal villus morphology and arrangement (). The levels of the serum inflammatory factors interleukin-1β (IL-1β), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) were significantly higher in the SAP group than in the SO group. After Qingyi granule and emodin treatment, the levels of these cytokines were significantly reduced (IL-1β, SO: 29.71 ± 0.85 pg/mL; SAP: 36.17 ± 1.87 pg/mL; Q: 29.48 ± 0.88 pg/mL; E: 30.29 ± 1.18 pg/mL, p < 0.01; IL-6, SO: 110.6 ± 3.52 pg/mL; SAP: 128.9 ± 9.09 pg/mL; Q: 112.2 ± 3.57 pg/mL; E: 111.5 ± 1.14 pg/mL, p < 0.05; TNF-α, SO: 210.5 ± 4.69 pg/mL; SAP: 266.4 ± 28.03 pg/mL; Q: 215.3 ± 8.67 pg/mL; E: 215.7 ± 10.02 pg/mL, p < 0.05; ). Importantly, the diamineoxidase (DAO) level in serum was higher in SAP rats than in SO rats (SO: 490.3 ± 17.03 U/L; SAP: 566.1 ± 26.83 U/L, p < 0.05, ), suggesting increased permeability of the intestinal barrier in SAP rats. After treatment with Qingyi granules or emodin, the serum DAO level was significantly reduced (Q: 492.8 ± 26.08 U/L; E: 497 ± 14.88 U/L, p < 0.05, ). These results suggested that both Qingyi granules and emodin can protect the intestinal barrier and inhibit systemic inflammation in SAP rats.
Figure 2. Both Qingyi granules and emodin alleviated intestinal damage and reduced serum inflammatory factors in SAP rats. (A) Histological observation of the intestine (HE, ×100) among the four groups. (B) IL-1β, IL-6 and TNF-α levels in the serum among the four groups. (C) Diamineoxidase levels in the serum among the four groups. SO, sham operation group; SAP, severe acute pancreatitis; Q, Qingyi granules; E, emodin. *p < 0.05; **p < 0.01.
Qingyi granules improved gut microbiota dysbiosis in SAP rats
A total of 3,311,288 sequence reads were obtained from 50 fecal samples (n = 12 in the SO group, n = 12 in the SAP group, n = 13 in the Q group, n = 13 in the E group), with an average of 64,927 reads for each sample. A total of 1413 OTUs were clustered based on a 97% nucleotide similarity cutoff. The Venn diagram shows the common and unique OTUs among the different groups (). The Shannon index and observed species index were calculated to further evaluate the community richness and diversity at the OTU level. Rats in the SAP group exhibited a lower Shannon index and observed species index than those in the SO group (p < 0.05), while rats in the Q and E groups exhibited improved community richness and diversity compared with those in the SAP group, with significant elevation observed in the Q group (p < 0.05, ). Therefore, both Qingyi granules and emodin had positive regulatory effects on SAP-induced gut microbiota structural dysbiosis.
Figure 3. Qingyi granules modulate the intestinal microbiota composition of SAP rats. (A) Venn diagram of the shared and unique bacterial OTUs in different experimental groups. (B) Alpha diversity reflected by the Shannon index and observed species. (C) Beta diversity reflected by nonmetric multidimensional scaling (NMDS) analysis among the four groups. (D-E) Relative abundance of the gut microbiota at the phylum and genus levels. (F) LEfSe diagram shows the differentially abundant bacteria between the SO and SAP groups. (G) The relative abundance of bacteria among the four groups at the genus level. n = 12 in the SO group, n = 12 in the SAP group, n = 13 in the Q group, n = 13 in the E group. SO, sham operation group; SAP, severe acute pancreatitis; Q, Qingyi granules; E, emodin. *p < 0.05, **p < 0.01.
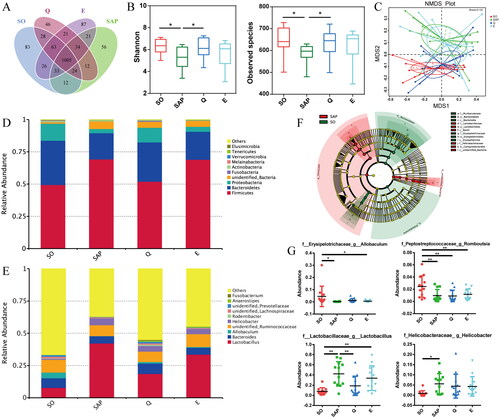
NMDS analysis was conducted to compare the bacterial community structure among the four groups. The SAP-induced gut microbiota composition markedly diverged from that of the SO group, but the composition was restored in the Q group. However, rats in the E group exhibited a similar microbiota structure to rats in the SAP group (). That is, compared with emodin, Qingyi granules had a much more significant effect on gut microflora restoration in SAP rats. The detailed gut microbiota distribution among the four groups is shown in terms of relative abundance in the histograms. At the phylum level, rats in both the SAP and E groups exhibited larger proportions of Firmicutes and lower proportions of Bacteroidetes and Proteobacteria than rats in the SO and Q groups (). At the genus level, rats in the SAP group showed increased Lactobacillus abundance and decreased Bacteroides abundance compared with rats in the SO group. After intervention with Qingyi granules, the relative abundance of Lactobacillus was decreased, and that of Bacteroides was increased (). LEfSe analysis showed the main differences between the SO and SAP groups; Muribaculaceae and Erysipelotrichaceae were enriched in the SO group, while Lactobacillaceae and Helicobacteraceae were enriched in the SAP group (). Furthermore, univariate analysis showed the detailed differences among the four groups (). The abundance of Lactobacillus and Helicobacter increased significantly in the SAP group compared with the SO group (p < 0.05), and they showed a decreasing trend in the Q and E groups compared with the SAP group, with the abundance of Lactobacillus decreasing significantly in the Q group (p < 0.01). In addition, SAP induced a significant reduction in the relative abundance of Allobaculum and Romboutsia, which remained unchanged in the Q and E groups. These results suggested that SAP induced changes in the gut microbiota composition, which could be partially repaired by Qingyi granule treatment.
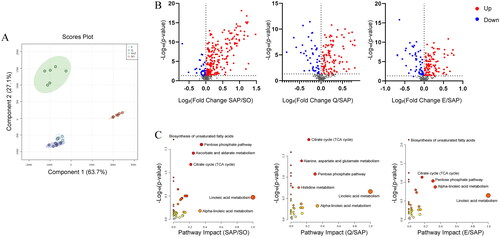
Qingyi granules and emodin partly reversed serum metabolic aberrations in SAP rats
As the microbe-host bridge, many intestinal flora derived metabolites can influence host physiology and pathology in the intestine and/or distant organs by entering the bloodstream. Six samples were randomly selected from each group for serum metabolomic analysis. A total of 560 metabolites were identified in positive (ES +) and negative (ES -) ion modes. Partial least squares discriminant analysis (PLS-DA) showed that the metabolic profiles of the SO and SAP groups were obviously separated from each other, while those of the Q and E groups were hardly separated and showed significant differences from that of the SAP group (). This suggested that there were significant differences in metabolites between the SO and SAP groups, and Qingyi granules and emodin treatment could change the SAP-induced serum metabolic profile. The volcano plots demonstrated significantly changed metabolites between each pair of groups (). A total of 284 differentially abundant metabolites were identified between the SAP and SO groups, 153 differentially abundant metabolites between the Q and SAP groups, and 157 differentially abundant metabolites between the E and SAP groups. There were 41 metabolites that changed significantly among the four groups, including amino acids, carbohydrates, fatty acids, and organic acids. Metabolomics pathway analysis was applied to explore the involved metabolic pathways. As shown in , linoleic acid metabolism, the pentose phosphate pathway (PPP), and the citrate cycle were the main affected metabolic pathways related to SAP and treatment response. In addition, ascorbate and aldarate metabolism was affected by SAP. Biosynthesis of unsaturated fatty acids was related to SAP and emodin treatment. Alanine, aspartate and glutamate metabolism was related to Qingyi granule treatment.
Figure 4. Analysis of serum metabolites among the four groups by UHPLC-HRMS-based metabolomics. (A) OPLS-DA plots of metabolite distribution among the four groups. (B) Volcano plots of metabolites between each pair of groups: SO and SAP, SAP and Q, and SAP and E. (C) Metabolic pathway enrichment analysis of each pair of groups. n = 6 in each group. SO, sham operation group; SAP, severe acute pancreatitis; Q, Qingyi granules; E, emodin.
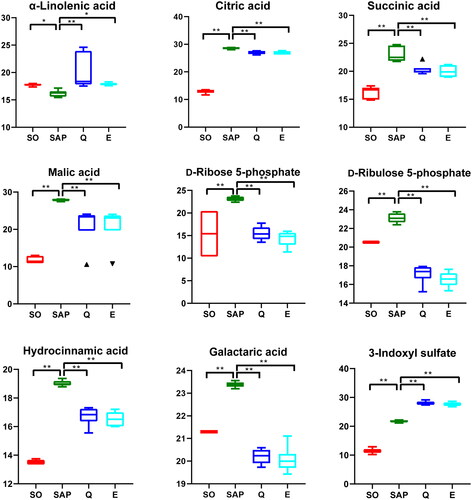
Representative metabolites with significant differences in levels are shown in the boxplot (). The level of α-linolenic acid in the SAP group was lower than that in the SO group, and after treatment with Qingyi granule or emodin, it increased to nearly normal levels [Fold change (FC) (SAP/SO) = 0.91, p < 0.05; FC (Q/SAP) = 1.24, p < 0.01; FC (E/SAP) = 1.1, p < 0.05]. The levels of three organic acids involved in the Krebs cycle, including citric acid [FC (SAP/SO) = 2.21, p < 0.01; FC (Q/SAP) = 0.94, p < 0.01; FC (E/SAP) = 0.94, p < 0.01], succinic acid [FC (SAP/SO) = 1.42, p < 0.01; FC (Q/SAP) = 0.88, p < 0.01; FC (E/SAP) = 0.87, p < 0.01] and malic acid [FC (SAP/SO) = 2.37, p < 0.01; FC (Q/SAP) = 0.76, p < 0.01; FC (E/SAP) = 0.76, p < 0.01], in the serum of SAP rats were significantly higher than those in the SO rats and partly restored to normal levels after treatment with Qingyi granule or emodin. Two intermediate metabolites in the PPP, d-ribose 5-phosphate [FC (SAP/SO) = 1.5, p < 0.01; FC (Q/SAP) = 0.67, p < 0.01; FC (E/SAP) = 0.62, p < 0.01] and d-ribulose 5-phosphate [FC (SAP/SO) = 1.13, p < 0.01; FC (Q/SAP) = 0.74, p < 0.01; FC (E/SAP) = 0.72, p < 0.01], were elevated in the SAP group and depleted significantly after Q and E treatment, respectively. The levels of hydrocinnamic acid [FC (SAP/SO) = 1.41, p < 0.01; FC (Q/SAP) = 0.88, p < 0.01; FC (E/SAP) = 0.87, p < 0.01] and 3-indoxyl sulfate [FC (SAP/SO) = 1.9, p < 0.01; FC (Q/SAP) = 1.29, p < 0.01; FC (E/SAP) = 1.27, p < 0.01] in serum were higher in rats in the SAP group than in rats in the SO group, and Qingyi granules reduced the serum content of hydrocinnamic acid but not 3-indoxyl sulfate in SAP rats. The levels of galactaric acid [FC (SAP/SO) = 1.1, p < 0.01; FC (Q/SAP) = 0.86, p < 0.01; FC (E/SAP) = 0.86, p < 0.01] increased significantly in the SAP group and then decreased significantly after Qingyi granule or emodin treatment. These results indicated that Qingyi granules and emodin can improve serum metabolic aberrations induced by SAP.
Figure 5. Relative concentrations of α-linolenic acid, citric acid, succinic acid, malic acid, d-ribose 5-phosphate, d-ribulose 5-phosphate, hydrocinnamic acid, galactaric acid, and 3-indoxyl sulfate among the four groups. n = 6 in each group. SO, sham operation group; SAP, severe acute pancreatitis; Q, Qingyi granules; E, emodin. *p < 0.05, **p < 0.01.
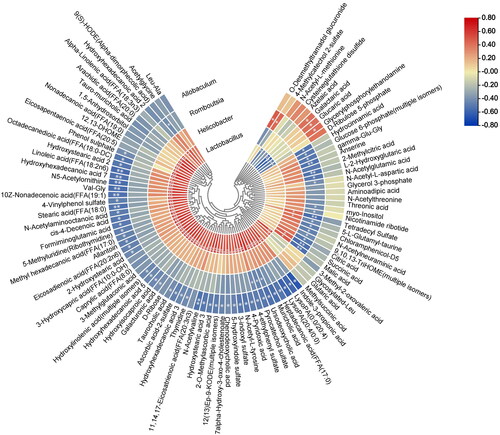
Serum metabolic aberrations in SAP rats had potential relationships with the SAP-induced gut microbiota dysbiosis
Correlation analysis between four key bacteria at the genus level, including Lactobacillus, Helicobacter, Romboutsia and Allobaculum, and differentially abundant metabolites was carried out to investigate the potential gut microbiota-mediated metabolic disturbance in SAP (). The level of Allobaculum and Romboutsia, which decreased in SAP rats, were negatively correlated with the levels of a variety of metabolites, including citric acid, malic acid, hydrocinnamic acid, succinic acid, and linoleic acid. In addition, the abundance of Allobaculum was positively correlated with the levels of galactaric acid, glucaric acid, and glycerylphosphorylethanolamine, and the abundance of Romboutsia was positively correlated with the levels of 4-methylcatechol 2-sulfate and o-desmethyltramadol glucuronide. The abundances of Lactobacillus and Helicobacter, which were increased in SAP rats, were positively correlated with the levels of most of the metabolites; in particular, Lactobacillus abundance was significantly correlated with the levels of metabolites such as indole-3-propionic acid, linoleic acid, and 3-indoxyl sulfate, while it was negatively correlated with the levels of galactaric acid, glucaric acid, glycerylphosphorylethanolamine and d-ribulose 5-phosphate. These results suggested that the four key bacteria have an intimate relationship with serum metabolite levels in SAP.
Discussion
Gut microbiota disturbance has been shown by many studies and our previous work to induce pancreatitis and contribute to its development. Oral administration of traditional Chinese medicines, especially those with low intestinal absorptivity, is likely to affect SAP through interaction with the gut microbiota. In this respect, our study demonstrated that Qingyi granules could improve SAP-associated pathological and enzymological indices, protect intestinal barrier integrity and alleviate systemic inflammation and metabolic disturbance in gut microbiota-dependent and gut microbiota-independent manners.
Using 16S rDNA sequencing, we assessed gut microbiota changes in rats with SAP. Consistent with a previous report (Brubaker et al. Citation2021), SAP was accompanied by decreased species diversity and an apparently different microbiota structure from that in the healthy state. The relative abundances of Lactobacillus and Helicobacter were elevated, and those of Allobaculum and Romboutsia were reduced. Lactobacillus is often regarded as a probiotic, but in SAP-associated microbiota imbalance, evidence to support its beneficial effects is lacking. No significant difference in Lactobacillus abundance among healthy, mild AP and severe AP patients was found in a clinical study (Tan et al. Citation2015). An earlier clinical trial even pointed out that a probiotic prophylaxis including mainly Lactobacillus was associated with an increased risk of death in AP patients (Besselink et al. Citation2008). Both our previous work (Jiao et al. Citation2022) and Zhu’s research (Zhu et al. Citation2019) confirmed that Lactobacillales was enriched in SAP rats and might be correlated with the increased intestinal permeability and systemic inflammation. Further studies need to be performed with ingenious research design and using more advanced technologies to obtain a deeper understanding of the complex role of Lactobacillus. Helicobacter has long been recognized as a pathogenic bacterium in the human gastrointestinal system (Smet and Menard Citation2020), so its increase in SAP can promote disease progression. On the other hand, the abundance of Romboutsia, as a previous study suggested, was inversely correlated with systemic inflammation (Dang et al. Citation2022). In addition, it favored chemoprevention in an animal model of colorectal cancer (Silva-Reis et al. Citation2022). Allobaculum is a kind of probiotic that produces short-chain fatty acids (Balakrishnan et al. Citation2021). It can also produce certain long-chain fatty acids to exert anti-inflammatory activity (Pujo et al. Citation2021). Accordingly, SAP shapes a biotope to support pathogenic bacterial growth but restrain potential anti-inflammatory probiotics.
Next, the regulatory effects of Qingyi granules and emodin on the gut microbiota of SAP rats were evaluated to compare the effects of the compound formula and monomer in parallel. Qingyi granules improved the species diversity of the gut microbiota and tended to restore the SAP-induced abnormal microbiota structure toward a normal distribution, while emodin failed to achieve a comparable effect. Notably, the emodin content in the administered dose of Qingyi granules was much lower than the administered monomer dose, so the relatively weak effect of emodin on the gut microbiota was not attributed to underdosage. Emodin was previously reported to regulate the microbiota for renal disease improvement (Zeng et al. Citation2016; Sun et al. Citation2019) and to have an anti-inflammatory role in SAP (Besselink et al. Citation2008). Although a consistent anti-inflammatory role of emodin was identified in this study, the effect seems to be less closely related to the gut microbiota. Furthermore, the increase in Lactobacillus abundance induced by SAP could be decreased by Qingyi granules but not emodin, suggesting a suppressive role of Qingyi granules on pathogenic bacteria. For the potential beneficial bacteria Allobaculum and Romboutsia, no promoting or inhibitory effects were observed with Qingyi granules or emodin. Therefore, the compound formula in Qingyi granules outperformed the monomer emodin, at least in maintaining gut microecological balance in SAP.
Intestinal microorganisms can cometabolize endogenous and exogenous substances to produce many absorbable metabolites that enter the host circulation and participate in host pathophysiological activities (Ye et al. Citation2021). Among the identified differentially abundant metabolites associated with SAP, α-linolenic acid, citric acid, succinic acid, galactaric acid, d-ribose 5-phosphate, hydrocinnamic acid and 3-indoxyl sulfate have been shown to have clinical importance in pancreatic and inflammatory diseases. α-Linolenic acid is an essential long-chain polyunsaturated fatty acid and has therapeutic value in metabolic syndrome, cancer, inflammation, oxidative stress, obesity, neuroprotection, and gut microbiota regulation (Yuan et al. Citation2022). The serum citric acid level in AP patients is significantly increased, with a higher level observed in SAP patients, indicating that it is a potential biomarker for AP (Xiao et al. Citation2017; Guo et al. Citation2019). Succinate, an intermediate in the Krebs cycle, can stabilize hypoxia-inducible factor-1 and stimulate dendritic cells to mediate inflammation (Mills and O'Neill Citation2014). It could promote IL-1β production in the inflammatory response (Tannahill et al. Citation2013). In addition, serum succinic acid levels were found to increase significantly in AP patients (Guo et al. Citation2019). Galactaric acid, a dicarboxylic acid produced by d-galacturonic acid oxidation, is closely involved in microflora metabolism (Watanabe et al. Citation2007). Serum galactaric acid was shown to be positively correlated with Ruminococcus abundance in hepatocellular carcinoma patients responding to immunotherapy (Wu et al. Citation2022). This suggests that high galactaric acid levels are related to abnormal microbiota activities in SAP. d-Ribose 5-phosphate is the end product of the PPP, and is produced by ribose-5-phosphate isomerase mediated isomerization of d-ribulose 5-phosphate (Chen et al. Citation2020). The increase in these two intermediates suggested enhancement of the PPP in SAP, which is supported by previous studies showing that PPP could be activated by lipopolysaccharide and that PPP inhibition could eliminate LPS-induced inflammatory cytokine secretion (Smith et al. Citation2014; Yu et al. Citation2019). Hydrocinnamic acid and 3-indoxyl sulfate are microbial metabolites. Hydrocinnamic acid is produced by Enterobacteriaceae, such as Escherichia coli (Sun et al. Citation2016; Sharma et al. Citation2019). It can act with PIP2 to trigger inflammation (Ren et al. Citation2017). 3-Indoxyl sulfate is a byproduct of indole metabolism by commensal gut bacteria and can induce intestinal barrier injury (Cheng et al. Citation2020). Mechanistically, indoxyl sulfate, as a kind of serotoxin, can induce oxidative stress to cause vascular damage and visceral dysfunction (Gao and Liu Citation2017).
Treatment with Qingyi granules and emodin restored the SAP-induced decrease in α-linolenic acid, which may contribute to both drugs’ therapeutic role via anti-inflammation and antioxidation effects. In contrast, the levels of the metabolites enriched in SAP, including citric acid, succinic acid, galactaric acid, d-ribose 5-phosphate, and hydrocinnamic acid, were decreased after Qingyi granules and emodin treatment, suggesting that both drugs can clear the inflammation-promoting metabolites in SAP. Taken together, the results show that Qingyi granules and emodin can counteract the disturbed serum metabolism to alleviate SAP. Interestingly, as intermediates of the Krebs cycle, the levels of succinic acid and malic acid both have a positive correlation with the abundance of Helicobacter and a negative correlation with Romboutsia, and citric acid has a negative correlation with Romboutsia. As microbiota-derived metabolites, the level of hydrocinnamic acid was negatively correlated with the abundance of Romboutsia, and 3-indoxyl sulfate concentration was negatively correlated with Allobaculum abundance but positively correlated with Lactobacillus abundance. According to these results, an increase in pathogenic bacteria and a decrease in beneficial bacteria are believed to be at least partially responsible for the abnormally increased metabolites in SAP. Qingyi granules can improve SAP-associated metabolic phenotypes by favoring gut microbiota homeostasis, and also through its main active ingredient emodin in a gut microbiota independent manner.
Conclusions
This study combined microbiome analysis and metabolomics to reveal that the compound formula in Qingyi granules can promote gut microbiota restoration, maintain gut barrier integrity, restrain the systemic inflammatory response and correct metabolic abnormalities in SAP (, created by Figdraw). Emodin, the main active ingredient of Qingyi granules, can also improve SAP-associated phenotypes and serum metabolism in a gut microbiota-independent manner. Therefore, although the dominant monomer underpins the efficacy of the compound formula, the compound formula in Qingyi granules has its own advantages in the holistic treatment of SAP in a multipronged fashion. Multidimensional action mechanism mining will provide a comprehensive understanding of the crosstalk among multifaceted pathological changes and the regulatory role of compound prescriptions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Balakrishnan B, Luckey D, Bodhke R, Chen J, Marietta E, Jeraldo P, Murray J, Taneja V. 2021. Prevotella histicola protects from arthritis by expansion of Allobaculum and augmenting butyrate production in humanized mice. Front Immunol. 12:609644. doi: 10.3389/fimmu.2021.609644.
- Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, Nieuwenhuijs VB, Bollen TL, van Ramshorst B, Witteman BJ, et al. 2008. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 371(9613):651–659. doi: 10.1016/S0140-6736(08)60207-X.
- Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, van Santvoort HC, Besselink MG. 2020. Acute pancreatitis. Lancet. 396(10252):726–734. doi: 10.1016/S0140-6736(20)31310-6.
- Brubaker L, Luu S, Hoffman K, Wood A, Navarro Cagigas M, Yao Q, Petrosino J, Fisher W, Van Buren G. 2021. Microbiome changes associated with acute and chronic pancreatitis: a systematic review. Pancreatology. 21(1):1–14. doi: 10.1016/j.pan.2020.12.013.
- Capurso G, Zerboni G, Signoretti M, Valente R, Stigliano S, Piciucchi M, Delle Fave G. 2012. Role of the gut barrier in acute pancreatitis. J Clin Gastroenterol. 46 Suppl: S46–51. doi: 10.1097/MCG.0b013e3182652096.
- Chen J, Wu H, Zhang W, Mu W. 2020. Ribose-5-phosphate isomerases: characteristics, structural features, and applications. Appl Microbiol Biotechnol. 104(15):6429–6441. doi: 10.1007/s00253-020-10735-4.
- Chen W, Yang X, Huang L, Xue P, Wan M, Guo J, Zhu L, Jin T, Huang Z, Chen G, et al. 2015. Qing-Yi decoction in participants with severe acute pancreatitis: a randomized controlled trial. Chin Med. 10:11. doi: 10.1186/s13020-015-0039-8.
- Cheng TH, Ma MC, Liao MT, Zheng CM, Lu KC, Liao CH, Hou YC, Liu WC, Lu CL. 2020. Indoxyl sulfate, a tubular toxin, contributes to the development of chronic kidney disease. Toxins. 12(11):684. doi: 10.3390/toxins12110684.
- Dang JT, Mocanu V, Park H, Laffin M, Hotte N, Karmali S, Birch DW, Madsen KL. 2022. Roux-en-Y gastric bypass and sleeve gastrectomy induce substantial and persistent changes in microbial communities and metabolic pathways. Gut Microbes. 14(1):2050636. doi: 10.1080/19490976.2022.2050636.
- Gao H, Liu S. 2017. Role of uremic toxin indoxyl sulfate in the progression of cardiovascular disease. Life Sci. 185:23–29. doi: 10.1016/j.lfs.2017.07.027.
- Guo J, Li X, Wang D, Guo Y, Cao T. 2019. Exploring metabolic biomarkers and regulation pathways of acute pancreatitis using ultra-performance liquid chromatography combined with a mass spectrometry-based metabolomics strategy. RSC Adv. 9(21):12162–12173. doi: 10.1039/c9ra02186h.
- Guo XJ, Guo WB. 2019. A clinical study of qingyi decoction in the treatment of acute pancreatitis. J Biol Regul Homeost Agents. 33(4):1197–1200.
- Hu Q, Yao J, Wu X, Li J, Li G, Tang W, Liu J, Wan M. 2022. Emodin attenuates severe acute pancreatitis-associated acute lung injury by suppressing pancreatic exosome-mediated alveolar macrophage activation. Acta Pharm Sin B. 12(10):3986–4003. doi: 10.1016/j.apsb.2021.10.008.
- Ji Y, Luo K, Zhang JM, Ni P, Xiong W, Luo X, Xu G, Liu H, Zeng Z. 2021. Obese rats intervened with Rhizoma coptidis revealed differential gene expression and microbiota by serum metabolomics. BMC Complement Med Ther. 21(1):208. doi: 10.1186/s12906-021-03382-3.
- Jiao J, Liu J, Li Q, Zhang G, Pan C, Luo F, Zhang Q, Qi B, Zhao L, Yin P, et al. 2022. Gut microbiota-derived diaminopimelic acid promotes the NOD1/RIP2 signaling pathway and plays a key role in the progression of severe acute pancreatitis. Front Cell Infect Microbiol. 12:838340. doi: 10.3389/fcimb.2022.838340.
- Lankisch PG, Apte M, Banks PA. 2015. Acute pancreatitis. Lancet. 386(9988):85–96. doi: 10.1016/S0140-6736(14)60649-8.
- Li Q, Wang C, Tang C, Zhao X, He Q, Li J. 2018. Identification and characterization of blood and neutrophil-associated microbiomes in patients with severe acute pancreatitis using next-generation sequencing. Front Cell Infect Microbiol. 8:5. doi: 10.3389/fcimb.2018.00005.
- Liu LW, Xie Y, Li GQ, Zhang T, Sui YH, Zhao ZJ, Zhang YY, Yang WB, Geng XL, Xue DB, et al. 2023. Gut microbiota-derived nicotinamide mononucleotide alleviates acute pancreatitis by activating pancreatic SIRT3 signalling. Br J Pharmacol. 180(5):647–666. doi: 10.1111/bph.15980.
- Mills E, O'Neill LA. 2014. Succinate: a metabolic signal in inflammation. Trends Cell Biol. 24(5):313–320. doi: 10.1016/j.tcb.2013.11.008.
- Pujo J, Petitfils C, Le Faouder P, Eeckhaut V, Payros G, Maurel S, Perez-Berezo T, Van Hul M, Barreau F, Blanpied C, et al. 2021. Bacteria-derived long chain fatty acid exhibits anti-inflammatory properties in colitis. Gut. 70(6):1088–1097. doi: 10.1136/gutjnl-2020-321173.
- Ren S, Pang C, Huang Y, Xing C, Zhan Y, An H. 2017. Hydrocinnamic acid inhibits the currents of WT and SQT3 syndrome-related mutants of Kir2.1 channel. J Membr Biol. 250(5):425–432. doi: 10.1007/s00232-017-9964-z.
- Rongione AJ, Kusske AM, Kwan K, Ashley SW, Reber HA, McFadden DW. 1997. Interleukin 10 reduces the severity of acute pancreatitis in rats. Gastroenterology. 112(3):960–967. doi: 10.1053/gast.1997.v112.pm9041259.
- Sharma S, Gopu V, Sivasankar C, Shetty PH. 2019. Hydrocinnamic acid produced by Enterobacter xiangfangensis impairs AHL-based quorum sensing and biofilm formation in Pseudomonas aeruginosa. RSC Adv. 9(49):28678–28687. doi: 10.1039/c9ra05725k.
- Silva-Reis R, Castro-Ribeiro C, Gonçalves M, Ferreira T, Pires MJ, Iglesias-Aguirre CE, Cortés-Martín A, Selma MV, Espín JC, Nascimento-Gonçalves E, et al. 2022. An integrative approach to characterize the early phases of dimethylhydrazine-induced colorectal carcinogenesis in the rat. Biomedicines. 10(2):409. doi: 10.3390/biomedicines10020409.
- Smet A, Menard A. 2020. Review: other Helicobacter species. Helicobacter. 25 Suppl 1:e12744. doi: 10.1111/hel.12744.
- Smith JA, Stallons LJ, Schnellmann RG. 2014. Renal cortical hexokinase and pentose phosphate pathway activation through the EGFR/Akt signaling pathway in endotoxin-induced acute kidney injury. Am J Physiol Renal Physiol. 307(4):F435–444. doi: 10.1152/ajprenal.00271.2014.
- Su S, Liang T, Zhou X, He K, Li B, Xia X. 2019. Qingyi decoction attenuates severe acute pancreatitis in rats via inhibition of inflammation and protection of the intestinal barrier. J Int Med Res. 47(5):2215–2227. doi: 10.1177/0300060518809289.
- Sun J, Lin Y, Shen X, Jain R, Sun X, Yuan Q, Yan Y. 2016. Aerobic biosynthesis of hydrocinnamic acids in Escherichia coli with a strictly oxygen-sensitive enoate reductase. Metab Eng. 35:75–82. doi: 10.1016/j.ymben.2016.02.002.
- Sun J, Luo JW, Yao WJ, Luo XT, Su CL, Wei YH. 2019. Effect of emodin on gut microbiota of rats with acute kidney failure. Zhongguo Zhong Yao za Zhi. 44:758–764. in Chinese)
- Sun Z, Li L, Qu J, Li H, Chen H. 2019. Proteomic analysis of therapeutic effects of Qingyi pellet on rodent severe acute pancreatitis-associated lung injury. Biomed Pharmacother. 118:109300. doi: 10.1016/j.biopha.2019.109300.
- Tan C, Ling Z, Huang Y, Cao Y, Liu Q, Cai T, Yuan H, Liu C, Li Y, Xu K. 2015. Dysbiosis of intestinal microbiota associated with inflammation involved in the progression of acute pancreatitis. Pancreas. 44(6):868–875. doi: 10.1097/MPA.0000000000000355.
- Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, et al. 2013. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 496(7444):238–242. doi: 10.1038/nature11986.
- Wang GJ, Wang Y, Teng YS, Sun FL, Xiang H, Liu JJ, Xia SL, Zhang GX, Chen HL, Shang D. 2016. Protective effects of emodin-induced neutrophil apoptosis via the Ca2+-caspase 12 pathway against SIRS in rats with severe acute pancreatitis. Biomed Res Int. 2016:1736024. doi: 10.1155/2016/1736024.
- Wang Z, Liu J, Li F, Luo Y, Ge P, Zhang Y, Wen H, Yang Q, Ma S, Chen H. 2022. The gut-lung axis in severe acute pancreatitis-associated lung injury: the protection by the gut microbiota through short-chain fatty acids. Pharmacol Res. 182:106321. doi: 10.1016/j.phrs.2022.106321.
- Watanabe S, Yamada M, Ohtsu I, Makino K. 2007. alpha-Ketoglutaric semialdehyde dehydrogenase isozymes involved in metabolic pathways of d-glucarate, d-galactarate, and hydroxy-l-proline. Molecular and metabolic convergent evolution. J Biol Chem. 282(9):6685–6695. doi: 10.1074/jbc.M611057200.
- Wei TF, Zhao L, Huang P, Hu FL, Jiao JY, Xiang KL, Wang ZZ, Qu JL, Shang D. 2021. Qing-Yi decoction in the treatment of acute pancreatitis: an integrated approach based on chemical profile, network pharmacology, molecular docking and experimental evaluation. Front Pharmacol. 12:590994. doi: 10.3389/fphar.2021.590994.
- Werge M, Novovic S, Schmidt PN, Gluud LL. 2016. Infection increases mortality in necrotizing pancreatitis: a systematic review and meta-analysis. Pancreatology. 16(5):698–707. doi: 10.1016/j.pan.2016.07.004.
- Wu H, Zheng X, Pan T, Yang X, Chen X, Zhang B, Peng L, Xie C. 2022. Dynamic microbiome and metabolome analyses reveal the interaction between gut microbiota and anti-PD-1 based immunotherapy in hepatocellular carcinoma. Int J Cancer. 151(8):1321–1334. doi: 10.1002/ijc.34118.
- Xia S, Ni Y, Zhou Q, Liu H, Xiang H, Sui H, Shang D. 2019. Emodin attenuates severe acute pancreatitis via antioxidant and anti-inflammatory activity. Inflammation. 42(6):2129–2138. doi: 10.1007/s10753-019-01077-z.
- Xiang H, Tao X, Xia S, Qu J, Song H, Liu J, Shang D. 2017. Emodin alleviates sodium taurocholate-induced pancreatic acinar cell injury via microRNA-30a-5p-mediated inhibition of high-temperature requirement A/transforming growth factor beta 1inflammatory signaling. Front Immunol. 8:1488. doi: 10.3389/fimmu.2017.01488.
- Xiao H, Huang JH, Zhang XW, Ahmed R, Xie QL, Li B, Zhu YM, Cai X, Peng QH, Qin YH, et al. 2017. Identification of potential diagnostic biomarkers of acute pancreatitis by serum metabolomic profiles. Pancreatology. 17(4):543–549. doi: 10.1016/j.pan.2017.04.015.
- Ye S, Si C, Deng J, Chen X, Kong L, Zhou X, Wang W. 2021. Understanding the effects of metabolites on the gut microbiome and severe acute pancreatitis. Biomed Res Int. 2021:1516855. doi: 10.1155/2021/1516855.
- Yu W, Wang Z, Zhang K, Chi Z, Xu T, Jiang D, Chen S, Li W, Yang X, Zhang X, et al. 2019. One-carbon metabolism supports S-adenosylmethionine and histone methylation to drive inflammatory macrophages. Mol Cell. 75(6):1147–1160.e5. doi: 10.1016/j.molcel.2019.06.039.
- Yuan Q, Xie F, Huang W, Hu M, Yan Q, Chen Z, Zheng Y, Liu L. 2022. The review of alpha-linolenic acid: sources, metabolism, and pharmacology. Phytother Res. 36(1):164–188. doi: 10.1002/ptr.7295.
- Zeng YQ, Dai Z, Lu F, Lu Z, Liu X, Chen C, Qu P, Li D, Hua Z, Qu Y, et al. 2016. Emodin via colonic irrigation modulates gut microbiota and reduces uremic toxins in rats with chronic kidney disease. Oncotarget. 7(14):17468–17478. doi: 10.18632/oncotarget.8160.
- Zhang GX, Zhan C, Wang K, Han J, Shang D, Chen HI. 2019. Qingyi decoction amerliorates acute biliary pancreatitis by targeting Gpbar1/NF-κb pathway. Front Biosci (Landmark Ed). 24(5):833–848. doi: 10.2741/4754.
- Zhang JW, Zhang GX, Chen HL, Liu GL, Owusu L, Wang YX, Wang GY, Xu CM. 2015. Therapeutic effect of Qingyi decoction in severe acute pancreatitis-induced intestinal barrier injury. World J Gastroenterol. 21(12):3537–3546. doi: 10.3748/wjg.v21.i12.3537.
- Zhang Q, Tao X, Xia S, Qu J, Song H, Liu J, Li H, Shang D. 2019. Emodin attenuated severe acute pancreatitis via the P2X ligand‑gated ion channel 7/NOD‑like receptor protein 3 signaling pathway. Oncol Rep. 41(1):270–278.
- Zheng J, Lou L, Fan J, Huang C, Mei Q, Wu J, Guo Y, Lu Y, Wang X, Zeng Y. 2019. Commensal Escherichia coli aggravates acute necrotizing pancreatitis through targeting of intestinal epithelial cells. Appl Environ Microbiol. 85(12):e00059-19. doi: 10.1128/AEM.00059-19.
- Zhu Y, He C, Li X, Cai Y, Hu J, Liao Y, Zhao J, Xia L, He W, Liu L, et al. 2019. Gut microbiota dysbiosis worsens the severity of acute pancreatitis in patients and mice. J Gastroenterol. 54(4):347–358. doi: 10.1007/s00535-018-1529-0.