Abstract
Context
The antidiabetic effects of flavonoids have been reported, but it is still unclear whether 5,7,3′,4′,5′-pentamethoxyflavone, isolated from Bauhinia championii Benth. (Fabaceae), also exhibits such properties.
Objective
To isolate 5,7,3′,4′,5′-pentamethoxyflavone from B. championii using high-speed countercurrent chromatography and examine its potential in treating diabetic nephropathy.
Materials and methods
The phytochemical constituents from the stems of B. championii were separated and purified with high-speed countercurrent chromatography; 5,7,3′,4′,5′-pentamethoxyflavone (PMF) was identified by mass spectrum, 1H-NMR, and 13C-NMR. After exposing mesangial cells to 30 mM glucose and either 5 μM or 10 μM PMF for 6 h, the levels of fibronectin (FN) and p-Smad2/3 were analyzed using Western blotting. Male Sprague–Dawley rats were injected intraperitoneally with 55 mg/kg streptozotocin to induce diabetes and then were randomized into three groups (n = 10): vehicle administration, low-dose (5 mg/kg) PMF, and high-dose (25 mg/kg) PMF by intragastric gavage for 3 months. A healthy group was included as the control.
Results
Compared to the diabetic group, low-dose and high-dose PMF treatment decreased the phosphorylation of Smad2/3 by 0.54- and 0.52-fold, and the accumulation of FN decreased by 0.82- and 0.77-fold in vitro; the phosphorylation of Smad2/3 was decreased by 0.39- and 0.37-fold, and the accumulation of FN decreased by 0.47- and 0.40-fold in vivo, respectively. Furthermore, PMF alleviated the glomerular basement membrane thickness and foot process fusion.
Conclusion
The findings suggest for the first time that PMF may be a promising treatment option for diabetic kidney fibrosis, which warrants additional clinical investigation.
Introduction
Diabetes mellitus (DM) is characterized by persistent hyperglycemia and the incidence of DM is increasing worldwide (Chung et al. Citation2021). According to the projections of the International Diabetes Federation (IDF) (2019), approximately 700 million adults will live with diabetes (Azemi et al. Citation2021). Long-term high blood glucose level is associated with multiple complications such as diabetic renal fibrosis, diabetic retinopathy, and diabetic cardiovascular disease (Guan et al. Citation2018). Diabetic renal fibrosis is one of the most serious of these complications (Jin et al. Citation2015). Overexpression and the excessive accumulation of extracellular matrix protein (ECM) such as fibronectin (FN) and collagen IV (Col IV) in mesangial cells as well as the glomerular basement membrane thickening are the hallmarks of diabetic nephropathy (DN) (Kanwar et al. Citation2008, Citation2011; Li et al. Citation2022). Currently, there are no commercially available drugs to cure DN. Insulin and antihypertensive drugs are the main alternative medicines for DN, however, they bring many side effects to the human body such as hypoglycemia, liver damage, lactic acidosis, diarrhea, etc. (Xu et al. Citation2012; Liang et al. Citation2018).
Traditional herbal medicine has been used for intractable and chronic diseases for thousands of years (Adam et al. Citation2016; de Moura Barbosa et al. Citation2018). Multiple studies have shown that flavonoids, as natural products from herbal medicine had few side effects. (Hashem et al. Citation2016; Li et al. Citation2019; Yaribeygi et al. Citation2019; Wei et al. Citation2021). Bauhinia championii Benth. (Fabaceae) is a folk medicine used for the treatment of rheumatoid arthritis, and its main active ingredient is flavonoids (Zheng et al. Citation2013; Aung et al. Citation2016; Chen et al. Citation2020). It has been found to alleviate collagen-induced arthritis in rats by inhibiting the TLR4/NF-κB signaling pathway (Xu et al. Citation2013). DM is also an inflammatory-related disease, and many traditional hypoglycemic herbs are also found to have anti-inflammatory effects (Xie and Du Citation2011). It is reported that B. championii could alleviate inflammation in the collagen-induced arthritis rat model (Xu et al. Citation2013, Citation2016a, Citation2016b). Flavones from B. championii could alleviate myocardial ischemia/reperfusion injury via antioxidant, anti-apoptotic and anti-inflammatory effects (Jian et al. Citation2016). However, it is unknown if B. championii can exert kidney protective effects on diabetic rats.
We separated four compounds (1–4) from B. championii by high-speed counter-current chromatography (HSCCC) and analyzed the purity of these compounds by high-performance liquid chromatography (HPLC). The purity of compound 2 is 96.78%, which was the highest among these four compounds. Then the structure of compound 2 was further identified by mass spectrum, 1H-NMR and 13C-NMR, and finally, compound 2 was identified to be 5,7,3′,4′,5′-pentamethoxyflavone. This study explored if PMF from B. championii could inhibit the phosphorylation of Smad2/3 and then reduce the expression of FN and Col IV in the diabetic cell models and animal models.
Materials and methods
Preparation of chloromethane extract from B. championii
The stems of B. championii were collected in the mountain woods of Xinfeng (E114.34, N25.20, Ganzhou, China) in June 2014 and identified by associated professor Jialin Li. A voucher specimen (No. 20140607) was deposited in the Chinese Medicinal Herbarium, School of Pharmacy, Gannan Medical University, China. All samples were powdered with a pulverizer and extracted with 70% ethanol (1:10, g/v) under reflux at 80 °C for 2 h (×3 times). Then, the extract was filtered under vacuum, and the filtrate was collected and concentrated under reduced pressure to remove the ethanol. The crude ethanol extract was further extracted with dichloromethane (1.107 g powder was obtained from 200 g of the stems of B. championii using dichloromethane).
Preparation of a two-phase solvent system for HSCCC
An n-hexane:ethyl acetate:methanol:water solvent system was selected based on previous publications (Han et al. Citation2007; Kamto et al. Citation2017) and the partition coefficients (K values) of the sample between the two-phase systems were calculated according to the equation: K = Peak area of upper phase (AU)/Peak area of lower phase (AL).
Separating and analyzing the constituents of dichloromethane extract by HSCCC
Finally, an n-hexane:ethyl acetate:methanol: water (V/V, 4:6:4:6) solvent system was selected as the two-phase solvent system for HSCCC (TBE-300B, Shanghai Tauto Biotech Co., Ltd., Shanghai, China). All solvents were placed in a separatory funnel, and then placed at room temperature for 2 h to establish equilibrium after vigorous shaking. The stationary phase (upper phase) and the mobile phase (lower phase) were separated before HSCCC separation. Dichloromethane extract (750 mg) was dissolved with a 50 mL lower phase (if it is difficult to dissolve, use an ultrasonic device to accelerate the dissolution) to obtain a concentration of 15 mg/mL sample solution for HSCCC.
Pump the stationary phase into the spiral tube of the HSCCC host at a flow rate of 8.00 mL/min, and fill it with the entire spiral tube, the apparatus rotating at 950 rpm, then pump the mobile phase at a flow rate of 2.00 mL/min until the two phases reach the dynamic balance. After that, injecting 15 mL of the sample solution into the separation column, the effluent was detected by the UV detector at a wavelength of 254 nm, and each peak fraction was collected according to the peak shape of the chromatogram.
Purity detection of the sample by HPLC
The purity of the HSCCC fractions was determined by HPLC. The chromatographic column was shim-pack VP-ODS (150 mm, 4.6 mm). The mobile phase consisted of two solvents: solvent A-0.2% acetic acid and solvent B-acetonitrile. The column oven temperature was 35 °C. The gradient elution program was set up as follows: increasing the concentration of mobile phase B from 10% to 90%, and then to 100% over a period of 0.01 min–25 min, with an additional 5 min at the end. The sample was dissolved in the same volume of acetonitrile and filtrated with a microporous membrane, then injected 20 μL sample and detected at 254 nm. The flow rate was 1 mL/min.
Structure identification of compound 2 by mass spectrum, 1H-NMR, and 13C-NMR
HR-ESI-MS spectra were measured on a Waters Xevo G2-XS Tof LC mass spectrometer (Waters Corporation, Milford, USA). NMR spectra were recorded using Bruker Avance III 400 MHz for 1H and 100 MHz for 13C (Bruker AG, Fällanden, Switzerland) with BBFO Smart Probe and Bruker 400 AEON Nitrogen-Free Magnet.
Cell culture and treatments
Primary rat mesangial cells (MCs) separated from the kidney of male Sprague–Dawley (SD) rats by the differential size of sieving (250, 106 and 75 mesh sieves, respectively) and identified with a specific assay were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Solarbio, #31600) supplemented with 20% fetal bovine serum (FBS, Gibco, #42A0378K), 100 μg/mL streptomycin, and100 U/mL penicillin at 37 °C in an environment of 95% air and 5% CO2. Pretreated the MCs with PMF for 1 h, then cotreated the MCs with high glucose (HG, 24.4 mM glucose was added to the media, DMEM contained 5.6 mM glucose, and the final concentration of 30 mM glucose was considered a high glucose level) (Sepahi et al. Citation2021) for 6 h, after which the expression of ECM and the phosphorylation of Smad2/3 was demonstrated by Western blotting.
Cell viability assay
Briefly, cells (2 × 104) were cultured in a 96-well cell culture plate, 48 h later, treated the cells with the indicated concentration of PMF for 24 h, after then, the cells were treated with 10 μL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide solution (MTT, 5 mg/mL) and incubated for another 4 h, then dissolve the purple crystal with 150 μL dimethyl sulfoxide. Then tested the absorbance with a microplate reader at wavelengths 570 and 690 nm. Normalized the absorbance to dimethyl sulfoxide control and then calculated the cell viability with GraphPad Prism 7.0 software.
Confocal
Seeded the cells in the confocal dish, 48 h later, treated the cells with PMF for 24 h, after then, washed the cells with PBS twice and then fixed the cells with 4% formaldehyde, washed with PBS again three times, and blocked with 5% BSA for 60 min at room temperature. Incubated the cells with the corresponding primary antibody overnight at 4 °C with gentle agitation. After which, incubated with a rhodamine-labeled secondary antibody. The nuclei were stained with DAPI. Then observed under a confocal microscope (Zeiss LSM880 META, Jena, Germany).
Animal experiments
Male Sprague-Dawley (SD) rats (about 8 weeks old, weighing 200–225 g) were chosen for the animal experiments. All the animal experiments were approved by the ethics committee of Gannan Medical University and adhered to the Animal Care and Use Committee of Gannan Medical University guidelines (License No. SYXK2018-0004). Intraperitoneally injected 55 mg/kg streptozotocin (STZ, Sigma Chemicals Co., Ltd., USA) which was dissolved in sodium citrate buffer at pH 4.2–4.5 to induce diabetes in experimental animals (Wu et al. Citation2014), and an equal volume of citrate buffer was administered as a corresponding non-diabetic control. All rats were fed a standard diet (21.1% protein, 60.6% carbohydrate, 4.5% fat) supplied by BiotechHD Co., Ltd. (Beijing, China). Random blood glucose was tested after the injection of STZ for 72 h, and animals with blood glucose levels >16.7 mmol/L were considered diabetic (Hu et al. Citation2018; Chen and Jin Citation2021). Then, the rats were divided into four groups (n = 10), and all rats were treated by intragastric administration once daily as follows:
Con group: nondiabetic rats, which received equal volumes of normal saline.
DM group: diabetic rats, which were treated with the same volume of normal saline.
DM + PMF-L group: diabetic rats, were treated with 5 mg/kg PMF.
DM + PMF-H group: diabetic rats, were treated with 25 mg/kg PMF.
Biochemical index analysis
The blood urea nitrogen and serum creatinine were tested using a commercially available kit (Nanjing Jiancheng Bioengineering Institute, #C013-2, #C011-2). The kidney-to-body weight ratio (mg/g) was calculated to assess renal hypertrophy. The blood glucose level was tested every month during the whole experimental period.
Histopathologic examination
Paraformaldehyde-fixed renal samples were dehydrated by using graded alcohol and then embedded in paraffin. The samples were cut into 4 μm slices and mounted on glass slides. Then, the slices were stained with hematoxylin and eosin, periodic acid-Schiff (PAS), and Masson′s trichrome staining reagent according to the standard procedure for the observation of renal morphological changes (Li et al. Citation2022).
Immunohistochemistry
The renal paraffin sections were deparaffinized in xylene and rehydrated in 100%, 95%, 80%, 70%, and 0% ethanol. After the slides were washed with PBS three times, they were dipped in citrate buffer, and the solution was boiled by using a microwave to retrieve the antigen. The slides were incubated with 3% H2O2 for 10 min to quench the endogenous peroxidase and were then blocked with serum. Then, several drops of primary antibodies were added, followed by incubation at 4 °C overnight. The primary antibodies are monoclonal FN antibody (1:200, BD Biosciences, 610077) and polyclonal type IV collagen antibody (1:200, Proteintech, 55131-1-AP). The mixture was then incubated with corresponding secondary antibodies. The slides were stained with diaminobenzidine and then counterstained with hematoxylin. Finally, the slides were observed with a light microscope. Three pictures per slide were randomly selected to analyze the relative expression of the target gene by using ImageJ.
Transmission electron microscopy
The renal cortex was cut into small pieces and then fixed with 2.5% glutaraldehyde for 2 h and 1% osmium tetroxide for 2 h, stained with uranyl acetate, dehydrated by graded ethanol, and embedded with resin. The renal ultrastructural changes of the rats were observed under a transmission electron microscope (JEOL, Japan). Three glomerular transmission electron micrographs of the kidney were randomly photographed per sample. Then, the thickness of the glomerular basement membrane (GBM) and the fusion of the podocyte foot process was analyzed.
Protein extraction and Western blotting
Lysed the renal cortex of SD rats with RIPA buffer (Beyotime Biotech, #P0013C) for 15 min, then centrifuged with 12,000 g at 4 °C for 10 min, collected the supernatant. The protein concentration was tested with a BCA kit (Beyotime Biotech, #P0012). Protein (50 µg) of the whole lysates per sample was separated by SDS-PAGE using different concentrations of polyacrylamide gels and identified by immunoblotting. Transferred protein from the gel to a nitrocellulose membrane, incubated the membrane with the primary antibodies as follows: monoclonal FN (1:10,000, Merck Millipore, #AB1954), monoclonal phospho-Smad2/3 (S465/467) antibody and monoclonal Smad2/3 antibody (1:1000, Cell Signaling Technology, 8828S and 8685S), monoclonal β-actin antibody (1:1000, Cell Signaling Technology, 3700S) at 4 °C overnight. The membranes were incubated with enhanced chemiluminescence and visualized the protein band with a Chemi-Doc MP imaging system (Bio-rad, CA, USA), then analyzed the intensity with Image J.
Statistical analysis
Results were presented as mean ± SE. Data among groups were analyzed by one-way ANOVA followed by Tukey′s post hoc test. The statistical analysis was performed using Graphpad Prism 7.0 software. p < 0.05 was considered to have a significant difference.
Results
Four fractions separated from B. championii by HSCCC
A suitable solvent system with moderate K values (0.5–2.0) is very important for HSCCC separation (Chen et al. Citation2018). A larger K value will result in a broad peak shape and an extended elution time, however, a smaller K value will lead to a lower resolution. As shown in , the K values of n-hexane:ethyl acetate:methanol:water (4:6:4:6, v/v/v/v) were the most suitable solvent system to separate PMF by HSCCC. Therefore, the two-phase solvent system consisting of n-hexane:ethyl acetate:methanol:water (4:6:4:6, v/v/v/v) was selected as the optimal solvent system. Four fractions were collected which were labelled compounds 1–4, respectively ().
Figure 1. Chromatogram for the separation of dichloromethane extract from B. championii by HSCCC in the two-phase solvent system of n-hexane:ethyl acetate:methanol:water (4:6:4:6, v/v/v/v). Four compounds (compounds 1–4) were collected.
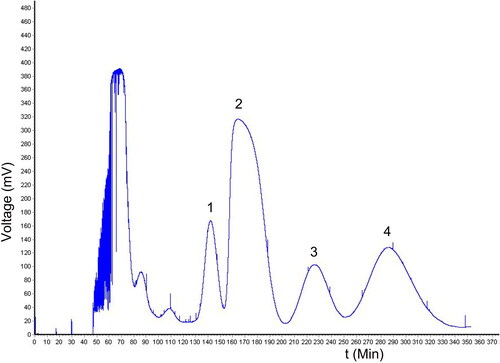
Table 1. The K values of the fractions calculated by HPLC in the solvent system of n-hexane:ethyl acetate:methanol:water.
Purity detection by HPLC
Compound 2 had the highest yield (6.22%) among them, as shown in . The purity of the four components was detected by HPLC, and compound 2 had the highest purity up to 96.78% among the four fractions, as shown in and .
Figure 2. HPLC chromatogram. The retetion time and the purity of the four fractions separated by HSCCC were calculated by the area normalized method. (A–D) are the HPLC peaks of compounds 1–4.
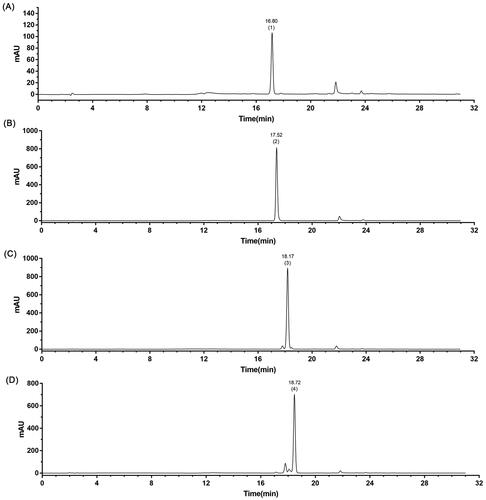
Table 2. Yield (the dry weight of the compound/the total dry weight of dichloromethane extract from Bauhinia championii) of the compounds separated by HSCCC.
Table 3. The retention time and purity of compounds (1–4) were tested by HPLC.
Structure identification of compound 2 by mass spectrum, 1H-NMR, and 13 C-NMR
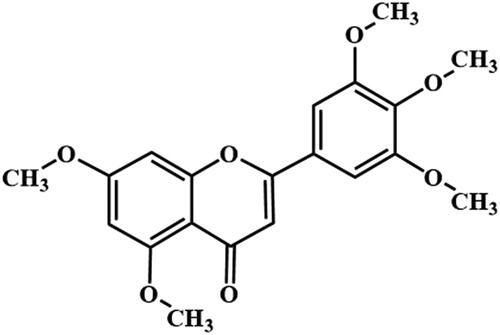
The structure of compound 2 was identified to be 5,7,3′,4′,5′-pentamethoxyflavone (): HCl-Mg reaction (+). C20H20O7, ESI-MS m/z: 373 [M + H]+. 1H NMR (400 MHz, CDCl3) δ = 7.07 (s, 2H), 6.62 (s, 1H), 6.57 (d, J = 2.3 Hz, 1H), 6.38 (d, J = 2.3 Hz, 1H), 3.96 (s, 9H), 3.93 (s, 3H), 3.92 (s, 3H). 13C NMR (101 MHz, CDCl3) δ = 177.65, 164.09, 160.84, 160.52, 159.82, 153.48, 140.72, 126.72, 109.10, 108.77, 103.27, 96.19, 92.87, 61.04, 56.43, 56.33, 55.8. These data are the same as those for 5,7,3′,4′,5′-pentamethoxyflavone (Zhang et al. Citation2018).
PMF could alleviate high glucose-induced upregulation of FN and phosphorylation of Smad2/3
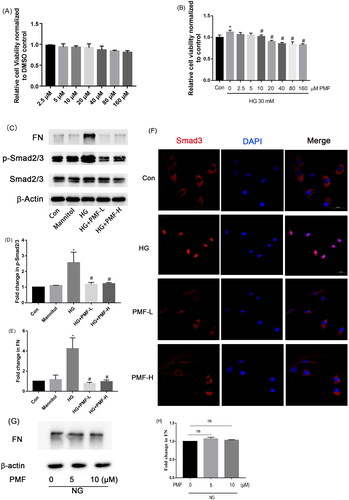
PMF is the main component of B. championii separated by HSCCC as aforementioned. First, MTT was performed to determine a suitable dosage range for MCs. As shown in , 5–20 μM PMF (IC50 = 304.29 μM) didn’t significantly inhibit the cell viability of MCs. Since the proliferation of MCs is one of the typical features of DN, we further investigated whether PMF affects the proliferation of MCs induced by high glucose via MTT. As shown in , HG could induce the proliferation of MCs, 10–160 μM PMF could significantly inhibit HG-induced cell proliferation of MCs. ECM accumulation is a hallmark of DN and long-term high glucose in diabetes could induce the activation of the TGF-β/Smads signal pathway and then lead to the expression of ECM. Then we evaluated the renal protective effects of PMF in an HG-induced diabetic mesangial cell model. Pretreated the mesangial cells with PMF for 1h, then incubated with HG for 6 h, the expression of FN and the phosphorylation of Smad2/3 were detected by Western blotting. The results showed that PMF could inhibit the TGF-β/Smads signal pathway and then downregulate the expression of FN as shown in . The immunofluorescence result indicated both low dosages of PMF and high dosage of PMF could suppress the translocation of Smad3 from the cytoplasm to the nucleus, these results showed that PMF could inhibit HG-induced activation of the TGF-β/Smads signal pathway (). Subsequently, MCs were solely exposed to PMF to determine whether it had any influence on the expression of FN. The findings demonstrated that PMF had no effect on FN expression in normal MCs ( and ).
Figure 4. Effects of PMF treatment on mesangial cell viability with or without the presence of HG. (A) MTT assay was performed to assess the cell viability of mesangial cells treated with various concentrations of PMF. (B) MTT assay was performed to assess the cell viability of HG-induced mesangial cells treated with various concentrations of PMF. (C) The effect of PMF on the protein expression of FN and the phosphorylation of Smad2/3 was demonstrated by Western blotting. (D and E) The relative density of p-Smad2/3 and FN were statistically analyzed, respectively. (F) The translocation of Smad3 from the cytoplasm to the nucleus was observed with laser confocal microscope (Scale bar = 50 μm, n = 3). (G) The effect of PMF on the expression of FN without HG was tested by Western blotting. (H) The relative expression of FN was calculated. *p < 0.05 vs. control, #p < 0.05 vs. HG. ns means no significant difference (p > 0.05). NG: normal glucose, which means DMEM contained 5.6 mM glucose. Con: nontreated group; HG:30 mM glucose treated group; Mannitol: the osmolality control; HG + PMF-L: 30 mM glucose + 5 μM PMF cotreated group; HG + PMF-H: 30 mM glucose +10 μM PMF cotreated group.
PMF could reduce the blood glucose level of type 1 diabetic rats and improve the renal function of STZ-induced diabetic rats
The diabetic rats were treated with 5 and 25 mg/kg PMF for three months, and the blood glucose levels were monitored during the whole experiment. Treatment with 5 mg/kg PMF could lower the blood glucose level compared with the DM group after 3 months of administration (p < 0.05). The low dosage of PMF (PMF-L) had no obvious effect on the body weight of the diabetic rats, however, the body weight of the rats in the high-dose group (PMF-H) was significantly lower than that in the control group. At the end of the experiment, we collected the kidney, urine, and serum of the rats and then tested the renal injuries indicators, such as albuminuria, serum creatinine level, and kidney-to-body weight ratio. These parameters of the diabetic rats treated with 5 mg/kg of PMF were all alleviated compared with those of the diabetic group (p < 0.05), as shown in .
Table 4. Blood glucose level, kidney/body weight ratio, serum creatinine level and 24 h total urine protein level was shown as the following table.
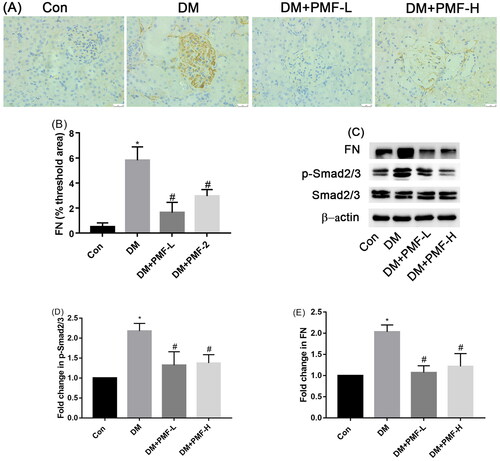
PMF could inhibit the TGF-β/Smads signal pathway and then alleviate the expression of ECM in the kidney of the diabetic rats
The TGF-β/Smads signal pathway was activated in the kidney of diabetic rats. Treating diabetic rats with PMF could inhibit the phosphorylation of Smad2/3 and then alleviate the expression of ECM in the kidney (p < 0.05), as shown in .
Figure 5. Effects of PMF on the ECM accumulation and phosphorylation of Smad2/3. (A) The effect of PMF on the accumulation of FN in the renal of diabetic rats was tested by IHC. Original magnification, ×400. (B) The quantification of FN was statistically analyzed. (C) Representative photographs of the phosphorylation of Smad2/3 and expression of FN. (D and E) Quantitative analysis of p- Smad2/3 and FN, respectively. *p < 0.05 vs. control, #p < 0.05 vs. DM. Con: nondiabetic rats without treatment; DM: diabetic rats treated with saline; DM + PMF-L: diabetic rats treated with 5 mg/kg PMF; DM + PMF-H: diabetic rats treated with 25 mg/kg PMF.
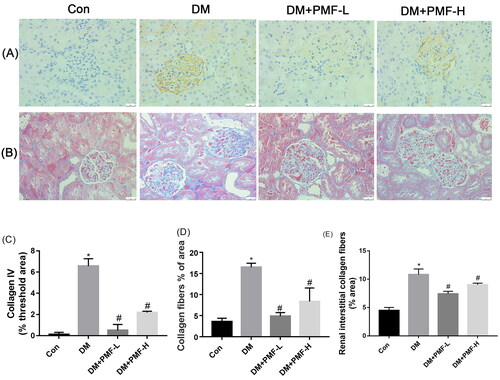
PMF could improve the accumulation of collagen fibers in the kidney of the diabetic rats
Our results showed that the expression of Col IV in the kidney of diabetic rats was upregulated, and the administration of 5 and 25 mg/kg PMF could inhibit the expression of Col IV and the accumulation of collagen fibers (p < 0.05), as shown in .
Figure 6. Effects of PMF on the accumulation and expression of collagen fibers in the kidney of diabetic rats. (A) The expression of collagen IV in the kidney of rats was detected by IHC. (B) The accumulation of collagen fibers in the kidney of rats was tested by Masson staining. (C) The quantification of collagen IV tested by IHC was statistically analyzed. (D) The quantification of collagen fibers (blue) accumulated in the kidney was statistically analyzed. (E) The renal interstitial collagen fibers were calculated by Image J software. Original magnification, ×400. *p < 0.05 vs. control, #p < 0.05 vs. Con: nondiabetic rats without treatment; DM: diabetic rats treated with saline; DM + PMF-L: diabetic rats treated with 5 mg/kg PMF; DM + PMF-H: diabetic rats treated with 25 mg/kg PMF.
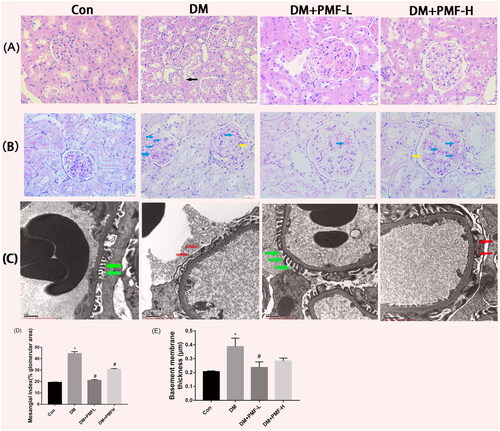
Effects of PMF on renal histopathology
Morphological changes in the kidney were analyzed by PAS staining and H&E staining. The rats in the diabetic group showed obvious renal morphological damage. For instance, the renal tubules showed a loss of the vacuolated epithelial cell brush border (black arrow), the glomeruli shrank slightly, the mesangial matrix increased (blue arrows) and the glomerular basement membrane (GBM) was diffusely thickened (yellow arrows). Kidney damage was significantly improved by low-dose PMF treatment. The glomerulus podocyte foot processes in the corresponding nondiabetic rats were arranged regularly and perpendicular to the basement membrane (green arrows). However, the podocyte foot processes in the diabetic group appeared fused or effaced (red arrows) along the surface of the GBM. Although high doses of PMF could also improve the aforementioned kidney damage, the effect was not as significant as low-dose as shown in .
Figure 7. Effects of PMF on the pathological changes in the kidney of rats. (A) H&E staining. The black arrow indicates the loss of brush border. (B) PAS staining of rat kidney. Original magnification, ×400. Blue arrows indicate mesangial matrix accumulation; Yellow arrows indicate GBM thickening. (C) Representative ultrastructure of GBM was demonstrated by TEM. Original magnification, ×10,000. Red arrows demonstrate foot process effacement. Green arrows indicate well-aligned foot processes. (D) The mesangial matrix index was calculated by Image J software. (E) Quantitative analysis of GBM thickness (µm). *p < 0.05 vs. control, #p < 0.05 vs. DM. Con: nondiabetic rats without treatment; DM: diabetic rats treated with saline; DM + PMF-L: diabetic rats treated with 5 mg/kg PMF; DM + PMF-H: diabetic rats treated with 25 mg/kg PMF.
Discussion
DM is a serious common health problem throughout the world, and many people die from DM every year (Maffei et al. Citation2018). Chronic hyperglycemia accompanied by DM will damage the kidney and lead to DN (Kanwar et al. Citation2011).
B. championii is a well-known traditional herbal medicine for the treatment of rheumatoid arthritis. The current study, for the first time, separated and purified PMF from B. championii by HSCCC and first explored its renal protective function in DN. We found that PMF from B. championii could alleviate the renal injury of type 1 diabetic rats. Renal morphological damage such as a thickness of GBM, renal tubular epithelial cell loss, etc., induced by diabetes, was alleviated by PMF treatment. The kidney/body weight ratio, serum creatinine level and albuminuria, indicators of kidney injury, were also obviously improved by the treatment of low-dose PMF. According to Xu et al. (Citation2012), B. championii primarily consists of flavonoids, which have been shown in previous research to alleviate insulin resistance and enhance glycemic control (Al-Ishaq et al. Citation2019; Choumessi et al. Citation2019; Ren et al. Citation2019). We also found that PMF, which was a kind of flavonoid from B. championii, could slightly lower the blood glucose of type 1 diabetic rats. However, a high dose of PMF was not as good as a low dose of PMF in lowering blood glucose and improving kidney damage index, which may be because the low-dose set in our experiment has achieved the maximum effect. Although the diabetic rats were treated with both low and high doses of PMF, their blood glucose levels were not restored to normal physiological levels. Therefore, PMF is not an effective drug for lowering glucose. It is worth noting that no drug can effectively lower the blood glucose of type 1 diabetes better than insulin until now because the islets of the pancreas are destroyed, and secretion of insulin is insufficient. If a drug can slightly reduce the blood glucose of type 1 diabetes, it may be because the damaged islets are repaired, or the islet progenitor cells are stimulated to differentiate into islet beta cells. Another possibility is that PMF can inhibit the absorption of dietary glucose in the intestine. Future in-depth research is suggested. Despite its limited efficacy as a hypoglycemic drug for type 1 diabetes, PMF can still be utilized as a lead compound for structural optimization in the creation of more effective blood glucose-lowering drugs for this disease in future studies.
Liquiritigenin, a flavonoid compound, has been found to have the potential to inhibit the accumulation of extracellular matrix (ECM) in high glucose-induced HBZY-1 mesangial cell line by the suppression of the NF-κB and NLRP3 inflammasome pathways (Zhu et al. Citation2018). The present study discovered that PMF derived from B. championii can prevent STZ-induced ECM accumulation via a different signal pathway known as the TGF-β/Smads signal pathway. Smad2 and Smad3 are important downstream effectors of this pathway, and their phosphorylation is triggered by high glucose and TGF-β. Then the phosphorylated Smad2 and Smad3 are translocated to the nucleus as transcriptional factors to promote the expression of FN and Col IV.
Numerous studies indicated that the TGF-β/Smads signal pathway was activated both in the diabetic animal model and cell model, then induced the deposition of ECM in the glomerulus, and finally developed glomerular sclerosis. However, inhibition of this pathway could improve the deposition of ECM in the glomerulus (Mason and Wahab Citation2003; Böttinger Citation2007; Meng et al. Citation2016; Han et al. Citation2017; Voelker et al. Citation2017; Li et al. Citation2020). The present study found that PMF obtained from B. championii could inhibit diabetes-induced phosphorylation of Smad2 and Smad3, and then alleviate the expression of FN and Col IV both in a diabetic animal model and cell model.
Conclusions
Our research has led us to the conclusion that PMF derived from B. championii has the potential to lower blood glucose levels in type 1 diabetic rats and can provide renal protection for STZ-induced diabetic rats. PMF can alleviate DN by reducing the expression of FN and Col IV via the inhibition of the TGF-β/Smads signal pathway. These findings suggest that PMF from B. championii could potentially be used as a therapeutic compound for treating diabetic kidney disease. Nevertheless, further research is required to ascertain the therapeutic effects of PMF on DN-related renal fibrosis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Adam S, Giribabu N, Kassim N, Kumar K, Brahmayya M, Arya A, Salleh N. 2016. Protective effect of aqueous seed extract of Vitis vinifera against oxidative stress, inflammation and apoptosis in the pancreas of adult male rats with diabetes mellitus. Biomed Pharmacother. 81:439–452. doi: 10.1016/j.biopha.2016.04.032.
- Al-Ishaq R, Abotaleb M, Kubatka P, Kajo K, Büsselberg D. 2019. Flavonoids and their anti-diabetic effects: cellular mechanisms and effects to improve blood sugar levels. Biomolecules. 9(9):430. doi: 10.3390/biom9090430.
- Aung H, Sein M, Aye M, Thu Z. 2016. A review of traditional medicinal plants from Kachin state, northern Myanmar. Nat Prod Commun. 11:353–364.
- Azemi AK, Mokhtar SS, Sharif SET, Rasool AHG. 2021. Clinacanthus nutans attenuates atherosclerosis progression in rats with type 2 diabetes by reducing vascular oxidative stress and inflammation. Pharm Biol. 59(1):1430–1438. doi: 10.1080/13880209.2021.1990357.
- Böttinger E. 2007. TGF-beta in renal injury and disease. Semin Nephrol. 27(3):309–320. doi: 10.1016/j.semnephrol.2007.02.009.
- Chen B, Liu Z, Zhang Y, Li W, Sun Y, Wang Y, Wang Y, Sun Y. 2018. Application of high-speed counter-current chromatography and HPLC to separate and purify of three polyacetylenes from Platycodon grandiflorum. J Sep Sci. 41(3):789–796. doi: 10.1002/jssc.201700767.
- Chen H, Jin G. 2021. Downregulation of salusin-β protects renal tubular epithelial cells against high glucose-induced inflammation, oxidative stress, apoptosis and lipid accumulation via suppressing miR-155-5p. Bioengineered. 12(1):6155–6165. doi: 10.1080/21655979.2021.1972900.
- Chen Y, Chen W, Chung C, Kuo C, Lee A. 2020. Cardiac protection of Bauhinia championii against reperfusion injury. Environ Toxicol. 35(7):774–782. doi: 10.1002/tox.22912.
- Choumessi A, Johanns M, Beaufay C, Herent M, Stroobant V, Vertommen D, Corbet C, Jacobs R, Herinckx G, Steinberg G, et al. 2019. Two isoprenylated flavonoids from Dorstenia psilurus activate AMPK, stimulate glucose uptake, inhibit glucose production and lower glycemia. Biochem J. 476(24):3687–3704. doi: 10.1042/BCJ20190326.
- Chung MY, Choi HK, Hwang JT. 2021. AMPK activity: a primary target for diabetes prevention with therapeutic phytochemicals. Nutrients. 13(11):4050. doi: 10.3390/nu13114050.
- de Moura Barbosa H, Amaral D, do Nascimento J, Machado D, de Sousa Araújo T, de Albuquerque U, Guedes da Silva Almeida J, Rolim L, Lopes N, Gomes D, et al. 2018. Spondias tuberosa inner bark extract exert antidiabetic effects in streptozotocin-induced diabetic rats. J Ethnopharmacol. 227:248–257. doi: 10.1016/j.jep.2018.08.038.
- Guan M, Li W, Xu L, Zeng Y, Wang D, Zheng Z, Lyv F, Xue Y. 2018. Metformin improves epithelial-to-mesenchymal transition induced by TGF-β1 in renal tubular epithelial NRK-52E cells via inhibiting Egr-1. J Diabetes Res. 2018:1031367. doi: 10.1155/2018/1031367.
- Han H, Cao A, Wang L, Guo H, Zang Y, Li Z, Zhang X, Peng W. 2017. Huangqi decoction ameliorates streptozotocin-induced rat diabetic nephropathy through antioxidant and regulation of the TGF-β/MAPK/PPAR-γ signaling. Cell Physiol Biochem. 42(5):1934–1944. doi: 10.1159/000479834.
- Han X, Ma X, Zhang T, Zhang Y, Liu Q, Ito Y. 2007. Isolation of high-purity casticin from Artemisia annua L. by high-speed counter-current chromatography. J Chromatogr A. 1151(1–2):180–182. doi: 10.1016/j.chroma.2007.02.105.
- Hashem A, Soliman M, Hamed M, Swilam N, Lindequist U, Nawwar M. 2016. Beta vulgaris subspecies cicla var. flavescens (Swiss chard): flavonoids, hepatoprotective and hypolipidemic activities. Die Pharmazie. 71:227–232.
- Hu L, Zhang K, Tian T, Zhang H, Fu Q. 2018. Probucol improves erectile function via activation of Nrf2 and coordinates the HO-1/DDAH/PPAR-γ/eNOS pathways in streptozotocin-induced diabetic rats. Biochem Biophys Res Commun. 507(1–4):9–14. doi: 10.1016/j.bbrc.2018.10.036.
- Jian J, Xuan F, Qin F, Huang R. 2016. The antioxidant, anti-inflammatory and anti-apoptotic activities of the Bauhinia championii flavone are connected with protection against myocardial ischemia/reperfusion injury. Cell Physiol Biochem. 38(4):1365–1375. doi: 10.1159/000443080.
- Jin J, Peng C, Wu S, Chen H, Zhang B. 2015. Blocking VEGF/Caveolin-1 signaling contributes to renal protection of fasudil in streptozotocin-induced diabetic rats. Acta Pharmacol Sin. 36(7):831–840. doi: 10.1038/aps.2015.23.
- Kamto E, Carvalho T, Mbing J, Matene M, Pegnyemb D, Leitão G. 2017. Alternating isocratic and step gradient elution high-speed counter-current chromatography for the isolation of minor phenolics from Ormocarpum kirkii bark. J Chromatogr A. 1480:50–61. doi: 10.1016/j.chroma.2016.12.026.
- Kanwar Y, Sun L, Xie P, Liu F, Chen S. 2011. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu Rev Pathol. 6:395–423. doi: 10.1146/annurev.pathol.4.110807.092150.
- Kanwar Y, Wada J, Sun L, Xie P, Wallner E, Chen S, Chugh S, Danesh F. 2008. Diabetic nephropathy: mechanisms of renal disease progression. Exp Biol Med. 233(1):4–11. doi: 10.3181/0705-MR-134.
- Li J, Wu B, Hu H, Fang X, Liu Z, Wu S. 2020. GdCl3 attenuates the glomerular sclerosis of streptozotocin (STZ) induced diabetic rats via inhibiting TGF-β/Smads signal pathway. J Pharmacol Sci. 142(2):41–49. doi: 10.1016/j.jphs.2019.06.008.
- Li J, Zhang J, Yang M, Huang X, Zhang M, Fang X, Wu S. 2022. Kirenol alleviates diabetic nephropathy via regulating TGF-β/Smads and the NF-κB signal pathway. Pharm Biol. 60(1):1690–1700. doi: 10.1080/13880209.2022.2112239.
- Li S, Zhang Y, Sun Y, Zhang G, Bai J, Guo J, Su X, Du H, Cao X, Yang J, et al. 2019. Naringenin improves insulin sensitivity in gestational diabetes mellitus mice through AMPK. Nutr Diabetes. 9(1):28. doi: 10.1038/s41387-019-0095-8.
- Li Y, Tian Z, Pan G, Zhao P, Pan D, Zhang J, Ye L, Zhang F, Xu X. 2022. Heidihuangwan alleviates renal fibrosis in rats with 5/6 nephrectomy by inhibiting autophagy. Front Pharmacol. 13:977284. doi: 10.3389/fphar.2022.977284.
- Liang G, Song L, Chen Z, Qian Y, Xie J, Zhao L, Lin Q, Zhu G, Tan Y, Li X, et al. 2018. Fibroblast growth factor 1 ameliorates diabetic nephropathy by an anti-inflammatory mechanism. Kidney Int. 93(1):95–109. doi: 10.1016/j.kint.2017.05.013.
- Maffei A, Lembo G, Carnevale D. 2018. PI3Kinases in diabetes mellitus and its related complications. IJMS. 19(12):4098. doi: 10.3390/ijms19124098.
- Mason R, Wahab N. 2003. Extracellular matrix metabolism in diabetic nephropathy. J Am Soc Nephrol. 14(5):1358–1373. doi: 10.1097/01.asn.0000065640.77499.d7.
- Meng X, Nikolic-Paterson D, Lan H. 2016. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 12(6):325–338. doi: 10.1038/nrneph.2016.48.
- Ren N, Kim E, Li B, Pan H, Tong T, Yang C, Tu Y. 2019. Flavonoids alleviating insulin resistance through inhibition of inflammatory signaling. J Agric Food Chem. 67(19):5361–5373. doi: 10.1021/acs.jafc.8b05348.
- Sepahi S, Soheili Z, Tavakkol-Afshari J, Mehri S, Hosseini S, Mohajeri S, Khodaverdi E. 2021. Retinoprotective effects of crocin and crocetin via anti-angiogenic mechanism in high glucose-induced human retinal pigment epithelium cells. Curr Mol Pharmacol. 14(5):883–893. doi: 10.2174/1874467214666210420111232.
- Voelker J, Berg P, Sheetz M, Duffin K, Shen T, Moser B, Greene T, Blumenthal S, Rychlik I, Yagil Y, et al. 2017. Anti-TGF-β1 antibody therapy in patients with diabetic nephropathy. J Am Soc Nephrol. 28(3):953–962. doi: 10.1681/ASN.2015111230.
- Wei X, Zhao Z, Zhong R, Tan X. 2021. A comprehensive review of herbacetin: from chemistry to pharmacological activities. J Ethnopharmacol. 279:114356. doi: 10.1016/j.jep.2021.114356.
- Wu S, Peng F, Li J, Ye F, Lei S, Zhang B. 2014. Akt and RhoA activation in response to high glucose require caveolin-1 phosphorylation in mesangial cells. Am J Physiol Renal Physiol. 306(11):F1308–1317. doi: 10.1152/ajprenal.00447.2013.
- Xie W, Du L. 2011. Diabetes is an inflammatory disease: evidence from traditional Chinese medicines. Diabetes Obes Metab. 13(4):289–301. doi: 10.1111/j.1463-1326.2010.01336.x.
- Xu W, Chu K, Li H, Zhang Y, Huang M, Zheng H, Sha M, Zhang X, Chen L. 2013. Bauhinia championii extraction treatment of collagen-induced arthritis via downregulation of the expression of TLR4, MyD88 and NF-κB. Am J Chin Med. 41(2):379–390. doi: 10.1142/S0192415X13500286.
- Xu W, Chu K, Li H, Zhang Y, Zheng H, Chen R, Chen L. 2012. Ionic liquid-based microwave-assisted extraction of flavonoids from Bauhinia championii (Benth.) Benth. Molecules. 17(12):14323–14335. doi: 10.3390/molecules171214323.
- Xu W, Huang M, Zhang Y, Li H, Zheng H, Yu L, Chu K. 2016a. Extracts of Bauhinia championii (Benth.) Benth. inhibit NF-κB-signaling in a rat model of collagen-induced arthritis and primary synovial cells. J Ethnopharmacol. 185:140–146. doi: 10.1016/j.jep.2016.03.035.
- Xu W, Huang M, Zhang Y, Li H, Zheng H, Yu L, Chu K, Lin Y, Chen L. 2016b. Extracts of Bauhinia championii (Benth.) Benth. attenuate the inflammatory response in a rat model of collagen-induced arthritis. Mol Med Rep. 13(5):4167–4174. doi: 10.3892/mmr.2016.5070.
- Yaribeygi H, Simental-Mendía L, Butler A, Sahebkar A. 2019. Protective effects of plant-derived natural products on renal complications. J Cell Physiol. 234(8):12161–12172. doi: 10.1002/jcp.27950.
- Zhang Y, Yan G, Sun C, Li H, Fu Y, Xu W. 2018. Apoptosis effects of dihydrokaempferol isolated from Bauhinia championii on synoviocytes. Evid Based Complement Alternat Med. 2018:9806160. doi: 10.1155/2018/9806160.
- Zheng H, Xu W, Li H, Chu K, Chen L, Zhang Y. 2013. Study on Bauhinia championi effective parts of anti-rheumatoid arthritis. Zhejiang Zhongyiyao Daxue Xuebao. 37:321–325.
- Zhu X, Shi J, Li H. 2018. Liquiritigenin attenuates high glucose-induced mesangial matrix accumulation, oxidative stress, and inflammation by suppression of the NF-κB and NLRP3 inflammasome pathways. Biomed Pharmacother. 106:976–982. doi: 10.1016/j.biopha.2018.07.045.