?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Context
Qing Cuo Formula (QCF) is a traditional Chinese medicine for treating acne, but its active compounds and molecular mechanisms are unclear.
Objective
To investigate the material basis and molecular mechanism of QCF.
Materials and methods
In vivo experiments were conducted on 60 male golden hamsters with damp-heat acne, with a blank group, a spironolactone group and 3 QCF administration groups (given high, medium and low doses) over a 30-day period. Serum androgen and inflammatory cytokine levels were tested by ELISA. In vitro, chemical compositions of QCF were investigated by UPLC-LTQ-Orbitrap-MS. Network pharmacology approaches were used to analyse the protein–protein interaction (PPI) network and QCF active compounds-intersection targets-acne network. GO enrichment and KEGG pathway analysis was conducted subsequently.
Results
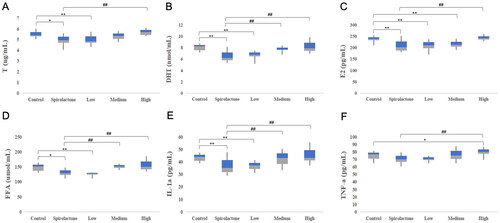
Low-dose QCF group (11.4 g/kg/day) showed significantly reduced levels of serum T (4.94 ± 0.36; 5.51 ± 0.36 ng/mL), DHT (6.67 ± 0.61; 8.09 ± 0.59 nmol/L), E2 (209.01 ± 20.92; 237.08 ± 13.94 pg/mL), IL-1α (36.84 ± 3.23; 44.07 ± 4.00 pg/mL) and FFA (128.32 ± 10.94; 148.00 ± 12.12 µmol/L) compared to the blank group (p < 0.05). In vitro experiments identified 75 compounds in QCF decoction, with 27 active compounds absorbed in serum. Network pharmacology identified 6 active components connecting 17 targets. GO enrichment and KEGG pathway analysis indicated that QCF’s anti-acne targets mainly regulate extracellular matrix function, inflammatory processes, immune response and endocrine function.
Conclusions
This study provides evidence of the molecular mechanism and material basis of QCF in treating androgen-related damp-heat acne, paving the way for further research on its potential in treating other conditions related to damp-heat constitution.
Introduction
Acne is a chronic inflammatory skin disease of hair follicles and sebaceous glands. Studies have found that up to 95% of the population have been effected by acne at different degrees (Ju Citation2019). It was reported in Europe that 95% of adolescents are affected by acne, causing serious physical and psychological damage (Zouboulis and Bettoli Citation2015). Modern medicine indicates that acne is a multifactorial disease, and its occurrence is mainly related to factors such as sex hormone levels, sebum secretion, Propionibacterium proliferation, keratinization of hair follicle sebaceous gland ducts and inflammatory response (Eichenfield et al. Citation2021). Modern medical treatments for acne mainly include general treatment, drug treatment and changes in lifestyle. Commonly used western medicines include spironolactone, cimetidine, glucocorticoids, retinoic acid drugs, antibiotics, benzoyl peroxide, etc. Some new drugs such as oral contraceptives, zinc products, and 5α-reductase inhibitors were also employed (Fox et al. Citation2016; Cervantes et al. Citation2018). However, western medicine treatment of acne has many side effects, including skin irritation, gastrointestinal irritation and teratogenicity; the rise of drug resistance has greatly reduced the utility (Fox et al. Citation2016; Mavranezouli et al. Citation2022). So safe and effective treatment options for acne are needed to address side effects and increasing rates of antibiotic resistance from current treatments.
Intervention from Traditional Chinese Medicine (TCM) constitution provides a new idea for the treatment of acne. The Chinese Medicine constitutional theory holds that there is a correlation between constitution and disease, and the constitution is the soil where diseases grow. On this basis, Wang Qi, the founder of the constitutional science of Chinese medicine, further put forward the ‘Skin-Body Correlation Theory’, which points out that constitution factors are the important material basis of skin type and characterization, and different constitution types are prone to different skin diseases (Wang J et al. Citation2013). People with damp-heat constitution have the characteristics of a greasy face, dirty complexion and rosacea, and these people are susceptible to acne, hair loss, halitosis and unpleasant body odour (Wang Q Citation2005). Numerous epidemiological investigations (Liu et al. Citation2015; Zhang Citation2017; Yang J et al. Citation2021) have consistently demonstrated that individuals with a damp-heat constitution are predominantly predisposed to acne. These findings have led to a conclusion that the damp-heat constitution is the principal constitution underlying the pathogenesis of acne (Li S, Yu, Jiang, et al. Citation2018). Treatment by correcting the damp-heat constitution can fundamentally improve the ‘soil’ environment of acne and prevent recurrence. Therefore, Professor Wang Qi created Qing Cuo Formula (QCF) as a specific prescription for the treatment of acne with damp-heat constitution. QCF is made from Weijing decoction, a famous and classic herbal formula in TCM. It is composed of 13 traditional Chinese medicines including Lonicerae Japonicae Flos, Phragmitis Rhizoma, Coicis Semen, Salvia miltiorrhiza, etc. (). Yu et al. (Citation2012) used self-controlled case series methods to observe the clinical efficacy of Wang Qi’s clinical application of this prescription in the treatment of acne and the total effective rate was 85.71%. At the same time, previous animal experiments showed that QCF can effectively inhibit the hyperplasia of sebaceous glands in golden hamsters (Li S, Yu, Xiao, et al. Citation2018). But the material basis and mechanism of QCF in the treatment of acne have not yet been elucidated.
Serum medicinal chemistry is a classic method to study the pharmacodynamic material basis of traditional Chinese medicines (TCMs). The transition components in human or animal blood after oral administration of TCMs are substances that directly exert medicinal effects in the body (Li and He Citation2018). Network pharmacology emphasizes the analysis of the molecular correlation between drugs and diseases from the overall perspective of systems and biological networks (World Federation of Chinese Medicine Societies Citation2021). It is widely used in the screening of TCM pharmacodynamic substances and the prediction of their mechanisms of action (Wang Y et al. Citation2019). In this study, the changes in serum sex hormones and inflammatory factors levels of golden hamsters were observed after intragastric administration of QCF. The ultra-performance liquid chromatography coupled with linear trap quadrupole-Orbitrap mass spectrometry (UPLC-LTQ-Orbitrap-MS) and network pharmacology were used to investigate the material basis and molecular mechanism of QCF in the treatment of acne.
Materials and methods
Reagents
ELISA kits of testosterone (T), oestradiol (E2), dihydrotestosterone (DHT), interleukin (IL)-1α, free fatty acid (FFA) and tumour necrosis factor (TNF)-α were provided by Beijing JinhaiKeyu Biological Science and Technology Development Co. Ltd. (Beijing, China). Methanol, acetonitrile and acetic acid were purchased from Fisher Company (HPLC grade; Fisher, Fair Lawn, NJ, USA). Purified water was acquired from a Millipore Synergy UV Ultrapure Water Machine (Millipore, Bedford, MA, USA).
Medicines
QCF is a decoction of 13 Chinese herbs as shown in . The 13 ingredients of QCF were bought from TCM Clinic, Beijing University of Chinese Medicine. The ingredients of QCF were mixed and soaked in distilled water for 30 min (QCF: water, 1:10 mL/mL), the mixture was heated to boil and held for 10 min and then held at low heat for 1 h. The same procedure was repeated after filtering out the aqueous phase. The two batches of filtrate and the mixture were mixed and used for intragastric administration of laboratory animals. Spironolactone tablets were provided by the Hangzhou Minsheng Pharmacy Co. Ltd. (Quasi-word: H33020070, batch number: T16E017).
Table 1. Composition of QCF.
Animals
The male golden hamsters were selected as damp-heat constitution acne model (Avci et al. Citation2013; Wang J et al. Citation2021). We used two batches of male golden hamsters, weighing between 110 and 130 g, both provided by the Beijing WeitongLihua Laboratory Animal Technique Co. Ltd. All golden hamsters were kept in a controlled environment (22 ± 2 °C, humidity of 59%∼65% and 12 h light/dark cycle). They were fed with complete nutritional pellet feed and provided with free access to drinking water. The handling of animals was approved by the Animal Ethics Committee of Beijing University of Chinese Medicine (protocol approval number: BUCM-2016071201-3006) and all experiments were performed in accordance with the institutional guidelines for the care and use of laboratory animals.
Pharmacodynamics animal experiment design of QCF
The sixty male golden hamsters were divided randomly into the following five groups (n = 12): blank control group, spironolactone group (7 mg/kg/day), QCF low-dose group (11.4 g/kg/day), QCF medium-dose group (22.8 g/kg/day) and QCF high-dose group (45.5 g/kg/day). Corresponding, normal saline was given to the blank control group. The gavage volume was 10 mL/kg. All the treatment drugs were given by gavage once a day for 30 consecutive days. Blood samples (3 ∼ 4 mL) from the five groups of hamsters were collected from the orbital vein in centrifuge tubes after 30 days of administration, respectively. The collected blood samples were then kept for 30 min at room temperature and centrifuged at 3000 rpm for 15 min, and the supernatant serums were stored at −20 °C for further analysis.
Biochemical analysis
Measurement of serum concentrations of three sex hormones (T, DHT and E2) and three cellular factors (TNF-α, IL-1α and FFA) levels was conducted with the ELISA method according to the kit’s instructions.
Preparation of QCF solution
The ingredients of QCF were mixed and soaked in distilled water for 30 min (QCF:water, 1:10 g/mL), the mixture was heated to boil and held for 10 min and then held at low heat for 1 h. The same procedure was repeated after filtering out the aqueous phase. The two batches of filtrate solutions were combined, concentrated to 39 mL and constant volume to 1 mL (containing 1.15 g QCF). The 1 mL QCF was placed in a 10 mL measuring bottle, and the volume was fixed with methanol. After that, ultrasonic treatment was conducted at room temperature for 30 min, and the sample was filtered with 0.45 µm microporous filter membrane before sample injection. The final sample solution was used for UPLC-LTQ-Orbitrap-MS analysis.
Preparation of golden hamsters serum samples
The 17 golden hamsters were divided into the blank group (n = 5) and the drug administration group (n = 12). The administration group was admitted aqueous solution of QCF by oral gavage once a day for seven consecutive days. Dose: 1.15 g/mL, 23 g/kg/day. Corresponding normal saline was given to the blank control group. On the eighth day, blood samples were collected from the fundus venous plexus of golden hamsters at 15, 30, 60, 90, 120, 150, 240, 480 and 720 min after administration, respectively, and placed in a heparinized test tube, centrifuged at 3000 rpm at 4 °C for 10 min. The serum from the same group was mixed and stored in the refrigerator at −80 °C.
Take 500 μL of blank group serum and administration group serum and place them in the 2 mL centrifuge tube respectively. Add three times (1.5 mL) acetonitrile and then vortex for 3 min. At a speed of 14,500 rpm, the protein was removed by centrifugation for 20 min. The supernatant was placed in a 1.5 mL centrifuge tube and dried with a nitrogen blower at room temperature. Each sample was then re-dissolved with 100 L methanol. Centrifugation was conducted at a speed of 14,500 rpm for 15 min, and 10 µL of supernatant was taken for injection analysis.
UPLC-LTQ-Orbitrap-MS analysis
UPLC analyses were carried out on a Thermo Scientific Dionex 3000 UHPLC Utimate Plus Focused ultra HPLC system coupled to an LTQ-linear ion trap Oribitrap XL-series electrostatic field orbit trap mass spectrometer (Thermo Scientific Company, USA). A Waters C-18 column (2.1 mm × 50 mm, 1.7 µm, ACQUITY UPLC BEH) was used to separate the compounds. The mobile phase was composed of water (with 0.1% formic acid) and acetonitrile. Gradient elution was employed 0–1 min, 4% B; 1–2.5 min, 4%–13% B; 2.5–7.5 min, 13%–22% B; 7.5–8.5 min, 22%–48% B; 8.5–12 min, 48%–60% B; 12–13 min, 60%–90% B; 13–14 min, 90% B. The injection volume was 2 μL the column temperature was 35 °C, and the flow rate was 0.4 mL/min.
The mass spectrometer utilized electrospray ionization, and positive and negative detection modes were separately used to analyse the samples. The ion source temperature was 350 °C, the ionization source voltage was set at 4 KV, and the capillary voltage was 35 V. The data adopted Fourier transform high resolution total scan (TF, Full scan, Resolution 30,000), data-dependent acquisition ddMS2 and Parent ion list PIL-MS2 (Duan et al. Citation2021; Yang L et al. Citation2022).
Xcalibur 2.2 and High Chem Mass frontier 7.0 software were used to analyse the characteristic fragments and structures of the related compounds and to infer and identifies of corresponding compounds based on the fragmentation pathways.
Screening of targets of QCF against acne
The effective compounds absorbed into blood of QCF identified by UPLC-LTQ-Orbitrap-MS were considered as the potential active components. The Binding Database and Stitch (5.0) database was used to screen the protein targets. ‘Acne’ was set as a keyword to screen related targets in GeneCards (https://www.genecards.org), TTD (http://db.idrblab.net/ttd/) and OMIM (https://www.omim.org) databases. Common targets for QCF and acne were analysed by Venn tool (https://bioinfogp.cnb.csic.es/tools/venny/) to determine the potential targets of QCF against acne.
Construction of PPI network
The potential therapeutic targets of QCF for acne were analysed by a PPI network, and the species were set to humans using the STRING database (http://string-db.org/) with a confidence level greater than 0.4 (Tao et al. Citation2020). To filter the key targets, this network was imported into Cytoscape 3.7.2. CytoHubba plug-in Cytoscape software was used to acquire the hub genes for QCF anti-acne.
QCF active compounds-intersection targets-acne network construction
The active compounds of QCF and the intersection targets of QCF and acne were sorted out and input into Cytoscape 3.7.2 to construct the network between QCF active compounds, intersection targets and acne (Qin et al. Citation2020).
GO function enrichment and KEGG pathway analysis
The GO biological function and KEGG signalling pathway enrichment analysis of the common target proteins of QCF and acne was performed using the R Studio. Both GO and KEGG analysis indicated a statistically significant difference at p < 0.05.
Statistical methods
Analysis was done with SPSS 20.0 statistical software. Data were expressed in the form of mean value standard deviation (
), with a 5% confidence interval.
Results
Effect of QCF on serum sex hormones and inflammatory factors in golden hamsters
As shown in , compared with the blank group, serum T (), DHT (), E2 (), FFA () and IL-lα () levels in the spironolactone group and the low-dose QCF group decreased significantly (p < 0.05), no significant difference in serum TNF-α level (). Except for the significant decrease in serum E2 level in the middle-dose QCF group (p < 0.05), there was no significant difference in the levels of serum inflammatory factors and sex hormones in the medium-dose and high-dose QCF groups compared with the blank group. Compared with the spironolactone group, serum T, E2, IL-lα, TNF-α and FFA levels in the low-dose group were lower, and the difference was not statistically significant; the levels of serum DHT, IL-lα and FFA in the middle-dose group of QCF increased significantly (p < 0.01); the level of all detection indicators in the high-dose QCF group was significantly increased (p < 0.05). The results of pharmacodynamic experiments showed that low-dose QCF can significantly reduce the serum concentrations of T, E2, DHT, IL-lα and FFA in golden hamsters, while the middle-dose and high-dose QCF have little effect on serum sex hormones and inflammatory factors of golden hamsters and may even aggravate hormone metabolism disorder and inflammation to a certain extent.
Analysis of in vitro chemical compositions of QCF
A database of the chemical constituents of various Chinese herbs in QCF, including name, molecular formula, molecular weight and other information was established. The typical total ion chromatograms (TICs) of compounds in QCF extract in negative and positive ion modes are shown in . Finally, a total of 75 compounds in QCF were identified or tentatively identified. Detailed information on the identification results and MS data are shown in .
Figure 2. Total ion chromatograms of QCF extract by UPLC-LTQ-Orbitrap-MS. (A) Total ion current graph in positive ion mode. (B) Total ion current graph in negative ion mode.
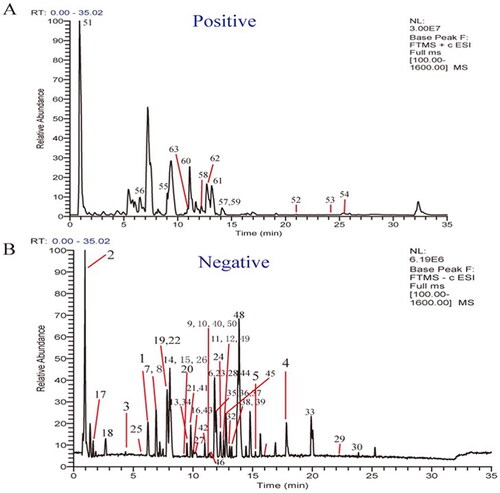
Table 2. Identification of in vitro constituents of QCF based on UPLC-LTQ-Orbitrap-MS.
Analysis of constituents absorbed into blood
Traditional Chinese medicines (TCMs) exhibit extremely high chemodiversity, and individual components affect synergistically. Effective components must use blood as a medium before being delivered to the target site to exert their pharmacodynamic effects (Li X et al. Citation2020). Therefore, the identification of in vitro drug components along cannot fully elucidate the mechanism of the effective ingredients of TCMs. Based on this, we further analysed the constituents absorbed into blood of golden hamsters after gastrointestinal administration of QCF. The TICs of compounds in rat sera in negative and positive ion modes are shown in . By comparing with the serum of blank group, a total of 27 compounds were detected in drug-containing serum, including 17 prototype compounds and 10 metabolites. The detailed information of the identification results and MS data are shown in .
Figure 3. Total ion chromatograms of serum after the administration of by UPLC-LTQ-Orbitrap-MS. (A) Total ion current graph in positive ion mode. (B) Total ion current graph in negative ion mode.
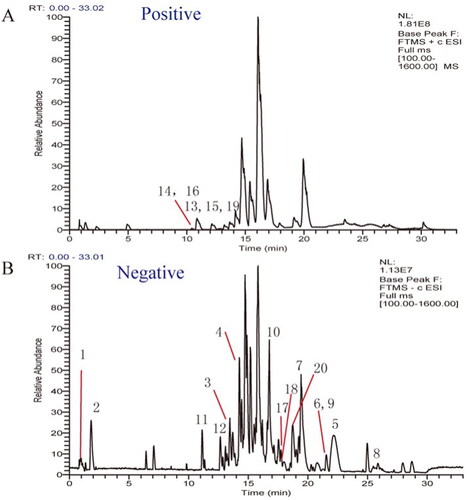
Table 3. Identification of constituents absorbed into blood of QCF based on UPLC-LTQ-Orbitrap-MS.
Potential QCF anti-acne molecular targets
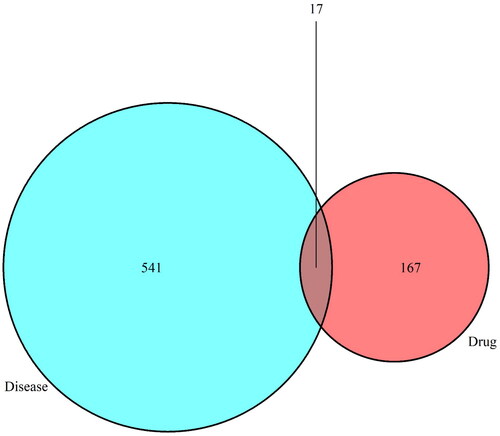
The components absorbed into blood identified by UPLC-LTQ-Orbitrap-MS were predicted by the Binding Database and Stitch (5.0) database, and finally 10 compounds had target records, with a total of 197 targets. With the keyword ‘Acne’, 1417 acne-targets were collected from GeneCards, TTD and OMIM. After removing the redundancy, a total of 184 drug targets and 558 disease targets were obtained. And the result of Venn showed that a total of 17 common targets were identified as potential therapeutic targets between QCF and acne ().
Protein–protein interaction (PPI) network construction
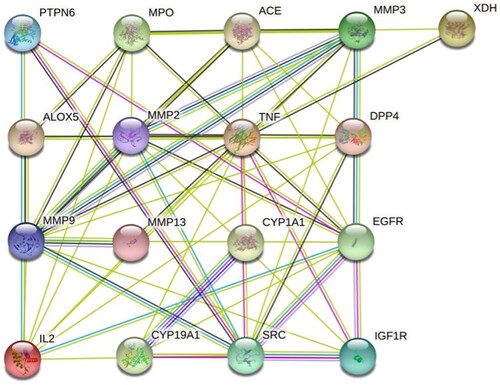
The interaction network of 17 potential targets was constructed by using the STRING database platform. The minimum interaction threshold was set to ‘0.4′, and the results are shown in . The nodes in the figure are intersection genes, the edges represent the association degree of the intersection genes, and the thickness represents the binding degree. As shown in the figure, there are 17 nodes and 60 edges (). Upload results to Cytoscape 3.7.2 and use the CytoHubba (an application in Cytoscape) to screen hub genes by MCC method. The top ten hub genes were TNF, MMP9, MMP2, MMP3, EGFR, SRC, IL2, MPO, ACE and DPP4.
QCF active compounds-intersection targets-acne network construction
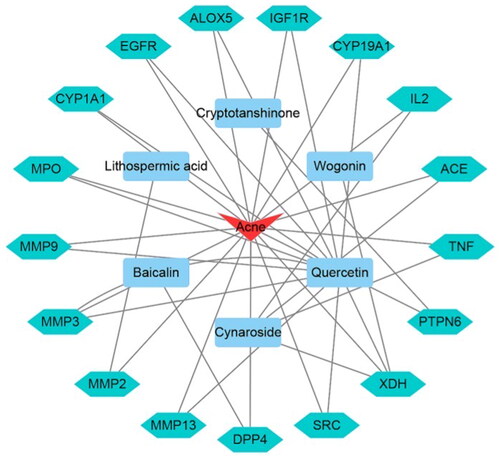
The 10 active compounds and 17 common targets were input into Cytoscape 3.7.2 software to build the compounds-intersection targets-acne network (), which included 24 nodes (6 for potential bioactive components, 17 for protein targets and 1 for acne disease) and 37 edges. The blue nodes represent the active components. The green nodes represent common targets of QCF and acne. The red node represents acne.
GO and KEGG pathways analysis
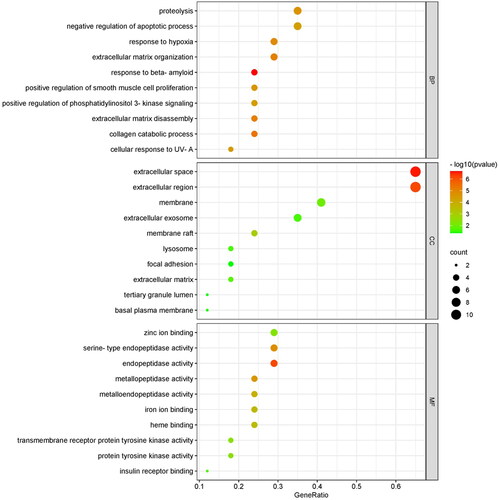
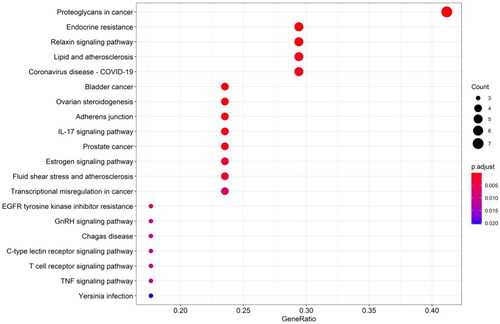
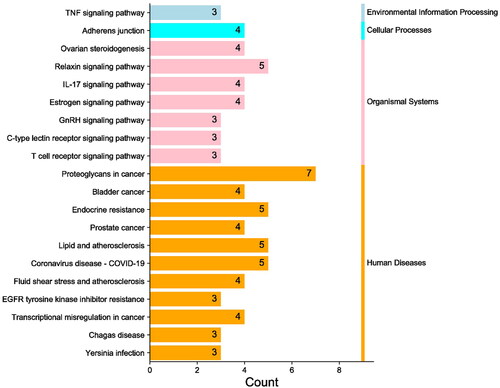
The results of GO analysis showed that there were 141 terms, including 103 terms for biological process (BP), 14 terms for cellular component (CC) and 24 terms for molecular function (MF). The results of each term were sorted by significance, and the top 10 findings of each analysis were selected for display (). Analyses using the GO database showed that the main enrichment of genes in the BP category was ‘response to beta-amyloid’, ‘collagen catabolic process’, ‘extracellular matrix disassembly’, ‘extracellular matrix organization’ and ‘response to hypoxia’. The main MF categories were ‘endopeptidase activity’, ‘serine-type endopeptidase activity’, ‘metallopeptidase activity’ and ‘metalloendopeptidase activity’. The main CC categories were ‘extracellular space’ and ‘extracellular region’. It can be discovered that these functional terms are highly relevant to extracellular matrix metabolism. KEGG metabolic pathway enrichment analysis obtained 48 enrichment results and information on the top 10 entries were shown in . According to the classifications and descriptions provided by KEGG, these terms can be roughly classified into metabolism, environmental information processing, cellular processes, organismal systems and human diseases (). Among them, organismal systems mainly involve the pathways related to the immune system, such as C-type lectin receptor signalling pathway, IL-17 signalling pathway and T cell receptor signalling pathway, and the pathways related to the endocrine system, such as endocrine system, ovarian steroidogenesis, relaxin signalling pathway, oestrogen signalling pathway and GnRH signalling pathway.
Discussion
Acne is a chronic inflammatory disease of the pilosebaceous unit resulting from androgen-induced increased sebum production, altered keratinization, inflammation and bacterial colonization of hair follicles on the face, neck, chest and back by Propionibacterium acnes (Dagnelie et al. Citation2022). Professor Wang Qi created Chinese medicinal formula QCF, starting from adjusting the damp-heat constitution and dedicated to improving the ‘soil’ environment of acne, so as to cure acne. In order to further clarify the material basis and mechanism of QCF against acne, we conducted pharmacodynamic experiments using golden hamsters as animal models and investigated the effective chemical constituents and targets of QCF in the treatment of acne using UPLC-LTQ-Orbitrap-MS combined with network pharmacology.
We observed that QCF can significantly reduce the serum level of T, E2, DHT, IL-lα and FFA in golden hamsters and the low-dose group is more advantageous. Compared with the spironolactone group, serum T, E2, IL-lα, TNF-α and FFA levels in the low-dose group were lower. Inflammation is one of the critical factors in the pathogenesis of acne. During inflammatory reaction, P. acnes induces the production of pro-inflammatory cytokines such as IL-1β, IL-6, IL-8 and TNF-α in monocytes and keratinocytes (Huang et al. Citation2014; Dréno et al. Citation2018). IL-1α can induce follicular hyperkeratinization, thereby promoting the occurrence and development of acne (Oh et al. Citation2020). P. acnes possess lipolytic activity, which is believed to be responsible for the hydrolysis of sebaceous lipids, liberating FFAs to the skin surface (Dessinioti and Katsambas Citation2017). At the same time, FFA can induce hair follicle sebaceous duct keratosis and acne inflammation, which leads to the occurrence of acne (Desbois and Smith Citation2010). In addition to inflammatory factors, male hormones in the body are also involved in the occurrence and development of acne. Evidence showed that T and DHT promoted sebocyte proliferation in vitro at concentrations higher than physiological levels (Lovászi et al. Citation2017). Therefore, we speculated that QCF could inhibit the inflammation by suppressing the production of IL-lα, FFA and TNF-α and at the same time inhibit the proliferation of sebaceous glands by reducing the level of androgen in golden hamsters.
We detected 75 in vitro chemical compounds and 27 compounds absorbed into blood. The network pharmacology study identified 17 active compounds found in the component of QCF that is absorbed into the blood. QCF active compounds-intersection targets-acne network showed that the main active compounds included lithospermic acid, wogonin, cryptotanshinone, cynaroside, quercetin and baicalin. Most of them are related to anti-inflammatory. For example, cymaroside (Wang W et al. Citation2020) and quercetin (Wang M et al. Citation2013) can inhibit the expression of inflammatory cytokines, including IL-6, IL-1β and TNF-α, etc. Wogonin, a naturally occurring flavonoid derived from the root extract of Scutellaria baicalensis Georgi (Lamiaceae), exerts anti-inflammatory effects through suppression of iNOS and COX-2 expression (Wei et al. Citation2017). Baicalin can inhibit multiple inflammatory pathways in the process of acne, such as TNF signalling pathway and pathway of Staphylococcus aureus infections (Yang X Citation2019). Some active chemical components of QCF also play an antibacterial effect. For example, cryptotanshinone can destroy the bacterial cell structure and affects the expression of intracellular proteins, which causes the normal physiological function of bacteria to be impaired (Li C et al. Citation2012). In addition to anti-inflammatory and antibacterial effects, some active compounds of QCF have oestrogen-like effects. Quercetin is one of flavones in phytoestrogen, as an oestrogen receptor modulator, quercetin restrains tumour cell growth by influencing down-stream substrate of oestrogen receptors and impacting signal pathway (Yang Y et al. Citation2011). Cryptotanshinone lessens the combination of androgen with the androgen-receptors present in the sebaceous glands and inhibits the sebaceous glands from secreting acne caused by the sebaceous glands of the hair follicle (Wang N Citation2015). In summary, constituents absorbed into blood of QCF contain antibacterial, anti-inflammatory and anti-androgen components.
PPI network analysis showed that the hub genes of QCF for the treatment of acne are TNF, MMP9, MMP2, MMP3, EGFR, SRC, IL2, MPO, ACE and DPP4. Most of these core genes are involved in inflammation, immune response and regulation of sebaceous gland function, thus playing a role in the pathogenesis of acne. GO enrichment analysis showed that the target proteins of QCF were related to extracellular matrix function. Aberrant extracellular matrix (ECM) remodelling has been associated with the presence of acne conditions (Emanuele et al. Citation2012). The remodelling of the ECM is regulated by a balance between matrix metalloproteinases (MMPs) and their inhibitors called tissue inhibitors of metalloproteinases (TIMPs) (Yaykasli et al. Citation2013). An imbalance in the ratio of MMPs to TIMPs has been suggested to play a key role in the development of atrophic or hypertrophic scars in patients with acne (Fabbrocini et al. Citation2010; Xu and Deng Citation2018). KEGG pathway enrichment analysis showed that many targets were enriched in signal pathways related to inflammation, immune response and endocrine function. Inflammation is a key factor in the pathogenesis of acne. P. acnes were found to stimulate monocytes to secrete proinflammatory cytokines including TNF-α, IL-1β, IL-6 and IL-8 (Said et al. Citation2020). Cutibacterium acnes interacts with the innate immune system to induce an inflammation via multiple pathways such as the activation of inflammasomes, the induction of the generation of MMPs and the stimulation of antimicrobial peptides (AMP) (Firlej et al. Citation2022). Endocrine disorders are known to involve all organ systems of the body, including the skin (Raj et al. Citation2021). The pathogenesis of acne involves several hormonal pathways, including androgens, insulin-like growth factor-1, oestrogens and corticosteroids (Rao et al. Citation2021).
Conclusions
The mechanisms that lead to the onset and persistence of acne involve many genes or signalling cascades, so it is clear that a multi-targeted approach is needed to overcome this skin disease. In the study, we found QCF could reduce the level of serum sex hormones and inflammatory factors in golden hamsters. In vitro, 75 compounds in QCF decoction and 27 active compounds absorbed in serum were identified using UPLC-LTQ-Orbitrap-MS. The constituents absorbed into blood of QCF can exert antibacterial, anti-inflammatory and anti-androgenic effects. Based on the network pharmacology analysis, 6 active components of QCF, connecting 17 targets. The GO enrichment analysis and KEGG pathway analysis results indicated that the anti-acne targets of QCF mainly regulate extracellular matrix function, inflammatory processes, immune response and endocrine function. Overall, this study showed multi-ingredient therapeutics of QCF to achieve multi-targets and multi-pathways regulation of acne. However, in vivo and in vitro experiments should be performed to verify the mechanism of QCF against acne in future studies.
Author contributions
Yanqi Cao: Data curation and analysis, Writing – Original draft. Jinfeng Liang: Methodology, Formal analysis, Writing – Original draft. Chunguo Wang: Data analysis, Writing – Review & Editing. Siqi Li and Xuejie Bao: Data curation. Bin Zeng: Visualization. David Humberto Lopez: Writing – Review & Editing. Ruoxi Yu: Conceptualization, Writing – Review & Editing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Avci P, Sadasivam M, Gupta A, De Melo WC, Huang Y-Y, Yin R, Chandran R, Kumar R, Otufowora A, Nyame T, et al. 2013. Animal models of skin disease for drug discovery. Expert Opin Drug Discov. 8(3):331–355. doi: 10.1517/17460441.2013.761202.
- Cervantes J, Eber AE, Perper M, Nascimento VM, Nouri K, Keri JE. 2018. The role of zinc in the treatment of acne: a review of the literature. Dermatologic Therapy. 31(1):e12576. doi: 10.1111/dth.12576.
- Dagnelie M-A, Poinas A, Dréno B. 2022. What is new in adult acne for the last 2 years: focus on acne pathophysiology and treatments. Int J Dermatol. 61(10):1205–1212. doi: 10.1111/ijd.16220.
- Desbois AP, Smith VJ. 2010. Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl Microbiol Biotechnol. 85(6):1629–1642. doi: 10.1007/s00253-009-2355-3.
- Dessinioti C, Katsambas A. 2017. Propionibacterium acnes and antimicrobial resistance in acne. Clin Dermatol. 35(2):163–167. doi: 10.1016/j.clindermatol.2016.10.008.
- Dréno B, Pécastaings S, Corvec S, Veraldi S, Khammari A, Roques C. 2018. Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: a brief look at the latest updates. J Eur Acad Dermatol Venereol. 32:5–14. doi: 10.1111/jdv.15043.
- Duan H, Wang G-C, Khan GJ, Su X-H, Guo S-L, Niu Y-M, Cao W-G, Wang W-T, Zhai K-F. 2021. Identification and characterization of potential antioxidant components in Isodon amethystoides (Benth.) Hara tea leaves by UPLC-LTQ-Orbitrap-MS. Food Chem Toxicol. 148:111961. doi: 10.1016/j.fct.2020.111961.
- Eichenfield DZ, Sprague J, Eichenfield LF. 2021. Management of acne vulgaris: a review. JAMA. 326(20):2055–2067. doi: 10.1001/jama.2021.17633.
- Emanuele E, Bertona M, Altabas K, Altabas V, Alessandrini G. 2012. Anti-inflammatory effects of a topical preparation containing nicotinamide, retinol, and 7-dehydrocholesterol in patients with acne: a gene expression study. Clin Cosmet Investig Dermatol. 5:33–37. doi: 10.2147/CCID.S29537.
- Fabbrocini G, Annunziata MC, D'Arco V, De Vita V, Lodi G, Mauriello MC, Pastore F, Monfrecola G. 2010. Acne scars: pathogenesis, classification and treatment. Dermatol Res Pract. 2010:893080. doi: 10.1155/2010/893080.
- Firlej E, Kowalska W, Szymaszek K, Roliński J, Bartosińska J. 2022. The role of skin immune system in acne. J Clin Med. 11(6):1579. doi: 10.3390/jcm11061579.
- Fox L, Csongradi C, Aucamp M, Du Plessis J, Gerber M. 2016. Treatment modalities for acne. Molecules. 21(8):1063. doi: 10.3390/molecules21081063.
- Huang WC, Tsai TH, Chuang LT, Li YY, Zouboulis CC, Tsai PJ. 2014. Anti-bacterial and anti-inflammatory properties of capric acid against Propionibacterium acnes: a comparative study with lauric acid. J Dermatol Sci. 73(3):232–240. doi: 10.1016/j.jdermsci.2013.10.010.
- Ju Q. 2019. Guideline for diagnosis and treatment of acne (the 2019 revised edition). J Clin Dermatol. 48:583–588.
- Li C, Zhao L, Xue Z, Kang W. 2012. Antibacterial mechanism of cryptotanshinone. Chin Pharm J. 47:1706–1710.
- Li G, He J. 2018. Serum medicinal chemistry study of traditional Chinese medicine and traditional Chinese medicine compound. Mod Med Health Res Elec J. 2(11):153.
- Li S, Yu R, Jiang H, Wang Q. 2018. Acne treatments from damp-heat constitution. World Chin Med. 13:236–240.
- Li S, Yu R, Xiao L, Jiang H, Wang Q. 2018. Experimental study on the effect of traditional Chinese medicine Qingcuo formula treating acne on the golden hamster model. J Xinjiang Med Univ. 41:781–784.
- Li X, Cao X, Liu J, Shi Y, Zhang Q, Zhang S, Zhu L, Du W. 2020. Exploration of research thoughts for pharmacological essence foundation and therapeutic mechanism of Chinese medicine. J Guangzhou Univ Trad Chinese Med. 37:366–370.
- Liu Y, Huang Q, Zhao H. 2015. Analysis on traditional Chinese medicine constitution types in 318 patients with acne vulgaris. J Trad Chinese Med. 56:223–227.
- Lovászi M, Szegedi A, Zouboulis CC, Törőcsik D. 2017. Sebaceous-immunobiology is orchestrated by sebum lipids. Dermatoendocrinol. 9(1):e1375636. doi: 10.1080/19381980.2017.1375636.
- Mavranezouli I, Daly CH, Welton NJ, Deshpande S, Berg L, Bromham N, Arnold S, Phillippo DM, Wilcock J, Xu J, et al. 2022. A systematic review and network meta-analysis of topical pharmacological, oral pharmacological, physical and combined treatments for acne vulgaris. Br J Dermatol. 187(5):639–649. doi: 10.1111/bjd.21739.
- Oh Y, Hwang HJ, Yang H, Kim JH, Park JHY, Kim J-E, Lee KW. 2020. Orobol, a derivative of genistein, inhibits heat-killed Propionibacterium acnes-induced inflammation in HaCaT keratinocytes. J Microbiol Biotechnol. 30(9):1379–1386. doi: 10.4014/jmb.2003.03063.
- Qin T, Wu L, Hua Q, Song Z, Pan Y, Liu T. 2020. Prediction of the mechanisms of action of Shenkang in chronic kidney disease: a network pharmacology study and experimental validation. J Ethnopharmacol. 246:112128. doi: 10.1016/j.jep.2019.112128.
- Raj R, Elshimy G, Mishra R, Jha N, Joseph V, Bratman R, Tella SH, Correa R. 2021. Dermatologic manifestations of endocrine disorders. Cureus. 13(9):e18327. doi: 10.7759/cureus.18327.
- Rao A, Douglas SC, Hall JM. 2021. Endocrine disrupting chemicals, hormone receptors, and acne vulgaris: a connecting hypothesis. Cells. 10(6):1439. doi: 10.3390/cells10061439.
- Said O, Khamaysi I, Kmail A, Fulder S, AboFarekh B, Amin R, Daraghmeh J, Saad B. 2020. In vitro and randomized, double-blind, placebo-controlled trial to determine the efficacy and safety of nine antiacne medicinal plants. Evid Based Complement Alternat Med. 2020:3231413. doi: 10.1155/2020/3231413.
- Tao Q, Du J, Li X, Zeng J, Tan B, Xu J, Lin W, Chen X-L. 2020. Network pharmacology and molecular docking analysis on molecular targets and mechanisms of Huashi Baidu formula in the treatment of COVID-19. Drug Dev Ind Pharm. 46(8):1345–1353. doi: 10.1080/03639045.2020.1788070.
- Wang J, Zhang H, Li L, Li Y, Zheng L, Yu R, Yang Y, Zhang Y, Bai M, Ni C, et al. 2013. Theory of skin relating to constitution put forward by Professor WANG Qi and its application in dermatology. J Beijing Univ Trad Chinese Med. 36:476–479.
- Wang J, Zhao X, Zhou Y, Wang Q. 2021. Establishing animal constitution models for research on nine classification of TCM constitution. J Beijing Univ Trad Chinese Med. 44:318–322. +357.
- Wang M, Liu B, Guo X. 2013. Quercetin and one of its metabolites inhibit reactive oxygen-species and inflammation. Food Sci. 34:256–260.
- Wang N. 2015. Effect of cryptotanshinone on glucose and lipid metabolism and sex hormone levels of rats with polycystic ovary syndrome. Matern Child Health Care China. 30:3490–3493.
- Wang Q. 2005. Classification and diagnosis basis of nine basic constitutions in Chinese medicine. J Beijing Univ Trad Chin Med. 28(04):1–8.
- Wang W, He P, Jiang X. 2020. Anti-inflammatory and antioxidant effects of luteolin and its flavone glycosides. Food Sci. 41:208–215.
- Wang Y, Cui T, Li Y, Liao M, Zhang H, Hou W, Zhang T, Liu L, Huang H, Liu C. 2019. Prediction of quality markers of traditional Chinese medicines based on network pharmacology. Chinese Herbal Med. 11(4):349–356. doi: 10.1016/j.chmed.2019.08.003.
- Wei C-Y, Sun H-L, Yang M-L, Yang C-P, Chen L-Y, Li Y-C, Lee C-Y, Kuan Y-H. 2017. Protective effect of wogonin on endotoxin-induced acute lung injury via reduction of p38 MAPK and JNK phosphorylation. Environ Toxicol. 32(2):397–403. doi: 10.1002/tox.22243.
- World Federation of Chinese Medicine Societies. 2021. Network pharmacology evaluation methodology guidance. World Chin Med. 16:527–532.
- Xu Y, Deng Y. 2018. Ablative fractional CO2 laser for facial atrophic acne scars. Facial Plast Surg. 34(2):205–219. doi: 10.1055/s-0037-1606096.
- Yang J, Cui B, Jiang G. 2021. A study on the distribution pattern of TCM constitution in 179 adult acne patients. Chinese J Dermatovenerol Integrated Trad Western Med. 20:214–216.
- Yang L, Liang J, Zheng Q, Zhou L, Xiong Y, Wang H, Yuan J. 2022. A comparative study of serum pharmacochemistry of Kai-Xin-San in normal and AD rats using UPLC-LTQ-Orbitrap-MS. Pharmaceuticals. 16(1):30. doi: 10.3390/ph16010030.
- Yang X. 2019. [Study on the molecular mechanism of baicalin in the treatment of acne caused by Propionibacterium acnes] [dissertation]. Beijing University of Chinese Medicine. Chinese. doi:10.26973/d.cnki.gbjzu.2019.000109.
- Yang Y, Cao Y, Zhang T, Zhu Y, Cao L. 2011. The pharmacologic actions progression of quercetin on estrogen related diseases. J Int Reprod Health/Family Planning. 30(01):69–72.
- Yaykasli KO, Turan H, Kaya E, Hatipoglu OF. 2013. Polymorphisms in the promoters of MMP-2 and TIMP-2 genes in patients with acne vulgaris. Int J Clin Exp Med. 6:967–972.
- Yu R, Wang Q, Ni C, Yang Y, Zhang H. 2012. Clinical observation on the acne treatment from the dampness-heat constitution. China J Trad Chin Med Pharm. 27:3240–3242.
- Zhang J. 2017. Analysis on types of traditional Chinese medicine constitution of 300 female patients with acne vulgaris. Chin J Rational Drug Use. 14(04):1–4.
- Zouboulis CC, Bettoli V. 2015. Management of severe acne. Br J Dermatol. 172:27–36. doi: 10.1111/bjd.13639.