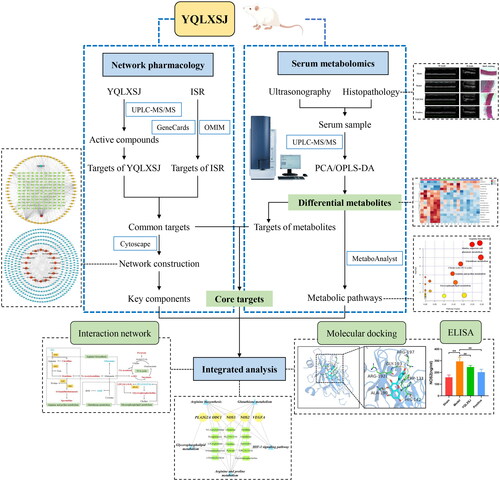
Abstract
Context
Yiqi Liangxue Shengji prescription (YQLXSJ) is a traditional Chinese medicine (TCM) formula that has long been used for treatment after percutaneous coronary intervention (PCI).
Objective
To investigate the putative pharmacological mechanism of YQLXSJ on restenosis through an integrated approach utilizing metabolomics and network pharmacology.
Materials and methods
Forty male Sprague–Dawley rats were divided into sham, model, YQLXSJ, and positive groups. YQLXSJ group received the treatment of YQLXSJ (6 g/kg/d, i.g.) and the positive group was treated with atorvastatin (2 mg/kg/d, i.g.). After 4 weeks, the improvement in intimal hyperplasia was evaluated by ultrasound, H&E staining, and immunofluorescence. UPLC–MS/MS technology was utilized to screen the differential metabolites. Network pharmacology was conducted using TCMSP, GeneCards, and Metascape, etc., in combination with metabolomics. Eventually, the core targets were acquired and validated.
Results
Compared to models, YQLXSJ exhibited decreased intima-media thickness on ultrasound (0.23 ± 0.02 mm vs. 0.20 ± 0.01 mm, p < 0.01) and reduced intima thickness by H&E (30.12 ± 6.05 μm vs. 14.32 ± 1.37 μm, p < 0.01). We identified 18 differential metabolites and 5 core targets such as inducible nitric oxide synthase (NOS2), endothelial nitric oxide synthase (NOS3), vascular endothelial growth factor-A (VEGFA), ornithine decarboxylase-1 (ODC1) and group IIA secretory phospholipase A2 (PLA2G2A). These targets were further confirmed by molecular docking and ELISA.
Discussion and conclusions
This study confirms the effects of YQLXSJ on restenosis and reveals some biomarkers. TCM has great potential in the prevention and treatment of restenosis by improving metabolic disorders.
Introduction
With the increase in interventional cardiology in recent decades, percutaneous coronary intervention (PCI) has developed into one of the most frequently performed revascularization techniques and has been of benefit to an increasing number of patients with coronary artery disease (CAD) (Neumann et al. Citation2019). Despite remarkable advances in coronary stents, the rate of in-stent restenosis (ISR) remains at approximately 10% (Giustino et al. Citation2022), which has become a major limitation in clinical practice. The occurrence of restenosis represents stent failure and is regarded as a predictor of an increase in 4-year mortality (Cassese et al. Citation2015). Extensive studies (Stefanini and Holmes Citation2013; Yang et al. Citation2021) have concluded that the inflammatory reaction in the vessel wall after mechanical injury during PCI could drive the proliferation of vascular smooth muscle cells (VSMCs), bringing about varying degrees of neointimal hyperplasia, which is the main cause of restenosis. As one class of drugs used for the secondary prevention of CAD, statins such as atorvastatin have been confirmed to have beneficial effects in preventing restenosis after PCI (Kamishirado et al. Citation2007). Nevertheless, the adverse reactions and poor repair of endothelial injury have not been effectively solved. Hence, searching for an efficient approach to inhibit the proliferation of VSMCs has become the focus of current research on restenosis.
In China, some traditional herbal preparations have been widely applied in the clinic with remarkable curative effects (Liu et al. Citation2018). Yiqi Liangxue Shengji prescription (YQLXSJ) is a traditional Chinese medicine (TCM) preparation created by Professor Jiazhen Liao to treat patients after PCI. A few previous studies have shown that YQLXSJ reduces the incidence of major adverse cardiac events (MACEs) (Ma et al. Citation2019) and has a tendency to reduce restenosis following PCI with better clinical efficacy and safety (Jia et al. Citation2021; Li et al. Citation2021). Additionally, YQLXSJ has also been granted a patent in China. The underlying mechanism by which YQLXSJ promotes reendothelialization might be associated with its ability to ameliorate energy metabolism, relieve oxidative stress and inhibit the inflammatory response (Su et al. Citation2015). Despite these findings, it is essential to fill the knowledge gap in the field of metabolomics.
As a subdiscipline of systems biology, metabolomics reflects the function and metabolic changes in organisms by systematically adopting a “top-down” strategy, which coincides with the holistic viewpoint of TCM (Johnson et al. Citation2016). Metabolomics has been extensively applied to modernized research on TCM and has become a conventional tool for biomarker discovery in recent years. However, metabolomics is unable to clearly display the endogenous mechanisms causing metabolite changes, such as the production of metabolites, upstream target proteins, and pathways. Fortunately, network pharmacology makes up for this deficiency. In network pharmacology, a multilevel network is constructed to link medicines, targets, and pathways with diseases through a series of public databases (Zhang et al. Citation2020). Therefore, metabolomics combined with network pharmacology provides new strategies to further explore the metabolic mechanisms of TCM.
The process of this study is shown schematically in . First, we performed animal experiments to confirm the inhibitory action of YQLXSJ against neointimal hyperplasia in rats experiencing balloon injury to the abdominal aorta. Subsequently, we applied widely targeted metabolomics to obtain the differential metabolites and identify a few relevant metabolic pathways. We used network pharmacology to elucidate the key components and predict the targets and enriched pathways. Moreover, using integrated pharmacology, we tried to comprehensively reveal the potential mechanisms of YQLXSJ in vascular intimal repair after balloon injury.
Materials and methods
Preparation and identification of YQLXSJ
YQLXSJ consists of four herbal medicines, including Radix Astragali (30 g), Salviae Miltiorrhizae (15 g), Cortex Moutan (10 g) and Flos Lonicerae (10 g) (Chen et al. Citation2021). YQLXSJ granules were produced by Beijing Tcmages Pharmaceutical Co., Ltd. (Beijing, China; batch number: 201005). The abovementioned four herbals were extracted with boiling water, concentrated, separated, dried, granulated by modern pharmaceutical technology, and finally packed in medical bags, which were stored at 4 °C.
Qualitative identification of the chemical components of YQLXSJ was conducted by ultra-performance liquid chromatography-tandem mass spectrometry (UPLC–MS/MS) analysis. Chromatographic separations were performed by a Waters HSS T3 column (1.8 µm, 2.1 mm × 50 mm; Waters, MA, USA). The mobile phases included 0.1% formic acid in water (solvent A) and 0.1% formic acid in acetonitrile (solvent B). The gradient program was as follows: 0–1.5 min, 5–20% B; 1.5–15 min, 20–90% B; 15–18 min, 90–100% B; 18–18.1 min, 100–5% B; and 18.1–20 min, 5% B. The flow rate was 0.3 mL/min. The column and autosampler temperatures were maintained at 40 °C and 10 °C, respectively. MS analysis was performed on a SCIEX Triple TOF 6600 instrument equipped with an electrospray ionization (ESI) source operating in positive and negative ion modes. The ESI source operation parameters were set as follows: source temperature, 500 °C; ion spray voltage, 5.5 kV (positive), −4.5 kV (negative); sheath gas 35 psi; gas 1, 50 psi; gas 2, 50 psi; curtain gas, 25 psi; and gas temperature 5000 °C. MS spectra were scanned in the range of m/z 100-1250.
Reagents
Atorvastatin calcium tablets (20 mg/tablet) were purchased from Dalian Pfizer Inc. (Dalian, China; batch number: EK5190). Methanol, acetonitrile, and formic acid (UPLC grade) were supplied by Merck (Darmstadt, Germany). Ammonium formate and ammonia (UPLC grade) were provided by Aladdin (Shanghai, China). Enzyme-linked immunosorbent assay (ELISA) kits for nitric oxide (NO), vascular endothelial growth factor A (VEGFA), inducible nitric oxide synthase (iNOS, NOS2), endothelial nitric oxide synthase (eNOS, NOS3), ornithine decarboxylase-1 (ODC1) and group IIA secretory phospholipase A2 (sPLA2-IIA, PLA2G2A) were provided by Beijing Reanta Biotechnology Co., Ltd. (Beijing, China). All other reagents were of UPLC grade.
Animal experiment
Healthy male Sprague–Dawley rats (250 ± 50 g) were acquired from Beijing Vital River Laboratory Animal Technology Co., Ltd. (licence number: SCXK 2016-0011/2021-0006). All rats were fed adaptively for one week before model establishment. Rats were bred with free access to food and water under a standard laboratory environment of 23 ± 2 °C temperature, 50-65% relative humidity and 12 h light/dark cycle. The animal experimental procedures were conducted following the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and the study protocol was approved by the Committee on Ethics of Experimental Research Center of China Academy of Chinese Medical Science (NO. ERCCACMS21-2208-02).
Forty rats were randomly divided into 4 groups: sham group, model group, YQLXSJ group and positive group (n = 10 in each group). The restenosis model was established by balloon-induced abdominal aorta injury according to a published study (Zou et al. Citation2019). Rats were anaesthetized by intraperitoneal injection of 0.3% sodium pentobarbital (1 mL/100 g). After making an incision along the midline of the neck, the left common carotid artery (1–1.5 cm) was separated, the distal end of the artery from the heart was ligated, and the proximal end was clipped with an artery clamp. To prevent acute thrombosis, an anticoagulant (heparin sodium, 100 u/kg) was injected. A V-shaped incision was made between the arterial clamp and ligation, and a balloon catheter (2.0 mm) was inserted. Then, guided by the guide wire, the balloon catheter was pushed down over the aortic arch to the abdominal aorta, with a depth of approximately 6–7 cm. After expanding with a pressure of 6–10 atm, the following procedure was repeated four times: the inflatable balloon was pulled back to the aortic arch and pushed forwards to the original location in the abdominal aorta. The proximal end of the left common carotid artery was ligated to stanch the bleeding, and the incision was sutured. A total of 40,000 U of gentamicin was injected intramuscularly for 3 days to prevent infection. The rats in the sham group underwent left carotid artery ligation only, without balloon injury.
Treatments were administered on the first day after operation. Our previous dosing study showed that 6 g/kg/day was the best dose to inhibit intimal hyperplasia (Jia Citation2020). Therefore, we did not conduct dose-response analysis again and directly selected the best dose. Rats in the YQLXSJ group were given intragastric administration at a dose of 6 g/kg/day. Moreover, rats in the sham and model groups were injected intragastrically with an equal amount of saline solution. Rats in the positive group were provided atorvastatin at a dosage of 2 mg/kg/day. All rats were fed for 4 weeks, and the body weights were measured weekly.
Ultrasonography and histopathology
Four weeks after surgery, the rats were fasted for 1 day, and then ultrasonography was performed to detect the related indices of the abdominal aorta. The intima-media thickness (IMT, mm), peak systolic velocity (PSV, mm/s) and end diastolic velocity (EDV, mm/s) were measured, and the resistance index (RI) was calculated as RI = (PSV–EDV)/PSV. In this study, triplicate measurements were performed on each rat.
As soon as ultrasonography was completed, the rats were sacrificed with excessive sodium pentobarbital anesthesia (90 mg/kg). Then, rat blood was directly drawn from the heart for metabolomics studies. The abdominal aorta was collected and washed with phosphate-buffered saline (PBS). Segments of the injured aorta (1 cm/per rat) were fixed in 4% paraformaldehyde for 24 h, dehydrated and embedded in paraffin. Each segment was cut to obtain three sections with a thickness of 5 μm. The slices were stained with hematoxylin and eosin (H&E) to observe intimal hyperplasia and photographed under a microscope for image acquisition. The thickness of the intima (IT, μm) and the thickness of the media (MT, μm) were measured by the imaging analysis software ImageJ v1.8.0. In turn, triplicate analyses were performed on each sample. Vascular endothelial cell (VEC) and VSMC proliferation were detected by immunofluorescence staining of von Willebrand factor (vWF) and alpha smooth muscle actin (α-SMA), respectively. DAPI was used for nuclear staining.
Metabolomics
Sample preparation
Blood samples taken from the heart were allowed to stand for 40 min, then the serum was separated by two centrifugation steps (3000 rpm/25 °C/10 min, 3000 rpm/4 °C/10 min). All samples were stored at −80 °C. Thawed serum (50 μL) and 300 μL of pure methanol were evenly mixed and vortexed for 3 min. Subsequently, each mixture was centrifuged (12,000 rpm/4 °C/10 min) and incubated at −20 °C for 30 min. After centrifugation again, 150 μL of supernatant was collected in an injection bottle for onboard testing. A quality control (QC) sample was prepared by extracting 20 μL from the mixed sample for method validation.
UPLC–MS/MS analysis
UPLC–MS/MS (SCIEX, Framingham, MA, USA) was used for metabolomics analysis. The metabolites were analyzed on an ACQUITY UPLC HSS T3 C18 column (1.8 µm, 2.1 mm × 100 mm; Waters, MA, USA). The mobile phases included 0.1% formic acid in water (solvent A) and 0.1% formic acid in acetonitrile (solvent B). At a flow rate of 0.4 mL/min, the gradient program was as follows: 0–11 min, 5–90% B; 11–12 min, 90% B; 12–12.1 min, 90-5% B; and 12.1–14 min, 5% B. The injection volume was 2 μL. MS analysis was performed in positive and negative ion modes by using a QTRAP system equipped with an ESI source. The parameters were set as follows: source temperature, 500 °C; ion spray voltage, 5.5 kV (positive), −4.5 kV (negative); curtain gas, 25 psi; collision gas, high; and ion source gas I and II, 55 psi and 60 psi, respectively. During analysis, a QC sample was inserted between every 10 analytical samples.
Data processing and analysis
UPLC–MS/MS analysis and data processing were conducted by Allwegene Tech (China). The raw mass spectral data were processed with Analyst 1.6.3 software. Unsupervised principal component analysis (PCA) was conducted using the R basic package (v3.5.1). Furthermore, orthogonal partial least squares discrimination analysis (OPLS-DA) was performed with the R package MetaboAnalystR (v1.0.1). For the t-test (p < 0.05), variable importance plot (VIP > 1) and fold change (FC > 1.2 or FC < 0.8) between the sham group and model group, the eligible metabolites were screened as differential metabolites which were appraised in HMDB (https://hmdb.ca/) and KEGG (https://www.kegg.jp/). The targets of the differential metabolites were obtained from HMDB. The HMDB IDs of the differential metabolites were imported into MetaboAnalyst 5.0. Metabolic pathways with p < 0.05 and impact > 0.1 were considered.
Integration of network pharmacology and metabolomics
Based on qualitative research on YQLXSJ, the targets of its major components were queried in TCMSP (https://www.tcmsp-e.com/). The disease-related targets were gathered from the gene map of GeneCards (https://www.genecards.org/) and OMIM (https://omim.org/) via the keywords ‘in-stent restenosis’. Finally, KEGG pathway enrichment analysis was performed in the Metascape database (https://metascape.org/), and pathways with p < 0.05 were selected for further research.
The targets of the differential metabolites and network pharmacology were overlapped to gain the core targets. Cytoscape (v3.9.0) was utilized to construct the following visualization networks: (1) active component-common target-disease network, (2) metabolite-target network, and (3) target-pathway-metabolite network. In addition, an interaction network was manually constructed to visually denote the relationships between metabolites, targets and metabolic pathways.
Validation
Molecular docking
Target protein accession
The 3D structures of the core target proteins were taken from the RCSB PDB database (https://www.rcsb.org/), which is currently the most important database for collecting the structures of biological macromolecules. The 3D structures of receptor proteins can be mostly searched and downloaded from the RCSB PDB. The experimental data were obtained by X–ray crystallography and the best refinement resolution value was chosen.
Preparation of the ligands
The molecular structures of the key components were downloaded from TCMSP and saved as mol2 files. Then, the components were optimized by adding hydrogens and the structures were saved as pdbqt files using AutoDockTools 1.5.7.
Preparation of the receptors
First, the target proteins were processed by PyMOL 2.5.0 to remove the water molecules and original ligands. Subsequently, the proteins were optimized by adding hydrogens with AutoDockTools and saved as pdbqt files.
Docking
AutoDock Vina 1.1.2 was used for molecular docking to evaluate the binding energies of the components to the core targets. Ligands were positioned correctly in each protein’s binding pocket during the docking process. The default docking parameters were used as follows: 1 CPU to use and an exhaustiveness of 9 poses to output.
Evaluation of the docking results
The lower the binding energy of a complex is, the more stable it is. It was reported that the most common binding energy values are less than −5 kcal/mol, which indicates good binding affinity between the receptor and ligand (Yang et al. Citation2022). Finally, the results were visualized by PyMOL.
Enzyme-linked immunosorbent assays
The plasma contents of NO, NOS2, NOS3, ODC1, PLA2G2A, and VEGFA in the different groups were measured with the appropriate ELISA kits.
Statistical analysis
Data are expressed as the mean ± standard deviation. In the present study, the software SPSS 22.0 was applied for experimental data analysis. Statistical analysis was performed by Student’s t-test for comparisons between two groups. One-way ANOVA was performed for more than two groups, with an LSD post hoc test. The Kruskal–Wallis followed by Dunnett’s post hoc test was used for data for which the normality test failed. A value of p < 0.05 represented statistical significance, and p < 0.01 was regarded as highly statistically significant. The experiments were performed in triplicate.
Results
Qualitative analysis of YQLXSJ
According to qualitative analysis of YQLXSJ by UPLC–MS/MS, a total of 95 major compounds were identified, as shown in Supplementary Table S1.
Effects of YQLXSJ on abdominal aorta balloon injury in rats
Establishment of intimal hyperplasia in rats
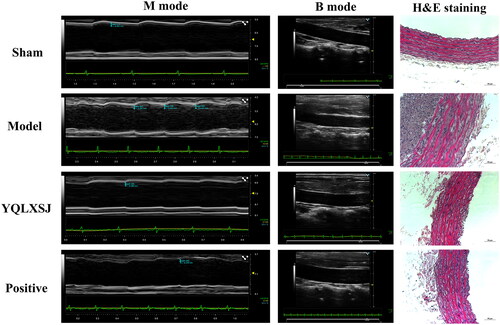
All rats were weighed weekly. In terms of body weight, there were no significant differences observed between the groups. Subsequently, ultrasound was performed to detect the intimal condition and verify the successful construction of the intimal hyperplasia model (). Intimal hyperplasia developed in the abdominal aorta 28 days after balloon injury. IMT was significantly increased in the model group (0.23 ± 0.02 mm) compared with the sham group (0.10 ± 0.01 mm, p < 0.01), while prominent decreases in the IMT were observed in the YQLXSJ group (0.20 ± 0.01 mm) and positive group (0.19 ± 0.01 mm) in comparison with the model group (p < 0.01). Regarding vascular function, the diastolic and systolic functions of the abdominal aorta in the model group (PSV 562.64 ± 58.24 mm/s, EDV 32.28 ± 7.92 mm/s, RI 0.94 ± 0.01) were notably worse than those in the sham group (PSV 386.61 ± 42.60 mm/s, EDV 80.28 ± 7.15 mm/s, RI 0.79 ± 0.02, p < 0.01). Both YQLXSJ (PSV 414.28 ± 13.65 mm/s, EDV 48.01 ± 4.11 mm/s, RI 0.88 ± 0.01) and atorvastatin (PSV 433.71 ± 29.68 mm/s, EDV 49.40 ± 43.99 mm/s, RI 0.88 ± 0.01) improved vasomotor function and reduced vascular resistance (p < 0.01).
Histopathological analysis
The H&E staining results from each group are also presented in . These images show a smooth vessel wall, clear structures of the intima-media and orderly arranged cells in the sham group. In contrast, the neointima and media were thickened, but intimal thickening was more significant in the model group. A large amount of smooth muscle cells (SMCs) proliferation and a disordered cell arrangement were observed in the neointima. Both YQLXSJ and atorvastatin attenuated the degree of intimal hyperplasia and proliferation of SMCs.
The quantitative indices IT, MT and IT/MT were utilized to compare the results of H&E staining, as shown in . Notably, no significant difference existed between the YQLXSJ and positive groups (p > 0.05).
Table 1. Quantitative indices of H&E staining. (n = 6).
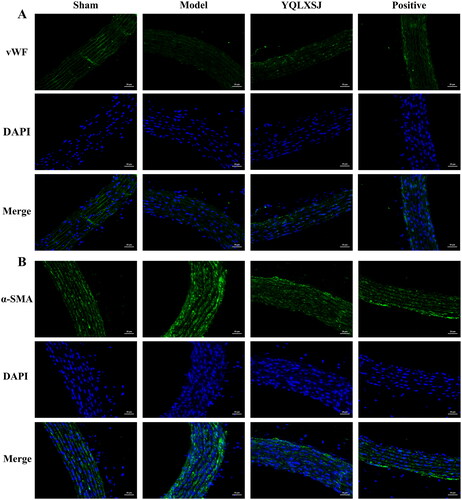
displays the immunofluorescence results. In the model group, the vWF fluorescence intensity was the weakest, but the intensity of α-SMA was the strongest, indicating that the number of endothelial cells (ECs) decreased but the number of SMCs increased. Compared with the model group, the fluorescence intensity of both the YQLXSJ and positive groups approached that of the sham group, suggesting that the degree of intimal hyperplasia had been improved in the treated groups.
Multivariate metabolomics analysis
A total of 771 metabolites were captured after matching with the database. The total ion chromatogram (TIC) showed that the LC–MS system behaved stably ( and ). As demonstrated in the PCA model (), all points were situated in ellipses, representing the 95% confidence interval. We observed that the samples in one group show clustering trend. Moreover, there was obvious separation of the metabolic profiles among the different groups, particularly between the sham group and model group, indicating that the metabolites in each group had changed. The YQLXSJ group was different from the model group and more similar to the positive group, which indicated that YQLXSJ had a positive regulatory effect on metabolic disorders.
Figure 4. Metabolomics profile for YQLXSJ intervention in rats. (A) TIC of QC samples. (B) TIC of all samples. (C) PCA of the sham, model, YQLXSJ, positive and QC groups. (D) OPLS-DA of the sham and model groups. (E) OPLS-DA of the model and YQLSXJ groups.
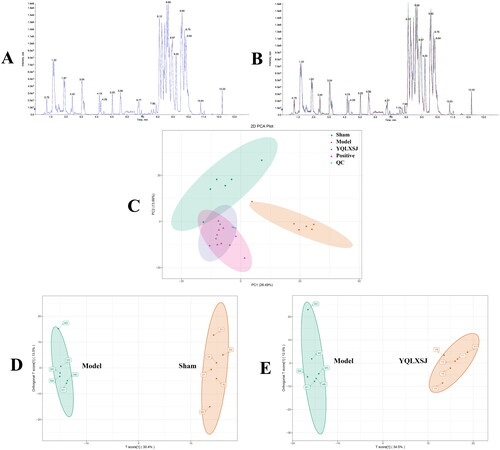
OPLS-DA was carried out to determine the metabolomic differences. As shown in , there were significant separations between different groups. Between the sham and model groups, the parameters of R2Y and Q2 were 0.997 and 0.79, while those of the comparison group were 0.991 and 0.844, respectively. This result indicated that the OPLS-DA models had great stability and predictive ability. Therefore, OPLS-DA can be used as an effective method for searching for differential metabolites.
Screening differential metabolites and metabolic pathways
Metabolites satisfying p < 0.05, VIP > 1 and FC > 1.2 or < 0.8 were considered to be differential metabolites in the OPLS-DA models. There were 68 compliant metabolites in the sham and model groups and 94 in the model and YQLXSJ groups. After intersection of the above metabolites, 18 differential metabolites in total were ascertained and matched with the HMDB and KEGG databases by comparison with the MS information. shows the detailed information. Furthermore, 369 targets of the differential metabolites were retrieved from the HMDB (Supplementary Table S3).
Table 2. The detailed information about 18 differential metabolites among the sham, model and YQLXSJ groups.
The metabolites in the model group changed in different ways. For example, the levels of pyruvate, ornithine, spermidine and LPC (16:1/0:0) were significantly increased, while the levels of glutamine, glutathione (GSH) and PC (15:0/15:0) decreased. Nevertheless, in contrast with the model group, the trends in the YQLXSJ group were reversed and tended to recover to those of the sham group. These results showed that YQLXSJ could correct metabolic disorders. A heatmap was constructed as shown in to directly observe the variations in the differential metabolites among the three groups.
Figure 5. Further studies on differential metabolites. (A) The clustering heatmap visualizing the contents of the 18 differential metabolites in the sham, model and YQLSXJ groups. (B) Metabolic pathway enrichment analysis.
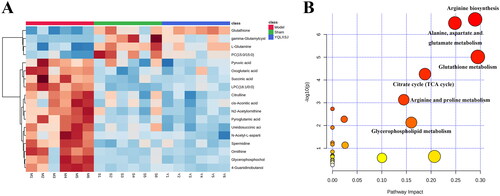
The above 18 differential metabolites were utilized to explore the possible metabolic pathways with MetaboAnalyst 5.0. As shown in , all enriched metabolic pathways were exhibited. By setting two criteria (p < 0.05 and impact > 0.1), 6 metabolic pathways were screened, and the detailed information is listed in .
Table 3. The detailed information about six metabolic pathways.
Results of network pharmacology
Using the identified compounds to find their targets in the TCMSP, we acquired 66 active components and 324 targets of YQLXSJ (Supplementary Table S2). Using ‘in-stent restenosis’ as the search term, 1160 disease targets were obtained. In total, 142 common targets were acquired. Ultimately, the common targets were uploaded to Metascape for pathway enrichment analysis. Among the pathways that closely corresponded to the pathophysiological processes of vascular injury and restenosis, the top 5 were hsa05417: lipid and atherosclerosis, hsa05418: fluid shear stress and atherosclerosis, hsa04151: PI3K-Akt signaling pathway, hsa04668: TNF signaling pathway, and hsa04066: HIF-1 signaling pathway, respectively.
Network construction
First, the active component-common target-disease network () was established, which consisted of 210 nodes (1 YQLXSJ, 66 active components, 142 common targets and 1 disease) and 717 edges. The node degrees were calculated by Network Analyzer in Cytoscape. A high degree value indicates that the component is vital. Among the active components, the top six nodes were quercetin (degree = 91), luteolin (degree = 39), fisetin (degree = 34), kaempferol (degree = 20), isorhamnetin (degree = 20) and tanshinone IIa (degree = 20). These compounds might be the major components in YQLXSJ that promote endothelial repair. Subsequently, the metabolite-target network () was constructed, which contained 387 nodes (18 metabolites and 369 target genes) and 449 edges. The above networks clearly displayed the complex relationship between the traditional Chinese medicine components, targets and metabolites. Thus, YQLXSJ plays a vital role during the repair process after vascular injury through multiple components, targets and pathways.
Figure 6. Network construction. (A) Active component-common target-disease network. Orange nodes represent active components, blue node represents YQLXSJ, green nodes represent common targets and red node represents disease. (B) Metabolite-target network. Blue nodes indicate targets of metabolites, and orange nodes indicate differential metabolites. (C) Venn diagram of 5 core targets. (D) Target-pathway-metabolite network. Yellow nodes represent core targets, green nodes represent differential metabolites, and blue nodes represent key pathways.
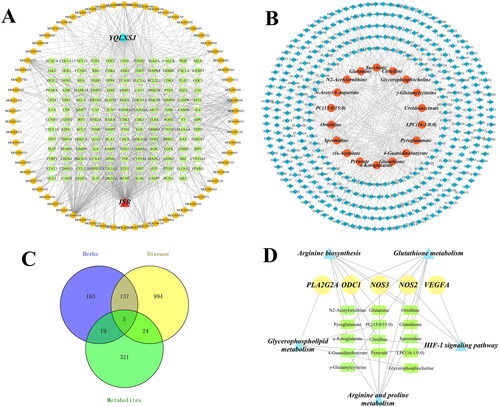
Integration of metabolomics and network pharmacology
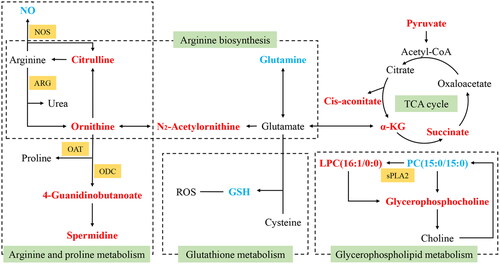
To gain insight into the overall relationship among the pathways, upstream targets and terminal metabolites, we integrated the results of metabolomics and network pharmacology, hoping to more comprehensively, systematically and clearly reveal the mechanism by which YQLXSJ reduces restenosis. Combining the targets from network pharmacology and differential metabolites, 5 core targets were found, namely, NOS2, NOS3, ODC1, PLA2G2A and VEGFA (). From this foundation, 5 crucial pathways and 14 affected metabolites were obtained. Moreover, the target-pathway-metabolite network () was constructed according to the five core targets, five key pathways and fourteen metabolites. An interaction network diagram is displayed in .
Figure 7. The interaction network of potential metabolic pathways. Red markers represent the upregulated metabolites in the model group. Blue markers represent the downregulated metabolites in the model group. Green tags represent the metabolic pathways, and orange tags represent corresponding enzymes. (α-KG: α-ketoglutarate; NO: nitric oxide; GSH: glutathione; ROS: reactive oxygen species; NOS: nitric oxide synthases; ARG: arginase; OAT: ornithine acetyltransferase; ODC: ornithine decarboxylase; sPLA2: secretory phospholipase A2)
Molecular docking
The 5 key components were simulated with 5 core targets NOS2 (PDB ID: 6JWM; resolution: 1.23 Å), NOS3 (PDB ID: 4D1P; resolution: 1.731 Å), ODC1 (PDB ID: 7S3G; resolution: 1.66 Å), PLA2G2A (PDB ID: 3U8I; resolution: 1.1 Å), and VEGFA (PDB ID: 1MKK; resolution: 1.32 Å). The ligand denotes the component of YQLXSJ and the receptor is the target protein. The binding energy results are shown in . The lower the binding energy of a complex is, the more stable it is. We considered that a binding energy value less than −5 kcal/mol indicates good binding between the component and target. The results showed that all binding energies were lower than −5 kcal/mol, indicating that the combinations of the key components with the core targets were stable. Ultimately, we selected portions of the optimal docking images with two representative components, quercetin and tanshinone IIa, to display in .
Figure 8. The docking model of quercetin and tanshinone IIa with core targets. (A) Quercetin-NOS2. (B) Quercetin-NOS3. (C) Quercetin-ODC1. (D) Tanshinone IIa-NOS3. (E) Tanshinone IIa-PLA2G2A. (F) Tanshinone IIa-VEGFA. Hydrogen bonds are shown as yellow dashed lines.
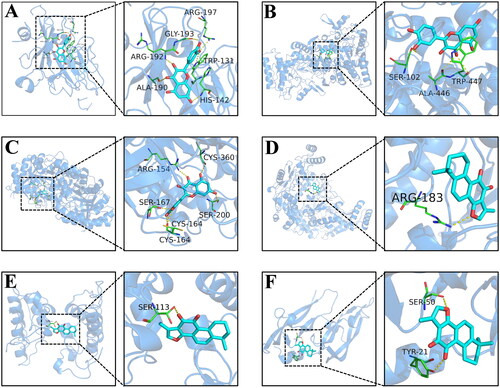
Table 4. Binding energy results of key compounds and core targets (kcal/mol).
For the quercetin-NOS2 complex (), quercetin clearly formed strong hydrogen bonding interactions with residues Arg-197, Arg-192, and His-142 of NOS2, suggesting that these hydrogen bonds are vital to stabilize quercetin in the NOS2 binding pocket. In addition, quercetin had strong hydrophobic interactions with Gly-193, Trp-131, and Ala-190 that could also stabilize the complex. In the quercetin-NOS3 complex (), quercetin was found to be associated with hydrophobic amino acids Trp-447 and Ala-446, showing that the binding of quercetin to NOS3 is primarily driven by hydrophobic interactions.
ELISA
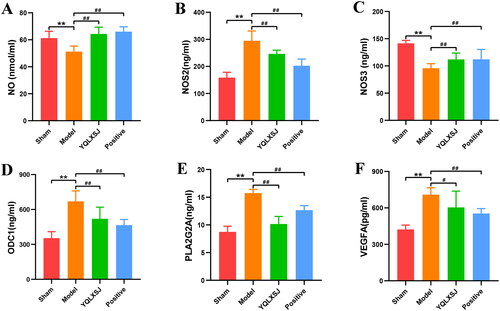
shows the ELISA results. Compared with the sham group, the levels of NO and NOS3 were significantly decreased in the model group (p < 0.01), while the levels of NOS2, PLA2G2A, ODC1 and VEGFA were obviously increased (p < 0.05). After treatment, the levels of NOS2, PLA2G2A, ODC1 and VEGFA were visibly reduced (p < 0.01), and the plasma contents of NO and NOS3 increased substantially (p < 0.01).
Figure 9. Results of ELISA analysis. (A-F) The serum levels of NO, NOS2, NOS3, ODC1, PLA2G2A, and VEGFA in each group. The results are expressed as the mean ± standard deviation. **p < 0.01 compared with the sham group; #p < 0.05 compared with the model group; ##p < 0.01 compared with the model group.
Discussion
After PCI, neointimal hyperplasia is the main cause of luminal obstruction and restenosis and is also aggravated by pathological processes including oxidative stress, inflammation and neoatherosclerosis (Li et al. Citation2018; Burtenshaw et al. Citation2019). The animal experiment strongly confirmed that 28 days after balloon-induced injury to the abdominal aorta, neointimal hyperplasia was dominated by abundant VSMC proliferation and migration. Moreover, we observed that both YQLXSJ and atorvastatin promoted the repair of ECs and inhibited the proliferation of SMCs to reduce the degree of intimal hyperplasia.
The network pharmacology results showed that the key components of YQLXSJ were quercetin, luteolin, kaempferol and tanshinone IIa. Among them, tanshinone IIa is recommended by the China State Food and Drug Administration for its therapeutic potential against cardiovascular diseases. Quercetin inhibits VSMC proliferation and migration and reduces EC dysfunction and apoptosis (Dagher et al. Citation2021). As one of the main components in Salviae Miltiorrhizae, tanshinone IIa can improve EC function and inhibit VSMC migration by suppressing the ERK1/2 and PI3K/AKT signaling pathways (Lu et al. Citation2018; Feng et al. Citation2021). KEGG analysis showed that the greatly related pathways included PI3K/AKT signaling pathway, TNF signaling pathway and HIF-1 signaling pathway, indicating that YQLXSJ probably contributes to the repair of endothelial injury by regulating inflammation, oxidative stress and cell proliferation.
In prior clinical trials, a few biomarkers have been selected by serum metabolomics from patients with restenosis after PCI. For example, Japanese scholars used GC/MS and discovered 8 serum metabolites (isobutylamine and glutamine, etc.) that could serve as biomarkers from restenosis patients with an amino acid metabolism disorder (Hasokawa et al. Citation2012). The disruption of phospholipid metabolism is associated with restenosis. Six phospholipid biomarkers may play essential roles in cell signaling that regulates the proliferation and migration of VSMCs (Cui et al. Citation2017). Similarly, our study discovered 18 differential metabolites by UPLC–MS, 5 core targets (NOS2, NOS3, ODC1, PLA2G2A and VEGFA) and 5 related key pathways (HIF-1 signaling pathway, arginine and proline metabolism, arginine biosynthesis, glutathione metabolism and glycerophospholipid metabolism).
In arginine and proline metabolism, l-arginine, as a donor of NO, is catabolized to citrulline and NO by nitric oxide synthases (NOS). NO released from ECs is a powerful signaling molecule with physiological activities, such as vasodilation, platelet blocking, and VSMC proliferation and collagen synthesis inhibition to protect against intimal thickening (Infante et al. Citation2021). Due to the increased levels of reactive oxygen species (ROS) that result after mechanical injury and oxidative stress, the uncoupling of NOS3 leads to aberrant function and synthesis, which results in the reduction of NO (Khaddaj Mallat et al. Citation2017). Reduced NO production or bioavailability is associated with endothelial dysfunction and neointimal hyperplasia. Our results showed a decrease in the level of NO but an increase in level of citrulline. It was revealed that greater citrulline levels are related to a higher risk of obstructive atherosclerosis and MACE incidence in CAD patients after coronary angiography (Tang et al. Citation2009).
In arginine biosynthesis, glutamine is one of the changed metabolites after YQLXSJ treatment. Glutamine participates in many metabolic and biosynthetic pathways, with a negative correlation between its levels in circulation and cardiometabolic diseases (Li et al. Citation2019). Glutamine fuels the proliferation of ECs (Kim et al. Citation2017). Hence, in the model group, decreased levels of glutamine inhibited VEC proliferation but promoted VSMC proliferation, leading to neointimal hyperplasia. After YQLXSJ treatment, these cells were restored to normal levels. It was also found that the level of glutamine is intimately connected to certain procoagulant factor activities in SMCs, indicating that glutamine may deliver information on stent thrombosis (Koyama et al. Citation2021).
Glutathione (GSH), a natural intracellular antioxidant, is the most important metabolite of glutathione metabolism. Its antioxidant, antiatherogenic, and anti-inflammatory properties have been proven in many previous studies (Matuz-Mares et al. Citation2021). In patients with CAD, a Mediterranean diet is positively correlated with higher plasma levels of GSH and regulates improved endothelial functions (Bettermann et al. Citation2018). GSH levels in the model group decreased but rebounded in the YQLSXJ group, suggesting that YQLXSJ can act on glutathione metabolism to alleviate oxidative stress and enhance endothelial function. It was proven that quercetin, a key ingredient of YQLXSJ, could neutralize ROS and promote GSH levels to protect against cardiovascular damage (Vanani et al. Citation2020).
Glycerophospholipid metabolism was proven to be relevant to vascular inflammation and injury. In this pathway, there are multiple enzymatic lipid metabolites, such as phosphatidylcholine (PC) and lyso-phosphatidylcholine (LPC), which play vital roles in inflammation and lipid oxidation (Lu et al. Citation2017). LPC, together with a proinflammatory factor, is the key constituent of oxidized low-density lipoprotein (ox-LDL). Under oxidative stress, the enzyme phospholipase A2 (PLA2) is activated. PC is converted into LPC via catalysis and degradation by PLA2. LPC shows a proatherogenic effect by promoting EC apoptosis and VSMC proliferation and migration to the endothelium and transforming lipids into foam cells (Schmitz and Ruebsaamen Citation2010). Interestingly, TNF signaling pathway could enhance the conversion of PC to LPC during glycerophospholipid metabolism (Su et al. Citation2022). In the model group, the level of LPC (16:1/0:0) increased, and that of PC (15:0/15:0) decreased. After treatment, the above two metabolites recovered to normal levels. It is thus suggested that YQLXSJ may inhibit VSMC proliferation and migration by decreasing PLA2 activity and decreasing LPC production through glycerophospholipid metabolism.
In addition, pyruvate and α-ketoglutarate levels were high in the model group. It was reported that the levels of plasma pyruvate were increased in patients suffering coronary artery bypass grafting (Santiago Hernandez et al. Citation2021). These organic acids participate in the TCA cycle, indicating that the effects of YQLXSJ should also be connected to energy metabolism.
Molecular docking predicts the interaction between a known small molecule compound with the selected target protein, and the binding energy indicates whether they could recognize each other to form a molecular complex, which allows prediction of a potential target. Our molecular docking results showed that the five key components of YQLXSJ and five core targets possessed good binding activities, indicating that they could form stable combinations. For example, the binding energy of quercetin to NOS3 was −8.9 kcal/mol, and quercetin formed hydrogen bonds with the residues Ser-102, Trp-447 and Ala-446. The stability of the quercetin-NOS3 complex was therefore verified. The binding modes of the other complexes gave similar results, and the key components were shown to form stable hydrogen bonds and hydrophobic interactions with the active sites of the core target proteins. It was preliminarily confirmed that our predicted targets were reliable, and the mechanism of the key components of YQLXSJ with the targets was clarified at the molecular level. Moreover, for the five core targets, the differences in expression levels were further validated by ELISA.
NOS2 is expressed in low levels in cells but is upregulated under inflammatory and oxidative stress conditions (Förstermann and Sessa Citation2012). Furthermore, the expression of NOS2 is relevant to pathological remodeling, especially in the cardiovascular system (Farah et al. Citation2018). NOS3 is mainly expressed in ECs. NO, produced via the decomposition of arginine by NOS3, diffuses to VSMCs to inhibit their proliferation. Secretory phospholipase A2 (sPLA2) is a precursor of LPC. PLA2G2A (sPLA2-IIA), the most abundant sPLA2 isoform, has proinflammatory and atherosclerotic effects. It was determined that elevated plasma PLA2G2A levels and activity were highly correlated with restenosis development after PTCA (Korotaeva et al. Citation2005). Moreover, previous studies reported that the serum PLA2G2A level improved in patients with unstable angina (UA) and AMI, and their prognosis were worse (Akinkuolie et al. Citation2019). ODC is the initial rate-limiting enzyme in polyamine synthesis and can participate in the control of cell proliferation by regulating intracellular polyamine levels (Pegg Citation2006). VEGFA (also called VEGF) is a crucial angiogenic factor. A prospective real-world study reported that 91.7% of patients with ISR caused by DES treatment displayed a significant increase in VEGF plasma levels (Katsaros et al. Citation2014). We obtained the same results. The mechanism may be that VEGF enhances the expression of monocyte chemoattractant protein-1 (MCP-1) via SMC VEGFR-2, thereby promoting monocyte aggregation and SMC proliferation and migration and resulting in neointimal hyperplasia (Simons Citation2009). Notably, the above five core target genes might be the crucial enzymes that regulate the differential metabolites.
Interestingly, the interactions between the key components of YQLXSJ and the core targets were mainly reflected in the aspects of anti-inflammation, antioxidative stress, antiplaque, and angiogenesis, which are closely related to the pathological processes of ISR. Quercetin plays an important anti-inflammatory role through classic pathways such as the nuclear factor kappa-B (NF-κB) signaling pathway and TNF signaling pathway. It was proven that quercetin reduced the activation of NF-κB in atherosclerotic rats and inhibited the expression of NOS2, thus reducing vascular inflammation (Bhaskar et al. Citation2016). Tanshinone IIa was reported to be involved in arachidonic acid metabolism by regulating PLA2G2A, while PLA2G2A can activate NF-κB (Lim et al. Citation2019). In terms of antioxidative stress, luteolin can directly act upon VECs, leading to rapid NOS3 activation and NO production, thereby protecting the vascular endothelium (Si et al. Citation2014). This is possible by the ROS-mediated downregulation of NF-κB, which ameliorates the suppression of NOS3 expression (Chen et al. Citation2020). VEGFs are potent angiogenic factors that can affect plaque neovascularization and may be associated with neoatherosclerosis after PCI. Tanshinone IIa can reduce the expression of VEGFA, perhaps by inhibiting angiogenesis in plaques through the VEGF/VEGFR-2 pathway.
In the present study, we used balloon catheters instead of stents to injure the artery. The degrees and mechanisms of restenosis between these two methods are slightly different. On the other hand, the indicators are inadequate. In future experiments, we will examine ductility based on the results and conduct in-depth research on the metabolites, targets and pathways.
Conclusions
This study confirmed the inhibitory effects of YQLXSJ on VSMC proliferation and intimal hyperplasia in balloon-injured aorta rats. Metabolomics analysis showed 18 differential metabolites, including glutamine, GSH and spermidine. Together with network pharmacology, these findings revealed that YQLXSJ mainly works on NOS2, NOS3, PLA2G2A, ODC1, and VEGFA through the active ingredients such as quercetin, luteolin, kaempferol, and tanshinone IIa. Finally, the potential mechanisms by which YQLXSJ reduces restenosis include regulating amino acid metabolism, lipid metabolism and energy metabolism, inhibiting inflammation and reducing oxidative stress. Our findings will provide a theoretical basis for the clinical application of YQLXSJ.
Author contributions
MTS performed experiments, data analysis, and wrote the manuscript. XL, GYQ and JX assisted in the experiments. WJ, CXY and FQ organized the validation. LW, WS, and HWB assisted in the data analysis. LQ and JWH contributed to conception and design of the study. All authors contributed to manuscript revision, read, and approved the submitted version.
Geolocation information
Anwai Xiaoguan Street No. 51, Chaoyang District, Beijing, China
Supplemental Material
Download MS Word (48.9 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All relevant data presented in the study are included in the supplementary material.
Additional information
Funding
References
- Akinkuolie AO, Lawler PR, Chu AY, Caulfield M, Mu J, Ding B, Nyberg F, Glynn RJ, Ridker PM, Hurt-Camejo E, et al. 2019. Group IIA secretory phospholipase A2, vascular inflammation, and incident cardiovascular disease. Arterioscler Thromb Vasc Biol. 39(6):1182–1190. doi: 10.1161/ATVBAHA.118.311894.
- Bettermann EL, Hartman TJ, Easley KA, Ferranti EP, Jones DP, Quyyumi AA, Vaccarino V, Ziegler TR, Alvarez JA. 2018. Higher Mediterranean diet quality scores and lower body mass index are associated with a less-oxidized plasma glutathione and cysteine redox status in adults. J Nutr. 148(2):245–253. doi: 10.1093/jn/nxx045.
- Bhaskar S, Sudhakaran PR, Helen A. 2016. Quercetin attenuates atherosclerotic inflammation and adhesion molecule expression by modulating TLR-NF-κB signaling pathway. Cell Immunol. 310:131–140. doi: 10.1016/j.cellimm.2016.08.011.
- Burtenshaw D, Kitching M, Redmond EM, Megson IL, Cahill PA. 2019. Reactive oxygen species (ROS), intimal thickening, and subclinical atherosclerotic disease. Front Cardiovasc Med. 6:89. doi: 10.3389/fcvm.2019.00089.
- Cassese S, Byrne RA, Schulz S, Hoppman P, Kreutzer J, Feuchtenberger A, Ibrahim T, Ott I, Fusaro M, Schunkert H, et al. 2015. Prognostic role of restenosis in 10 004 patients undergoing routine control angiography after coronary stenting. Eur Heart J. 36(2):94–99. doi: 10.1093/eurheartj/ehu383.
- Chen C, Xia J, Feng R, Wan J, Zhou K, Lin Q, Li D. 2021. Randomized controlled clinical study on Yiqi Liangxue Shengji prescription for intervention cardiac function of acute myocardial infarction with ischemia-reperfusion injury. Medicine (Baltimore). 100(10):e24944. doi: 10.1097/MD.0000000000024944.
- Chen HI, Hu WS, Hung MY, Ou HC, Huang SH, Hsu PT, Day CH, Lin KH, Viswanadha VP, Kuo WW, et al. 2020. Protective effects of luteolin against oxidative stress and mitochondrial dysfunction in endothelial cells. Nutr Metab Cardiovasc Dis. 30(6):1032–1043. doi: 10.1016/j.numecd.2020.02.014.
- Cui S, Li K, Ang L, Liu J, Cui L, Song X, Lv S, Mahmud E. 2017. Plasma phospholipids and sphingolipids identify stent restenosis after percutaneous coronary intervention. JACC Cardiovasc Interv. 10(13):1307–1316. doi: 10.1016/j.jcin.2017.04.007.
- Dagher O, Mury P, Thorin-Trescases N, Noly PE, Thorin E, Carrier M. 2021. Therapeutic potential of quercetin to alleviate endothelial dysfunction in age-related cardiovascular diseases. Front Cardiovasc Med. 8:658400. doi: 10.3389/fcvm.2021.658400.
- Farah C, Michel LYM, Balligand JL. 2018. Nitric oxide signalling in cardiovascular health and disease. Nat Rev Cardiol. 15(5):292–316. doi: 10.1038/nrcardio.2017.224.
- Feng J, Liu L, Yao F, Zhou D, He Y, Wang J. 2021. The protective effect of tanshinone IIa on endothelial cells: a generalist among clinical therapeutics. Expert Rev Clin Pharmacol. 14(2):239–248. doi: 10.1080/17512433.2021.1878877.
- Förstermann U, Sessa WC. 2012. Nitric oxide synthases: regulation and function. Eur Heart J. 33(7):829–837d. doi: 10.1093/eurheartj/ehr304.
- Giustino G, Colombo A, Camaj A, Yasumura K, Mehran R, Stone GW, Kini A, Sharma SK. 2022. Coronary in-stent restenosis: JACC State-of-the-Art Review. J Am Coll Cardiol. 80(4):348–372. doi: 10.1016/j.jacc.2022.05.017.
- Hasokawa M, Shinohara M, Tsugawa H, Bamba T, Fukusaki E, Nishiumi S, Nishimura K, Yoshida M, Ishida T, Hirata KI. 2012. Identification of biomarkers of stent restenosis with serum metabolomic profiling using gas chromatography/mass spectrometry. Circ J. 76(8):1864–1873. doi: 10.1253/circj.cj-11-0622.
- Infante T, Costa D, Napoli C. 2021. Novel insights regarding nitric oxide and cardiovascular diseases. Angiology. 72(5):411–425. doi: 10.1177/0003319720979243.
- Jia WH. 2020. P.R. China: Beijing University of Chinese Medicine. Expore the effect and mechanism of Yiqi Liangxue Shengji recipe on rabbit carotid artery after balloon injury based on NF-κB pathway. Ph.D, Chinese
- Jia WH, Wan J, Ma Z, Jin JL, Cai Y, Cui XY, Lin Q. 2021. Effects of Yiqi Liangxue Shengji prescription on TCM symptoms and syndrome elements after PCI. World J Integrated Tradit West Med. 16:229–232. Chinese
- Johnson CH, Ivanisevic J, Siuzdak G. 2016. Metabolomics: beyond biomarkers and towards mechanisms. Nat Rev Mol Cell Biol. 17(7):451–459. doi: 10.1038/nrm.2016.25.
- Kamishirado H, Inoue T, Sakuma M, Tsuda T, Hayashi T, Takayanagi K, Node K. 2007. Effects of statins on restenosis after coronary stent implantation. Angiology. 58(1):55–60. doi: 10.1177/0003319706295223.
- Katsaros KM, Kastl SP, Krychtiuk KA, Hutter R, Zorn G, Maurer G, Huber K, Wojta J, Christ G, Speidl WS. 2014. An increase of VEGF plasma levels is associated with restenosis of drug-eluting stents. EuroIntervention. 10(2):224–230. doi: 10.4244/EIJV10I2A36.
- Khaddaj Mallat R, Mathew John C, Kendrick DJ, Braun AP. 2017. The vascular endothelium: a regulator of arterial tone and interface for the immune system. Crit Rev Clin Lab Sci. 54(7-8):458–470. doi: 10.1080/10408363.2017.1394267.
- Kim B, Li J, Jang C, Arany Z. 2017. Glutamine fuels proliferation but not migration of endothelial cells. Embo J. 36(16):2321–2333. doi: 10.15252/embj.201796436.
- Korotaeva AA, Samoilova EV, Kaminny AI, Pirkova AA, Resink TJ, Erne P, Prokazova NV, Tkachuk VA, Chazov EI. 2005. The catalytically active secretory phospholipase A2 type IIA is involved in restenosis development after PTCA in human coronary arteries and generation of atherogenic LDL. Mol Cell Biochem. 270(1-2):107–113. doi: 10.1007/s11010-005-5266-3.
- Koyama S, Yamashita A, Matsuura Y, Saito Y, Maekawa K, Gi T, Kitamura K, Asada Y. 2021. Intracellular glutamine level determines vascular smooth muscle cell-derived thrombogenicity. Atherosclerosis. 328:62–73. doi: 10.1016/j.atherosclerosis.2021.05.012.
- Li DJ, Fu H, Tong J, Li YH, Qu LF, Wang P, Shen FM. 2018. Cholinergic anti-inflammatory pathway inhibits neointimal hyperplasia by suppressing inflammation and oxidative stress. Redox Biol. 15:22–33. doi: 10.1016/j.redox.2017.11.013.
- Li X, Kumar A, Carmeliet P. 2019. Metabolic pathways fueling the endothelial cell drive. Annu Rev Physiol. 81:483–503. doi: 10.1146/annurev-physiol-020518-114731.
- Li XX, Li LY, Fan ZJ, Cui J, Zhuang R, Liu RP, Wu Y. 2021. Effects of Yiqi Liangxue Shengji prescription on VEGF and hs-CRP in patients with coronary heart disease after PCI. J Tradit Chin Med and Pharm. 36:6898–6900. Chinese
- Lim Y, Hwang W, Kim JY, Lee CH, Kim YJ, Lee D, Kwon O. 2019. Synergistic mechanisms of Sanghuang-Danshen phytochemicals on postprandial vascular dysfunction in healthy subjects: a network biology approach based on a clinical trial. Sci Rep. 9(1):9746–9754. doi: 10.1038/s41598-019-46289-3.
- Liu L, Liu J, Gao Q, Wu Y, Lu J, Wan J, Li Y, Cui X, Zhou K, Jia W, et al. 2018. Effectiveness and safety of compound Chinese medicine plus routine western medicine in in-stent restenosis: a meta-analysis and systematic review. Evid Based Complement Alternat Med. 2018:6207524. doi: 10.1155/2018/6207524.
- Lu J, Chen B, Chen T, Guo S, Xue X, Chen Q, Zhao M, Xia L, Zhu Z, Zheng L, et al. 2017. Comprehensive metabolomics identified lipid peroxidation as a prominent feature in human plasma of patients with coronary heart diseases. Redox Biol. 12:899–907. doi: 10.1016/j.redox.2017.04.032.
- Lu M, Luo Y, Hu P, Dou L, Huang S. 2018. Tanshinone IIA inhibits AGEs-induced proliferation and migration of cultured vascular smooth muscle cells by suppressing ERK1/2 MAPK signaling. Iran J Basic Med Sci. 21:83–88.
- Ma Z, Long J, Jia WH, Cui XY, Li Y, Lu JJ, Li D, Wan J, Lin Q. 2019. Randomize-controlled trial of Yiqi Liangxue Shengji formula in preventing major adverse cardiovascular events after percutaneous coronary intervention for coronary heart disease. J Tradi Chin Med. 60:1837–1842. Chinese
- Matuz-Mares D, Riveros-Rosas H, Vilchis-Landeros MM, Vázquez-Meza H. 2021. Glutathione participation in the prevention of cardiovascular diseases. Antioxidants (Basel). 10:1220–1238. doi: 10.3390/antiox10081220.
- Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet J-P, Falk V, Head SJ, et al. 2019. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 40(2):87–165. doi: 10.1093/eurheartj/ehy394.
- Pegg AE. 2006. Regulation of ornithine decarboxylase. J Biol Chem. 281(21):14529–14532. doi: 10.1074/jbc.R500031200.
- Santiago Hernandez A, Martinez PJ, Agudiez M, Heredero A, Gonzalez-Calero L, Yuste-Montalvo A, Esteban V, Aldamiz Echevarria G, Martin Lorenzo M, Alvarez Llamas G. 2021. Metabolic alterations identified in urine, plasma and aortic smooth muscle cells reflect cardiovascular risk in patients with programmed coronary artery bypass grafting. Antioxidants. 10:1369–1379. doi: 10.3390/antiox10091369.
- Schmitz G, Ruebsaamen K. 2010. Metabolism and atherogenic disease association of lysophosphatidylcholine. Atherosclerosis. 208(1):10–18. doi: 10.1016/j.atherosclerosis.2009.05.029.
- Si H, Wyeth RP, Liu D. 2014. The flavonoid luteolin induces nitric oxide production and arterial relaxation. Eur J Nutr. 53(1):269–275. doi: 10.1007/s00394-013-0525-7.
- Simons M. 2009. VEGF and restenosis: the rest of the story. Arterioscler Thromb Vasc Biol. 29(4):439–440. doi: 10.1161/ATVBAHA.109.183970.
- Stefanini GG, Holmes DR. 2013. Drug-eluting coronary-artery stents. N Engl J Med. 368(3):254–265. doi: 10.1056/NEJMra1210816.
- Su D, Liao L, Zeng Q, Liao Z, Liu Y, Jin C, Zhu G, Chen C, Yang M, Ai Z, et al. 2022. Study on the new anti-atherosclerosis activity of different Herba patriniae through down-regulating lysophosphatidylcholine of the glycerophospholipid metabolism pathway. Phytomedicine. 94:153833. doi: 10.1016/j.phymed.2021.153833.
- Su F, Sun YN, Cui XY, Lin Q. 2015. The effects of Yiqi Liangxue Shengji Formula on cell morphology of injured vascular endothelial cells. J Tradi Chin Med. 56:1691–1694. Chinese
- Tang WHW, Wang Z, Cho L, Brennan DM, Hazen SL. 2009. Diminished global arginine bioavailability and increased arginine catabolism as metabolic profile of increased cardiovascular risk. J Am Coll Cardiol. 53(22):2061–2067. doi: 10.1016/j.jacc.2009.02.036.
- Vanani AR, Mahdavinia M, Shirani M, Alizadeh S, Dehghani MA. 2020. Protective effects of quercetin against oxidative stress induced by bisphenol – a in rat cardiac mitochondria. Environ Sci Pollut Res Int. 27(13):15093–15102. doi: 10.1007/s11356-020-08048-0.
- Yang X, Yang Y, Guo J, Meng Y, Li M, Yang P, Liu X, Aung LHH, Yu T, Li Y. 2021. Targeting the epigenome in in-stent restenosis: from mechanisms to therapy. Mol Ther Nucleic Acids. 23:1136–1160. doi: 10.1016/j.omtn.2021.01.024.
- Yang YF, Yan XR, Wu RX, Li N, Chu M, Dong Y, Fu SP, Shi JR, Liu Q. 2022. Network pharmacology and experimental evidence reveal the protective mechanism of Yi-Qi Cong-Ming decoction on age-related hearing loss. Pharm Biol. 60(1):1478–1490. doi: 10.1080/13880209.2022.2101671.
- Zhang W, Chen Y, Jiang H, Yang J, Wang Q, Du Y, Xu H. 2020. Integrated strategy for accurately screening biomarkers based on metabolomics coupled with network pharmacology. Talanta. 211:120710. doi: 10.1016/j.talanta.2020.120710.
- Zou G, Zhu J, Liu Z, Wu L, Xu R, Chen H, Deng P, Deng C. 2019. Detoxification and activating blood circulation decoction reduces restenosis involving the TLR4/NF-κB pathway after balloon injury. Prostaglandins Other Lipid Mediat. 140:1–8. doi: 10.1016/j.prostaglandins.2018.11.002.