Abstract
Context
Styrax is used for prevention and treatment of cerebrovascular diseases. However, the underlying mechanism remains unclear.
Objective
To elucidate styrax’s anti-ischemic stroke protective effects and underlying mechanisms.
Materials and methods
An ischemic-stroke rat model was established based on middle cerebral artery occlusion (MCAO). Sprague-Dawley rats were randomly assigned to the following groups (n = 10) and administered intragastrically once a day for 7 consecutive days: sham, model, nimodipine (24 mg/kg), styrax-L (0.1 g/kg), styrax-M (0.2 g/kg) and styrax-H (0.4 g/kg). Neurological function, biochemical assessment, and ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry (UPLC-Q/TOF-MS)-based serum metabonomics were used to elucidate styrax’s cerebral protective effects and mechanisms. Pearson correlation and western blot analyses were performed to verify.
Results
The addition of 0.4 g/kg styrax significantly reduced cerebral infarct volume and neurobehavioral abnormality score. Different doses of styrax also decrease MDA, TNF-α, IL-6, and IL-1β, and increase SOD and GSH-Px in ischemic-stroke rats (p < 0.05; MDA, p < 0.05 only at 0.4 g/kg dose). Biochemical indicators and metabolic-profile analyses (PCA, PLS-DA, and OPLS-DA) also supported styrax’s protective effects. Endogenous metabolites (22) were identified in ischemic-stroke rats, and these perturbations were reversible via styrax intervention, which is predominantly involved in energy metabolism, glutathione and glutamine metabolism, and other metabolic processes. Additionally, styrax significantly upregulated phosphorylated AMP-activated protein kinase and glutaminase brain-tissue expression.
Conclusion
Styrax treatment could ameliorate ischemic-stroke rats by intervening with energy metabolism and glutamine metabolism. This can help us understand the mechanism of styrax, inspiring more clinical application and promotion.
Introduction
Stroke is the second leading cause of death and the third leading cause of disability worldwide (GBD 2016 DALYs and HALE Collaborators Citation2017). What is more alarming is that China has the highest stroke incidence and mortality rates globally, with approximately one million new patients diagnosed with ischemic stroke annually (Sun et al. Citation2019), and the number is constantly increasing. Various processes are considered to contribute to ischemic stroke damage, including excitotoxicity, acid toxicity, ionic imbalance, apoptosis, oxidative/nitrative stress, and inflammation (Brouns and De Deyn Citation2009). In most instances, the blockade of these pathways leads to the protection of cellular functions. To date, although a variety of well-known drugs (e.g., anticoagulants, antiplatelet drugs, free radical scavengers, and neuroprotective agents) are used in stroke therapy, their side effects and unknown mechanisms of interaction present an immense challenge. Thrombolysis remains the most effective treatment for ischemic stroke, as it attempts to dissolve blood clots, restore blood flow, and protect the surrounding brain tissue (Berkhemer et al. Citation2015). However, this strategy is only effective if implemented within a narrow time window. Urgent unmet needs exist in developing a range of neuroprotective strategies for restraining the damage that occurs hours or days after a stroke.
Numerous studies have suggested that traditional Chinese medicine (TCM) has been used for thousands of years to treat various diseases and disorders. Noteworthily, TCM medications are usually prescribed as a combination of multiple herbs to treat stroke and neurological disorders (Chen et al. Citation2009; Liu et al. Citation2018; Xu et al. Citation2018; Xu et al. Citation2019). Several Chinese patent drugs have been approved and are widely used in clinical practice for the treatment and prevention of ischemic stroke (Wu et al. Citation2007), including styrax pills (Su-Hexiang pill), which contain various traditional Chinese herbs, among which styrax plays a major role in their physiological effects (Zhang et al. Citation2019). Styrax (Su-Hexiang), a natural resin extracted from the wounded bark of Liquidambar orientalis Mill. (Altingiaceae), is one of the main components and the oldest traditional medicine used in India and East Asia for the treatment of cerebrovascular and cardiovascular disorders (Zhang et al. Citation2019; Xu et al. Citation2021; Zhang et al. Citation2022). Gas chromatography–mass spectrometry (GC-MS) analysis of styrax revealed that its chief components were free cinnamic acid; cinnamyl cinnamate; phenylpropyl cinnamate; styrene, a resin containing terpenoids (caryophyllene and caryophyllene oxide); and cinnamic acid esters (Kim et al. Citation2008; Zhang et al. Citation2019; Xu et al. Citation2021). Notably, studies have shown that styrax inhibits inflammatory responses and neuronal apoptosis (Zhang et al. Citation2019; Xu et al. Citation2021; Li et al. Citation2022). Furthermore, styrax treatment after focal ischemic stroke improves long-term neurological outcomes, reduces cerebral infarct volume, and inhibits caveolae-mediated transcytosis in the blood-brain barrier (Zhou et al. Citation2021, Citation2022). However, the exact mechanisms underlying the cerebral protective effects of styrax at the metabolite level are yet to be elucidated.
Metabonomics is a valuable tool for the comprehensive analysis of all small molecules metabolized within a given biological system and has been extensively used in drug discovery and redevelopment (Mu et al. Citation2017; Weng et al. Citation2019; Chen et al. Citation2022). Since TCM is based on a ‘holistic’ philosophical view, metabonomic techniques possibly provide valuable information regarding TCM from a philosophical standpoint. In this study, a metabonomic approach based on ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry (UPLC-Q/TOF-MS) coupled with integrative pharmacology was used to explore the biochemical changes triggered by middle cerebral artery occlusion (MCAO) in rats and the cerebral protective effects of styrax. The mechanism behind styrax’s concentration-response relationship is potentially revealed from the perspective of metabolic regulation. This study investigates changes in serum metabolites in ischemic stroke rats and the effect of styrax on metabolic pathways and new metabolite production.
Materials and methods
Experimental drugs and reagents
Styrax (Latin: storax; Greek: στύραξ, stúrax), also known as Storax or Su-Hexiang, is a natural resin. In this study, refined styrax was purchased from Shanghai Lei Yun Shang Pharmaceutical Co., Ltd. (Batch No. 2012120, date of purchase: 20180927, Shanghai, China) and conformed to the standard of the China Pharmacopeia (version 2020).
2,3,5-Triphenyltetrazolium chloride (TTC) was obtained from Sigma (St. Louis, MO, USA). Superoxide dismutase (SOD), malondialdehyde (MDA), glutathione peroxidase (GSH-Px), citrate synthase (CS), glutamine synthetase (GS), and glutaminase (GLS) were acquired from Jiancheng Biological Engineering Institute (Nanjing, China), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and interleukin-1β (IL-1β) were obtained from Shanghai Westang Bio-tech Co., Ltd. (Shanghai, China). We obtained nimodipine tablets from Bayer Healthcare Company Ltd. (Batch No. BJ29160, Leverkusen, Germany). The antibodies for phosphorylated AMP-activated protein kinase (p-AMPK), AMP-activated protein kinase (AMPK), and β-actin were obtained from Cell Signaling Technologies, Inc. (Danvers, MA, USA), the antibody of glutaminase 1 (GLS1) was obtained from Beijing Biosynthesis Biotechnology Co., Ltd. (Beijing, China). A secondary antibody for anti-rabbit IgG-B (Cat. No.sc-53804) was acquired from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Animal treatment
Sprague-Dawley male rats weighing 250 ± 20 g were acquired from the laboratory animal center of the Academy of Fourth Military Medical University [the corresponding qualified production number was SCXK-(Shan)-2014-002]. In accordance with the ARRIVE guidelines and the NIH Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978), all procedures involving animals were approved by the Animal Experimentation Ethics Committee of the Fourth Military Medical University [No. IACUC20220196]. All rats were kept in a specific pathogen-free colony of the laboratory (23 ± 2 °C), humidity (55 ± 4%), a 12 h light/dark cycle, and free access to pure water and standard rat chow. After acclimatization of two weeks, they were randomly divided into the following six groups (n = 10): sham operation group, model group, nimodipine group (as positive group, 24 mg/kg/d), low dosage group of styrax (styrax-L, 0.1 g/kg), medium dosage group of styrax (styrax-M, 0.2 g/kg) and high dosage group of styrax (styrax-H, 0.4 g/kg).
Before surgery for MCAO-induced ischemic stroke, the rats were kept without food for 12 h, but allowed access to water. Anesthesia was achieved by using 2.0% to 3.0% isoflurane, followed by a maintenance dose of 1.0% to 1.5% isoflurane (both in 70% N2O/30% O2). The procedures on the MCAO model rats were carried out in accordance with the operating procedure described previously (Ding et al. Citation2014; Liu et al. Citation2018). Induced ischemia for 3 h was followed by slow filament removal and neck incision closure (Liu et al. Citation2018). Changes in rat behavior were used to judge the successful establishment of the model, such as the inability to stretch the contralateral forelimb or to chase the tail to the opposite side while walking (Han et al. Citation2020). Rats in the sham group received no filament insertion and otherwise the same surgical procedures as the model group. After surgery, rats were allowed free access to water and food. Specifically, the sham and model group were intragastrically administered 0.25% Tween-80 solution, and the positive group (nimodipine-treated group) was intragastrically administered nimodipine for 7 days (24 mg/kg/d), while three treatment groups were intragastrically administered styrax for 7 days (0.1, 0.2 and 0.4 g/kg, respectively).
Sample collection
After 7 days, all the rats were sacrificed by deeply anaesthetized with 5% isoflurane and euthanized by cervical dislocation, and whole blood samples were collected from the abdominal aorta, allowed to clot for 30 min, and then centrifuged at 3500 rpm for 10 min at 4 °C. The supernatant was carefully removed with a pipette, dispensed into new EP tubes, and then stored at −20 °C for approximately 20 min before being transferred to −80 °C for storage. Moreover, the brain was removed immediately after the sacrifice of the rats, and the tissue was rinsed with a saline buffer for further analysis.
Measurement of cerebral edema and infarct volume
Six coronal sections of the rat brain were cut at a thickness of 2 mm, stained with 0.5% TTC for 15 min at 37 °C, and then fixed with 4% polyformaldehyde solution overnight at 4 °C. By staining, normal tissue was rendered rose red and infarcted tissue white. Additionally, numeric images of the infarct volumes were taken, and each slice’s infarct volume was calculated by multiplying the infarct area by the thickness, with the sum of infarct volumes of all brain slices being used to determine the total infarct volume.
Evaluation of neurobehavioral abnormality
All rats were weighed and scored for neurobehavioral abnormality score on the 7th day after gavage administration. Neurological function was evaluated according to the Zea Longa standard by an observer blinded to the experimental groups with a 5-point scale system as follows: 0 points, no motor deficits (normal); 1 point, forelimb weakness and torso turning to the ipsilateral side when held by tail (mild); 2 point, circling to the contralateral side but normal posture at rest (moderate); 3 point, falling over to on the affected side at rest (severe); 4 point, no spontaneous locomotor activity or unable to walk (critical) (Longa et al. Citation1989). At 0 h after reperfusion rats dying were rejected, and other rats were recruited. The score was calculated for each rat within 1 min and it was repeated three times.
Assessment of histopathological damages
The brains were removed and fixed with 4% paraformaldehyde for 24 h. The brain tissue was embedded in paraffin and then cut into sections (5 μm thickness). The sections were stained with hematoxylin and eosin (H&E) for histopathological observation.
Assessment of oxidative stress and Inflammation
The serum samples were taken out from the −80 °C freezer and thawed. Antioxidant properties of styrax treatment were evaluated by analyzing SOD, MDA and GSH-Px activities and the contents of IL-1β, IL-6, and TNF-α in serum. The TNF-α or IL-6 protein standards with an ELISA reader (Model ELX800, BioTek, USA). The detailed experimental manipulations were carried out according to the manufacturer’s instructions. Further, the key pathway was validated using commercial kits (ATP, Ca++-ATPase, Na+K+-ATPase, Ca++Mg++-ATPase, Caspase-3, CS, GS, and GLS).
Preparation of metabonomic samples
Serum samples were thawed on ice at 4 °C for 30–60 min prior to analysis. Aliquot 100 µL of serum into a microcentrifuge tube labeled 1.5 mL, add 160 µL of internal standard solution, and then 140 µL of methanol. Mix thoroughly for 15 s on a vortex mixer and centrifuge the protein precipitate pellet for 10 min at 4 °C and 12,000 rpm. Transferring 100 µL of supernatant into a 200 µL vial insert for metabonomic analysis. Also, aliquots of 10 µL of each serum sample were mixed as quality controls (QCs) to determine whether the method and system were stable.
UPLC-Q/TOF-MS analysis
The analysis was performed on a Waters ACQUITY UPLC CSH C18 column (2.1 mm × 100 mm, 1.7 µm, UK) with chromatographic separation at 45 °C. The mobile phase A was acetonitrile/water (60:40) and mobile phase B was isopropanol/acetonitrile (10:90), where both A and B contained 10 mmol/L ammonium acetate and 0.1% formic acid. The optimized gradient conditions were as follows: 0.0–10.0 min, 20% B; 10.0–16.0 min, 20.0–100.0% B; 16.0–18.0 min, 100.0% B; 18.0–20.0 min, 100–20% B; 20.0–22.0 min, 20% B, with a flow rate of 300 µL/min and an injection volume of 1.0 µL each.
Mass spectrometry was performed on a Waters Xevo G2-XS Q/TOF mass spectrometer instrument equipped with a HESI-II probe. The positive and negative ion source voltages were 3.7 kV and 3.5 kV, respectively. The capillary heating temperature was 320 °C, and the vaporizer heating temperature was 300 °C. The sheath gas and the auxiliary gas were nitrogen with a sheath gas pressure of 30 psi and auxiliary gas pressure of 10 psi. The collision gas was nitrogen with a pressure of 1.5 mTorr. The full mass scan parameters were as follows: resolution of 70,000, maximum isolation time of 50 ms, auto gain control target under 1 × 106, and m/z range of 150–1500. The calibration was modified to maintain a mass tolerance of 5 ppm for Q Exactive. Accordingly, the dd-MS2 scan parameters were as follows: resolution of 17,500, maximum isolation time of 50 ms, auto gain control target under 1 × 105, loop count of the top 10 peaks, isolation window of m/z 2, normalized collision energy of 30 v and intensity threshold of 1 × 105. In addition, to provide robust quality assurance for each metabolic feature, seven injections of the QCs sample were performed throughout the analytical run and one injection was analyzed every tenth analytical run.
Western blotting analysis
Total proteins were extracted from the brain tissue, and the concentrations were determined according to the bicinchoninic acid method. Electrophoretic fractionation was performed with 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The separated proteins were transferred to PVDF membranes, blocked with 5% nonfat dried milk for 1 h at 37 °C, washed three times with Tween-Tris-buffered saline, and incubated overnight at 4 °C with primary antibodies (p-AMPK, AMPK, GLS1, and β-actin) diluted 1:1000. After washing three times as described above, incubated with secondary antibody (1:5000) at 37 °C. Finally, a chemiluminescence-based method was used to detect immunoreactive bands, followed by semi-quantification using an Image J software program (NIH, Bethesda, MD, USA).
Statistical analysis
After raw data were converted to mzXML format by ProteoWizard software, peak detection, extraction, alignment, and integration were performed using an in-house written R program package (core XCMS). The data preprocessed were then introduced to SIMCA 14.1 (Umetrics AB, Umea, Sweden), which was used to perform principal component analysis (PCA), partial least squares discriminant analysis (PLS-DA), and orthogonal partial least squares discriminant analysis (OPLS-DA) after mean centering and pareto-scaling. We analyzed metabolic peaks with MSE (E represents collision energy) or interpreted their significance with available biochemical databases, such as HMDB (http://www.hmdb.cn), ChemSpider (http://www.chemspider.com), KEGG (http://www.kegg.com) and Lipidmaps (http://www.lipidmaps.org). Variable importance in the projection (VIP) greater than 1 influenced classification more strongly, which can be used to select potential biomarkers. Owing to the possible fragmentation mechanism, items without a given mass fragmentation information were removed from the biomarker candidate list in this study, and only the most likely items were retained. In addition, MetaboAnalyst 5.0 (McGill University, Montreal, QC, Canada) and KEGG were used for the enrichment analysis of metabolic pathways.
All continuity variable values were expressed as mean ± standard deviation. The difference among the groups was compared primarily using one-way ANOVA followed by Tukey’s post hoc test. Rank data (e.g., neurobehavioral abnormality score) was analyzed using the Kruskal-Wallis test, followed by Dunn’s test for multiple comparisons (GraphPad Prism 9.0, La Jolla, CA, USA). A critical p value of < 0.05 was considered as a statistical difference. Meanwhile, Pearson correlation analysis was performed using SPSS 23.0 program (IBM, NY, USA). A correlation coefficient r with an absolute value > 0.7 was considered as a strong correlation.
Results
MCAO-induced neuronal injury assessment
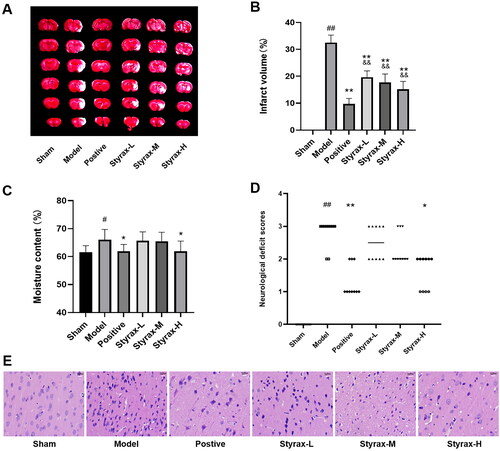
The results of infarct volume, degree of brain moisture, neurobehavioral abnormality score, and histopathological changes are shown in . When compared with the sham group, it significantly increased the cerebral infarct volume, moisture content, and neurobehavioral abnormality score in the model group (p < 0.05, p < 0.01), and its effect was significantly attenuated in the positive and styrax treatment groups compared to the model group (p < 0.05, p < 0.01). Treatment with styrax ameliorated histopathological damage, and the high-dose group exhibited fewer pathological changes. On 7th day, the mortality rates of the vehicle-treated rats of the MCAO group and styrax-H-treated rats were 30% and 15%, respectively. These results prove that styrax can attenuate cerebral ischemic injury, especially in high doses.
Figure 1. Anti-ischemic cerebral effects of different doses of styrax in MCAO rats (n = 10). (A) TTC staining of the brain slices. (B) Quantitative analysis of the infarct volume. (C) The results of cerebral infarct area moisture content. (D) Neurobehavioral abnormality score. (E) H&E staining of ischemic cortex. #p < 0.05, ##p < 0.01 vs. Sham group; *p < 0.05, **p < 0.01 vs. Model group; &&p < 0.01 vs. Positive group.
Assessment of oxidative stress and inflammation
The levels of the most important antioxidant enzymes and anti-inflammatory indicators were significantly altered in the model group compared with those in the sham group (p < 0.01). However, the indicators returned to near-sham-operated levels after styrax administration. Styrax significantly reduced the concentrations of MDA, TNF-α, IL-6, and IL-1β, and increased the activity of SOD and GSH-Px (p < 0.05, p < 0.01), as shown in . These results indicate that styrax potentially alleviates cerebral ischemic injury by exerting antioxidant and anti-inflammatory effects.
Table 1. Biochemical indicator levels of serum in different groups.
Method development and validation
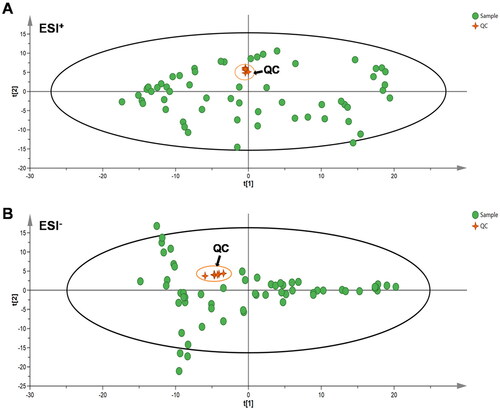
As evidenced by the acquisition of UPLC-QTOF/MS-based data in positive and negative ion modes, the QC samples exhibited favorable overlap (), and the relative standard deviations of the retention time and peak area were smaller than 0.85% and 4.2%, respectively, indicating that the instrument was stable during the sequence operation, and the reproducibility and precision of the method were satisfactory. These results indicate that the experimental method was suitable for the detection of serum samples.
Metabolomic profiling and multivariate data analysis
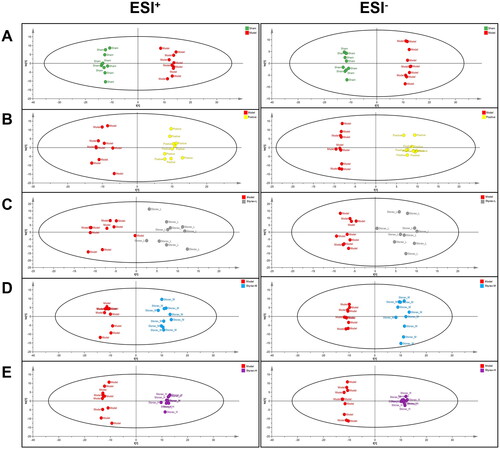
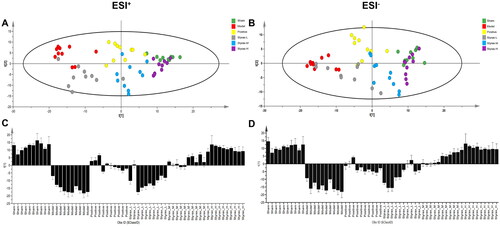
To determine styrax’s ability to influence the metabolic pattern of MCAO rats and identify metabolites that change significantly in concentration (i.e., potential biomarkers), multivariate data-analysis techniques were applied to the UPLC-Q/TOF-MS analysis data of serum samples in the positive and negative ion modes, and they included PCA, PLS-DA, and OPLS-DA. As shown in , the score plot of the PLS-DA revealed that the sham, model, positive, and styrax groups were clearly separated, while the styrax-L, styrax-M, and styrax-H groups were located between the model and sham groups, suggesting that their metabolic profiles had altered and styrax had potentially resolved the pathological process of MCAO-induced cerebral ischemic injury.
Figure 3. Differential metabolic profiles in rat serum among the sham, model, positive and styrax groups (n = 10). (A) 2D PLS-DA score plot in the ESI+ mode. (B) 2D PLS-DA score plot in the ESI- mode. (C) PLS-DA scores column plot in the ESI+ mode. (D) PLS-DA scores column plot in the ESI– mode. PLS-DA, partial least squares discriminant analysis.
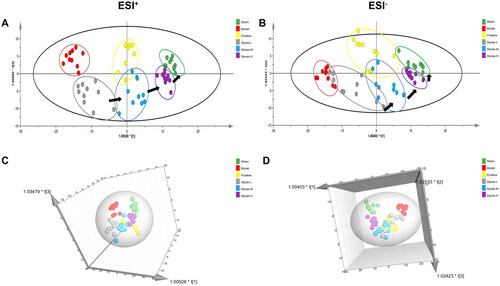
In addition, OPLS-DA was applied to maximize the difference between the groups ( and ). The results suggest that the MCAO model was successful and that styrax had a dose-dependent protective effect in MCAO rats. In addition, the styrax-H group was closer to the sham group than both the styrax-M and styrax-L groups along the first principal component, implying that styrax-H was more effective in treating MCAO-induced cerebral ischemic injury than styrax-M and styrax-L, exhibiting consistency with the results of previous pharmacodynamic evaluations and biochemical assessments ( and ).
Figure 4. Distinct metabolic profiles of OPLS-DA models in rat serum among the sham, model, positive, and styrax groups (n = 10). (A) 2D score plot in the ESI+ mode. (B) 2D score plot in the ESI- mode. (C) 3D score plot in the ESI+ mode. (D) 3D score plot in the ESI– mode. OPLS-DA, orthogonal partial least squares discriminant analysis; 2D: two dimensional; 3D: three dimensional.
Identification of potential biomarkers
As observed in the score plot created by the OPLS-DA model, all sample scatterers could be clearly classified into six groups, indicating that the pattern accurately explained the metabolite changes in the different groups and that their serum metabolic patterns were significantly altered after nimodipine and styrax treatment. Typically, OPLS-DA was also established to identify individual differences and potential biomarkers that effectively reflect the differences among the sham, model, and different-dose styrax groups. Finally, 22 potential MCAO-related metabolites were identified according to a VIP > 1.0 and p < 0.05. To identify these metabolites, candidate metabolites were searched from MSE data as well as the ChemSpider, HMDB, and KEGG databases. By comparing the retention times and mass spectra of the authentic chemicals, some of the metabolites associated with ischemic stroke were identified, including arachidonic acid, leukotriene A4, l-lactic acid, citric acid, fumaric acid, oxoglutarate, succinic acid, 3-hydroxybutyric acid, palmitic acid, stearic acid, glutathione, l-glutamine, pyroglutamic acid, l-isoleucine, 2-ketobutyric acid, lysoPC [20:4(5Z,8Z,11Z,14Z)], lysoPC (18:0), lysoPC (16:0), lysoPE [0:0/20:2(11Z,14Z)], lysoPE [0:0/22:4(7Z,10Z,13Z,16Z)], lysoPE [24:6(6Z,9Z,12Z,15Z,18Z,21Z)/0:0], and cholesterol acetate. The results are shown in .
Table 2. Characterization and trends of metabolites as potential biomarkers in serum profiles.
Nimodipine is an l-type calcium channel antagonist that reduces excessive calcium influx during pathological conditions, contributing to its neuroprotective properties, it has been reported that nimodipine may play a protective role in the brain by regulating energy metabolism (Chen Y et al. Citation2018). Therefore, nimodipine was used as a positive control group in this study. According to , there were 15 significantly changed metabolites between nimodipine-treated rats and model rats. Increased consumption of citric acid, fumaric acid, l-isoleucine, lysoPC (18:0), lysoPC (16:0), lysoPE [0:0/20:2(11Z,14Z)], lysoPE [0:0/22:4 (7Z,10Z,13Z,16Z)], lysoPE [24:6(6Z,9Z,12Z,15Z,18Z,21Z)/0:0] and cholesterol acetate, together with reduced excretion of arachidonic acid, leukotriene A4, l-lactic acid, 3-hydroxybutyric acid, palmitic acid and stearic acid were found. In addition, there were 22 significantly changed metabolites between styrax-treated rats and model rats, indicating that the characterized metabolites were partially altered from normal with high-dose styrax treatment. The specific change trends are shown in .
Metabolic pathway and function analysis
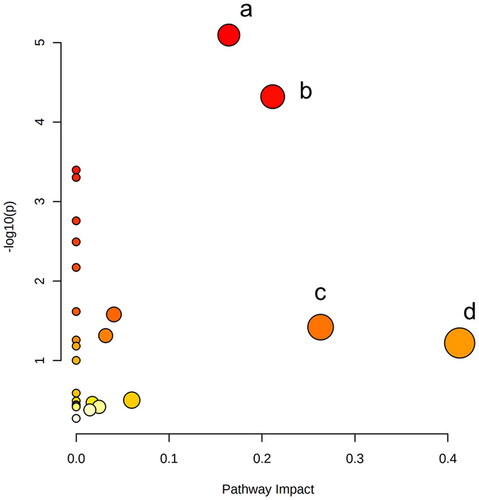
To gain a deeper understanding of the potential biomarkers, MCAO-associated metabolic perturbations were examined from a pathway analysis perspective, and 10 disturbed metabolic pathways were identified by topology analysis using MetaboAnalyst 5.0. Moreover, four important metabolic pathways, including alanine metabolism, aspartate arid glutamate metabolism, the citrate cycle, glutathione metabolism, and arachidonic acid metabolism, were revealed by filtering for pathway impact values ≥0.1 ().
Correlation analysis of potential biomarkers and biochemical indicators
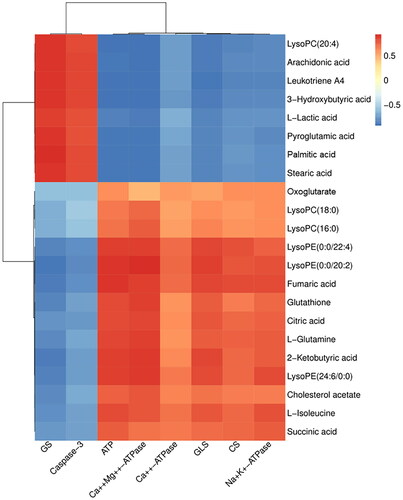
To further investigate styrax’s overall mode of action in MCAO rats, we conducted an association analysis and mechanism study of the high-dose styrax group, which demonstrated the greatest efficacy. As shown in , compared with those in the sham group, the levels of arachidonic acid, leukotriene A4, l-lactic acid, 3-hydroxybutyric acid, palmitic acid, stearic acid, pyroglutamic acid, and lysoPC [20:4(5Z,8Z,11Z,14Z)] were increased, whereas those of citric acid, fumaric acid, oxoglutarate, succinic acid, glutathione, l-glutamine, l-isoleucine, 2-ketobutyric acid, lysoPC (18:0), lysoPC (16:0), lysoPE [0:0/20:2(11Z,14Z)] lysoPE [0:0/22:4(7Z,10Z,13Z,16Z)], lysoPE [24:6 (6Z,9Z,12Z,15Z,18Z,21Z)/0:0], and cholesterol acetate were reduced. However, when MCAO rats were administered styrax, the above metabolites shifted to near-normal levels. In addition, the results of the biochemical indicators are shown in , the levels of caspase-3 and GS were significantly elevated in the model group compared with those in the sham group, whereas those of energy-related indicators, such as adenosine triphosphate (ATP), Ca++Mg++-ATPase, Na+K+-ATPase, and Ca++-ATPase, were significantly decreased (p < 0.01). However, when MCAO rats were administered styrax, the levels of the above biochemical indicators were significantly reversed (p < 0.01).
Figure 7. Box plots of the normalized relative contents of these metabolites among the sham, model, and styrax-H groups (n = 10). #p < 0.01 vs. Sham group; *p < 0.01 vs. Model group.
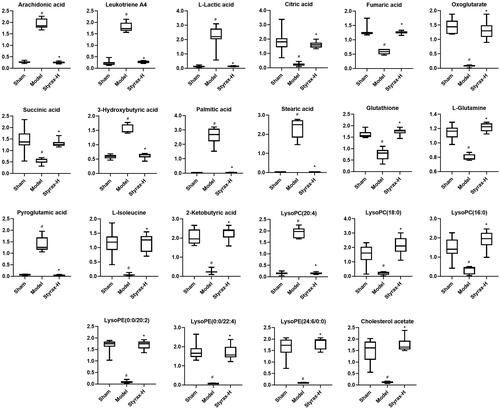
Figure 8. Biochemical indicators of brain tissue among the sham, model, and styrax-H groups (n = 10). #p < 0.01 vs. Sham group; *p < 0.01 vs. Model group.
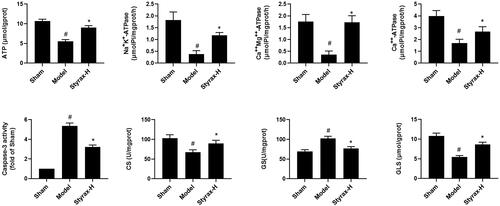
A correlation matrix was generated by calculating Pearson’s correlation coefficient to investigate the relationship between potential biomarkers and biochemical efficacy indicators. When the absolute value of the correlation coefficient, r, exceeded 0.7, the correlation was considered strong. As shown in , the levels of ATP, Ca++Mg++-ATPase, Na+K+-ATPase, GLS, and CS exhibited a significantly positive association with the metabolites lysoPE [0:0/20:2(11Z,14Z)], lysoPE [0:0/22:4 (7Z,10Z,13Z,16Z)], lysoPE [24:6(6Z,9Z,12Z,15Z,18Z,21Z)/0:0], fumaric acid, glutathione, citric acid, l-glutamine, 2-ketobutyric acid, l-isoleucine, and succinic acid (r > 0.7). An evidently negative correlation was observed between the biochemical indicators (ATP, Na+K+-ATPase, Ca++Mg++-ATPase) and metabolic substance {lysoPC [20:4(5Z,8Z,11Z,14Z)], arachidonic acid, leukotriene A4, l-lactic acid, 3-hydroxybutyric acid, palmitic acid, stearic acid, and pyroglutamic acid} (r < − 0.7). Moreover, GS and caspase-3 levels demonstrated a significantly positive association with the metabolites of lysoPC [20:4(5Z,8Z,11Z,14Z)], arachidonic acid, leukotriene A4, 3-hydroxybutyric acid, l-lactic acid, pyroglutamic acid, palmitic acid, and stearic acid (r > 0.8). The analysis of these intrinsic relationships is considered to have important implications for the understanding of cerebral ischemic injury, mainly in relation to energy metabolism and glutamine metabolism.
Western blot validation
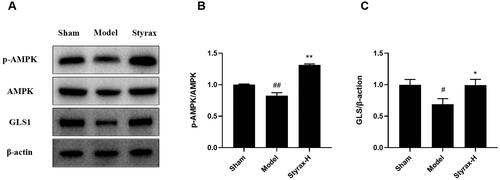
To verify whether styrax’s cerebral protective effect on MCAO injury was mediated by improving energy metabolism and glutamine metabolism, we detected the protein expression of p-AMPK, AMPK, and GLS by western blotting. As shown in , no significant difference in the AMPK-expression level was noted between the groups; however, the styrax group demonstrated significantly upregulated p-AMPK/AMPK expression levels compared with the model group (p < 0.01). Moreover, the results demonstrate that the GLS expression level was reduced in the model group compared with that in the sham group (p < 0.05). The styrax group exhibited significantly increased GLS expression compared with the model group (p < 0.05).
Figure 10. Validation of the effect of styrax on AMPK/GLS pathway in rat brain tissue based on Western blot (n = 3). (A) representative protein levels of p-AMPK, AMPK, GLS, and β-actin. (B) Semi-quantification of the density ratio of p-AMPK/AMPK. (C) Semi-quantification of the density ratio of GLS/β-actin. The graph outlines their relative levels, #p < 0.05, ##p < 0.01 vs. Sham group; *p < 0.05, **p < 0.01 vs. Model group.
Discussion
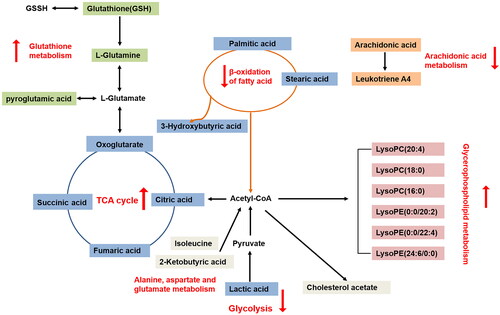
Metabolite perturbations are considered important factors that contribute to ischemic stroke. Metabonomics, a quantitative measurement of the dynamic, multiparametric metabolic response of living systems, can help identify important metabolic biomarkers with potential diagnostic and prognostic value (Au Citation2018). The present study investigated the potential biomarkers of ischemic stroke and explored the potential protective effect of styrax against MCAO injury in rats. The styrax-treatment groups, especially the styrax-H group, had a smaller cerebral infarct size than the MCAO group. Biochemical indicator measurements and metabolic profile analyses (PCA, PLS-DA, and OPLS-DA score plots) yielded results that also support the cerebral protective effects of styrax treatment. More importantly, in response to MCAO-induced cerebral ischemia, 22 endogenous metabolites involved in metabolic pathways and physiological functions were identified. These alterations were reversible via treatment with styrax. As shown in , most of them were involved in energy metabolism, glutathione and glutamine metabolism, glycerophospholipid metabolism, and other metabolic processes.
Figure 11. The network of potential biomarkers of styrax treated on MCAO-induced cerebral ischemic injury rats. Different colors represent different metabolic pathways, with red arrows pointing upward, indicating upregulation, and red arrows, pointing downward, indicating downregulation.
To further examine styrax’s overall mode of action in MCAO-induced cerebral ischemic injury rats, we conducted a correlation analysis and mechanism-validation study of the high-dose styrax group, which exhibited the greatest efficacy. The results of the correlation analysis, as shown in , suggest that it may be significant for understanding styrax’s effect on ischemic stroke, primarily arising from energy metabolism and glutamine metabolism. Furthermore, western blot analysis also revealed that the styrax-H group had significantly upregulated p-AMPK and GLS expression levels compared with the model group.
Energy metabolism
The brain is known to have high metabolic requirements, consuming 20% of the glucose-derived energy while only constituting 2% of the body’s weight. The tricarboxylic acid (TCA) cycle is the most important component of energy metabolism, producing >90% of the total energy in the body (Mergenthaler et al. Citation2013). In this study, the levels of citric acid, fumaric acid, oxoglutarate, and succinic acid decreased in the model group, indicating that the TCA cycle was downregulated in ischemic stroke rats. When glycometabolism is reduced, glycolysis is upregulated to rapidly provide energy and thus manifests as lactate elevation, consistent with published findings (Mu et al. Citation2017). However, the levels of citric acid, fumaric acid, oxoglutarate, and succinic acid in the styrax group exhibited an upward trend, and the level of lactic acid displayed a downward trend, which is predominantly related to improved energy-supply patterns.
In addition, fatty acids can also provide energy to the body through β-oxidation in mitochondria; thus, β-oxidation of fatty acids is an indirect source of energy when glucose metabolism is disordered. In the present study, we found that 3-hydroxybutyric acid, palmitic acid, and stearic acid levels were significantly increased in the model group, indicating that the β-oxidation of fatty acid metabolism was increased. More importantly, the levels of palmitic acid, stearic acid, and 3-hydroxybutyric acid exhibited a significantly negative association with the energy metabolism of ATP, Na+K+-ATPase, Ca++Mg++ATPase, and Ca++-ATPase (), indicating an increase in brain-energy consumption in ischemic stroke rats. In addition, the mobilization of fatty acid β-oxidation was moderated by decreasing the levels of palmitic acid, stearic acid, and 3-hydroxybutyric acid in the styrax group compared with that in the model group, thus reducing the amount of oxygen consumed and restoring normal mitochondrial function to protect the brain.
Based on the above analysis, we concluded that styrax potentially affects glycolysis (lactic acid), the TCA cycle (citric acid, fumaric acid, oxoglutarate, and succinic acid), and β-oxidation of fatty acids (3-hydroxybutyric acid, palmitic acid, and stearic acid), all of which are directly related to energy metabolism. In addition, these results were also consistent with the augmentation of energy-related indicators, such as ATP, Na+K+-ATPase, Ca++Mg++-ATPase, and Ca++-ATPase, as shown in .
Glutathione and glutamine metabolism
Glutathione, a core component of glutathione metabolism, is widely distributed in various body organs and rich in sulfhydryl (-SH), which plays an important role in maintaining cellular biological functions, with neurocytes being no exception (Bains and Shaw Citation1997). GSH depletion may result in oxidative stress and increase the level of excitotoxic molecules that potentially cause cell death in diverse neuron populations (Bains and Shaw Citation1997). In this study, the MCAO model group might have experienced decreased GSH-Px activity and serum glutathione levels due to oxidative stress; nonetheless, after styrax treatment, GSH-Px activity increased, thus promoting glutathione production and alleviating oxidative stress-induced nerve damage.
In the brain, glutamine not only maintains normal glutamate levels but also synthesizes glutamine via glutamine-synthase action to ensure sufficient raw materials for glutathione synthesis, maintain a high glutathione content in the neuron, remove reactive oxygen species, and provide antioxidant protection for neurons (Kleinkauf-Rocha et al. Citation2013; Wang J et al. Citation2020). Moreover, glutamine is catalyzed by GLS to form glutamate and ammonia in the mitochondria. Thereafter, glutamate is converted to α-ketoglutarate by the action of enzymes, and α-ketoglutarate enters the TCA cycle to provide energy for the body; moreover, glutamine may indirectly participate in the TCA cycle via 2-oxoglutarate (Tapiero et al. Citation2002). In addition, glutamate excitotoxicity is considered an important factor in neuronal cell death and brain damage under conditions of cerebral hypoxia and/or ischemia (Moskowitz et al. Citation2010). The results revealed that GLS-expression levels were elevated in the styrax-treated group, suggesting that styrax’s therapeutic effects on ischemic stroke may be partially due to its interference with glutamine metabolism (). Moreover, some studies have demonstrated increased glutamate and glutamic acid levels in ischemic stroke as well as decreased glutamine levels, suggesting impaired glutamate-glutamine cycling in the brain (Jeitner et al. Citation2015). Based on these results, the significantly lower levels of pyroglutamic acid and higher levels of glutamine in the styrax-treated group than those in the model group potentially explain styrax’s protective effects in brain tissue exposed to ischemia or oxidative stress. These results corroborate those of previous studies in terms of antioxidant biochemical indicators ().
Glycerophospholipid metabolism
Lipids are elementary components of biological membranes, and the synthesis and degradation of many lipids are perceived to be closely related to the mechanisms of neurocyte impairment and repair during cerebral ischemia, which are associated with the risk of stroke (Yang et al. Citation2017; Han et al. Citation2020). LysoPC is known to play a crucial role in a variety of motility-related functions, such as angiogenesis and neurite outgrowth (Block et al. Citation2013). Increased lysoPC (20:4) and decreased lysoPC (16:0 and 18:0) levels are reportedly associated with an increased risk of ischemic stroke (Block et al. Citation2013). Moreover, several lipids have been implicated in cell proliferation, and some studies have demonstrated that lysoPC and lysoPE protect ischemic neurons (Blondeau et al. Citation2002). In this study, the levels of lipids, including lysoPC and lysoPE, were all distinctly decreased in the model group compared with those in the sham group, except for lysoPC (20:4). A tendency toward normalization was observed in the styrax-treated group for the following biomarkers: lysoPC (18:0), lysoPC (16:0), lysoPE (0:0/20:2), lysoPE (0:0/22:4), and lysoPE (24:6/0:0). The therapeutic effects of styrax on ischemic stroke have been suggested to be potentially related to its interference with glycerophospholipid metabolism. However, the specific role of the protein warrants further verification.
Other metabolic pathways
Numerous free radicals are produced after ischemic stroke by arachidonic acid metabolism and other processes, and they exert neurotoxic effects on intracellular proteins, nucleotides, and lipids, resulting in damage to neuronal cells (Wang C et al. Citation2018). Following treatment with styrax, arachidonic acid and leukotriene A4 levels tended toward normalcy, exhibiting consistency with the decrease in serum inflammatory indicators such as TNF-α, IL-6, and IL-1β compared with those in the model group (). These findings suggest that styrax may protect the brain against MCAO-induced ischemic stroke via anti-inflammatory effects.
These findings suggest that styrax potentially regulates the above potential biomarkers and metabolic pathways, particularly those related to energy metabolism and glutamine metabolism. Furthermore, this study also provides a useful method for exploring the pathogenesis of ischemic stroke and evaluating the effectiveness of styrax or other traditional remedies. However, the present study has several limitations. First, styrax is a natural medicine containing multiple ingredients. Considering the complexity of styrax’s composition, further experiments aimed at defining its neuroprotective role and identifying effective components would be translationally significant. In addition, the distribution of styrax throughout the body is currently unclear. In future studies, the metabolic profiles of rat blood, urine, cerebrospinal fluid, and brain tissue should be compared by combining multiple omics techniques (Cheng et al. Citation2020). Based on our efforts, we expect to attain a deeper understanding of the neuroprotective effects and underlying molecular mechanisms of styrax, which will lay a strong foundation for the international promotion and secondary development of styrax and its constituent prescriptions.
Conclusion
This study successfully established a metabonomic method based on UPLC-Q/TOF-MS combined with an integrative pharmacological approach for investigating the perturbed metabolic pathways of ischemic stroke and holistic efficacies of styrax treatment, thus contributing to the elucidation of the possible therapeutic mechanisms by which styrax exerts cerebral protection. Styrax markedly reduced infarct volumes, alleviated neurobehavioral abnormality score, improved brain function, altered oxidative and inflammatory biochemical indicators, and partially reversed abnormalities in serum metabonomics. Moreover, styrax treatment demonstrated an overall benefit in reducing cerebral ischemia injury and partially regulating perturbations in energy metabolism, glutathione and glutamine metabolism, glycerophospholipid metabolism, and other metabolic processes. Finally, protein expression in this experiment suggested that energy metabolism (upregulation of p-AMPK/AMPK expression) and glutamine metabolism (upregulation of GLS expression) may serve as potential therapeutic targets for MCAO-induced cerebral ischemic injury. These findings provide additional insight into the metabolic changes associated with ischemic stroke and may augment current evidence regarding the mechanisms of stroke.
Author contributions
FM, RL, XL, and MZ equally contributed to this work. HT, JW, and FM conceived the idea and revised the manuscript. FM, XL, RL, and SH designed the research, conducted most of the experiments, and wrote the paper. JZ, MZ, CG, YG, and MX assisted experiments. FM, HZ, and RL collated and analyzed data.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Au A. 2018. Metabolomics and lipidomics of ischemic stroke. Adv Clin Chem. 85:31–69.
- Bains JS, Shaw CA. 1997. Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death. Brain Res Brain Res Rev. 25(3):335–358. doi: 10.1016/s0165-0173(97)00045-3.
- Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, et al. 2015. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 372(1):11–20. doi: 10.1056/NEJMoa1411587.
- Block RC, Yalcin M, Srinivasan M, Georas S, Mousa SA. 2013. Angiogenesis modulation by arachidonic acid-derived lipids: positive and negative regulators of aangiogenesis. In: Mousa S, Davis P. (eds). Angiogenesis Modulations in Health and Disease. Springer: Dordrecht.
- Blondeau N, Lauritzen I, Widmann C, Lazdunski M, Heurteaux C. 2002. A potent protective role of lysophospholipids against global cerebral ischemia and glutamate excitotoxicity in neuronal cultures. J Cereb Blood Flow Metab. 22(7):821–834. doi: 10.1097/00004647-200207000-00007.
- Brouns R, De Deyn PP. 2009. The complexity of neurobiological processes in acute ischemic stroke. Clin Neurol Neurosurg. 111(6):483–495. doi: 10.1016/j.clineuro.2009.04.001.
- Chen C, Venketasubramanian N, Gan RN, Lambert C, Picard D, Chan BPL, Chan E, Bousser MG, Xuemin S. 2009. Danqi piantang jiaonang, a traditional Chinese medicine, in poststroke recovery. Stroke. 40(3):859–863. doi: 10.1161/STROKEAHA.108.531616.
- Chen S, Zhou M, Zhao X, Han Y, Huang Y, Zhang L, Wang J, Xiao X, Li P. 2022. Metabolomics coupled with network pharmacology study on the protective effect of keguan-1 granules in LPS-induced acute lung injury. Pharm Biol. 60(1):525–534. doi: 10.1080/13880209.2022.2040544.
- Chen Y, Guo Z, Peng X, Xie W, Chen L, Tan Z. 2018. Nimodipine represses AMPK phosphorylation and excessive autophagy after chronic cerebral hypoperfusion in rats. Brain Res Bull. 140:88–96. doi: 10.1016/j.brainresbull.2018.03.019.
- Cheng X, Yang Y, Li W, Liu M, Zhang S, Wang Y, Du G. 2020. Dynamic alterations of brain injury, functional recovery, and metabolites profile after cerebral ischemia/reperfusion in rats contributes to potential biomarkers. J Mol Neurosci. 70(5):667–676. doi: 10.1007/s12031-019-01474-x.
- Ding Y, Chen M, Wang M, Wang M, Zhang T, Park J, Zhu Y, Guo C, Jia Y, Li Y, et al. 2014. Neuroprotection by acetyl-11-keto-β-boswellic acid, in ischemic brain injury involves the Nrf2/HO-1 defense pathway. Sci Rep. 4:7002. doi: 10.1038/srep07002.
- GBD 2016 DALYs and HALE Collaborators. 2017. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (hale) for 195 countries and territories, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet. 390(10100):1260–1344. doi: 10.1016/S0140-6736(17)32130-X.
- Han K, Rong W, Wang Q, Qu J, Li Q, Bi K, Liu R. 2020. Time-dependent metabolomics study of cerebral ischemia–reperfusion and its treatment: focus on the combination of traditional Chinese medicine and western medicine. Anal Bioanal Chem. 412(26):7195–7209. doi: 10.1007/s00216-020-02852-w.
- Jeitner TM, Battaile K, Cooper AJL, Rae C, Sonnewald U. 2015. Critical evaluation of the changes in glutamine synthetase activity in models of cerebral stroke. Neurochem Res. 40(12):2544–2556. doi: 10.1007/s11064-015-1667-1.
- Kim J, Seo S, Lee S, Shin S, Park I. 2008. Nematicidal activity of plant essential oils and components from coriander (Coriandrum sativum), oriental sweetgum (Liquidambar orientalis), and valerian (Valeriana wallichii) essential oils against pine wood nematode (Bursaphelenchus xylophilus). J Agric Food Chem. 56(16):7316–7320. doi: 10.1021/jf800780f.
- Kleinkauf-Rocha J, Bobermin LD, Machado PDM, Gonçalves C, Gottfried C, Quincozes-Santos A. 2013. Lipoic acid increases glutamate uptake, glutamine synthetase activity and glutathione content in C6 astrocyte cell line. Int J Dev Neurosci. 31(3):165–170. doi: 10.1016/j.ijdevneu.2012.12.006.
- Li L, Yue S, Han R, Yu Y, Zhang P, Lv L, Zhu J, Zhou M, Fan X, Zhang H. 2022. Storax protected primary cortical neurons from oxygen-glucose deprivation/reoxygenation injury via inhibiting the TLR4/TRAF6/NF-κB signaling pathway. Brain Res. 1792:148021. doi: 10.1016/j.brainres.2022.148021.
- Liu P, Tang Y, Yang X, Dai J, Yang M, Zhang H, Liu Y, Yan H, Song X. 2018. Validation of a preclinical animal model to assess brain recovery after acute stroke. Eur J Pharmacol. 835:75–81. doi: 10.1016/j.ejphar.2018.07.035.
- Liu W, Mu F, Liu T, Xu H, Chen J, Jia N, Zhang Y, Dou F, Shi L, Li Y, et al. 2018. Ultra performance liquid chromatography/quadrupole time-of-flight mass spectrometry–based metabonomics reveal protective effect of Terminalia chebula extract on ischemic stroke rats. Rejuvenation Res. 21(6):541–552. doi: 10.1089/rej.2018.2082.
- Longa EZ, Weinstein PR, Carlson S, Cummins R. 1989. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 20(1):84–91. doi: 10.1161/01.str.20.1.84.
- Mergenthaler P, Lindauer U, Dienel GA, Meisel A. 2013. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 36(10):587–597. doi: 10.1016/j.tins.2013.07.001.
- Moskowitz MA, Lo EH, Iadecola C. 2010. The science of stroke: mechanisms in search of treatments. Neuron. 67(2):181–198. doi: 10.1016/j.neuron.2010.07.002.
- Mu F, Duan J, Bian H, Zhai X, Shang P, Lin R, Zhao M, Hu D, Yin Y, Wen A, et al. 2017. Metabonomic strategy for the evaluation of Chinese medicine Salvia miltiorrhiza and Dalbergia odorifera interfering with myocardial ischemia/reperfusion injury in rats. Rejuvenation Res. 20(4):263–277. doi: 10.1089/rej.2016.1884.
- Sun R, Li Y, Cai M, Cao Y, Piao X. 2019. Discovery of a new biomarker pattern for differential diagnosis of acute ischemic stroke using targeted metabolomics. Front Neurol. 10:1011. doi: 10.3389/fneur.2019.01011.
- Tapiero H, Mathé G, Couvreur P, Tew KD. 2002. II. Glutamine and glutamate. Biomed Pharmacother. 56(9):446–457. doi: 10.1016/s0753-3322(02)00285-8.
- Wang C, Liu C, Wang M, Ma Q, Li Y, Wang T, Zhao B. 2018. UPLC-HRMS-based plasma metabolomic profiling of novel biomarkers by treatment with kdzi in cerebral ischemia reperfusion rats. Molecules. 23:1315. doi: 10.3390/molecules23061315.
- Wang J, Wang F, Mai D, Qu S. 2020. Molecular mechanisms of glutamate toxicity in Parkinson’s disease. Front Neurosci. 14:585584. doi: 10.3389/fnins.2020.585584.
- Weng J, Zhou J, Liang L, Li L. 2019. UHPLC/QTOF-MS-based metabolomics reveal the effect of Melastoma dodecandrum extract in type 2 diabetic rats. Pharm Biol. 57(1):807–815. doi: 10.1080/13880209.2019.1693605.
- Wu B, Liu M, Liu H, Li W, Tan S, Zhang S, Fang Y. 2007. Meta-analysis of traditional Chinese patent medicine for ischemic stroke. Stroke. 38(6):1973–1979. doi: 10.1161/STROKEAHA.106.473165.
- Xu J, Liu X, Luo L, Tang L, Guo N, Liu M, Li H, Zhang F, Zhang Y, Li D, et al. 2019. A metabonomics investigation into the therapeutic effects of buchang naoxintong capsules on reversing the amino acid-protein interaction network of cerebral ischemia. Oxid Med Cell Longev. 2019:7258624. doi: 10.1155/2019/7258624.
- Xu W, Zhang Y, Yu Y, Li B, Liu J, Wang P, Wu H, Liu Q, Wei Z, Xiao H, et al. 2018. Dose-dependent target diversion of danhong injection on the glu-glt-1/gly-glyralpha dynamic balance module of cerebral ischemia. Pharmacol Res. 135:80–88. doi: 10.1016/j.phrs.2018.07.020.
- Xu Z, Lu D, Yuan J, Ren M, Ma R, Xie Q, Li Y, Li J, Wang J. 2021. Storax, a promising botanical medicine for treating cardio-cerebrovascular diseases: a review. Front Pharmacol. 12:785598. doi: 10.3389/fphar.2021.785598.
- Yang L, Lv P, Ai W, Li L, Shen S, Nie H, Shan Y, Bai Y, Huang Y, Liu H. 2017. Lipidomic analysis of plasma in patients with lacunar infarction using normal-phase/reversed-phase two-dimensional liquid chromatography-quadrupole time-of-flight mass spectrometry. Anal Bioanal Chem. 409(12):3211–3222. doi: 10.1007/s00216-017-0261-6.
- Zhang F, Zhang T, Gong J, Fang Q, Qi S, Li M, Han Y, Liu W, Ge G. 2022. The Chinese herb styrax triggers pharmacokinetic herb-drug interactions via inhibiting intestinal CYP3A. Front Pharmacol. 13:974578. doi: 10.3389/fphar.2022.974578.
- Zhang M, Ma Y, Chai L, Mao H, Zhang J, Fan X. 2019. Storax protected oxygen-glucose deprivation/reoxygenation induced primary astrocyte injury by inhibiting NF-κB activation in vitro. Front Pharmacol. 9:1527. doi: 10.3389/fphar.2018.01527.
- Zhou M, Li D, Li L, Zhao P, Yue S, Li X, Du Y, Fan X, Zhang M. 2021. Post-stroke treatment of storax improves long-term outcomes of stroke in rats. J Ethnopharmacol. 280:114467. doi: 10.1016/j.jep.2021.114467.
- Zhou M, Li D, Shen Q, Gao L, Zhuang P, Zhang Y, Guo H. 2022. Storax inhibits caveolae-mediated transcytosis at blood-brain barrier after ischemic stroke in rats. Front Pharmacol. 13:876235. doi: 10.3389/fphar.2022.876235.