Abstract
Context
Crocin exhibits anti-depressant properties. However, its underlying mechanisms and its relationship with metabolomics remain unclear.
Objective
This study elucidates the mechanism of action and potential targets of crocin in treating chronic unexpected mild stress (CUMS)-induced depression in rats.
Materials and methods
Male Sprague-Dawley (SD) rats underwent 4 weeks of CUMS to establish the depression model. The normal control (distilled water), crocin (25 mg/kg), and fluoxetine (5.4 mg/kg) groups were orally administered for 4-weeks. Behavioural tests evaluated the effects of crocin, while liquid chromatography-mass spectrometry metabolomics identified differential metabolites and their associated metabolic pathways. Subsequently, network pharmacology was utilized to predict the targets of crocin.
Results
Crocin significantly increased body weight (from 319.16 ± 4.84 g to 325.67 ± 2.84 g), sucrose preference (from 0.46 ± 0.09 to 0.70 ± 0.09), vertical activity (from 2.83 ± 1.94 to 8 ± 2.36), horizontal activity (from 1 ± 0.63 to 4.5 ± 3.08) and decreased immobilization time (from 13.16 ± 2.69 to 3.97 ± 3.00). Metabolomics analysis identified 7 metabolites and 5 associated metabolic pathways. From the combined analysis of network pharmacology and metabolomics, three targets (PRMT1, CYP3A4, and GLB1) are the overlapping targets and the two most important metabolic pathways are tryptophan metabolism and glycerolipid metabolism.
Discussion and conclusions
This study provides insights into the antidepressant therapeutic effect of crocin and its underlying mechanisms. The findings contribute to a better understanding of the metabolic mechanism involved in the anti-depressant effect of crocin, establishing a strong foundation for future research in this area.
Introduction
Depression is a common and severe mental illness, characterized by feelings of pessimism, restlessness, anhedonia, despondency, and even suicidal thoughts, and it has considerable social and economic impact worldwide (Stachowicz and Sowa-Kućma Citation2022). According to the World Health Organization (WHO), over 300 million people globally suffer from severe depression and approximately 800,000 depression-induced suicide-related deaths occur annually (Liu et al. Citation2021). Numerous experimental and clinical studies have demonstrated that the function of serotonin and norepinephrine in the central nervous system was somewhat altered in patients with depression (Nemeroff Citation2002; Meyer et al. Citation2003; Kao et al. Citation2018). Another major theory about the neurobiological basis of depression indicates that brain-derived neurotrophic factor (BDNF) plays a significant role in the brain (Gonul et al. Citation2005). Hyperactivity of the hypothalamic-pituitary adrenal (HPA) axis is also considered an important discovery in the field of psychoneuroendocrinology (Heim et al. Citation2008). In clinical practice, only ⅓ of patients respond positively to medication (Peng et al. Citation2015). Although these antidepressant medications can be effective, they frequently have adverse effects and are typically expensive (Thanacoody and Thomas Citation2005). Therefore, the development of high-efficiency, low-toxicity, and multi-target antidepressants is required.
Herbal remedies, with their low toxicity and multi-targeted effects, can be a good substitute for the pharmaceutical treatment of depression. Additionally, over 20 herbs have been proposed as potential antidepressants (Yeung et al. Citation2018). Crocin, a valuable herb obtained from the stigmas of Crocus sativus Linn. (Iridaceae) (Zhang et al. Citation2022), has anticancer, antioxidant, anti-inflammatory, and antiplatelet activities (Siddiqui et al. Citation2022). Several studies have assessed the effects of crocin on patients with depression. For example, one clinical trial found that crocin improved anxiety and depression in breast cancer patients undergoing chemotherapy (Salek et al. Citation2021). Additionally, there is evidence to suggest that crocin can be used to treat major depressive disorder (Talaei et al. Citation2015). However, the metabolic mechanism and key targets of crocin’s antidepressant effects are still unknown.
Metabolomics, a branch of omics science, has greatly enhanced our understanding of the underlying mechanisms of diseases and drug treatments (Lin et al. Citation2022). By providing a comprehensive view of multiple biochemical pathways, metabolomics can help to elucidate the mechanisms of disease pathogenesis and the effects of multi-targeted medicines (Zhou et al. Citation2020). In recent years, metabolomics has been used to investigate the molecular mechanisms and scientific basis of traditional Chinese medicine (TCM) (Chen et al. Citation2022).
Network pharmacology, an emerging approach in modern Chinese medicine research, studies mechanistic connections between compounds, their targets, and diseases (Chi et al. Citation2022). This new approach, therefore, provides a holistic view of Chinese herbal medicine and syndrome differentiation in TCM theory and may provide alternative research methods for exploring the pharmacological mechanisms of TCM (Yu et al. Citation2022). An effective method of investigating the function of antidepressants in TCM is to integrate the synthetic strategy of metabolomics with network pharmacology (Gu et al. Citation2022). This combined strategy addresses the lack of experimental validation in network pharmacology and the lack of elucidation of upstream molecular mechanisms and drug-binding targets in metabolomics (Lei et al. Citation2022).
In this study, we focused on metabolomics, network pharmacology, and molecular docking to evaluate the antidepressant effect of crocin. Firstly, the antidepressant effect of crocin was evaluated in rats exposed to chronic unexpected mild stress (CUMS), changes in the rat weight were monitored and behavioural tests were conducted. Then, metabolites and metabolic pathways were investigated using untargeted metabolic analysis. Additionally, network pharmacology was used to predict potential targets and pathways associated with crocin against depression. Finally, using untargeted metabolomics and network pharmacology, we constructed an interactive network of differentially expressed metabolites, potential targets, and metabolic pathways, and validated the core targets of crocin using molecular docking. The results of the present study may lead to the administration of crocin to treat depression and could guide the modernization of TCM monomers for application in healthcare.
Materials and methods
Animals and treatment
Twenty-four male Sprague-Dawley rats (200–220 g) were acquired from the China Hunan SJA Laboratory Animal Co., Ltd., Hunan, China. The animals were adaptively fed based on their circadian rhythm. All processes were undertaken in compliance with the Regulations of Experimental Animal Administration published by the State Committee of Science and Technology of the People’s Republic of China (Approval No. LL2020111001). The rats were housed with free access to food and water under constant temperature conditions (22 ± 2 °C), humidity of 55% ± 10%, and a 12 h light/dark period [light cycle (6:00–18:00); dark cycle (18:00–6:00)] (Spulber et al. Citation2019). During the adaptation period, three rats were housed in each cage. Then rats were divided into four groups, with six rats in each group: a normal control group (NC), a model group (CUMS), a crocin group (25 mg/kg) (Vahdati Hassani et al. Citation2014), and a fluoxetine group (5.4 mg/kg) (Nair et al. Citation2018). The rats were administered (intragastric) crocin or fluoxetine 1 h before modelling daily for four weeks. Drug administration was started on the first day of modelling and continued throughout the experiment.
Establishment of CUMS model
The CUMS rat model was established using: food deprivation (24 h), water deprivation (24 h), damp bedding (24 h), tail clamping (1 min), ice water swimming (5 min at 5 °C), electric shock (3 min), and noise (4 h). Rats were exposed to one of the stressors above daily and were not subjected to the same stressor for three consecutive days. The details of CUMS were as follows Electric shock: the rat was placed in a cloth sleeve with both hind legs exposed and the electroshock device was clamped to the palm of the hind paws for 3 min of electroshock intervention (Shen et al. Citation2022). Tail clamping: the rat was placed in a cloth sleeve with the tail exposed and a needle holder was clamped to the root of the tail (Wu et al. Citation2017). Noise: an intervention using an alarm sound on a loudspeaker for 4 h (Shen et al. Citation2022). Ice water swimming: a container with a depth higher than the rat’s body length, and 4 °C ice water was filled in the container up to the height of the rat’s neck (Albrakati et al. Citation2021). Rats in the normal control group had free access to food and water and were not subjected to any stress. The rats were returned to the housing facility once the CUMS procedure was completed.
Body weight monitoring of rats
Rats were weighed weekly to monitor weight changes in each group (without fasting). The body weight of each rat was recorded one day prior to the initiation of the experiments (day 0), as well as on days 7, 14, 21 and 28 of the CUMS procedure.
Behavioural testing
Each test was measured on each group of rats in sequence after the 4-week-long CUMS exposure. Then the open field test (OFT), forced swim test (FST), and sucrose preference test (SPT) were each conducted on the following three days, respectively. All behavioural tests were carried out in a sound-attenuated room and only one behavioural test was arranged per day to avoid causing errors due to the physiological and psychological effects on rats.
Open field test
To assess behavioural characteristics in a quiet setting, an opaque box (100 × 100 × 40 cm, Beijing Zongshi Technology, Beijing, China) was used for an Open field test (OFT) (Abel Citation1991). During the test, each rat was positioned at the centre of the box and permitted to navigate the area freely for 5 min. Tracking Master v3.0 software was used to evaluate horizontal and vertical activity in the middle region (⅔ of the open field). After each test, the open-field area was cleaned with 75% ethyl alcohol and clean water (Song et al. Citation2008). The animal’s fear of the new open environment motivates it to stay mainly in the peripheral area and spend less time in the middle region, but the animal’s natural curiosity motivates it to move to the middle region, and depressive/anxious behaviour can also be observed (Kuniishi et al. Citation2017). Thus, the OFT is an environment for observing and studying the neuropsychological changes in animals.
Forced swimming test
For the forced swimming test (FST), rats were placed in an open, transparent cylinder vessel (40 cm high and 30 cm in diameter) with a water depth of approximately 15 cm and temperature of approximately 25 °C. Every rat was made to swim for 4 min. The swimming behaviour of each rat was recorded (Abuelezz et al. Citation2017). Containers were carefully cleaned before introducing new rats to reduce any disturbance from previous tests. Each test was recorded using a video camera. The criterion for determination of immobility was that all four of the rat’s limbs and its head were immobile, its head was suspended above the horizontal plane (Kuniishi et al. Citation2017). The FST was performed by placing the experimental animal in a confined environment (e.g. water) from which the animal struggles desperately to escape, thus creating an inescapable oppressive environment, and the animal exhibits a typical ‘immobile state’ after a period of attempting to escape. These performances illustrate that animals eventually give up the expectation of escaping, which is a behaviour related to despair, reflecting the animal’s ‘desperate depressive psychological state’ (Detke et al. Citation1995).
Sucrose preference test
The sucrose preference test (SPT) consists of an adaptation training phase and a testing phase (Abuelezz et al. Citation2017). During the adaptation phase, every rat was individually housed. Two bottles of 1% sucrose solution were given on the first day. On the second day, this was replaced with one vial of 1% sucrose solution and one vial of distilled water. On the third day, the water bottle was exchanged. Rats were then tested after 12 h of food and water fasting. During the test, each rat was exposed to two 100 mL bottles, one containing distilled water and another with 1% sucrose solution for 4 h, and the locations of the bottles were switched after 2 h. The ratio of sucrose preference was estimated by tracking and recording the volume of sucrose solution and distilled water consumed by the end of the 4 h long test (Li et al. Citation2021). Sucrose preference ratio = sucrose water intake/(sucrose water intake + distilled water intake) × 100.
LC-MS/MS analysis
We used LC-MS/MS to detect changes in most small molecule metabolites in the rats, focusing on metabolites that showed significant changes in different groups. An Ultra-High-Performance Liquid Chromatography (UHPLC) system (Vanquish, Thermo Fisher Scientific, Waltham, CA, USA) and a UPLC BEH amide column (2.1 100 mm, 1.7 m) linked to a Q Executive HFX mass spectrometer were used to conduct LC-MS/MS analysis (Orbitrap MS, Thermo Fisher Scientific). Ammonium acetate (25 mmol/L) and ammonia hydroxide (25 mmol/L) in water (pH = 9.75) constituted the mobile phase (A), and acetonitrile constituted the mobile phase (B). The temperature of the automatic sampling device was 4 °C, and the sample size was 2 μL. The information-dependent acquisition (IDA) mode was employed with a QE HFX mass spectrometer to acquire MS/MS spectra using the direction of acquisition software (Xcalibur, Thermo Fisher Scientific). The acquisition software continually assessed the full-scan MS spectrum. The next set of parameters selected for the ESI source was sheath gas flow rate, 30 arb; auxiliary gas flow rate, 25 arb; complete MS resolution, 60,000; and MS/MS resolution, 7500.
Serum sample preparation
After the behavioural assessment, blood was collected from the abdominal aorta. Serum was separated using a centrifuge (3500 rpm, 15 min) after clotting at 4 °C for 1 h. Then the serum was mixed with extraction solution (methanol: acetonitrile = 1:1 (V/V), containing isotope-labelled internal standard mixture) at a ratio of 1:4, respectively, vortexed for 30 s, sonicated for 10 min in an ice-water bath, and incubated for 1 h at −40 °C. Then, the sample was centrifuged at 12,000 rpm for 15 min at 4 °C. The resulting supernatant was transferred to the injection cup for analysis. The quality control (QC) samples were prepared by mixing equal amounts of supernatant from all samples.
Data pre-processing and annotation
ProteoWizard software was used to transform the initial data to mzXML format, and a custom-built R package was applied for peak identification, extraction, and integration. The threshold value selected for the method score was 0.3, and the peaks were matched against the BiotreeDB (v2.1) custom-built secondary mass spectral database for material descriptive annotations. Principal component analysis (PCA) and dimensional descent were employed to collect the metabolic data that significantly affected fitness and illness susceptibility. Variations in metabolites were identified using orthogonal partial least squares discriminant analysis (OPLS-DA).
Biomarker identification and metabolic pathway analysis
After standardization, LC-MS/MS data were input into SIMCA 16.0.2 software. PCA and OPLS-DA were conducted. The variable importance in projection (VIP) values produced by OPLS-DA was employed to appraise the impact of each metabolite on the identification precision and explanatory power. The higher the VIP value, the greater the contribution of the metabolite to sample differentiation. Metabolites were considered for metabolic pathway analysis if their variable importance was > 1 and their p-value for the Student’s t-test was < 0.05. Metabolic pathway analysis of the various metabolites between the CUMS and crocin groups was carried out using the KEGG (http://www.kegg.com) and MetaboAnalyst databases (http://www.metaboanalyst.com).
Network pharmacology analysis
Collection of relevant targets
The targets of crocin were predicted using the following databases: Similarity Ensemble Approach (SEA) (accessed: 8 November 2022, www.sea.bkslab.org/), SuperPred (accessed: 8 November 2022, https://prediction.charite.de/), and STITCH (accessed: 8 November 2022, http://stitch.embl.de/). After removing duplicates, we obtained 309 genes. The official names of the medicine targets were searched using the UniProt database (http://www.uniprot.org/). The known targets for depression were obtained from five databases: DisGeNet (accessed: 11 November 2022, https://www.disgenet.org/), Online Mendelian Inheritance in Man (accessed: 11 November 2022: OMIM, http://www.omim.org/), Therapeutic Target Database (accessed: 11 November 2022, TTD, http://database.idrb.cqu.edu.cn/TTD/), GeneCards (accessed: 11 November 2022, https://www.genecards.Org/), and Drug Bank (accessed: 11 November 2022, http://www.drugbank.ca/). The terms ‘depressive’, ‘depressed’ and ‘anti-depressant’ were used as the keywords to acquire depression-related targets (Yan et al. Citation2021). We searched these databases and obtained 2445 genes after removing duplicates.
Protein–protein interaction network construction
The Venny 2.1.0 (https://bioinfogp.cnb.csic.es/tools/venny/) website was used to identify proteins that were both depression-related targets and predicted targets of crocin. The target information obtained was used to search the STRING database (https://string-db.org/) to identify the connections between potential targets (Yuan et al. Citation2020). The results of the Protein–protein interaction (PPI) analysis of the STRING database were visually assessed using Cytoscape v3.9.1 software, and a PPI network was built.
Gene ontology and Kyoto encyclopedia of genes and genomes (KEGG) analyses
The Database for Annotation, Visualization, and Integrated Discovery (DAVID, https://david.ncifcrf.gov/) was used to perform Gene ontology (GO) and Kyoto encyclopedia of genes and genomes (KEGG) functional enrichment analyses on all target genes. Significant enrichment entries for GO and KEGG were those with a p-value <0.05. The top 20 pathways and top 10 cell components, molecular functions, and biological processes were selected and loaded onto the online website (http://www.bioinformatics.com.cn/) to plot the data (Tang et al. Citation2021).
Comprehensive analysis by metabolomics and network pharmacology
To more fully understand the antidepressant mechanism of crocin, we constructed an interactive network based on serum metabolomics and network pharmacology. Overlapping targets were obtained from the intersection of targets corresponding to the main metabolic pathways identified in the metabolomics analysis and targets related to crocin from the network pharmacology analysis. These interactions were used to appraise key biomarkers and metabolic pathways (Gu et al. Citation2022).
Molecular docking
The Protein Data Bank (PDB) file for the crystal structure of the receptors of proteins was acquired from the Research Collaboratory for Structural Bioinformatics (RCSB) PDB. Chemical Book (https://www.chemicalbook.com/) and Openbabel 2.4.1 were used to predict the 3D structure of the ligand of the active component of crocin, producing 3D conformations with reduced energy that were saved in ‘. pdb’ format. The ‘.pdb’ format was transformed to ‘.pdbqt’ format using AutoDockTools 1.5.7 (https://autodock.scripps.edu/). Subsequently, the ligand molecularly docked to the receptors using AutoDock, and PyMOL software (https://pymol.org/2/) was used for network visualization (Zhang et al. Citation2022).
Statistical analysis
The experimental results were analyzed using IBM SPSS Statistics 25 (IBM Corp., Armonk, NY, USA). All data are presented as mean ± SD. For comparisons between groups, t-tests and one-way analysis of variance (ANOVA) were used. In the one-way ANOVA for group comparisons, we used the LSD method and Dunnett’s T3 method for post hoc analyses. Statistical significance was set as p < 0.05.
Results
Crocin ameliorated CUMS-induced depressive-like behaviour
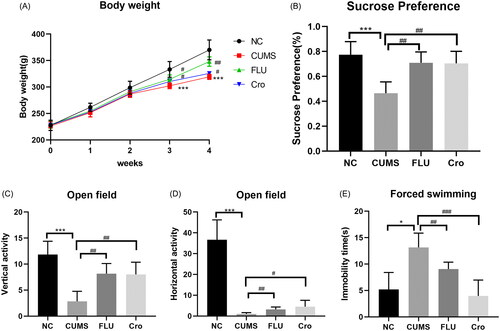
First, we established a rat model of depression using CUMS. Before the experiments, there were no marked differences in the initial weights of each group. We selected OFT, FST, and SPT as they are the three classic behavioural tests for evaluating animal emotions (Zhang et al. Citation2017). The results showed that crocin and fluoxetine significantly decreased weight loss () in CUMS-induced model rats (p < 0.05, p < 0.01, respectively). Additionally, the CUMS group consumed considerably less sucrose than the control group (p < 0.001), whereas the crocin and fluoxetine treatments significantly increased sucrose consumption (p < 0.01, p < 0.01, respectively). Furthermore, CUMS resulted in poorer performance in the OFT (decreased horizontal (p < 0.001) and vertical exploration (p < 0.001)) and FST (prolonged immobility time (p < 0.05)). However, after crocin or fluoxetine treatments, both horizontal (p < 0.05, p < 0.01, respectively) and vertical activity (p < 0.01, p < 0.01, respectively) increased in the open field experiment while immobility time (p < 0.001, p < 0.01, respectively) was reduced in the FST (). The findings demonstrate that crocin can notably decrease CUMS-induced depression in rats.
Figure 1. Crocin attenuates CUMS-induced depressive symptoms in depression-like rats. (A) Body weight in various groups. (B) Sugar consumption rates in various groups. (C) Vertical activity in various groups. (D) Horizontal activity in various groups. (E) Immobility time in various groups. Data are mean ± standard deviation, n = 6. *p < 0.05, **p < 0.01, ***p < 0.001 versus NC group; #p < 0.05, ##p < 0.01, ###p < 0.001 versus the CUMS group.
Effect of crocin treatment on metabolism in rats
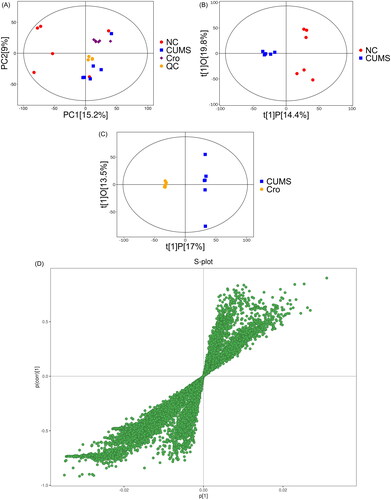
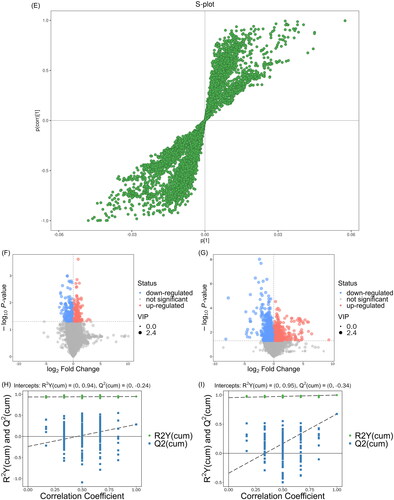
To further understand the treatment mechanisms of crocin in CUMS-induced rats, we performed an untargeted metabolomic analysis. LC-MS-based metabolomics technology combined with multivariate statistical analysis was used to obtain and analyse changes in metabolites in the NC, CUMS, and crocin groups. After centre scaling and log transformation of the data, an unsupervised clustering PCA was used to appraise the performance of the different groups. The PCA score chart () showed that the groups were distributed within Hotelling’s T-square ellipses at 95% confidence intervals. The NC group had a good separation from the CUMS group, suggesting that the serum metabolic profile of the rats after CUMS induction was obviously altered. Additionally, the crocin and CUMS groups were clearly separated, indicating that the levels of some metabolites in the CUMS rats were reversed after crocin treatment. Relevant biomarkers were further defined between group pairs using OPLS-DA. Notably, the six samples in every group were grouped closely, indicating low within-group variability, and there was considerable variability between samples from various groups, as illustrated in . The S-plot of the OPLS-DA displays various metabolites (). The V-plot of OPLS-DA indicates significant variations in multiple metabolites (). Using the data for the CUMS and NC groups, R2Y (cum) and Q2 (cum) in OPLS-DA were 0.877 and 0.542, respectively, and the values of the crocin and model groups were 0.853 and 0.596, respectively. All groups in the OPLS-DA module showed significant separation and fit the 95% Hotelling’s T-squared ellipse. A permutation test was run 200 times to assess the precision and obtain probability values for the predictions. The model had good accuracy, as demonstrated by the cross-validation results ().
Figure 2. Multivariate data analysis of serum metabolites of rat samples. (A) PCA score plot. (B) OPLS-DA score plot of the NC and CUMS groups. (C) OPLS-DA score plot of the CUMS and Cro groups. (D) S-plot of the NC and CUMS groups. (E) S-plot of the CUMS and Cro groups. (F) V-plot of the NC and CUMS groups. (G) V-plot of the CUMS and Cro groups. (H) Permutation test of the NC and CUMS groups. (I) Permutation test of the CUMS and Cro groups.
Identification of potential biomarkers
VIP parameters have been broadly used in biomarker screening and can indirectly reflect the relevance of metabolic products and diseases; all candidate metabolites with a VIP value > 1 and p < 0.05 were considered differential metabolites. Using a cluster formation analysis heat map, unstructured clustering was used to further investigate the distributions of various metabolites among groups (). Compared with those in the NC group, 30 metabolites were differentially expressed in CUMS rats (). The levels of five metabolites were reduced in CUMS rats, including cholic acid, antibiotic SB 202742, and (3R, 6′Z)-3,4-dihydro-8-hydroxy-3-(6-pentadecenyl)-1H-2-benzopyran-1-one and those of 25 metabolites, including d-malic acid, l-valine, and 3-hydroxybutyric acid, were increased in CUMS rats. Following the treatments, crocin clearly reversed the levels of seven metabolites, including 3-hydroxybutyric acid, cholic acid, 17-phenyl-18,19,20-trinor-prostaglandin E2, and (3R, 6′Z)-3,4-dihydro-8-hydroxy-3-(6-pentadecenyl)-1H-2-benzopyran-1-one in CUMS rats. The comparative contents of all distinct metabolites found in all groups were normalized using z-scores to analyze variations in metabolites in various groups. Subsequently, a k-means clustering analysis was carried out ().
Figure 3. Comparison of differentially expressed metabolite levels between different groups. Each row corresponds to data for a specific metabolite, and each column represents the NC, CUMS, or Cro group. Colors indicate the relative expression levels of metabolites in this group of samples.
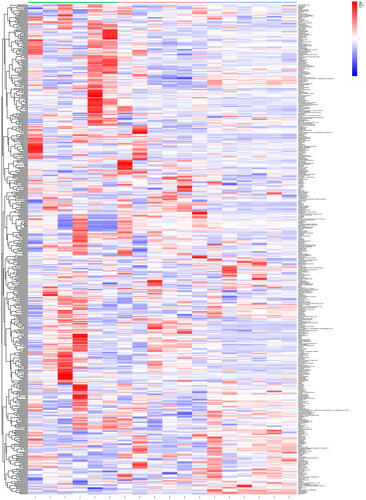
Figure 4. K-Means analysis. Cluster 1 includes cholic acid, Cluster 2 includes 17-phenyl-18,19,20-trinor-prostaglandin E2 and PS [22:0/22:6(4Z,7Z,10Z,13Z,16Z,19Z)], Cluster 4 includes 3-hydroxybutyric acid, Cluster 5 includes (3R, 6′Z)-3, 4-dihydro-8-hydroxy-3-(6-pentadecenyl)-1H-2-benzopyran-1-one, PC(15:0/14:0), antibiotic SB 202742.
![Figure 4. K-Means analysis. Cluster 1 includes cholic acid, Cluster 2 includes 17-phenyl-18,19,20-trinor-prostaglandin E2 and PS [22:0/22:6(4Z,7Z,10Z,13Z,16Z,19Z)], Cluster 4 includes 3-hydroxybutyric acid, Cluster 5 includes (3R, 6′Z)-3, 4-dihydro-8-hydroxy-3-(6-pentadecenyl)-1H-2-benzopyran-1-one, PC(15:0/14:0), antibiotic SB 202742.](/cms/asset/3b6b09f7-e751-4e6e-a6c4-bd29ac7cec74/iphb_a_2246531_f0004_c.jpg)
Table 1. Different endogenous metabolites in rat serum. Trend 1 is NC group compared with CUMS group; Trend 2 is CUMS group compared with Cro group.
Metabolic pathway analysis
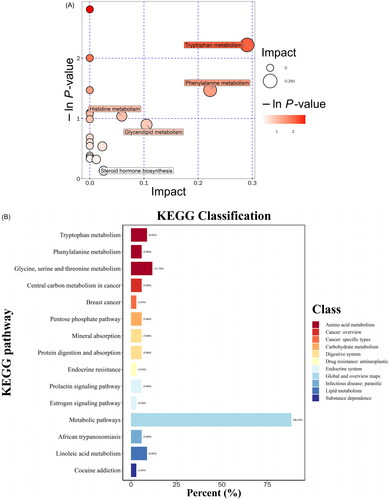
The metabolic pathway information for the crocin treatment is shown in . The main metabolic pathways included tryptophan, phenylalanine, histidine, glycerolipid, and steroid hormone biosynthesis. l-Tryptophan, 3-hydroxyanthranilic acid and l-kynurenine were mainly involved in tryptophan metabolism; phenylethylamine is mainly involved in phenylalanine metabolism and imidazole-4-acetaldehyde mainly participates in histidine metabolism. The KEGG pathway annotation classifications () revealed the associated pathways to be amino acid metabolism, carbohydrate metabolism, and the endocrine system. These results indicate that complex metabolic pathway disorders, including lipid, amino acid, and glucose metabolism disorders, can be triggered by depression. By modifying the metabolism and metabolic pathways involved in CUMS-induced depression in rats, crocin can achieve a therapeutic effect.
Gene targets associated with crocin and depression
All crocin targets were obtained from the Super Pred, STITCH, and SEA databases, resulting in the identification of 309 crocin-related targets after removing duplicates. To obtain targets related to depression, a total of 2445 depression targets were acquired from the GeneCards, OMIM, and TTD databases using the keywords ‘depression’, ‘depressive’, ‘depressed’, and ‘anti-depressant’. By utilizing a Venn diagram, we identified 65 overlapping targets between crocin and depression, which potentially represent latent targets for crocin in depression treatment.
Protein–protein interaction network construction
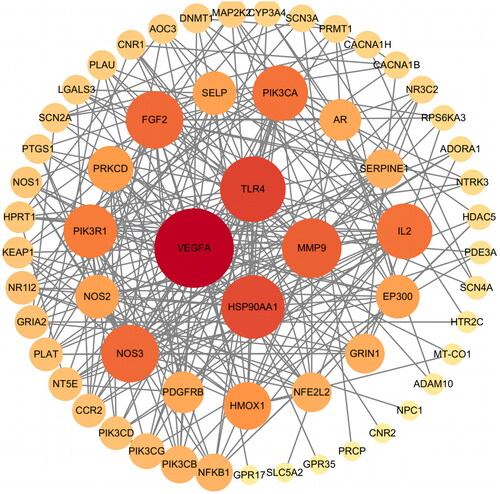
We utilized the STRING database to analyze the interactions among the overlapping targets and construct a PPI network. The PPI network was generated using Cytoscape v3.9.1, and the data were arranged as depicted in . The network consisted of 60 nodes and 256 edges, where larger nodes indicated higher connectivity and darker colours represented a greater role in the network. Similarly, darker edge colours indicated stronger connectivity. The screening criterion for identifying key targets was based on the median value of the connectivity degree. As a result, the key targets identified in this analysis were VEGFA, HSP90AA1, TLR4, MMP9, PIK3R1, FGF2, NOS3, IL2, PIK3CA, and HMOX1.
GO and KEGG enrichment analyses of crocin against depression
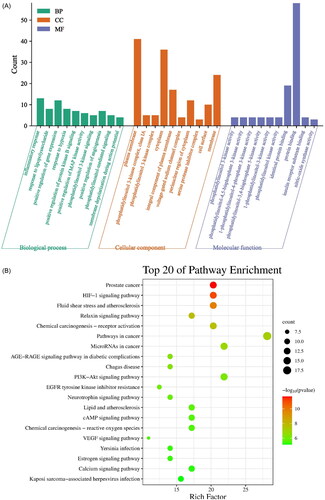
The 65 overlapping crocin antidepressant genes were subjected to GO annotation and KEGG pathway enrichment analyses using the DAVID electronic database. GO enrichment analysis yielded 37 cell components (CCs), 52 molecular functions (MFs), and 198 biological processes (BPs), with a p-value cutoff of 0.05. The top 10 results for BPs, CCs, and MFs were obtained and displayed as a Laplace diagram (). Significant BPs included the inflammatory response and response to lipopolysaccharides, while significant CC GO terms comprised plasma membrane, cytoplasm, and membrane. Notably, significant MF GO terms were associated with phosphatidylinositol 3-kinase activity, phosphatidylinositol-4,5-bisphosphate 3-kinase activity, and nitric-oxide synthase activity. Moreover, KEGG pathway enrichment analysis revealed that the 65 overlapping crocin antidepressant genes were significantly enriched in 120 signaling pathways. The first 20 KEGG-enriched pathways, based on p-values, were depicted in a bubble diagram to highlight the key mechanisms of crocin’s action against depression (). Notably, crocin mainly regulates the HIF-1, PI3K-Akt, and cAMP signaling pathways to exert its effect on depression.
Integrated analysis of metabolomics and network pharmacology
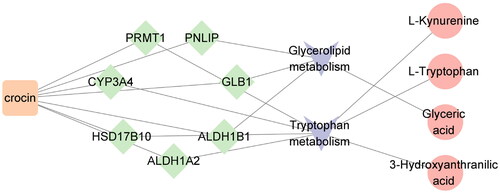
By integrating 151 targets enriched in five pathways with the targets related to crocin, we identified seven overlapping targets enriched in two pathways: tryptophan metabolism and glycerolipid metabolism. These targets include protein arginine methyltransferase 1 (PRMT1), galactosidase beta 1 (GLB1), hydroxysteroid 17-β-dehydrogenase 10 (HSD17B10), aldehyde dehydrogenase 1 family member A2 (ALDH1A2), aldehyde dehydrogenase 1 family member B1 (ALDH1B1), pancreatic lipase (PNLIP), and CYP3A4. Cytoscape (https://cytoscape.org/) was used to visualize the network of crocin, overlapping targets, pathways, and related metabolites (). Moreover, tryptophan metabolism was associated with three metabolites: l-tryptophan, 3-hydroxyanthranilic acid, and l-kynurenine, while glyceric acid was associated with glycerolipid metabolism.
Molecular docking
We further analyzed the results of integrated network pharmacology and metabolomics. This analysis revealed three intersection targets (PRMT1, CYP3A4, and GLB1) of crocin for antidepressant effects and their association with five major metabolic pathways. To validate the connections between crocin and these targets, molecular docking was employed. The binding energy and final values were utilized to evaluate the strength of the interactions between protein molecules and crocin molecules. Lower binding free energy and final values indicate stronger and more stable interactions between the ligand and the receptor. The binding energies of three crocin-related targets were calculated (). All targets exhibited binding energies less than 0, indicating autonomous binding of the ligand to the receptor. Furthermore, illustrates the interaction of crocin with PRMT1, CYP34A, and GLB1, showcasing 10 hydrophobic interactions with PRMT1, nine hydrogen bonds with CYP3A4, and six hydrogen bonds with GLB1.
Figure 9. Molecular docking-predicted binding mode. (A) Molecule docking of crocin binding to PRMT1. (B) Molecule docking of crocin binding to CYP3A4. (C) Molecule docking of crocin binding to GLB1.
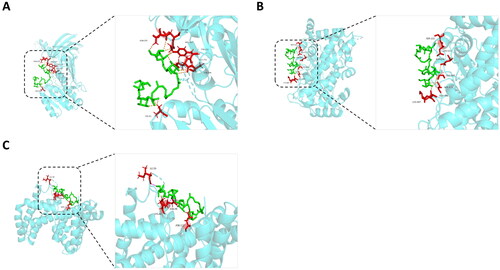
Table 2. Molecular docking score.
Discussion
Depression imposes a significant burden on both society and individuals (Li et al. Citation2022) and crocin is recognized as a natural solution for combating depressive disorders (Siddiqui et al. Citation2022). Although several studies have examined the therapeutic effects of crocin on depression, few have explored its metabolite variations through a metabolic approach.
In this study, we employed serum metabolomics and network pharmacology to investigate the potential targets and metabolic pathways associated with crocin’s antidepressant effects. Our results demonstrated comparable efficacy between crocin and fluoxetine in altering depressive behaviour. The metabolic analysis identified 101 differential metabolites, with significant alterations in the metabolic pathways of tryptophan, phenylalanine, histidine, glycerolipid metabolism, and steroid hormone biosynthesis. Additionally, employing network pharmacology analysis, we obtained 65 core targets and 120 related pathways. By combining these two approaches, we identified two core metabolic pathways (tryptophan metabolism and glycerolipid metabolism) and three core targets (PRMT1, CYP3A4, and GLB1) that likely play an essential role in the antidepressant crocin.
Depression is characterized by various behavioural phenotypes, including anhedonia, the inability to experience pleasure from rewarding or enjoyable activities (Liu et al. Citation2018). In animal experiments, SPT is commonly used to assess pleasure deficit symptoms (Verharen et al. Citation2023). The OFT evaluates the autonomous and exploratory behaviour and nervousness of experimental animals in a novel environment, which is used to observe voluntary locomotor ability. Additionally, because FST creates an inescapable oppressive environment, the animals exhibit ‘immobility’ which reflects their ‘desperate depressive state’ (Nadeau et al. Citation2022). We found that crocin increased the rate of sucrose preference and enhanced vertical and horizontal activity in CUMS-treated rats while reducing immobility time. This observation indicates that crocin improves depressive-like mood and alleviates despair in CUMS-treated rats and does not inhibit their activity. Therefore, crocin shows potential as a highly safe and effective antidepressant.
Utilizing untargeted serum metabolomic analysis based on LC-MS, we identified 101 metabolic biomarkers. Following crocin administration, seven metabolites exhibited reversed levels in CUMS-treated rats, including 3-hydroxybutyric acid, cholic acid (3R,6'Z)-3,4-dihydro-8-hydroxy-3-(6-pentadecenyl)-1H-2-benzopyran-1-one, 17-phenyl-18,19,20-trinor-prostaglandin E2. These metabolites primarily exerted antidepressant effects by regulating the metabolism of tryptophan, phenylalanine, histidine, glycerolipids, and biosynthesis of steroid hormones.
Metabolomics and network pharmacology analyses of crocin revealed its influence on five key metabolic pathways involving PRMT1, CYP3A4, HSD17B10, ALDH1A2, PNLIP, GLB1 and ALDH1B1. By integrating the metabolomics and network pharmacology data, three overlapping targets—PRMT1, CYP3A4, and GLB1—were identified as crucial for the antidepressant effects of crocin. These targets primarily act on tryptophan metabolism and glycerolipid metabolism pathways.
Tryptophan plays a pivotal role in depression as it is metabolized through two key pathways: serotonin and kynurenine. These tryptophan metabolic pathways have crucial implications for several depression-related processes (Correia and Vale Citation2022). Tryptophan is the only precursor of 5-hydroxytryptamine, a monoamine neurotransmitter implicated in depression (Xie et al. Citation2023). Notably, supplementation with this tryptophan is considered a potential treatment method for depression due to its association with the synthesis of tryptophan, 5-hydroxytryptamine and melatonin (Kałużna-Czaplińska et al. Citation2019).
Lipids are increasingly recognized for their significant role in the pathophysiology of depression, although only a few studies have been conducted (Pinto et al. Citation2022). Lipids serve various functions, including energy storage, microenvironment alteration, and influencing biofilm structure, all of which may contribute to the development of depression (Tsui-Pierchala et al. Citation2002; Ledesma et al. Citation2012; Schneider et al. Citation2017). A recent study indicated that differential products of lipid metabolism in the hippocampus, the memory hub of the brain, are primarily involved in glycerolipid and sphingolipid metabolism, potentially shedding light on the pathophysiological process of depression (Geng et al. Citation2020).
Crocin demonstrated strong binding activity to its target proteins (PRMT1, CYP3A4, and GLB1) predicted by molecular docking, thus might establish its material basis for alleviating depression. The mechanism of action of PRMT1 involves the methylation of key cellular proteins, including histones, and acts as a regulator in various cellular processes such as signal transduction, PPIs, and transcriptional regulation (Zhao et al. Citation2008). A previous study reported that PRMT1 not only regulates Nrf-2 expression, affecting inflammation and oxidative stress induced by LPS, but also affects anxiety and depression-like behaviour (Liu et al. Citation2019), which concurs with our study. Inhibiting or inducing Cytochrome P450 enzymes leads to changes in the plasma concentration of drugs (Červeňová Citation2019). These enzymes, particularly CYP2D6, CYP3A4, and P-glycoprotein, play a role in metabolizing antidepressant and anxiolytic drugs through various chemical reactions after absorption and distribution in the body (Zemanova et al. Citation2022). Further, a genome-wide meta-analysis on male depression identified a significant locus on 3p22.3, with the GLB1 gene in this region showing a significant correlation in gene-based tests (Hall et al. Citation2018). Therefore, based on the aforementioned literature evidence, the dependability of our findings can be well illustrated.
However, our study still has some limitations that need to be addressed in future work. The specific mechanisms underlying the regulation of metabolites associated with tryptophan and glycerolipid metabolism by crocin remain to be elucidated. In addition, regarding target validation, we only use molecular docking to verify the targets (PRMT1, CYP3A4, and GLB1) interacted with crocin. In the subsequent studies, we will not only perform in vivo but also in vitro experiments to further clarify the antidepressant mechanism of crocin in modulating these biomarkers and potential targets.
Conclusions
The antidepressant effect of crocin is likely mediated through the regulation of PRMT1, CYP3A4, and GLB1 targets, resulting in the modulation of tryptophan and glycerolipid metabolism. This integrated study utilizing untargeted serum metabolomics and network pharmacology provides novel perspectives and insights into crocin as an antidepressant. Additionally, our paper supplies a new reference for the study of active ingredients in traditional Chinese medicine.
Author contributions
Y.L, Z.Z, P.M designed the experiments; Y.L, Z.Z, H.L, L.W performed experiments; M.X, M.W, analysis the data; Y.L, Z.Z, D.G, X.D, J.L, writing and review of the manuscript; Y.L, Z.Z,Y.X, X.Z, P.M contributed to the study supervision. All authors have reviewed and approved it for publication.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The original data presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Additional information
Funding
References
- Abel EL. 1991. Behavior and corticosteroid response of maudsley reactive and nonreactive rats in the open field and forced swimming test. Physiol Behav. 50(1):151–153. doi: 10.1016/0031-9384(91)90513-n.
- Abuelezz SA, Hendawy N, Magdy Y. 2017. Targeting oxidative stress, cytokines and serotonin interactions via indoleamine 2, 3 dioxygenase by coenzyme Q10: role in suppressing depressive like behavior in rats. J Neuroimmune Pharmacol. 12(2):277–291. doi: 10.1007/s11481-016-9712-7.
- Albrakati A, Alsharif KF, Al Omairi NE, Alsanie WF, Almalki A, Abd Elmageed ZY, Elshopakey GE, Lokman MS, Bauomy AA, Abdel Moneim AE, et al. 2021. Neuroprotective efficiency of prodigiosins conjugated with selenium nanoparticles in rats exposed to chronic unpredictable mild stress is mediated through antioxidative, anti-inflammatory, anti-apoptotic, and neuromodulatory activities. Int J Nanomedicine. 16:8447–8464. doi: 10.2147/IJN.S323436.
- Červeňová J. 2019. Combination of mirtazapine and paroxetine: possible clinically demonstrated interaction. Cas Lek Cesk. 158:310–313.
- Chen S, Zhou M, Zhao X, Han Y, Huang Y, Zhang L, Wang J, Xiao X, Li P. 2022. Metabolomics coupled with network pharmacology study on the protective effect of Keguan-1 granules in LPS-induced acute lung injury. Pharm Biol. 60(1):525–534. doi: 10.1080/13880209.2022.2040544.
- Chi X, Xue X, Pan J, Wu J, Shi H, Wang Y, Lu Y, Zhang Z, Ma K. 2022. Mechanism of lily bulb and Rehmannia decoction in the treatment of lipopolysaccharide-induced depression-like rats based on metabolomics study and network pharmacology. Pharm Biol. 60(1):1850–1864. doi: 10.1080/13880209.2022.2121843.
- Correia AS, Vale N. 2022. Tryptophan metabolism in depression: a narrative review with a focus on serotonin and kynurenine pathways. Int J Mol Sci. 23:8493. doi: 10.3390/ijms23158493.
- Detke MJ, Rickels M, Lucki I. 1995. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl). 121(1):66–72. doi: 10.1007/BF02245592.
- Geng C, Hao G, Yi Q, Guo Y, Chen D, Han W, Zhang J, Yang M, Jiang P. 2020. The impact of Dl-3-n-butylphthalide on the lipidomics of the hippocampus in a rat model of lipopolysaccharide-induced depression. Prostaglandins Other Lipid Mediat. 150:106464. doi: 10.1016/j.prostaglandins.2020.106464.
- Gu X, Zhang G, Wang Q, Song J, Li Y, Xia C, Zhang T, Yang L, Sun J, Zhou M. 2022. Integrated network pharmacology and hepatic metabolomics to reveal the mechanism of Acanthopanax senticosus against major depressive disorder. Front Cell Dev Biol. 10:900637. doi: 10.3389/fcell.2022.900637.
- Gonul AS, Akdeniz F, Taneli F, Donat O, Eker C, Vahip S. 2005. Effect of treatment on serum brain-derived neurotrophic factor levels in depressed patients. Eur Arch Psychiatry Clin Neurosci. 255(6):381–386. doi: 10.1007/s00406-005-0578-6.
- Hall LS, Adams MJ, Arnau-Soler A, Clarke TK, Howard DM, Zeng Y, Davies G, Hagenaars SP, Maria Fernandez-Pujals A, Gibson J, et al. 2018. Genome-wide meta-analyses of stratified depression in generation Scotland and UK Biobank. Transl Psychiatry. 8(1):9. doi: 10.1038/s41398-017-0034-1.
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. 2008. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 33(6):693–710. doi: 10.1016/j.psyneuen.2008.03.008.
- Kałużna-Czaplińska J, Gątarek P, Chirumbolo S, Chartrand MS, Bjørklund G. 2019. How important is tryptophan in human health. Crit Rev Food Sci Nutr. 59(1):72–88. doi: 10.1080/10408398.2017.1357534.
- Kao WT, Chang CL, Lung FW. 2018. 5-HTT mRNA level as a potential biomarker of treatment response in patients with major depression in a clinical trial. J Affect Disord. 238:597–608. doi: 10.1016/j.jad.2018.06.035.
- Kuniishi H, Ichisaka S, Yamamoto M, Ikubo N, Matsuda S, Futora E, Harada R, Ishihara K, Hata Y. 2017. Early deprivation increases high-leaning behavior, a novel anxiety-like behavior, in the open field test in rats. Neurosci Res. 123:27–35. doi: 10.1016/j.neures.2017.04.012.
- Ledesma MD, Martin MG, Dotti CG. 2012. Lipid changes in the aged brain: effect on synaptic function and neuronal survival. Prog Lipid Res. 51(1):23–35. doi:10.1016/j.plipres.2011.11.004.22142854.
- Lei C, Chen Z, Fan L, Xue Z, Chen J, Wang X, Huang Z, Men Y, Yu M, Liu Y, et al. 2022. Integrating metabolomics and network analysis for exploring the mechanism underlying the antidepressant activity of paeoniflorin in rats with CUMS-induced depression. Front Pharmacol. 13:904190. doi: 10.3389/fphar.2022.904190.
- Li H, Xiang Y, Zhu Z, Wang W, Jiang Z, Zhao M, Cheng S, Pan F, Liu D, Ho R, et al. 2021. Rifaximin-mediated gut microbiota regulation modulates the function of microglia and protects against CUMS-induced depression-like behaviors in adolescent rat. J Neuroinflammation. 18(1):254. doi: 10.1186/s12974-021-02303-y.
- Li J, Gao W, Zhao Z, Li Y, Yang L, Wei W, Ren F, Li Y, Yu Y, Duan W, et al. 2022. Ginsenoside Rg1 reduced microglial activation and mitochondrial dysfunction to alleviate depression-like behaviour via the GAS5/EZH2/SOCS3/NRF2 axis. Mol Neurobiol. 59(5):2855–2873. doi: 10.1007/s12035-022-02740-7.
- Lin S, Li Q, Xu Z, Chen Z, Tao Y, Tong Y, Wang T, Chen S, Wang P. 2022. Detection of the role of intestinal flora and tryptophan metabolism involved in antidepressant-like actions of crocetin based on a multi-omics approach. Psychopharmacology (Berl). 239(11):3657–3677. doi: 10.1007/s00213-022-06239-w.
- Liu H, Jiang J, Zhao L. 2019. Protein arginine methyltransferase-1 deficiency restrains depression-like behavior of mice by inhibiting inflammation and oxidative stress via Nrf-2. Biochem Biophys Res Commun. 518(3):430–437. doi: 10.1016/j.bbrc.2019.08.032.
- Liu MY, Yin CY, Zhu LJ, Zhu XH, Xu C, Luo CX, Chen H, Zhu DY, Zhou QG. 2018. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat Protoc. 13(7):1686–1698. doi: 10.1038/s41596-018-0011-z.
- Liu T, Song Y, Hu A. 2021. Neuroprotective mechanisms of mangiferin in neurodegenerative diseases. Drug Dev Res. 82(4):494–502. doi: 10.1002/ddr.21783.
- Meyer JH, McMain S, Kennedy SH, Korman L, Brown GM, DaSilva JN, Wilson AA, Blak T, Eynan-Harvey R, Goulding VS, et al. 2003. Dysfunctional attitudes and 5-HT2 receptors during depression and self-harm. Am J Psychiatry. 160(1):90–99. doi: 10.1176/appi.ajp.160.1.90.
- Nadeau BG, Marchant EG, Amir S, Mistlberger RE. 2022. Thermoregulatory significance of immobility in the forced swim test. Physiol Behav. 247:113709. doi: 10.1016/j.physbeh.2022.113709.
- Nair A, Morsy MA, Jacob S. 2018. Dose translation between laboratory animals and human in preclinical and clinical phases of drug development. Drug Dev Res. 79(8):373–382. doi: 10.1002/ddr.21461.
- Nemeroff CB. 2002. Recent advances in the neurobiology of depression. Psychopharmacol Bull. 36 (2):6–23.
- Peng GJ, Tian JS, Gao XX, Zhou YZ, Qin XM. 2015. Research on the pathological mechanism and drug treatment mechanism of depression. Curr Neuropharmacol. 13(4):514–523. doi: 10.2174/1570159x1304150831120428.
- Pinto B, Conde T, Domingues I, Domingues MR. 2022. Adaptation of lipid profiling in depression disease and treatment: a Critical Review. Int J Mol Sci. 23:2032. doi: 10.3390/ijms23042032.
- Salek R, Dehghani M, Mohajeri SA, Talaei A, Fanipakdel A, Javadinia SA. 2021. Amelioration of anxiety, depression, and chemotherapy related toxicity after crocin administration during chemotherapy of breast cancer: a double blind, randomized clinical trial. Phytother Res. 35(9):5143–5153. doi: 10.1002/ptr.7180.
- Schneider M, Levant B, Reichel M, Gulbins E, Kornhuber J, Müller CP. 2017. Lipids in psychiatric disorders and preventive medicine. Neurosci Biobehav Rev. 76(Pt B):336–362. doi: 10.1016/j.neubiorev.2016.06.002.27317860.
- Shen F, Xie P, Li C, Bian Z, Wang X, Peng D, Zhu G. 2022. Polysaccharides from Polygonatum cyrtonema Hua reduce depression-like behavior in mice by inhibiting oxidative stress-Calpain-1-NLRP3 signaling axis. Oxid Med Cell Longev. 2022:2566917. doi: 10.1155/2022/2566917.
- Siddiqui SA, Ali Redha A, Snoeck ER, Singh S, Simal-Gandara J, Ibrahim SA, Jafari SM. 2022. Anti-depressant properties of crocin molecules in saffron. Molecules. 27:2076. doi: 10.3390/molecules27072076.
- Song C, Manku MS, Horrobin DF. 2008. Long-chain polyunsaturated fatty acids modulate interleukin-1beta-induced changes in behavior, monoaminergic neurotransmitters, and brain inflammation in rats. J Nutr. 138(5):954–963. doi: 10.1093/jn/138.5.954.
- Spulber S, Conti M, Elberling F, Raciti M, Borroto-Escuela DO, Fuxe K, Ceccatelli S. 2019. Desipramine restores the alterations in circadian entrainment induced by prenatal exposure to glucocorticoids. Transl Psychiatry. 9(1):263. doi: 10.1038/s41398-019-0594-3.
- Stachowicz K, Sowa-Kućma M. 2022. The treatment of depression - searching for new ideas. Front Pharmacol. 13:988648. doi: 10.3389/fphar.2022.988648.
- Talaei A, Hassanpour Moghadam M, Sajadi Tabassi SA, Mohajeri SA. 2015. Crocin, the main active saffron constituent, as an adjunctive treatment in major depressive disorder: a randomized, double-blind, placebo-controlled, pilot clinical trial. J Affect Disord. 174:51–56. doi: 10.1016/j.jad.2014.11.035.
- Tang Y, Su H, Wang H, Lu F, Nie K, Wang Z, Huang W, Dong H. 2021. The effect and mechanism of Jiao-tai-wan in the treatment of diabetes mellitus with depression based on network pharmacology and experimental analysis. Mol Med. 27(1):154. doi: 10.1186/s10020-021-00414-z.
- Thanacoody HK, Thomas SH. 2005. Tricyclic antidepressant poisoning: cardiovascular toxicity. Toxicol Rev. 24(3):205–214. doi: 10.2165/00139709-200524030-00013.
- Tsui-Pierchala BA, Encinas M, Milbrandt J, Johnson EM. Jr. 2002. Lipid rafts in neuronal signaling and function. Trends Neurosci. 25(8):412–417. doi: 10.1016/s0166-2236(02)02215-4.
- Vahdati Hassani F, Naseri V, Razavi BM, Mehri S, Abnous K, Hosseinzadeh H. 2014. Antidepressant effects of crocin and its effects on transcript and protein levels of CREB, BDNF, and VGF in rat hippocampus. Daru. 22:16.
- Verharen J, de Jong JW, Zhu Y, Lammel S. 2023. A computational analysis of mouse behavior in the sucrose preference test. Nat Commun. 14(1):2419. doi: 10.1038/s41467-023-38028-0.
- Wu GF, Ren S, Tang RY, Xu C, Zhou JQ, Lin SM, Feng Y, Yang QH, Hu JM, Yang JC. 2017. Antidepressant effect of taurine in chronic unpredictable mild stress-induced depressive rats. Sci Rep. 7(1):4989. doi: 10.1038/s41598-017-05051-3.
- Xie J, Wu WT, Chen JJ, Zhong Q, Wu D, Niu L, Wang S, Zeng Y, Wang Y. 2023. Tryptophan metabolism as bridge between gut microbiota and brain in chronic social defeat stress-induced depression mice. Front Cell Infect Microbiol. 13:1121445. doi: 10.3389/fcimb.2023.1121445.
- Yan ZY, Jiao HY, Chen JB, Zhang KW, Wang XH, Jiang YM, Liu YY, Xue Z, Ma QY, Li XJ, et al. 2021. Antidepressant mechanism of traditional Chinese medicine formula Xiaoyaosan in CUMS-induced depressed mouse model via RIPK1-RIPK3-MLKL mediated necroptosis based on network pharmacology analysis. Front Pharmacol. 12:773562. doi: 10.3389/fphar.2021.773562.
- Yeung KS, Hernandez M, Mao JJ, Haviland I, Gubili J. 2018. Herbal medicine for depression and anxiety: a systematic review with assessment of potential psycho-oncologic relevance. Phytother Res. 32(5):865–891. doi: 10.1002/ptr.6033.
- Yu C, Fu J, Guo L, Yu M, Yu D. 2022. Integrating metabolomics and network pharmacology to explore the protective effect of ginsenoside Re against radiotherapy injury in mice. Evid Based Complement Alternat Med. 2022:5436979.
- Yuan N, Gong L, Tang K, He L, Hao W, Li X, Ma Q, Chen J. 2020. An integrated pharmacology-based analysis for antidepressant mechanism of Chinese herbal formula Xiao-Yao-San. Front Pharmacol. 11:284. doi: 10.3389/fphar.2020.00284.
- Zemanova N, Anzenbacher P, Anzenbacherova E. 2022. The role of cytochromes P450 in the metabolism of selected antidepressants and anxiolytics under psychological stress. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 166(2):140–149. doi: 10.5507/bp.2022.019.
- Zhang F, Zhu X, Yu P, Sheng T, Wang Y, Ye Y. 2022. Crocin ameliorates depressive-like behaviors induced by chronic restraint stress via the NAMPT-NAD(+)-SIRT1 pathway in mice. Neurochem Int. 157:105343. doi: 10.1016/j.neuint.2022.105343.
- Zhang M, Liu Y, Zhao M, Tang W, Wang X, Dong Z, Yu S. 2017. Depression and anxiety behaviour in a rat model of chronic migraine. J Headache Pain. 18(1):27. doi: 10.1186/s10194-017-0736-z.
- Zhang Y, Liu X, Long J, Cheng X, Wang X, Feng X. 2022. Exploring active compounds and mechanisms of angong Niuhuang Wan on ischemic stroke based on network pharmacology and molecular docking. Evid Based Complement Alternat Med. 2022:2443615. doi: 10.1155/2022/2443615.
- Zhao X, Jankovic V, Gural A, Huang G, Pardanani A, Menendez S, Zhang J, Dunne R, Xiao A, Erdjument-Bromage H, et al. 2008. Methylation of RUNX1 by PRMT1 abrogates SIN3A binding and potentiates its transcriptional activity. Genes Dev. 22(5):640–653. doi: 10.1101/gad.1632608.
- Zhou X, Wang J, Lu Y, Chen C, Hu Y, Liu P, Dong X. 2020. Anti-depressive effects of Kai-Xin-San on lipid metabolism in depressed patients and CUMS rats using metabolomic analysis. J Ethnopharmacol. 252:112615. doi: 10.1016/j.jep.2020.112615.