?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Context
Qinggong Shoutao Wan (QGSTW) is a pill used as a traditional medicine to treat age-associated memory decline (AAMI). However, its potential mechanisms are unclear.
Objective
This study elucidates the possible mechanisms of QGSTW in treating AAMI.
Materials and methods
Network pharmacology and molecular docking approaches were utilized to identify the potential pathway by which QGSTW alleviates AAMI. C57BL/6J mice were divided randomly into control, model, and QGSTW groups. A mouse model of AAMI was established by d-galactose, and the pathways that QGSTW acts on to ameliorate AAMI were determined by ELISA, immunofluorescence staining and Western blotting after treatment with d-gal (100 mg/kg) and QGSTW (20 mL/kg) for 12 weeks.
Results
Network pharmacology demonstrated that the targets of the active components were significantly enriched in the cAMP signaling pathway. AKT1, FOS, GRIN2B, and GRIN1 were the core target proteins. QGSTW treatment increased the discrimination index from −16.92 ± 7.06 to 23.88 ± 15.94% in the novel location test and from −19.54 ± 5.71 to 17.55 ± 6.73% in the novel object recognition test. ELISA showed that QGSTW could increase the levels of cAMP. Western blot analysis revealed that QGSTW could upregulate the expression of PKA, CREB, c-Fos, GluN1, GluA1, CaMKII-α, and SYN. Immunostaining revealed that the expression of SYN was decreased in the CA1 and DG.
Discussion and conclusions
This study not only provides new insights into the mechanism of QGSTW in the treatment of AAMI but also provides important information and new research ideas for the discovery of traditional Chinese medicine compounds that can treat AAMI.
Introduction
Aging is associated with the decline of various physiologic and behavioral functions, the most obvious of which is cognitive function (Bishop et al. Citation2010; Harada et al. Citation2013). The geroscience hypothesis states that brain degeneration accelerates aging and that cognitive decline leads to disability and loss of independence in older adults. Enormous pressure is brought to bear by family and society. Extensive evidence from animal and human studies demonstrates that brain structure, network connections, neuronal morphology and function, and neurotransmitter levels are altered in different brain regions (Schmid et al. Citation2016). The prefrontal cortex and hippocampus are the two regions most affected by aging, and they are especially relevant to cognitive and behavioral deterioration during aging. Calcium antagonists, cholinesterase inhibitors, and ionic glutamate receptor antagonists have been proposed as potential therapies for treating aging-related cognitive dysfunction (León et al. Citation2013). However, there is no Food and Drug Administration (FDA)-approved treatment available. More importantly, traditional therapies with a single target are unsuitable for the treatment of cognitive decline. Therefore, identifying novel multitarget drugs underlying the regulation of brain function is essential for developing effective treatments.
There is general agreement in the scientific community that long-term potentiation (LTP) represents the neurophysiological substrate, or at least one of the substrates, of learning and memory (Nicoll Citation2017). Using a variety of behavioral tasks, it has been consistently demonstrated that pharmacological, genetic, or disease-induced disruption of LTP impairs memory formation and/or consolidation, whereas enhancement of LTP improves cognitive performance, at least in healthy animals and in transgenic mouse models of human neurodegenerative diseases (Bliss et al. Citation2013). Since its discovery, thousands of studies have been performed on LTP, especially in the hippocampus, and many cellular and molecular determinants have been identified as being involved in its expression (Park et al. Citation2021; Jin et al. Citation2022; Laha et al. Citation2022). cAMP is a well-established second messenger required for LTP and memory formation/consolidation. The evidence accumulated following the pioneering studies on Aplysia and Drosophila (Kandel Citation2012) clearly demonstrates that the cAMP pathway is critically involved in the process of memory consolidation. In particular, pharmacological and genetic manipulation of the cAMP/PKA/CREB pathway were found to affect late LTP (L-LTP) and long-term memory (LTM) in a variety of specific behavioral tasks (Ricciarelli and Fedele Citation2015).
Qinggong Shoutao Wan (QGSTW) is an antiaging prescription that was used by the Chinese emperor Qianlong of the Qing Dynasty and included in the third national nonmaterial cultural heritage list in 2011. QGSTW comprises traditional Chinese medicines, including Panax ginseng C. A. Mey. [Araliaceae] (the root and the rhizome), Ziziphus jujuba Mill. var. spinosa (Bunge) Hu ex H. F. Chou. [Rhamnaceae] (the seed), Rehmannia glutinosa Libosch. [Scrophulariaceae] (the root tuber), Lycium barbarum L. [Solanaceae] (the fruit), Asparagus cochinchinensis (Lour.) Merr. [Liliaceae] (the root tuber), Ophiopogon japonicus (L. f.) Ker-Gawl. [Liliaceae] (the root tuber), Angelica sinensis (Oliv.) Diels [Apiaceae] (the root), Alpinia oxyphylla Miq. [Zingiberaceae] (the fruit), Testis et Penis Canis [Canidae] (the penis), Penis et Testis Equi Asini [Equidae] (the penis and the testicles), Penis et Testis Cervi [Cervidae] (the penis and the testicles), Bombyx mori Linnaeus. [Bombycidae] (the feces) and Juglans regia L. [Juglandaceae] (the wooden septum within the fruit core). QGSTW is an effective treatment for memory decline, dizziness, fatigue, tinnitus, deafness, and nocturia resulting from aging in older adults (Chen et al. Citation1984, Citation1985). Clinical trials have shown that QGSTW can significantly alleviate amnestic mild cognitive impairment and prevent 8.85% of amnestic mild cognitive impairment patients from developing Alzheimer’s disease (Tian et al. Citation2019). QGSTW can significantly ameliorate fatigue, dizziness, tinnitus, deafness, and nocturia in patients (≥ 45 years old), improve short-term and long-term memory, decrease plasma lipid peroxide levels, and elevate plasma estradiol and testosterone concentrations (Chen et al. Citation1984, Citation1985). It has been confirmed that QGSTW markedly enhances the survival rate of quails and inhibits the formation of lipid peroxide in the rat liver (Chen et al. Citation1984, Citation1985). However, the mechanism of QGSTW in memory maintenance in older adults remains unexplored.
Traditional Chinese medicine (TCM) compounds act on multiple molecular targets or multiple biochemical pathways. Therefore, it is difficult to study their pharmacological mechanism using conventional methods. Network pharmacology is an efficient strategy for TCM research and development and is used to study drug actions and interactions with multiple targets. Currently, network pharmacology methods are widely used in new drug development, and computer network analysis software is used to fully display the relationships between drugs and targets, targets and diseases, diseases and diseases, and drugs and drugs (Buriani et al. Citation2020). Molecular docking is a kind of molecular simulation technology used to predict the best binding mode between the ligand and the receptor molecule (Satpathy Citation2020), and it can be used for the development of new drugs. This study reveals a previously unknown mechanism of QGSTW in treating d-galactose-injured mice by network pharmacology, molecular docking, and experimental verification and presents a new therapeutic strategy for treating age-related memory impairment.
Materials and methods
Collection of chemical components of QGSTW and screening of active compounds
The active ingredients of QGSTW were screened according to the absorption, distribution, metabolism and enhancement (ADME) of drugs, while the oral bioavailability (OB), drug likeness (DL) and gastrointestinal absorption (GA) were used as screening parameters (Vugmeyster et al. Citation2012). According to the ADME data (OB ≥30% and DL ≥0.18), the active ingredients of QGSTW were collected from traditional Chinese medicine databases, including the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) (Ru et al. Citation2014) and the High-Throughput Experiment-and Reference-Guided Database of Traditional Chinese Medicine (HERB) (Fang et al. Citation2021). Next, the structures of the compounds for which information was collected from HERB were obtained from PubChem (Kim et al. Citation2016). Compounds with both a high possibility of GA and a high DL score were detected using Swiss ADME (Daina et al. Citation2017) and were defined as the bioactive compounds. Eventually, the targets of the bioactive compounds in QGSTW were identified from the TCMSP and Swiss Target Prediction databases (Daina et al. Citation2019).
Identification of gene targets associated with memory disorder
The genes related to memory disorder were collected from the GeneCards database (Stelzer et al. Citation2016), Online Mendelian Inheritance in Man (OMIM) (Amberger et al. Citation2015) and DrugBank database (Wishart et al. Citation2018) using ‘memory disorder’ or ‘memory impairment’ as the search term. Then, unified UniProt gene IDs were obtained for the targets of QGSTW components and memory disorder-related targets by searching the Universal Protein Resource (UniProt) database. The targets of the active ingredients of QGSTW and memory disorder-related targets were intersected to obtain the potential targets of QGSTW in the treatment of memory disorder, and these targets were imported into VENNY2.1 for visualization.
Gene ontology (GO) and Kyoto encyclopedia of genes and genomes (KEGG) enrichment analysis
To investigate the key signaling pathways and cell functions in which the targets of memory disorder and QGSTW are involved, we used the Database for Annotation, Visualization and Integrated Discovery (DAVID) v6.8 (Huang da et al. Citation2009) to perform KEGG pathway enrichment analysis of the potential targets. The results are expressed as a bubble chart (p < 0.05). Next, the pathways in which the targets were most strongly enriched according to KEGG pathway enrichment analysis were identified, bubble diagrams of the GO enrichment analysis results were drawn, and enriched GO terms in the biological process (BP), molecular function (MF) and cell component (CC) categories were determined; terms and pathway with p values <0.05 were considered significantly enriched.
Construction of a protein–protein interaction (PPI) network
The intersection of the drug targets and memory disorder-related genes was depicted using a Venn diagram. Furthermore, the STRING database (Szklarczyk et al. Citation2019) was used to construct a protein–protein interaction (PPI) network of the intersected targets; for construction of the network, the species was limited to humans (Homo sapiens) and the interaction score was ≥0.4. The TSV file was downloaded from the STRING database, imported into Cytoscape 3.6.0 (Shannon et al. Citation2003) to construct a PPI network, and analyzed with Network Analyzer.
Cluster analysis
Cluster analysis is used to screen out identical or similar nodes and protein complexes from complex PPI networks (Anitha et al. Citation2016). The MCODE plug-in in Cytoscape was used for cluster analysis of the PPI networks (Deng et al. Citation2019). The node score cutoff was 0.2, the k core was 2, the maximum depth was 100, and the degree cutoff was 2.
Construction of an active compound-disease-target network
The active compounds of QGSTW and their potential targets were imported into Cytoscape 3.6.0 software to construct a compound-disease-target interaction network. The network was visualized with Cytoscape software and analyzed with Network Analyzer.
Molecular docking of key ingredients to key targets
The top 5 protein targets and key active ingredients of QGSTW were selected as receptors and ligands, respectively. The protein structures were dewatered and deliganded using PyMOL 2.5 software (Seeliger and de Groot Citation2010). Then, the core gene targets were hydrogenated, the charge was calculated using AutoDockTools (Cosconati et al. Citation2010), and the results were saved in .pdbqt format. The docking pocket is a possible binding site for ligands in the receptor. We set up a docking box large enough to cover the entire receiver so that it contained all possible docking pockets. The size of the docking box varied for different receptors. Upon completion, ligand–receptor molecular docking was performed using AutoDockVina 1.1.2. The stability of the receptor–ligand complex depends on the binding energy. The binding model was visualized using PyMol 2.5 software.
Preparation and quality control of QGSTW
Refined QGSTW powder (batch number P004) was provided by Darentang Pharmaceutical Factory (Tianjin, China). The refined QGSTW powder was prepared as follows: raw traditional Chinese medicine ingredients were mixed, dried, and crushed into fine powders; then, the fine powders were sieved and sterilized to obtain a refined powder. Details about the quality control method for the prepared QGSTW can be found in the China Patent File (CN102048990B) (see https://patents.google.com/patent/CN102048990B/en).
Animal experiments
Specific-pathogen free (SPF) male C57BL/6J mice aged 8 weeks (SYXK (Jing) 2016-0006, Beijing Vital River Laboratory Animal Technology Limited Company, Beijing, China) were housed separately in a standard animal housing room (room temperature: 22 ± 2 °C; relative humidity: 30-40%; 12 h dark/light cycle), and standard rodent chow and water were provided ad libitum (SYXK 2020-0005). The animals were adapted to their new environment for 7 days before the experiment. All experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA) and approved by the Ethics Committee of Tianjin University of Traditional Chinese Medicine (serial number: TCM-LAEC2021240, Tianjin, China). The experimental animals were divided randomly into the following three groups: control (n = 6 mice), d-galactose model group (model group, n = 6 mice) and d-galactose + QGSTW group (QGSTW group, n = 4 mice). d-galactose was dissolved in sterile saline. QGSTW was dissolved in CmC-Na solution.
Mice in the control group were injected with saline (s.c.) into the neck and administered CmC-Na solution (20 mL/kg/day, p.o.) for 12 weeks.
Mice in the model group were injected with d-gal (100 mg/kg/day, s.c.) into the neck and administered CmC-Na solution (20 mL/kg/day, p.o.) for 12 weeks.
Mice in the QGSTW group were injected with d-gal (100 mg/kg/day, s.c.) into the neck and administered QGSTW (20 mL/kg/day, p.o.) for 12 weeks.
Behavioral tests
After treatment with d-gal and QGSTW for 12 weeks, the behavior of each mouse was assessed in the novel location test (Heyser and Chemero Citation2012), novel object recognition test (Busquets-Garcia et al. Citation2013) and active avoidance test (Ghafarimoghadam et al. Citation2022) (control/model group: n = 6 mice per group; QGSTW group: n = 4 mice).
Novel location test
This test is used to measure the spatial memory of mice through evaluation of their ability to recognize the new location of a familiar object with respect to spatial cues. The experimental procedure was developed by the core facility of the Waisman Center at UW-Madison based on the tendency of mice to explore novel objects (Heyser and Chemero Citation2012). All procedures were conducted during the light cycle between 9 am and 5 pm. Before the test session, the mice were allowed to acclimate to the testing room for at least 30 min. The test consisted of five 6 min trials with a 3 min intertrial interval. During the intertrial interval, the mice remained inside the testing room in a holding cage. In the first trial, each mouse was placed in the center of an otherwise empty open arena (38.5 cm long × 38.5 cm wide × 25.5 cm high walls) for 6 min. In the next three trials (the training trials), two identical objects were placed in different corners of the arena equidistant from the walls, which were marked with colored decals. The objects were taped to the floor of the arena. Then, each mouse was placed in the center of the arena and allowed to explore for 6 min. At the end of the trial, the mouse was removed and returned to its home cage for 3 min. In the last trial (the test trial), one of the objects was moved to a new location, and the mouse was allowed to explore the objects for 6 min; the total time spent exploring each object was measured. During the test trial, exploration was defined as sniffing within <2 cm of an object or other motivated direct contact with the object. To control for odor cues, the objects and the floor of the arena were cleaned with 75% ethanol solution before each trial.
The discrimination index was calculated as follows:
(Contestabile et al. Citation2013). A higher discrimination index is considered to reflect greater memory retention of the object that was moved to a novel location. All trials were recorded and scored by researchers who were blinded to the experimental conditions to ensure accuracy.
Novel object recognition (NOR) test
This test is based on the natural propensity of rodents to preferentially explore novel objects over familiar ones. The experimental procedure was performed as described previously (Busquets-Garcia et al. Citation2013) and conducted during the light cycle from 9 am to 5 pm. Before the test, the mice were allowed to acclimate to the testing room for at least 30 min. On the first day, the mice were habituated for 10 min to an empty open arena (38.5 cm long × 38.5 cm wide × 25.5 cm high walls). On the second day, two identical objects were placed in different corners of the arena equidistant from the walls, which were marked with colored decals, and the mice were placed in the arena for another 10 min. After 24 h, one of the familiar objects was replaced with a novel object, and the mice were again placed in the arena and allowed to explore for 10 min. The total time spent exploring each object (novel and familiar) was measured. Before the testing phase, the novel and familiar objects were wiped down so that the objects had the same odor. Exploration was defined as the orientation of the nose toward an object at a distance of less than 2 cm. The discrimination index was calculated as the difference between the percentages of time spent investigating the novel object and the time spent investigating the familiar object as follows:
A higher discrimination index indicated greater memory retention for the novel object. All trials were videotaped and scored by researchers who were blinded to the experimental conditions to ensure accuracy.
Active avoidance test
This test is based on the natural propensity of rodents to preferentially avoid light and fearful stimuli (such as electric shocks to the feet) (Ghafarimoghadam et al. Citation2022). The experimental procedure was conducted during the light cycle between 9 am and 5 pm. Before the trial, the mice were allowed to acclimate to the testing room for at least 30 min. The test was performed in a shuttle box with two compartments separated by a guillotine door and a floor made of steel grids. On the first day, the house light was turned off, the guillotine door was closed, and each mouse was placed in the dark box and allowed to habituate for 60 s. Then, the house light was turned on, a 10,000 Hz 50 dB tone (conditioned stimulus, CS) was delivered, and the guillotine door was opened. A 0.2 mA electric foot shock (unconditioned stimulus, US) was applied for 5 s after 15 s. Immediately after the mouse entered the light compartment, the connecting door was closed, and both the CS and US were discontinued. An intertrial interval of 20 s was applied between each session. If the mouse crossed the guillotine door before the presentation of the US, its behavior was defined as ‘avoidance’ (conditioned reaction, CR). If it crossed the guillotine door during delivery of the US, its behavior was defined as ‘escaping’ (unconditioned reaction, UR). If the mouse did not display either of these actions, its behavior was classified as ‘failure to escape’, and the latency was recorded as 20 s. During the test phase, the compartments were wiped down between each trial so that the compartments had the same odor. The number of CRs and mean latency to the CR were measured over 50 sessions by TSE Systems software. After 24 h, the above tests were repeated, and behaviors were recorded continuously for 2 days.
Collection of brain tissue and protein extraction
After drug administration and behavior tests, the mice were sacrificed by decapitation under general anesthesia (intraperitoneally injected urethanes), and the brains were removed immediately. The hippocampus was carefully dissected from the brain, and all tissues were frozen in liquid nitrogen and stored at −80 °C until use. The hippocampus was homogenized in a mixture of PBS and phosphatase and protease inhibitors and then centrifuged at 12,s000 rpm at 4 °C for 10 min (Allegra™ X-22R centrifuge, Beckman Coulter, Brea, CA, USA). The supernatants were collected and stored at −80 °C.
Western blot analysis
The protein levels of AKT, c-Fos, NMDA receptor (GluN1), AMPA receptor (GluA1), CaMKII-α, SYN, PKA and CREB were measured by Western blot analysis. Mouse hippocampal tissues were lysed in ice-cold RIPA buffer mixed with a cocktail of protease and phosphatase inhibitors. The supernatant was collected after centrifugation, and the BCA assay was performed to measure the protein concentration. Equal amounts of protein from each sample were separated by 4-12% SDS–PAGE and then transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were cut into pieces in accordance with the sizes of the target proteins and blocked with 5% nonfat dry milk in 10% Tris buffered saline Tween (TBST) at room temperature for 2 h to block nonspecific binding. The membranes were incubated with different primary antibodies at 4 °C overnight (AKT, 1:1,000 dilution, #4685S, Cell Signaling Technology, Danvers, MA, USA; c-Fos, 1:1,000 dilution, #2250, Cell Signaling Technology; NMDA receptor (GluN1), 1:1,000 dilution, #5704, Cell Signaling Technology; AMPA receptor (GluA1), 1:1,000 dilution, #13185, Cell Signaling Technology; CaMKII-α, 1:1,000 dilution, #50049, Cell Signaling Technology; PKA C-α, 1:1,000 dilution, #5842, Cell Signaling Technology; CREB, 1:500 dilution, #9197, Cell Signaling Technology; β-tubulin, 1:1,000 dilution, #2128, Cell Signaling Technology; and Synapsin 1 (SYN), 1:2,000 dilution, CPA7127, Cohesionbio, London, UK). After being washed with 10% TBST solution, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody for 2 h at room temperature, and the protein bands were visualized with an enhanced electrochemiluminescence (ECL) system (Tanon ss Tanon, Shanghai, China). The data were analyzed with ImageJ software.
Enzyme-linked immunosorbent assay (ELISA)
The concentration of cAMP in the hippocampus was measured with a commercial ELISA kit (Elabscience Biotechnology Co., Ltd., Wuhan, China) in accordance with the manufacturer’s instructions. In brief, standard working solution and hippocampal tissue lysates were added to the wells of micro-ELISA plates and immediately mixed with biotinylated antibody (Ab) detection working solution. Then, the plate was covered and incubated at 37 °C for 45 min. After removing the solution from each well, the plate was washed three times. Afterward, horseradish peroxidase (HRP)-conjugated working solution was added to each well, and the plate was again covered and incubated at 37 °C for 30 min. The solution was then removed from each well, and the plate was washed five times. After the addition of substrate reagent, the plate was covered and incubated at 37 °C for 15 min. Stop solution was added to each well, and the optical density at 450 nm was determined using a microplate reader (Bio-Tek Elx800; BioTek Instruments, Inc., Winooski, VT, USA).
Tissue processing and immunofluorescence staining
To prepare brain sections, mice were transcardially perfused with ice-cold PBS followed by ice-cold 4% paraformaldehyde. Brain tissues were fixed for more than 24 h before being dehydrated in a graded alcohol series. The samples were sequentially soaked in alcohol–benzene and xylene and then embedded in paraffin. The wax-soaked tissues were cut into slices with a thickness of 4 μm. Finally, the sections were stored at room temperature. They were deparaffinized in xylene and rehydrated by successive incubation with decreasing ethanol concentrations. The hippocampal slices were subjected to antigen retrieval by incubation in Tris-EDTA buffer at 100 °C for 20 min and washed with TBS for 3 min × 3. Subsequently, the sections were permeabilized with 0.3% Triton X-100 and incubated with 10% normal goat serum for 1 h at room temperature to block nonspecific binding. After blocking, the sections were incubated overnight at 4 °C with primary antibody (Synapsin-1 (SYN), 1:200 dilution, #5297, Cell Signaling Technology, Danvers, MA, USA), followed by incubation with secondary antibody (goat-anti mouse conjugated to Alexa Fluor 488, 1:100 dilution, CSA3211, Cohesionbio, London, UK) at room temperature for 1 h in the dark. The nuclei were counterstained with DAPI. Finally, the brain sections were washed in TBS, incubated with spontaneous fluorescence quenching reagent for 5 min, and sealed with antifade mounting medium. Images were collected by fluorescence microscopy (Olympus). DAPI appeared blue, and FITC appeared green.
Statistical analysis
All experimental results are expressed as the mean ± SEM. Statistical differences among different groups were assessed with one-way analysis of variance (ANOVA) using SPSS software (version 24.0, Chicago, IL, USA). p Values less than 0.05 were considered to indicate significance. Graphing was performed using GraphPad Prism 8.0 software (San Diego, CA, USA).
Results
Identification of the potential targets of QGSTW in the treatment of memory disorder
OB ≥30%, DL ≥0.18 or both were used as the criteria for a high possibility of GA and a high DL score, and 206 active components of QGSTW were identified from TCMSP, HERB, and the literature; 2 of these components were from the root of Chinese angelica (DG), 43 were from Juglans regia (FXM), 22 were from ginseng (RS), 9 were from spine date seed (SZR), 9 were from Asparagus cochinchinensis root (TD), 43 were from the fruit of Chinese wolfberry (GQZ), 30 were from Liriope equivalent plant (MD), 15 from fresh Rehmannia root (SD), 1 was from dog testis (GS), and 32 were from sharpleaf galangal (YZR). The targets of the active ingredients were predicted by the aforementioned target prediction web servers (TCMSP and Swiss Target Prediction). After removing redundant targets, 1310 potential targets were obtained altogether.
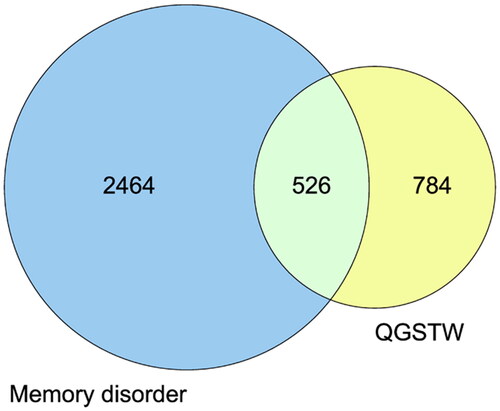
A total of 5049 memory disorder-related targets were collected from the GeneCards database as disease targets, and 2525 targets with a relevance score ≥ 5.87 (the median value) were selected for subsequent analysis. Additionally, 38 and 634 memory disorder-related targets were collected from DrugBank and OMIM, respectively. After the removal of duplicates, 2990 disease target genes were obtained. A Venn diagram of the 1310 active compound targets and the 2990 disease targets was drawn (). A total of 526 overlapping targets were obtained, and they were considered the core genes for subsequent research.
KEGG pathway and GO enrichment analysis
KEGG pathway enrichment analysis of the 526 common targets was performed. A total of 133 pathways (p < 0.05) were identified. The top 20 enriched signaling pathways were selected for visualization (). The results showed that the targets were enriched mainly in the neuroactive ligand–receptor interaction, cAMP signaling pathway, calcium signaling pathway, PI3K-Akt signaling pathway and MAPK signaling pathway. Most of the enriched signaling pathways were associated with neurotransmitters, synaptic function, neuronal morphology and physiological characteristics, suggesting that QGSTW may treat age-related memory disorder by intervening in these signaling pathways.
Figure 2. Functional enrichment analysis. (A) KEGG pathway analysis. The Y-axis represents the main pathway, and the X-axis represents the enrichment score. (B) GO biological processes. (C) GO molecular functions. (D) GO cellular components. The Y-axis represents the enrichment count of the target, and the X-axis represents the enrichment score.
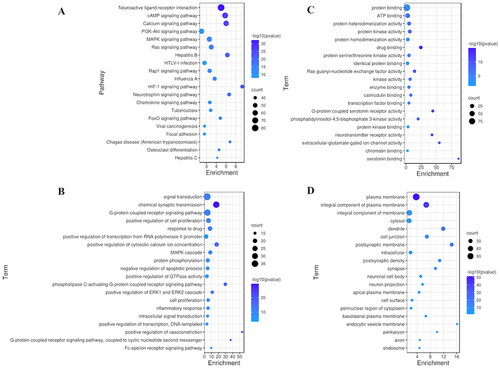
In addition, we analyzed the biological process (BP), molecular function (MF), and cellular component (CC) terms in which the 137 common targets associated with the top three KEGG pathways (the neuroactive ligand–receptor interaction, cAMP signaling pathway, and calcium signaling pathway) were enriched. The common targets were significantly enriched in 524 GO terms (p < 0.05), including 368 BP terms, 107 MF terms, and 49 CC terms. The top 20 significantly enriched terms in the BP, MF and CC categories are displayed visually (). The results showed that the main BP terms were signal transduction, chemical synaptic transmission, G-protein coupled receptor signaling pathway, positive regulation of cell proliferation, and response to drugs; the main MF terms were protein binding, ATP binding, protein heterodimerization activity, protein kinase activity, and protein homodimerization activity; and the main CC terms were plasma membrane, integral component of plasma membrane, integral component of membrane, cytosol, dendrite, cell junction, postsynaptic membrane, intracellular, postsynaptic density, synapse, neuronal cell body, and neuron projection.
PPI network analyses
A total of 137 common targets were imported into the STRING database, and their relationships were visualized as a PPI network that contained 137 nodes and 288 edges. For a better explanation of the relationships among targets, data in tab-separated value (TSV) format were imported into Cytoscape 3.6.0. The proteins are depicted as network nodes of various sizes and colors based on the number of combined targets (the degree value) (). The orange nodes in the middle represent the 10 proteins encoded by genes with the largest degree values, i.e., those that play the most important roles in the development and progression of memory disorder; these proteins are RAC-α serine/threonine-protein kinase (AKT1), the proto-oncogene c-Fos (FOS), glutamate receptor ionotropic NMDA 2B (GRIN2B), glutamate receptor ionotropic NMDA 1 (GRIN1), metabotropic glutamate receptor 5 (GRM5), epidermal growth factor receptor (EGFR), calcium/calmodulin-dependent protein kinase type II subunit alpha (CAMKII-α), mitogen-activated protein kinase 1 (MAPK1), cannabinoid receptor 1 (CNR1), and glutamate receptor 1 (GRIA1).
Figure 3. Protein–protein interaction (PPI) network and cluster analysis of the disease targets. (A) PPI network of the common targets. The orange nodes in the middle represent the target genes; the darker the color and the larger the node is, the greater the degree value. (B–E) Top four clustering graphs for the PPI network of common targets.
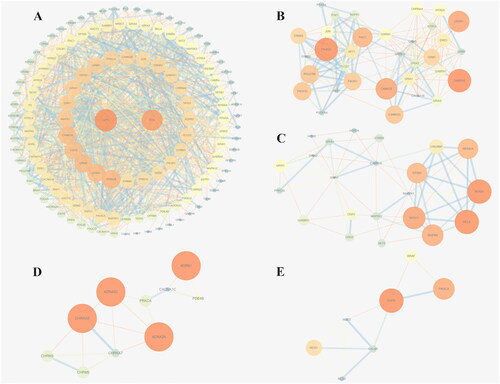
The MCODE plug-in in Cytoscape was then used to cluster the memory disorder-related targets in the PPI network. Six clusters are shown in . The top 4 clusters according to score are shown in .
Table 1. Cluster information for the memory disorder protein–protein interaction (PPI) network.
Construction of a botanical drug-active ingredient-potential target network
As QGSTW has multiple components, an active compound-disease-target network was constructed by using Cytoscape 3.6.0 () to further determine the main active components of QGSTW in the treatment of memory disorder. The network contained 334 nodes and 2707 edges. The top 10 active components in terms of degree value, including stigmasterol, β-sitosterol, zizyphusine, deoxyharringtonine, girinimbin, celabenzine, (S)-coclaurine, sanjoinenine, acetic acid, and stigmasterol-β-d-glucoside, were identified as pivotal components of QGSTW in the common targets–active ingredients network, indicating that they might be the active ingredients of QGSTW in treating memory disorders ().
Figure 4. Compound-disease-target network. The yellow triangle represents QGSTW, the yellow V represents memory disorder, the hexagons represent herbs in QGSTW, the blue round rectangles represent the active components of QGSTW, the dark blue circles represent the potential targets, and the purple diamonds represent the top 10 enriched KEGG pathways.
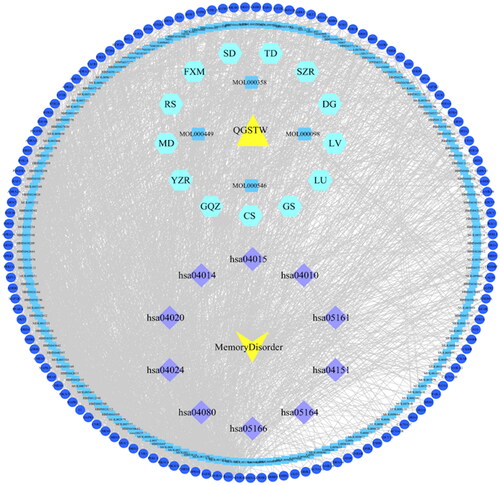
Table 2. The top 10 active components in terms of degree value.
Validation by molecular docking
We employed a molecular docking approach to explore the potential binding modes and interactions of the top 5 targets (AKT1, FOS, GRIN2B, GRIN1, and GRM5) with the top 10 active components of QGSTW. The stability of the binding conformations was negatively correlated with the binding energy. The molecular docking results indicated that three active compounds of QGSTW [(S)-coclaurine, girinimbin, and acetic acid] could enter the binding pocket of and bind to AKT1, GRIN2B, GRIN1 and GRM5. These proteins showed a higher affinity for QGSTW, providing important insight ( and ).
Figure 5. Molecular docking study of the top 5 target genes with the active components predicted by network pharmacology. The docking of AKT1 (a), GRIN1 (B), GRIN2B (C), GRM5 (D) and QGSTW. The protein targets that bind to the ligands are in the shape of blue rods, and the binding sites are connected by purple hydrogen bonds. The length of the hydrogen bond is indicated next to the bond.
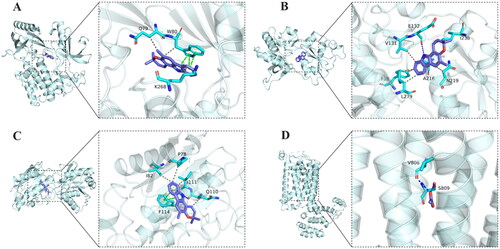
Table 3. The docking information of targets with QGSTW.
Experimental validation
Effects of QGSTW on cognitive decline in d-galactose-injured mice
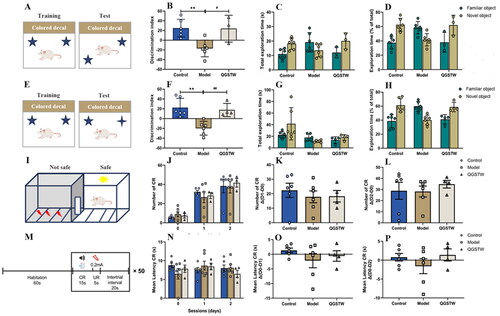
It has been confirmed that d-galactose can induce brain aging accompanied by age-associated decreases in learning and memory in mice (Xie et al. Citation2021; Yu et al. Citation2021; Zhang et al. Citation2021). We therefore investigated whether QGSTW can restore cognitive functions in d-galactose-injured mice. We treated mice with QGSTW and assessed their cognitive functions using the NLT (), NOR test () and AA test ( after treatment. Compared with the mice in the control group, the d-galactose-injured mice exhibited impaired spatial learning in the NLT, defective learning in the NOR test and deficient long-term memory in the AA test, as indicated by a reduced discrimination index (), reduced percentage of time spent exploring the novel location/object (), reduced number of CR (), and increased mean latency of CR (). QGSTW treatment alleviated cognitive deficits in d-galactose-injured mice according to the NLT (), NOR test () and AA test () compared with the model mice. Together, these results suggested that QGSTW treatment alleviates cognitive decline in d-galactose-injured mice.
Figure 6. QGSTW alleviates cognitive decline in d-galactose-injured mice. (A) Schematic of the novel location test (NLT) for assessing spatial learning. The discrimination index (B), total exploration time (C) and percentage exploration time (D) in the novel location test are shown. (E) Schematic of the novel object recognition (nor) test. The discrimination index (F), total exploration time (G) and percentage exploration time (H) in the novel object recognition test are shown. (I, M) Schematic of the active avoidance (AA) test. The number of CRs (J), the mean latency to a CR (N), and the difference in CR number (K, L) and the mean latency (O, P) between the first and second days are shown. Compared with the control group: *p < 0.05; **p < 0.01; ***p < 0.001. Compared with the model group: #p < 0.05; ##p < 0.01; ###p < 0.001. One-way ANOVA and two-way ANOVA were used for data analyses. The data are presented as the mean ± SEM (n = 3–6 mice per group).
The key target and molecular mechanism of QGSTW in treating cognitive decline in d-galactose-injured mice
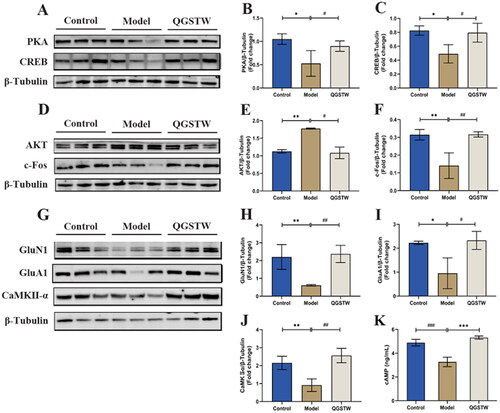
Based on the top 10 targets and the results of KEGG pathway enrichment analysis, we chose to study the core targets AKT, c-Fos, GluN1, GluA1, CaMKII-α and cAMP signaling pathway-related proteins (cAMP, PKA, and CREB) to identify the mechanisms underlying the neuroprotective effects of QGSTW. We first discovered that the protein levels of c-Fos, GluN1, GluA1, CaMKII-α, cAMP, PKA, and CREB were significantly decreased and that the protein level of AKT was significantly increased in d-galactose-injured mice compared with control mice. We found that compared with model mice, QGSTW-treated mice had significantly increased protein levels of PKA (), CREB (), c-Fos (), GluN1 (), GluA1 (), CaMKII-α (), and cAMP () and reduced protein levels of AKT (€). Therefore, our data suggested that QGSTW can alleviate cognitive decline by regulating the cAMP signaling pathway and the core targets AKT, c-Fos, GluN1, GluA1, and CaMKII-α in d-galactose-injured mice.
Figure 7. Effects of QGSTW on the protein expression levels of AKT, c-Fos, GluN1, GluA1, CaMKII-α, PKA, CREB, and cAMP in d-galactose-injured mice. (A) Western blotting was performed to measure the protein expression of PKA and CREB. Quantitative analysis of the protein expression of PKA (B) and CREB (C). Representative immunoblot (D) and quantification of AKT (E) and c-Fos (F) levels in mice. (G) Representative Western blots and histograms showing the total protein levels of GluN1 (H), GluA1 (I), and CaMKII-α (J) in mice. (K) cAMP levels were analyzed by ELISA. Compared with the control group: *p < 0.05; **p < 0.01; ***p < 0.001. Compared with the model group: #p < 0.05; ##p < 0.01; ###p < 0.001. One-way ANOVA was used for all data analyses. The data are presented as the mean ± SEM (n = 3 mice per group).
The effects of QGSTW on the protein expression level of synapsin (SYN) in the hippocampus of d-galactose-injured mice
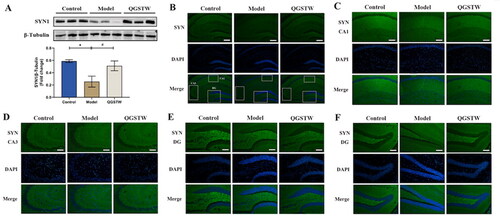
Most of the top 10 cellular component terms were associated with synapse-related structures. We chose to use the presynaptic marker SYN to identify the site at which QGSTW acts to determine whether QGSTW increases the synaptic number in the hippocampus. We first found that the protein level of SYN was significantly decreased in d-galactose-injured mice compared with control mice, and the protein levels of SYN () was significantly increased in QGSTW-treated mice compared with model mice. This result indicated that QGSTW increases the number of synapses in the hippocampus of mice. Next, we performed immunostaining with antibodies against SYN, which showed a punctate staining pattern. We found that the expression of SYN was decreased in the CA1 region and DG region of d-galactose-injured mice, while QGSTW treatment markedly elevated the expression of SYN in the CA1 and DG subregions of the mouse hippocampus. However, no significant difference was found in the CA3 region, showing that QGSTW mainly acts on the CA1 and DG regions of the hippocampus to ameliorate synaptic loss induced by d-galactose.
Figure 8. Effects of QGSTW on the protein expression level of SYN in d-galactose-injured mice. (A) Representative Western blot and quantitative analysis of the protein expression level of SYN in the mouse hippocampus. (B–F) Representative immunofluorescence images of SYN (green, FITC) in different subregions of the hippocampus (scale bars: B = 100 μm; C–F = 50 μm). The nuclei were stained blue (DAPI). CA1: cornu ammonis 1; CA3: cornu ammonis 3; DG: dentate gyrus. Compared with the control group: *p < 0.05 and **p < 0.01. Compared with the model group: #p < 0.05 and ##p < 0.01. One-way ANOVA was used for all data analyses. The data are presented as the mean ± SEM (n = 3 mice per group).
Discussion
Aging is a common problem faced by human beings. Many people experience a rapid decline in learning and memory upon aging, and these changes can greatly disrupt their lives. This decline is categorized as age-related learning and memory impairment (Lamberty and Gower Citation1990; Tong et al. Citation2007). Age-related learning and memory impairment is one of the manifestations of aging and a natural physiological change; methods to prevent early aging and alleviate the symptoms of age-related learning and memory impairment are of great significance. QGSTW comprises a variety of traditional Chinese medicines that can act on multiple organs and ameliorate multiple symptoms of aging, including memory loss. In our studies, we explored the specific mechanism and target of QGSTW in alleviating age-associated memory loss by network pharmacology, discovered the active ingredients responsible for its effect, and verified these findings in a d-galactose-injured mouse model of aging.
The use of systematic network pharmacology for the identification of biological targets, functional processes and molecular pathways is an attractive research approach for determining how QGSTW treats age-related memory impairment prior to preclinical or clinical research. The GO and KEGG pathway enrichment analysis results revealed that the effects of QGSTW are mechanistically linked to pharmacological modulation of the neuroactive ligand–receptor interaction, cAMP signaling pathway and calcium signaling pathway. According to the detailed bioinformatics data, QGSTW alleviates memory disorder via the regulation of signal transduction, chemical synaptic transmission and the G-protein coupled receptor signaling pathway. Some of these pathways and processes are critical for neuronal transmission, and their dysfunction may ultimately lead to disruption of neuronal plasticity and memory deficits. Neuroactive ligand–receptor interactions, which mediate the transduction of signals from the extracellular environment into cells, may be involved in the modulation of synaptic plasticity (Xiang et al. Citation2019). The cAMP signaling pathway is closely related to glutamate synaptic plasticity (Kim et al. Citation2021; Yuan et al. Citation2021). cAMP activates protein kinase A (PKA) and exchanges proteins activated by cAMP. Activation of the cAMP pathway can ultimately lead to activation of cAMP response element binding protein (CREB) to facilitate the transcription of CRE-dependent genes. Traditional Chinese medicine therapy enhances neuroplasticity in SAMP8 mice via regulation of the cAMP/PKA/CREB signaling pathway, thus improving cognitive function (Hou et al. Citation2022). Calcium signaling is involved in neurotransmitter release, synaptic plasticity, gene expression and other important neuronal functions (Lynch and Seubert Citation1989; Briggs et al. Citation2017; Pchitskaya et al. Citation2018). According to recent research, age-related calcium/cAMP/PKA dysregulation in the dorsolateral prefrontal cortex (dlPFC) is highly correlated with early-stage tau phosphorylation (Datta et al. Citation2021). Calcium dysregulation in the entorhinal cortex has been observed early in the aging process in rodents, consistent with the vulnerability of this area to AD-related tau pathology.
Using a PPI network assay, all potential biological targets of QGSTW in the treatment of age-related memory impairment were methodically identified. Bioinformatics analysis clearly showed that the six identified core targets, FOS (c-Fos), GRIN2B (GluN2B), GRIN1 (GluN1), GRM5 (mGluR5), CAMK2A (CaMKII-α) and GRIA1 (GluA1), were involved in the modulation of synaptic plasticity. Rapid and transient expression of c-Fos is associated with the initial steps of long-term memory formation in different learning tasks (Izquierdo and Medina Citation1997; Tischmeyer and Grimm Citation1999; Guzowski Citation2002; Kubik et al. Citation2007), and deletion of the c-Fos gene or inhibition of its expression at the time of training impairs memory (Lamprecht and Dudai Citation1996; Tischmeyer and Grimm Citation1999; He et al. Citation2002). Ionotropic glutamate receptors mediate the majority of excitatory synaptic transmission in vertebrate brains (Traynelis et al. Citation2010). Their activation by presynaptically released l-glutamate results in rapid depolarization of the postsynaptic membrane and signaling events that mediate synaptic plasticity, the substrate for learning and memory (Kessels and Malinow Citation2009; Bliss and Collingridge Citation2013; Huganir and Nicoll Citation2013). CaMKII is regarded as the central regulator of synaptic plasticity with strong control over learning and memory processes in the brain. CaMKII activity is linked to NMDAR channels. Upon activation, NMDARs facilitate the influx of extracellular Ca2+ into postsynaptic neurons. Ca2+ binds to its receptor calmodulin, forming a calcium/calmodulin (Ca2+/CaM) complex and further phosphorylating CaMKII (Baltaci et al. Citation2019). mGluR5 is ubiquitously expressed in brain regions that are responsible for memory and learning. It plays a key role in modulating rapid changes in synaptic transmission and plasticity. mGluR5 supports long-term changes in synaptic strength by regulating the transcription and translation of essential synaptic proteins (Abd-Elrahman and Ferguson Citation2022). Other identified core targets, AKT1, EGFR, MAPK1 and CNR1 (CB1), have been shown to participate in enhancing learning and memory performance through regulation (Grasing et al. Citation2021; Amini and Abdolmaleki Citation2022; Mo et al. Citation2022; Yang et al. Citation2022).
To predict the active pharmacological ingredient of QGSTW in memory disorder treatment, we identified the top 10 active components by constructing a compound-disease-target network. Stigmasterol and β-sitosterol are present in Chinese angelica root, Chinese wolfberry fruit, ginseng and Asparagus cochinchinensis root. 3(R)-Deoxyharringtonine, girinimbin and celabenzine are present in ginseng. Zizyphusine, (S)-coclaurine and sanjoinenine are present in spine date seed. Acetic acid is found in Juglans regia, and stigmasterol-β-d-glucoside is found in Liriope spicata var. prolifera.
Molecular docking is a method for simulating the interaction between a ligand and receptor and predicting binding patterns and affinity. A molecular docking assay was performed to investigate the interactions between the bioactive components of QGSTW and target proteins. (S)-Coclaurine had a potentially high binding affinity for AKT1, and girinimbin had potentially a high binding affinity for AKT1, GRIN1, and GRIN2B. (S)-Coclaurine is found in spine date seed, and girinimbin is found in ginseng; both are well-known neuroprotective components. At present, there are few studies on the effects of (S)-coclaurine and girinimbin on memory deficits. However, the beneficial effects derived from the action of ginseng on NMDA-type glutamate receptors, including its ability to inhibit NMDA-triggered Ca2+ influx, protect cultured hippocampal neurons against glutamate-induced excitotoxicity, and exert a neuroprotective effect, are now being studied in animal models (Lee et al. Citation2006; Peng et al. Citation2009; Zhang et al. Citation2012).
Long-term treatment with high-dose d-galactose can induce memory impairment in rodents. Superoxide anions produced during d-galactose metabolism directly destroy tissues and organs and promote the generation of ROS (Guo et al. Citation2019; Remigante et al. Citation2020). In addition, d-galactose can also elevate the levels of advanced glycosylation end products (AGEs) and MDA through nonenzymatic glycosylation reactions (Zeng et al. Citation2020), causing oxidative damage and neuroinflammation in the brain and eventually leading to neurodegenerative symptoms (Qian et al. Citation2008; Lu et al. Citation2010). Therefore, the d-galactose-induced aging model can partially mimic the human aging process and is widely used for the pharmacodynamic evaluation of anti-dementia drugs. In our study, a mouse model of aging was successfully constructed by d-galactose treatment and was applied to explore the effects of QGSTW in treating memory decline and its potential mechanism. A series of tests, including the novel location test, novel object recognition test, and active avoidance test, were used to comprehensively evaluate the effects of QGSTW in improving learning, memory, and cognitive functions in mice. The results showed that memory and learning were significantly impaired in d-galactose-induced mice and that QGSTW could efficiently reverse memory impairment in the behavior tests.
Finally, to verify the bioinformatics analysis results, a series of experiments were conducted. Bioinformatics suggested that the effect of QGSTW in alleviating memory decline might be related to its regulation of the cAMP signaling pathway. Western blot analysis of hippocampal tissues showed that QGSTW elevated the expression of proteins related to this signaling pathway, including AKT, c-Fos, GluN1, GluA1, CaMKII-α, cAMP, PKA and CREB. AKT activation is increased in the hippocampal and cortical neurons of AD patients, and the subcellular localization of AKT is changed with concomitant inactivation of PTEN (Griffin et al. Citation2005; Moloney et al. Citation2010; Sonoda et al. Citation2010). Incessant activation of the PI3K/Akt pathway suppresses mTOR inhibition and the protective effect of FOXO signaling, thus aggravating the effects of tau hyperphosphorylation and Aβ deposition, cognitive impairment, and synaptic damage (O’Neill et al. Citation2012). IEGs such as c-Fos is commonly as indicators learning-related neuronal activation and are involved in memory encoding (Josselyn et al. Citation2015; Tonegawa et al. Citation2015; Salery et al. Citation2021). C-Fos encodes a transcription factor that regulates the activity of effector genes and subsequent long-term neuronal plasticity (Miyashita et al. Citation2018). Glutamate is released from presynaptic neurons and transmits signals from presynaptic neurons to postsynaptic neurons through two ionic glutamate receptors, namely, NMDARs and AMPARs, which have various postsynaptic effects (Borroto-Escuela et al. Citation2018). The NMDAR subunit GluN1 and the AMPAR subunit GluA1 are the major markers of synaptic plasticity in glutamate neurons (Sanderson et al. Citation2016; Purkey and Dell’Acqua Citation2020). In this study, QGSTW treatment promoted the protein expression of GluN1 and GluA1. Furthermore, neural activity enhances CaM acetylation through NMDAR activation. CaM acetylation is important for the full activation of CaMKII-α and phosphorylation of GluA1 during LTP (Zhang et al. Citation2021). In addition to the GluA1 subunit of the AMPA receptor, CaMKII-α can interact with and phosphorylate a variety of synaptic proteins, contributing to synaptic plasticity and learning (Strack et al. Citation2000; Shonesy et al. Citation2014; Marks et al. Citation2018; Perfitt et al. Citation2020). Moreover, cAMP second messenger signaling pathways lead to the activation of PKA, which mediates signals regulating neurotransmission and, via transcription factors such as CREB, regulates genes involved in neuronal development, plasticity, and survival (Kim et al. Citation2021; Yuan et al. Citation2021). Previous studies have indicated that PKA/CREB is a vital upstream factor of NMDAR and thus the formation of new synapses (Wei et al. Citation2021; Wu et al. Citation2021). It has been reported that the predominant function of CREB is its function in the synaptic plasticity associated with long-term memory (Bartsch et al. Citation1998; Altarejos and Montminy Citation2011; Del Blanco et al. Citation2019), and disruption of CREB (both the α and δ isoforms) in mice causes defects in long-term potentiation and long-term memory (Bourtchuladze et al. Citation1994). In contrast, expression of the dominant-active CREB polypeptide accelerates the learning process (Yin et al. Citation1994, Citation1995). Our study found that QGSTW upregulates the expression of cAMP signaling pathway-related proteins, which is consistent with the results of previous studies. Synapsin 1 is associated with the regulation of chemical synaptic transmission, neurotransmitter secretion, the regulation of neurotransmitter secretion, synaptic vesicle exocytosis, and synaptic vesicle clustering. The levels of SYN 1 are significantly lower in various brain regions, notably the hippocampus, dentate gyrus, and posterior cingulate gyrus, in AD patients (Jovanovic et al. Citation2000; Mirza and Zahid Citation2018). This study found that QGSTW can improve synaptic function by upregulating the expression of SYN in the hippocampal CA1 and DG regions.
Conclusion
This study first indicates that QGSTW alleviated memory decline in d-galactose-injured mice through regulating the cAMP signaling pathway and synaptic plasticity and suggests the potential of QGSTW for AAMI therapy.
Author contributions
Guiyun Pan and Lijuan Chai are co-first authors. Yingqiang Zhao and Yi Wang were involved in the study design and coordination, provided material support, obtained funding, and supervised the study. Guiyun Pan, Zhihui Song and Wanying Feng performed the network analysis. Lijuan Chai, Rui Chen and Qing Yuan designed the validation experiment. Jinna Wei, Zhihua Yang, Yuhang Zhang and Guinan Xie performed the experiments. Guiyun Pan and Lijuan Chai wrote and revised the manuscript. An Yan, Qingbo Lv and Caijun Wang performed most of the experiments and statistical analyses. All authors approved the final version of the paper.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abd-Elrahman KS, Ferguson SSG. 2022. Noncanonical metabotropic glutamate receptor 5 signaling in Alzheimer’s disease. Annu Rev Pharmacol Toxicol. 62(1):235–254. doi: 10.1146/annurev-pharmtox-021821-091747.
- Altarejos JY, Montminy M. 2011. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol. 12(3):141–151. doi: 10.1038/nrm3072.
- Amberger JS, Bocchini CA, Schiettecatte F, Scott AF, Hamosh A. 2015. OMIM.org: online Mendelian inheritance in man (OMIM®), an online catalog of human genes and genetic disorders. Nucleic Acids Res. 43(Database issue):D789–98. doi: 10.1093/nar/gku1205.
- Amini M, Abdolmaleki Z. 2022. The effect of cannabidiol coated by nano-chitosan on learning and memory, hippocampal CB1 and CB2 levels, and amyloid plaques in an Alzheimer’s disease rat model. Neuropsychobiology. 81(3):171–183. doi: 10.1159/000519534.
- Anitha P, Anbarasu A, Ramaiah S. 2016. Gene network analysis reveals the association of important functional partners involved in antibiotic resistance: a report on an important pathogenic bacterium Staphylococcus aureus. Gene. 575(2 Pt 1):253–263. doi: 10.1016/j.gene.2015.08.068.
- Baltaci SB, Mogulkoc R, Baltaci AK. 2019. Molecular mechanisms of early and late LTP. Neurochem Res. 44(2):281–296. doi: 10.1007/s11064-018-2695-4.
- Bartsch D, Casadio A, Karl KA, Serodio P, Kandel ER. 1998. CREB1 encodes a nuclear activator, a repressor, and a cytoplasmic modulator that form a regulatory unit critical for long-term facilitation. Cell. 95(2):211–223. doi: 10.1016/s0092-8674(00)81752-3.
- Bishop NA, Lu T, Yankner BA. 2010. Neural mechanisms of ageing and cognitive decline. Nature. 464(7288):529–535. doi: 10.1038/nature08983.
- Bliss TV, Collingridge GL. 2013. Expression of NMDA receptor-dependent LTP in the hippocampus: bridging the divide. Mol Brain. 6(1):5–18. doi: 10.1186/1756-6606-6-5.
- Bliss TV, Collingridge GL, Morris RG. 2013. Synaptic plasticity in health and disease: introduction and overview. Philos Trans R Soc Lond B Biol Sci. 369(1633):20130129. doi: 10.1098/rstb.2013.0129.
- Borroto-Escuela DO, Tarakanov AO, Brito I, Fuxe K. 2018. Glutamate heteroreceptor complexes in the brain. Pharmacol Rep. 70(5):936–950. doi: 10.1016/j.pharep.2018.04.002.
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. 1994. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 79(1):59–68. doi: 10.1016/0092-8674(94)90400-6.
- Briggs CA, Chakroborty S, Stutzmann GE. 2017. Emerging pathways driving early synaptic pathology in Alzheimer’s disease. Biochem Biophys Res Commun. 483(4):988–997. doi: 10.1016/j.bbrc.2016.09.088.
- Buriani A, Fortinguerra S, Sorrenti V, Caudullo G, Carrara M. 2020. Essential oil phytocomplex activity, a review with a focus on multivariate analysis for a network pharmacology-informed phytogenomic approach. Molecules. 25(8):1833. doi: 10.3390/molecules25081833.
- Busquets-Garcia A, Gomis-González M, Guegan T, Agustín-Pavón C, Pastor A, Mato S, Pérez-Samartín A, Matute C, de la Torre R, Dierssen M, et al. 2013. Targeting the endocannabinoid system in the treatment of fragile X syndrome. Nat Med. 19(5):603–607. doi: 10.1038/nm.3127.
- Chen KJ, Zhou WQ, Li CS, Shi TR, Lei SP, Li XH, et al. 1984. Clinical study of Qinggong Shoutao Pill on delaying senility – clinical effect and effect on plasma lipid peroxidation level. Chin J Integr Trad West Med. 4(11):658–659 + 642. Chinese.
- Chen KJ, Zhou WQ, Li CS, Shi TR, Wang W, Wang JS, et al. 1985. Clinical and experimental study of Qinggong Shoutao Pill on delaying senility. J Trad Chin Med. 26:25–28.
- Contestabile A, Greco B, Ghezzi D, Tucci V, Benfenati F, Gasparini L. 2013. Lithium rescues synaptic plasticity and memory in Down syndrome mice. J Clin Invest. 123(1):348–361. doi: 10.1172/JCI64650.
- Cosconati S, Forli S, Perryman AL, Harris R, Goodsell DS, Olson AJ. 2010. Virtual screening with AutoDock: theory and practice. Expert Opin Drug Discov. 5(6):597–607. doi: 10.1517/17460441.2010.484460.
- Daina A, Michielin O, Zoete V. 2017. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 7(1):42717. doi: 10.1038/srep42717.
- Daina A, Michielin O, Zoete V. 2019. SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 47(W1):W357–W364. doi: 10.1093/nar/gkz382.
- Datta D, Leslie SN, Wang M, Morozov YM, Yang S, Mentone S, Zeiss C, Duque A, Rakic P, Horvath TL, et al. 2021. Age-related calcium dysregulation linked with tau pathology and impaired cognition in non-human primates. Alzheimers Dement. 17(6):920–932. doi: 10.1002/alz.12325.
- Del Blanco B, Guiretti D, Tomasoni R, Lopez-Cascales MT, Muñoz-Viana R, Lipinski M, Scandaglia M, Coca Y, Olivares R, Valor LM, et al. 2019. CBP and SRF co-regulate dendritic growth and synaptic maturation. Cell Death Differ. 26(11):2208–2222. doi: 10.1038/s41418-019-0285-x.
- Deng JL, Xu YH, Wang G. 2019. Identification of potential crucial genes and key pathways in breast cancer using bioinformatic analysis. Front Genet. 10:695–711. doi: 10.3389/fgene.2019.00695.
- Fang S, Dong L, Liu L, Guo J, Zhao L, Zhang J, Bu D, Liu X, Huo P, Cao W, et al. 2021. HERB: a high-throughput experiment- and reference-guided database of traditional Chinese medicine. Nucleic Acids Res. 49(D1):D1197–D1206. doi: 10.1093/nar/gkaa1063.
- Ghafarimoghadam M, Mashayekh R, Gholami M, Fereydani P, Shelley-Tremblay J, Kandezi N, Sabouri E, Motaghinejad M. 2022. A review of behavioral methods for the evaluation of cognitive performance in animal models: current techniques and links to human cognition. Physiol Behav. 244:113652. doi: 10.1016/j.physbeh.2021.113652.
- Grasing M, Kenned K, Sarnak MJ, Burns JM, Gupta A. 2021. Mild to moderate decrease in eGFR and cognitive decline in older adults. Nephrol Dial Transplant. 37(8):1499–1506. doi: 10.1093/ndt/gfab226.
- Griffin RJ, Moloney A, Kelliher M, Johnston JA, Ravid R, Dockery P, O’Connor R, O’Neill C. 2005. Activation of Akt/P KB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and P T EN are features of Alzheimer’s disease pathology. J Neurochem. 93(1):105–117. doi: 10.1111/j.1471-4159.2004.02949.x.
- Guo S, Wang J, Xu H, Rong W, Gao C, Yuan Z, Xie F, Bi K, Zhang Z, Li Q. 2019. Classic prescription, Kai-Xin-San, ameliorates Alzheimer’s disease as an effective multitarget treatment: from neurotransmitter to protein signaling pathway. Oxid Med Cell Longev. 2019:9096409.
- Guzowski JF. 2002. Insights into immediate-early gene function in hippocampal memory consolidation using antisense oligonucleotide and fluorescent imaging approaches. Hippocampus. 12(1):86–104. doi: 10.1002/hipo.10010.
- Harada CN, Natelson Love MC, Triebel KL. 2013. Normal cognitive aging. Clin Geriatr Med. 29(4):737–752. doi: 10.1016/j.cger.2013.07.002.
- He J, Yamada K, Nabeshima T. 2002. A role of Fos expression in the CA3 region of the hippocampus in spatial memory formation in rats. Neuropsychopharmacology. 26(2):259–268. doi: 10.1016/S0893-133X(01)00332-3.
- Heyser CJ, Chemero A. 2012. Novel object exploration in mice: not all objects are created equal. Behav Processes. 89(3):232–238. doi: 10.1016/j.beproc.2011.12.004.
- Hou Z, Yang X, Li Y, Chen J, Shang H. 2022. Electroacupuncture enhances neuroplasticity by regulating the orexin a-mediated cAMP/PKA/CREB signaling pathway in senescence-accelerated mouse prone 8 (SAMP8) mice. Oxid Med Cell Longev. 2022:8694462.
- Huang da W, Sherman BT, Lempicki RA. 2009. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 4(1):44–57. doi: 10.1038/nprot.2008.211.
- Huganir RL, Nicoll RA. 2013. AMPARs and synaptic plasticity: the last 25 years. Neuron. 80(3):704–717. doi: 10.1016/j.neuron.2013.10.025.
- Izquierdo I, Medina JH. 1997. Memory formation: the sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol Learn Mem. 68(3):285–316. doi: 10.1006/nlme.1997.3799.
- Jin SX, Liu L, Li S, Meunier AL, Selkoe DJ. 2022. Aβ oligomers from human brain impair mossy fiber LTP in CA3 of hippocampus, but activating cAMP-PKA and cGMP-PKG prevents this. Neurobiol Dis. 172:105816. doi: 10.1016/j.nbd.2022.105816.
- Josselyn SA, Köhler S, Frankland PW. 2015. Finding the engram. Nat Rev Neurosci. 16(9):521–534. doi: 10.1038/nrn4000.
- Jovanovic JN, Czernik AJ, Fienberg AA, Greengard P, Sihra T S. 2000. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat Neurosci. 3(4):323–329. doi: 10.1038/73888.
- Kandel ER. 2012. The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol Brain. 5(1):14–25. doi: 10.1186/1756-6606-5-14.
- Kessels HW, Malinow R. 2009. Synaptic AMPA receptor plasticity and behavior. Neuron. 61(3):340–350. doi: 10.1016/j.neuron.2009.01.015.
- Kim YJ, Lee JS, Kim H, Jang JH, Choung YH. 2021. Gap junction-mediated intercellular communication of cAMP prevents CDDP-induced ototoxicity via cAMP/PKA/CREB pathway. Int J Mol Sci. 22(12):6327. doi: 10.3390/ijms22126327.
- Kim S, Thiessen PA, Bolton EE, Chen J, Fu G, Gindulyte A, Han L, He J, He S, Shoemaker BA, et al. 2016. PubChem substance and compound databases. Nucleic Acids Res. 44(D1):D1202–13. doi: 10.1093/nar/gkv951.
- Kubik S, Miyashita T, Guzowski JF. 2007. Using immediate-early genes to map hippocampal subregional functions. Learn Mem. 14(11):758–770. doi: 10.1101/lm.698107.
- Laha K, Zhu M, Gemperline E, Rau V, Li L, Fanselow MS, Lennertz R, Pearce RA. 2022. CPP impairs contextual learning at concentrations below those that block pyramidal neuron NMDARs and LTP in the CA1 region of the hippocampus. Neuropharmacology. 202:108846. doi: 10.1016/j.neuropharm.2021.108846.
- Lamberty Y, Gower AJ. 1990. Age-related changes in spontaneous behavior and learning in NMRI mice from maturity to middle age. Physiol Behav. 47(6):1137–1144. doi: 10.1016/0031-9384(90)90364-a.
- Lamprecht R, Dudai Y. 1996. Transient expression of c-Fos in rat amygdala during training is required for encoding conditioned taste aversion memory. Learn Mem. 3(1):31–41. doi: 10.1101/lm.3.1.31.
- Lee E, Kim S, Chung KC, Choo MK, Kim DH, Nam G, Rhim H. 2006. 20(S)-ginsenoside Rh2, a newly identified active ingredient of ginseng, inhibits NMDA receptors in cultured rat hippocampal neurons. Eur J Pharmacol. 536(1–2):69–77. doi: 10.1016/j.ejphar.2006.02.038.
- León R, Garcia AG, Marco-Contelles J. 2013. Recent advances in the multitarget-directed ligands approach for the treatment of Alzheimer’s disease. Med Res Rev. 33(1):139–189. doi: 10.1002/med.20248.
- Lu J, Wu DM, Hu B, Zheng YL, Zhang ZF, Wang YJ. 2010. NGF-dependent activation of TrkA pathway: a mechanism for the neuroprotective effect of troxerutin in d-galactose-treated mice. Brain Pathol. 20(5):952–965. doi: 10.1111/j.1750-3639.2010.00397.x.
- Lynch G, Seubert P. 1989. Links between long-term potentiation and neuropathology. An hypothesis involving calcium-activated proteases. Ann N Y Acad Sci. 568(1):171–180. doi: 10.1111/j.1749-6632.1989.tb12505.x.
- Marks CR, Shonesy BC, Wang X, Stephenson JR, Niswender CM, Colbran RJ. 2018. Activated CaMKIIα binds to the mGlu5 metabotropic glutamate receptor and modulates calcium mobilization. Mol Pharmacol. 94(6):1352–1362. doi: 10.1124/mol.118.113142.
- Mirza FJ, Zahid S. 2018. The Role of Synapsins in Neurological Disorders. Neurosci Bull. 34(2):349–358. doi: 10.1007/s12264-017-0201-7.
- Miyashita T, Kikuchi E, Horiuchi J, Saitoe M. 2018. Long-term memory engram cells are established by c-Fos/CREB transcriptional cycling. Cell Rep. 25(10):2716–2728.e3. doi: 10.1016/j.celrep.2018.11.022.
- Mo H, Wang L, Chen Y, Zhang X, Huang N, Liu T, Hu W, Zhong Y, Li Q. 2022. Age-related memory vulnerability to interfering stimuli is caused by gradual loss of MAPK-dependent protection in Drosophila. Aging Cell. 21(6):e13628. doi: 10.1111/acel.13628.
- Moloney AM, Griffin RJ, Timmons S, O’Connor R, Ravid R, O’Neill C. 2010. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and insulin signaling. Neurobiol Aging. 31(2):224–243. doi: 10.1016/j.neurobiolaging.2008.04.002.
- Nicoll RA. 2017. A brief history of long-term potentiation. Neuron. 93(2):281–290. doi: 10.1016/j.neuron.2016.12.015.
- O’Neill C, Kiely AP, Coakley MF, Manning S, Long-Smith CM. 2012. Insulin and IGF-1 signalling: longevity, protein homoeostasis and Alzheimer’s disease. Biochem Soc Trans. 40(4):721–727. doi: 10.1042/BST20120080.
- Park P, Georgiou J, Sanderson TM, Ko KH, Kang H, Kim JI, Bradley CA, Bortolotto ZA, Zhuo M, Kaang BK, et al. 2021. PKA drives an increase in AMPA receptor unitary conductance during LTP in the hippocampus. Nat Commun. 12(1):413–427. doi: 10.1038/s41467-020-20523-3.
- Pchitskaya E, Popugaeva E, Bezprozvanny I. 2018. Calcium signaling and molecular mechanisms underlying neurodegenerative diseases. Cell Calcium. 70:87–94. doi: 10.1016/j.ceca.2017.06.008.
- Peng LL, Shen HM, Jiang ZL, Li X, Wang GH, Zhang YF, Ke KF. 2009. Inhibition of NMDA receptors underlies the neuroprotective effect of ginsenoside Rb3. Am J Chin Med. 37(4):759–770. doi: 10.1142/S0192415X09007223.
- Perfitt TL, Wang X, Dickerson MT, Stephenson JR, Nakagawa T, Jacobson DA, Colbran RJ. 2020. Neuronal L-type calcium channel signaling to the nucleus requires a novel CaMKIIα-shank3 interaction. J Neurosci. 40(10):2000–2014. doi: 10.1523/JNEUROSCI.0893-19.2020.
- Purkey AM, Dell’Acqua ML. 2020. Phosphorylation-dependent regulation of Ca2+-permeable AMPA receptors during hippocampal synaptic plasticity. Front Synaptic Neurosci. 12:8–31. doi: 10.3389/fnsyn.2020.00008.
- Qian YF, Wang H, Yao WB, Gao XD. 2008. Aqueous extract of the Chinese medicine, Danggui-Shaoyao-San, inhibits apoptosis in hydrogen peroxide-induced PC12 cells by preventing cytochrome c release and inactivating of caspase cascade. Cell Biol Int. 32(2):304–311. doi: 10.1016/j.cellbi.2007.10.004.
- Remigante A, Morabito R, Spinelli S, Trichilo V, Loddo S, Sarikas A, Dossena S, Marino A. 2020. d-galactose decreases anion exchange capability through band 3 protein in human erythrocytes. Antioxidants. 9(8):689–705. doi: 10.3390/antiox9080689.
- Ricciarelli R, Fedele E. 2015. Phosphodiesterase 4D: an enzyme to remember. Br J Pharmacol. 172(20):4785–4789. doi: 10.1111/bph.13257.
- Ru J, Li P, Wang J, Zhou W, Li B, Huang C, Li P, Guo Z, Tao W, Yang Y, et al. 2014. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 6(1):13–18. doi: 10.1186/1758-2946-6-13.
- Salery M, Godino A, Nestler EJ. 2021. Drug-activated cells: from immediate early genes to neuronal ensembles in addiction. Adv Pharmacol. 90:173–216. doi: 10.1016/bs.apha.2020.09.006.
- Sanderson JL, Gorski JA, Dell’Acqua ML. 2016. NMDA receptor-dependent LTD requires transient synaptic incorporation of Ca2+-permeable AMPARs mediated by AKAP150-anchored PKA and calcineurin. Neuron. 89(5):1000–1015. doi: 10.1016/j.neuron.2016.01.043.
- Satpathy R. 2020. Application of molecular docking methods on endocrine disrupting chemicals: a review. J Appl Biotechnol Rep. 7:74–80.
- Schmid LC, Mittag M, Poll S, Steffen J, Wagner J, Geis HR, Schwarz I, Schmidt B, Schwarz MK, Remy S, et al. 2016. Dysfunction of somatostatin-positive interneurons associated with memory deficits in an Alzheimer’s disease model. Neuron. 92(1):114–125. doi: 10.1016/j.neuron.2016.08.034.
- Seeliger D, de Groot BL. 2010. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J Comput Aided Mol Des. 24(5):417–422. doi: 10.1007/s10822-010-9352-6.
- Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. 2003. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13(11):2498–2504. doi: 10.1101/gr.1239303.
- Shonesy BC, Jalan-Sakrikar N, Cavener VS, Colbran RJ. 2014. CaMKII: a molecular substrate for synaptic plasticity and memory. Prog Mol Biol Transl Sci. 122:61–87. doi: 10.1016/B978-0-12-420170-5.00003-9.
- Sonoda Y, Mukai H, Matsuo K, Takahashi M, Ono Y, Maeda K, et al. 2016. Accumulation of tumor-suppressor P T EN in Alzheimer neurofibrillary tangles. Neurosci Lett. 471(1):20–24. doi: 10.1016/j.neulet.2009.12.078.
- Stelzer G, Rosen N, Plaschkes I, Zimmerman S, Twik M, Fishilevich S, Stein TI, Nudel R, Lieder I, Mazor Y, et al. 2016. The GeneCards suite: from gene data mining to disease genome sequence analyses. Curr Protoc Bioinformatics. 54:1.30.1–1.30.33.
- Strack S, McNeill RB, Colbran RJ. 2000. Mechanism and regulation of calcium/calmodulin-dependent protein kinase II targeting to the NR2B subunit of the N-methyl-d-aspartate receptor. J Biol Chem. 275(31):23798–23806. doi: 10.1074/jbc.M001471200.
- Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, et al. 2019. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 47(D1):D607–D613. doi: 10.1093/nar/gky1131.
- Tian J, Shi J, Wei M, Ni J, Fang Z, Gao J, Wang H, Yao H, Zhang J, Li J, et al. 2019. Chinese herbal medicine Qinggongshoutao for the treatment of amnestic mild cognitive impairment: a 52-week randomized controlled trial. Alzheimers Dement. 5(1):441–449. doi: 10.1016/j.trci.2019.03.001.
- Tischmeyer W, Grimm R. 1999. Activation of immediate early genes and memory formation. Cell Mol Life Sci. 55(4):564–574. doi: 10.1007/s000180050315.
- Tonegawa S, Pignatelli M, Roy DS, Ryan TJ. 2015. Memory engram storage and retrieval. Curr Opin Neurobiol. 35:101–109. doi: 10.1016/j.conb.2015.07.009.
- Tong H, Chen GH, Liu RY, Zhou JN. 2007. Age-related learning and memory impairments in adult-onset hypothyroidism in Kunming mice. Physiol Behav. 91(2–3):290–298. doi: 10.1016/j.physbeh.2007.03.008.
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. 2010. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 62(3):405–496. doi: 10.1124/pr.109.002451.
- Vugmeyster Y, Harrold J, Xu X. 2012. Absorption, distribution, metabolism, and excretion (ADME) studies of biotherapeutics for autoimmune and inflammatory conditions. AAPS J. 14(4):714–727. doi: 10.1208/s12248-012-9385-y.
- Wei W, Dong Q, Jiang W, Wang Y, Chen Y, Han T, Sun C. 2021. Dichloroacetic acid-induced dysfunction in rat hippocampus and the protective effect of curcumin. Metab Brain Dis. 36(4):545–556. doi: 10.1007/s11011-020-00657-5.
- Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, et al. 2018. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 46(D1):D1074–D1082. doi: 10.1093/nar/gkx1037.
- Wu L, Zhang T, Chen K, Lu C, Liu XF, Zhou JL, Huang YK, Yan H, Chen Y, Zhang CJ, et al. 2021. Rapid antidepressant-like effect of Fructus Aurantii depends on cAMP-response element binding protein/brain-derived neurotrophic facto by mediating synaptic transmission. Phytother Res. 35(1):404–414. doi: 10.1002/ptr.6812.
- Xiang X, Yu Y, Tang X, Chen M, Zheng Y, Zhu S. 2019. Transcriptome profile in hippocampus during acute inflammatory response to surgery: toward early stage of PND. Front Immunol. 10:149–157. doi: 10.3389/fimmu.2019.00149.
- Xie Y, Song A, Zhu Y, Jiang A, Peng W, Zhang C, Meng X. 2021. Effects and mechanisms of probucol on aging-related hippocampus-dependent cognitive impairment. Biomed Pharmacother. 144:112266. doi: 10.1016/j.biopha.2021.112266.
- Yang S, Du Y, Zhao X, Wu C, Yu P. 2022. Reducing PDK1/Akt activity: an effective therapeutic target in the treatment of Alzheimer’s disease. Cells. 11(11):1735–1755. doi: 10.3390/cells11111735.
- Yin JC, Del Vecchio M, Zhou H, Tully T. 1995. CREB as a memory modulator: induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell. 81(1):107–115. doi: 10.1016/0092-8674(95)90375-5.
- Yin JC, Wallach JS, Del Vecchio M, Wilder EL, Zhou H, Quinn WG, Tully T. 1994. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 79(1):49–58. doi: 10.1016/0092-8674(94)90399-9.
- Yu CC, He C, Du YJ, Gao S, Lin YF, Wang SQ, Wang L, Wang J, Wang XS, Jiang T, et al. 2021. Preventive electroacupuncture reduces cognitive deficits in a rat model of d-galactose-induced aging. Neural Regen Res. 16(5):916–923. doi: 10.4103/1673-5374.297090.
- Yuan L, Zhang J, Guo JH, Holscher C, Yang JT, Wu MN, Wang ZJ, Cai HY, Han LN, Shi H, et al. 2021. DAla2-GIP-GLU-PAL protects against cognitive deficits and pathology in APP/PS1 mice by inhibiting neuroinflammation and upregulating cAMP/PKA/CREB signaling pathways. J Alzheimers Dis. 80(2):695–713. doi: 10.3233/JAD-201262.
- Zeng L, Lin L, Peng Y, Yuan D, Zhang S, Gong Z, Xiao W. 2020. L-Theanine attenuates liver aging by inhibiting advanced glycation end products in d-galactose-induced rats and reversing an imbalance of oxidative stress and inflammation. Exp Gerontol. 131:110823. doi: 10.1016/j.exger.2019.110823.
- Zhang C, Du F, Shi M, Ye R, Cheng H, Han J, Ma L, Cao R, Rao Z, Zhao G. 2012. Ginsenoside Rd protects neurons against glutamate-induced excitotoxicity by inhibiting Ca2+ influx. Cell Mol Neurobiol. 32(1):121–128. doi: 10.1007/s10571-011-9742-x.
- Zhang B, Lian W, Zhao J, Wang Z, Liu A, Du G. 2021. DL0410 alleviates memory impairment in d-galactose-induced aging rats by suppressing neuroinflammation via the TLR4/MyD88/NF-κB pathway. Oxid Med Cell Longev. 2021:6521146. doi: 10.1155/2021/6521146.
- Zhang HL, Zhao B, Han W, Sun YB, Yang P, Chen Y, Ni D, Zhang J, Yin DM. 2021. Acetylation of calmodulin regulates synaptic plasticity and fear learning. J Biol Chem. 297(3):101034. doi: 10.1016/j.jbc.2021.101034.