Abstract
Context
Cervicitis is a common gynecological inflammatory disease. The Chinese herbal prescription Kang-Gong-Yan (KGY) is clinically effective against cervicitis; however, the chemical constituents and therapeutic mechanism of KGY remain elusive.
Objective
To analyze the chemical constituents of KGY and explore the potential mechanism of KGY in treating cervicitis.
Materials and methods
UHPLC-Q-Exactive Plus Orbitrap MS was used to identify the active compounds of KGY; Sprague-Dawley (SD) female rats were randomly divided into the control, model, and KGY groups. Phenol mucilage (25%) was slowly injected into the vagina and cervix of the rats to establish the cervicitis model. Then, rats in the KGY groups (low dose: 1 g/kg/d; medium dose: 5 g/kg/d; high dose: 10 g/kg/d) were continuously gavaged KGY for one week. HE staining was used to observe the cervical tissues of rats; ELISA was used to detect inflammatory factors in plasma; non-targeted metabolomics was used to analyze metabolites; 16S rRNA sequencing was used to analyze intestinal microorganisms.
Results
KGY exerted anti-cervicitis effects and decreased the levels of IL-6, IL-1β, and TNF-α. The mechanism of KGY in treating cervicitis is mainly associated with betaine, amino acid, pyrimidine, and phospholipid metabolism by regulating fifteen metabolites. Moreover, KGY reversed cervicitis-induced gut dysbiosis by mediating five bacteria.
Discussion and conclusions
The Chinese herbal prescription KGY may alleviate cervicitis by modulating metabolites and gut microbiota disorders. These findings provide a scientific basis for the clinical application of KGY and a new strategy for treating cervicitis in Chinese medicine.
Introduction
Cervicitis is a common gynecological inflammatory disease, a clinical syndrome that often presents as inflammation of the columnar epithelium of the endometrium, primarily in women of childbearing age. Many factors contribute to cervicitis, including female age, physical or chemical irritants, behavioral or hormonal factors, and pathogenic microorganisms. The most common causes of cervicitis are Chlamydia trachomatis and Neisseria gonorrhoeae infections (Ortiz-de la Tabla & Gutiérrez Citation2019; Holubekova et al. Citation2022). Cervicitis causes complications (Jayakumar Citation2015), including tubal infection, endometritis, pelvic inflammatory disease, and infertility.
Currently, cervicitis is usually treated with antibiotics, including azithromycin, ceftriaxone, and quinolones, but drug resistance is an inevitable problem (Miranda et al. Citation2021). In recent years, Chinese medicine has shown great potential for the treatment of gynecological inflammatory diseases. Kang-Gong-Yan soft capsule, an oral proprietary Chinese medicine, has often been used in the clinical treatment of gynecological inflammatory diseases including cervicitis, chronic pelvic inflammation, cervical erosion, and other gynecological disorders (Yuan et al. Citation2022).
The Chinese herbal prescription Kang-Gong-Yan has remarkable clinical efficacy and is included in the Chinese Pharmacopoeia, consisting of Callicarpa kwangtungensis Chun (Lamiaceae) (CK), Lindera aggregata (Sims) Kosterm (Lauraceae) (LA), and Leonurus japonicus Houttuyn (Lamiaceae) (LJ). The dried rhizomes and leaves of CK exert anti-inflammatory, antioxidant, antibacterial, and hemostatic effects (Tu et al. Citation2013). The root tuber of LA inhibits inflammation and oxidation, as well as the growth of cancer cells. It has analgesic and intestinal flora improvement effects (Lv et al. Citation2022). LJ is used to treat dysmenorrhea, amenorrhea, menoxenia, and other gynecological diseases, and it helps to activate blood circulation and remove blood stasis (Miao et al. Citation2019).
KGY is made from a mixture of dried infusions of CK, LA, and LJ with polyethylene glycol 400 (PEG 400) as an excipient. Our previous study found that norisoboldine, leonurine hydrochloride, isoacteoside, forsythoside B, acteoside, and poliumoside are quality markers of KGY (Yuan et al. Citation2022). In addition, we found that AKT1, TNF, and EGFR were crucial targets for KGY in treating cervicitis and were mainly related to the inflammatory response and immune regulation through network pharmacology research and molecular docking. However, these studies are insufficient to elucidate the active chemical constituents and the underlying therapeutic mechanisms of KGY.
Metabolomics is a key tool for the discovery of endogenous biomarkers, and the gut flora is an important factor affecting the immune and metabolic processes of the body. They provide new ideas for the diagnosis and treatment of diseases (Johnson et al. Citation2016; Strandwitz Citation2018). In this study, we conducted a qualitative analysis of the components of KGY and investigated the effects of KGY on cervicitis rats and combined metabolomics and gut microbiota to explore the potential mechanism.
Materials and methods
Reagents and materials
Kang-Gong-Yan soft capsules (batch number: 200101, national medicine permission number: Z20120005, Guizhou Huizheng Pharmaceutical Co., Ltd.), rat IL-1β kit (production batch number: 211210-007a), rat TNF-α Kit (production batch number: 211210-003a), and rat IL-6 kit (production batch number: R211210-102a) were purchased from NeoBioscience Technology Co., Ltd. Formic acid (Tianjin Kemio Chemical Reagent Co., Ltd., Tianjin, China), acetonitrile, methanol (Merck Company, Darmstadt, Germany), phenol kit (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China), PEG 400, arabic gum (Solarbio Company, Beijing, China), and other reagents were stored and used according to the manufacturer’s instructions.
The preparation method of 25% phenol mucilage (Li JM Citation2021): 5 mL liquid phenol, 20 mL distilled water, and 8 g Arabic gum were taken and mixed thoroughly in a water bath at 50 °C to produce 25% phenol mucilage, which was sealed and stored at 4 °C.
Identification of chemical constituents of KGY
KGY (1 g) was placed in a round-bottomed flask. Methanol aqueous solution (1:1, v/v) (50 mL) was added and weighed. After refluxing for 1 h, the lost weight was made up with methanol aqueous solution (1:1, v/v), mixed, and filtered for use.
The 2 μL KGY sample was detected by UHPLC-Q-Exactive Plus Orbitrap MS (Thermo Fisher Scientific, USA). The flow rate was set at 300 μL/min. The mobile phase gradients (A: acetonitrile, 0.1% formic acid; B: H2O, 0.1% formic acid) were as follows: 2% A at 0–2 min; 2–15% A at 2–3 min; 15–25% A at 3–8 min; 25–40% A at 8–9 min; 40–98% A at 9–11 min; 98% A at 11–15 min; 98–2% A at 15–18 min and 2% A at 18–20 min. The heated electrospray ion source (HESI) was used for simultaneous detection in positive and negative ion modes, with high purity N2 as the nebulizing gas and high purity He as the collision gas. The sheath gas flow rate was 35 arb; the auxiliary gas flow rate was 10 arb; the spray voltage was 3500 V/2500 V (±); the capillary temperature was 320 °C; the heater temperature was 350 °C; the scanning mode was Full MS/dd-MS2; the dd-MS2 resolution was 17500; the Full MS resolution was 70,000; the scanning range: 100–1500 m/z; the step collision energy: 20 V, 40 V, 60 V.
Experimental animals
SPF-grade female Sprague Dawley (SD) rats with a body mass of 220 ± 20 g were supplied by the Experimental Animal Center of Guizhou Medical University (License No. SCXK (Xiang) 2019-0014). The rats were acclimatized and fed for one week at 25 ± 1 °C and relative humidity of 55 ± 5%. In this study, 35 rats were randomly set up in 5 groups of 7 rats each, including control group, model group and KGY group (low/medium/high dose). Except for the control group, the remaining four groups were modelled with phenol mucilage (0.1 mL 25% phenol mucilage was slowly injected into the vagina and cervix of the rats once a day for 5 consecutive days) (Li JM Citation2021). The status of the rats (body weight, mental and behavioral conditions, etc.) was observed daily, and timely interventions were taken according to the status of the rats to ensure animal welfare. The cervicitis model was considered successful when the vaginal opening of the rats showed obvious redness, swelling and increased secretion. After the successful establishment of the rat model of cervicitis, all groups were continuously gavaged for one week. The gavage dose for rats was calculated from human equivalent doses based on body surface area; KGYL 1 g/kg (low dose), KGYM 5 g/kg (medium dose), and KGYH 10 g/kg (high dose). Control rats were given equivalent physiological saline, and the model group was assigned PEG 400, the excipient of KGY. The condition of the rats was observed after the last dose, mainly including whether the vaginal opening of the rats was clean and dry, and whether the mucous membrane of the vulva and vaginal opening were congested. Blood, feces, and cervical tissues were collected for subsequent experiments. All animal experiments were conducted in strict accordance with relevant laws and standards and were approved by the Animal Ethics Committee of Guizhou Medical University (ethics approval number: 2100437).
Histopathological analysis
All rats were anesthetized with isoflurane and then blood samples were obtained from the abdominal aorta, followed by execution and dissection to obtain cervical tissues. The cervical tissues of rats were fixed in 4% paraformaldehyde solution, followed by paraffin embedding, and the samples were made into sections of 5 μm thickness. The samples were dewaxed by xylene immersion, soaked in ethanol at different concentrations, then stained with hematoxylin and eosin staining solution, and sealed with neutral gum. The pathological changes were observed under a light microscope.
Measurement of cytokines
The blood samples of rats were centrifuged for 10 min at 4 °C at 4000 rpm, and the supernatant liquid obtained was stored at −80 °C for the next experiments. Inflammatory factors (IL-1β, IL-6, and TNF-α) in plasma samples were quantified using ELISA kits, according to the manufacturer’s instructions. According to the double antibody sandwich method, samples were added to the wells of the enzyme labeling plate, incubated for 1.5 h at 37 °C protected from light and then washed. The biotinylated antibody working solution, enzyme conjugate working solution and chromogenic substrate were added respectively, and the reaction termination solution was finally added after incubation and washing. The absorbance was measured at 450 nm. The concentration of cytokines was proportional to the OD450 value.
Sample preparation for metabolomics
Plasma sample (100 μL) was added to 400 μL acetonitrile, vortexed, mixed for 5 min, placed at −20 °C for 30 min, and collected by centrifugation for 15 min at 4 °C at 15,000 rpm. The liquid obtained after centrifugation was passed through a 0.22 μm filter membrane, and 4 μL was used for UHPLC-Q-Exactive Plus Orbitrap MS analysis. Quality control (QC) samples were mixed with equal volumes of plasma samples. The mobile phase gradients (A: acetonitrile, 0.1% formic acid; B: H2O, 0.1% formic acid) were as follows: 2% A at 0–2.5 min; 2–40% A at 2.5–5 min; 40–100% A at 5–12 min; 100% A at 12–16 min; 100–2% A at 16–16.1 min; 2% A at 16.1–19 min. The Mass Spectrometry conditions were set as follows: ion source, HESI; the sheath gas flow rate, 40 arb; the auxiliary gas flow rate, 10 arb; the spray voltage was 3500 V/2800V (±); the temperature was 320 °C (capillary) and 350 °C (gas heater); the scanning range: 100–1500 m/z; the step collision energy: 20 V, 40 V, 60 V; S-Lens RF Level, 50.
Gut microbiota analysis
The genomic DNA of rat feces from the appendix was obtained using the CTAB operation steps. The DNA sample content was adjusted to 1 ng/µL using sterile water after the concentration and purity of DNA were determined by agarose gel electrophoresis.
The 16S V4 region was amplified using diluted DNA as a template and specific primers (16S V4:515F- 806 R) with the barcode to identify bacterial diversity in the samples. The PCR products that passed the assay were purified by magnetic beads, quantified by enzyme labelling, mixed in equal amounts, and then detected using 2% agarose gel electrophoresis; recovered using a Qiagen Gel Extraction Kit (Qiagen, Germany), and then sequenced using NovaSeq6000.
The samples were clustered using the Uparse algorithm, and the sequence with the highest frequency of occurrence in OTUs (Operational Taxonomic Units) was used as the representative sequence; PCoA map was drawn using R software (Version 2.15.3); Qiime software (Version 1.9.1) was used to calculate the Alpha diversity index (Observed species, Chao1, Shannon, Simpson); species annotation analysis was performed using the Mothur method with the SSUrRNA database, and the community composition of each sample at each taxonomic level was counted.
Data analysis
SPSS 23.0, GraphPad Prism 9.0, Origin 2022, and QIIME (version 1.9.1) were used to analyze the data. Statistical significance was calculated by ANOVA and Student’s t-test, and P values less than 0.05 were considered significant. Raw data files generated by UHPLC-Q-Exactive Plus Orbitrap MS were processed using Thermo Compound Discover 2.0 analysis software for relative quantification. Metabolomics analysis was performed using SIMCA 14.1 software and MetaboAnalyst 5.0.
Results
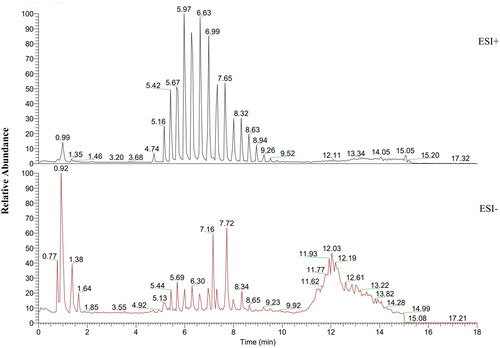
Identification of chemical constituents of KGY
To identify the chemical constituents of KGY, we qualitatively analyzed its components (). Combined with MZ cloud library and MZ vault library of Theromo Compound Discover 2.0 analysis software, 103 compounds were identified from the extract of KGY, including 33 flavonoids (luteolin, rutin, quercetin, apigenin, etc.), 5 phenylethanol glycoside (forsythoside B, acteoside, poliumoside, isoacteoside, etc.), 18 alkaloids (stachydrine, leonurine, norisoboldine, etc.), 11 terpenes (linderane, ferulic acid, etc.), 7 phenolic acids (gallic acid, gentisic acid, epicatechin, etc.) and 29 other class compounds (organic acids, chlorogenic acids, amino acids, etc.), as shown in .
Table 1. Chemical compounds identification of Kang-Gong-Yan soft capsule.
Effect of KGY on cervical tissues
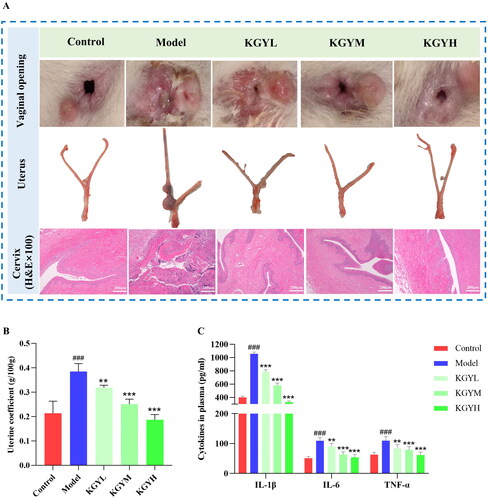
To investigate the influence of KGY on cervicitis in rats, we observed the cervical tissues (the vaginal opening and uterus) of rats and H&E-stained sections (). Compared with the control group, the vaginal opening of the model rats was red, swollen, and congested with mucopurulent discharge; the cervical tissue was edematous with blood clots, and the uterine coefficient (ratio of the mass of the uterus to the body mass of rat) was significantly increased (). Histopathological examination of the cervix showed that the cervical tissue structure of the model group rats was destroyed, and there was obvious infiltration of inflammatory cells and interstitial hyperplasia; the squamous epithelium of the mucosal layer appeared to be hyperplastic and thickened; the mucosal epithelial cells were degenerated, necrotic, and edema; and capillaries were congested. Additionally, fewer histopathological changes were observed in the control group. These results indicate that 25% phenol mucilage successfully caused rats to exhibit obvious signs of cervicitis, and that surgical manipulation had no significant effect on the control group. Compared to the model group, the cervical tissues in each KGY group showed different degrees of improvement as shown in . The mucosa of KGY rats was obviously improved with reduced edema, and there was no clear capillary congestion or inflammatory cell infiltration. In conclusion, KGY reduced the degree of cervical tissue inflammation, repaired the mucosa, and had a certain therapeutic effect on cervicitis.
Figure 2. KGY improves cervical tissue congestion, edema, and inflammatory cell infiltration in cervicitis rats, and decreased plasma levels of inflammatory factors, including IL-6, IL-1β and TNF-α. (A) Effects of KGY on cervical tissue of rats (vaginal opening and uterus). (H&E staining images, original magnification, ×100). (B) Uterine coefficient of rats (n = 6). (C) Cytokines in plasma (n = 6). The model group is compared with the control group, ###p < 0.001; the KGY (low/medium/high dose) groups were compared with the model group, **p < 0.01 and ***p < 0.001.
Effect of KGY on plasma cytokines
To explore the effect of KGY on pro-inflammatory factors, we assayed plasma using ELISA kits. Treatment with different doses of KGY greatly decreased the concentration of IL-1β, TNF-α, and IL-6 in the plasma, with significant differences, indicating that KGY relieved the inflammation of cervicitis rats, as shown in .
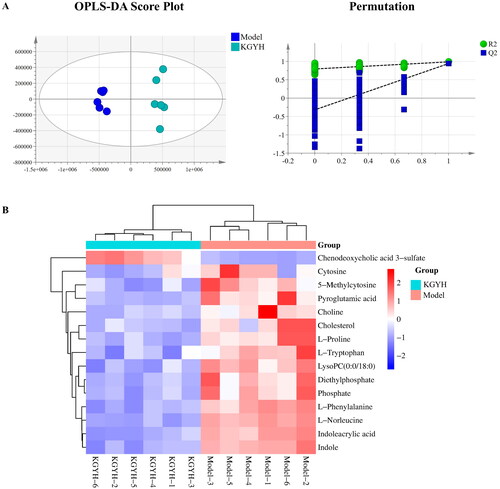
Effects of KGY on metabolites
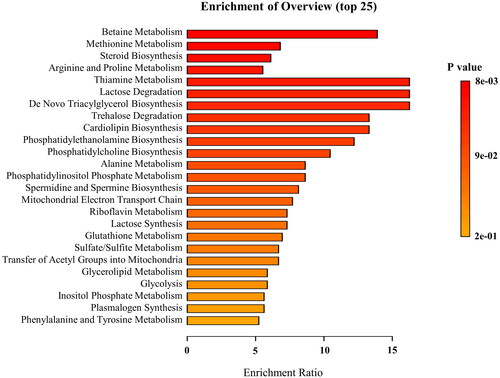
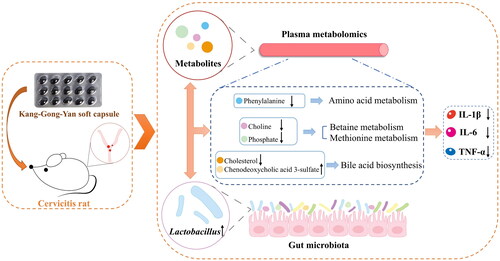
To explore changes in metabolites in cervicitis rats after KGY administration, we selected the KGYH group with the most obvious treatment effects for comparison with the model group. SIMCA-P 14.1 software was used for statistical analysis of OPLS-DA and permutation tests (). OPLS-DA analysis showed that the separation between groups was apparent, indicating differences in endogenous metabolites. In addition, the results of 200 random permutation tests (the Q2 regression line intersected the negative semiaxis of y; the value on the left side of R2 was less than the value on the right side) indicated that the model was reasonable and there was no overfitting. The differential metabolites of the KGYH and model groups were further screened by satisfying the parameters (FC > 1.5 or FC < 1/1.5, and p < 0.05). Heatmap analysis () intuitively showed the changes in metabolites in the model and KGYH groups. Fifteen differential metabolites were identified in the KGYH group and 25 metabolite pathways were identified using MetaboAnalyst 5.0. (). 14 metabolites were decreased, including L-phenylalanine, cholesterol, L-proline, pyroglutamic acid, L-tryptophan, L-norleucine, phosphate, diethylphosphate, indole, indoleacrylic acid, lysopc (0:0/18:0), choline, cytosine, 5-methylcytosine, but chenodeoxycholic acid 3-sulfate was increased. These potential metabolites are mainly involved in betaine, amino acid, pyrimidine, and phospholipid metabolism.
Effects of KGY on gut microbiota
To explore the effects of KGY on gut microbiota, we used 16S rRNA sequencing to identify bacterial diversity in rat appendix fecal samples. Principal coordinate analysis (PCoA) was used to analyze the differences in species composition and structure of each group (). The intestinal flora of rats with cervicitis significantly changed after KGY administration. A Venn diagram revealed that the control group contained 682 unique OTUs, the model group had 230 unique OTUs, the KGYH group had 115 unique OTUs, and there were 859 common OTUs (). We analyzed alpha diversities to clarify the microbial community diversity within the samples. The Shannon and Simpson indices indicated that the diversity and homogeneity of the distribution of microbial communities in the control and KGYH groups showed the same trend, but the difference between all groups was insignificant. Compared with the control group, the observed species and Chao1 indices were reduced in the model group, and the same trend was observed in the KGYH group (). The above results showed that KGY had no significant effect on the biodiversity of the gut microbiota but was able to significantly change the structure of the microbial community species composition compared to the model group.
Figure 5. Gut microbiota analysis. (A) PCoA analysis of all groups. (B) The Venn diagram. (C) Alpha diversity (Shannon, Simpson, Chao1 indexes, and observed-species) of gut microbiota in each group; the model group was compared with the control group, #p < 0.05. (D) The relative abundance on phylum level (top 10). (E) The relative abundance on genus level (top 30).
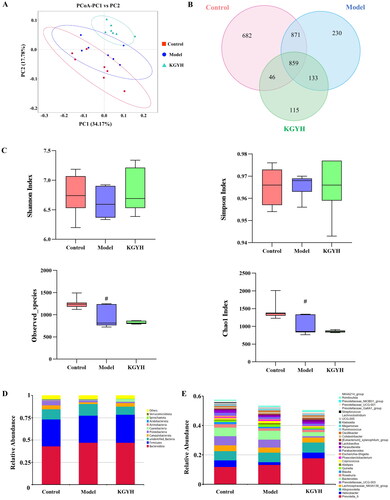
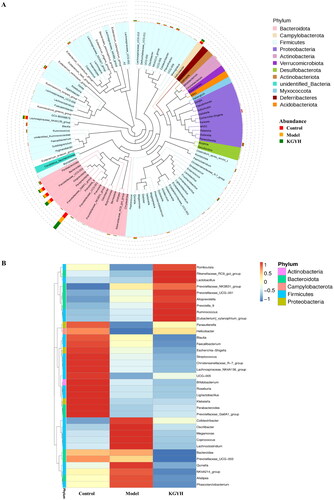
To study the differential microbial species in each group, we selected the top 10 species ranked for maximum abundance at the phylum level and the top 30 species ranked for maximum abundance at the genus level (). At the phylum level, Bacteroidota, Firmicutes, and Campylobacterota were primarily found in all groups. The abundances of Campylobacterota and Spirochaetota increased after KGY treatment. A phylogenetic tree of species-by-group analysis at the genus level was used to show the phylogenetic relationships of the top 100 genera (). The phylogenetic relationships of Bacteroidota, Campylobacterota, Firmicutes, Proteobacteria, and Actinobacteria were closer among the gut microorganisms of the three groups.
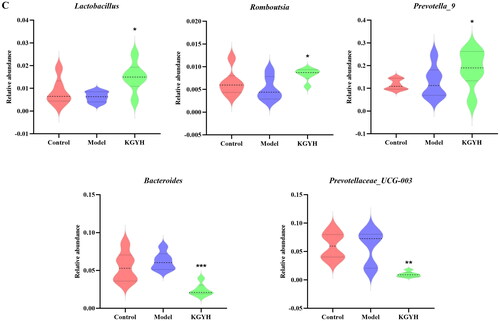
Figure 6. (A) Phylogenetic tree of species (top 100). (B) Heatmap of species at the top 35 genus level. (C) Five bacteria with significant changes in abundance. The KGYH group was compared with the model group, *p < 0.05, **p < 0.01 and ***p < 0.001.
Compared with the model group, 9 bacteria (Lactobacillus, Romboutsia, Prevotella_9, Rikenellaceae_RC9_gut_group, Alloprevotella, Prevotellaceae_NK3B31_group, Ruminococcus, Prevotellaceae_UCG-001, and [Eubacterium]_xylanophilum_group) were upregulated and 11 bacteria (Prevotellaceae_UCG-003, Bacteroides, Alistipes, Colidextribacter, Oscillibacter, Megamonas, Coprococcus, Lachnoclostridium, Quinella, NK4A214_group, and Phascolarctobacterium) were downregulated in the KGYH group, as shown in the abundance clustering heatmap of species in the top 35 genera (). Five bacteria (Lactobacillus, Romboutsia, Prevotella_9, Bacteroides and Prevotellaceae_UCG-003) showed significant changes in abundance after KGY administration ().
Discussion
Cervicitis has long been a health hazard for women, and it is essential to explore new treatment methods. Chinese medicine has achieved good clinical efficacy in preventing and treating cervicitis, and KGY exerts significant efficacy in the clinical treatment of gynecological diseases. Our study explores the chemical constituents of KGY and its mechanism of action in treating cervicitis by integrating the analysis of pharmacological effects, metabolomics, and gut microbiota.
Elucidation of the specific components of proprietary Chinese medicine is crucial for their clinical application and development. The identification of the chemical composition of KGY provides a material basis for improving its quality standards and studying the mechanism of its pharmacological effects. Cheng et al. (Citation2020) used the UPLC-Q-TOF-MS technique to study the chemical compounds of the Kang-Gong-Yan prescription and identified 70 compounds. In our study, we used UHPLC-Q-Exactive Plus Orbitrap MS to characterize the composition of KGY, identifying 103 compounds, including flavonoids, phenylethanol glycoside, alkaloids, and terpenes, which laid the foundation for future experiments.
Modern pharmacological research has shown that most of these chemical compounds have different degrees of anti-inflammatory, antibacterial, and antiviral effects. For example, luteolin reduces TNF-α, IL-17, IL-6, and IL-1β levels in the plasma of a model of acute gout inflammation induced by sodium urate (Zuo et al. Citation2021). Quercetin exerts anti-inflammatory, anticancer, and oxidative stress-inhibiting pharmacological effects (Li Y et al. Citation2016). Rutin, acteoside, norisoboldine, and forsythoside B exert anti-inflammatory impacts (Chen et al. Citation2021; Sun et al. Citation2021). Stachydrine and leonurine are the most critical alkaloids in Leonurus japonicus. Stachydrine exerts anti-inflammatory, antibacterial, antioxidative, and antitumor effects (Sun X et al. Citation2022). Leonurine has anti-inflammatory effects and treats platelet aggregation and uterine contractions (Shi XD et al. Citation2022). Gentisic acid inhibits inflammation via the Raf/ERK signaling pathway (Dong et al. Citation2022). Gallic acid exerts anti-inflammatory, antibacterial, and antiviral effects, significantly affecting pro-inflammatory cell factors (IL-6 and TNF-α) in rats (Dludla et al. Citation2018). Chlorogenic acid has antioxidant and anti-inflammatory effects and effectively reduces inflammatory factors, such as TNF-α, IL-1β, and IL-6, produced by CCl4-induced polar liver injury (Shi A et al. Citation2018). In addition, chlorogenic acid alleviates the inflammatory response and maintains the health of the intestinal barrier by improving glucose metabolism and increasing the number of gut microbes that produce short-chain fatty acids, such as Romboutsia (Ye X et al. Citation2021), which were enriched in the KGYH group in our study.
To explore the effects of KGY, we constructed a rat model of cervicitis by using phenol mucilage, and this modeling method is often used to establish a cervicitis model because of its simplicity and reproducibility. After KGY treatment, the appearance of cervical tissues, H&E-stained sections, and inflammatory factor levels significantly improved in cervicitis rats. Research on metabolites helps to identify and diagnose reproductive diseases such as cervicitis (Ye N et al. Citation2015). To reveal metabolite changes in cervicitis rats and elucidate the mechanisms of KGY therapy for cervicitis, we performed metabolomic studies on the plasma of each group. Metabolomic analysis indicated obvious differences between the model and KGYH groups. KGY may exert therapeutic effects by regulating 15 metabolites and 25 metabolic pathways. These metabolites were mainly involved in the betaine, amino acid, pyrimidine, and phospholipid metabolism pathways.
Choline and phosphate are closely related to the betaine metabolic pathways. Betaine, also known as trimethylglycine, is a significant methyl donor in the human body that can be synthesized from phosphatidylcholine and choline (Wang C et al. Citation2021). Betaine has critical physiological roles in anti-inflammatory, anti-stress, hypolipidemic, cardiovascular, and osmotic protection (Rosas-Rodríguez and Valenzuela-Soto Citation2021). Betaine inhibits the NF-κB signaling pathway, thereby regulating the secretion of TNF-α and IL-1β and exerting anti-inflammatory effects by inhibiting the NLRP3 inflammasome (Shi H et al. Citation2019). In this study, the choline and phosphate contents in the KGYH group were reduced, and TNF-α, IL-6, and IL-1β in plasma were significantly down-regulated. The inflammatory response was alleviated, indicating that choline and phosphate may be converted to betaine to inhibit the secretion of inflammatory factors, thereby inhibiting inflammation, and playing an essential role in cervicitis treatment.
Amino acids are essential active molecules in the body that can participate in metabolism (Tomé Citation2021). Changes in amino acid content suggest that the body is disordered, which affects metabolism. Previous studies have shown that inflammatory reactions are closely involved in amino acid metabolism (Gar et al. Citation2018; Zhao et al. Citation2020). Our experiments revealed several amino acid metabolic pathways, including phenylalanine, tyrosine, methionine, arginine, proline, and alanine metabolism. Phenylalanine is a biomarker of cervicitis (Zhang, Li et al. Citation2018) and is closely related to tryptophan. Tryptophan is metabolized by intestinal microorganisms to produce indole and its derivatives (Qayed et al. Citation2021). In this study, rats in the model group had higher levels of L-phenylalanine, L-tryptophan, and indole. Tryptophan indirectly affects the activity of prostaglandin F2α, which causes bleeding and pain in local uterine tissue (Li JM Citation2021). After treatment with KGY, the concentrations of L-phenylalanine, L-tryptophan, and indole significantly decreased, indicating that the prostaglandin F2α might be down-regulated, thereby reducing hemorrhage and edema in the rats. High levels of phenylalanine impair the physical ability of the mitochondria, thereby causing oxidative and inflammatory responses (Wyse et al. Citation2021). In the KGYH group, the amount of L-phenylalanine was lower than that in the model group, indicating that KGY might function in the treatment of cervicitis by regulating the phenylalanine metabolic pathway, repairing mitochondrial function, and reducing the inflammatory response.
Pyrimidine metabolism has an important impact on tumor progression. The proliferation of tumor cells has a greater demand for nucleotides, and pyrimidine is an essential component of nucleotides. Pyrimidine metabolism plays an essential role in the progression of several cancers and the development of drug resistance development (Zhu and Thompson Citation2019; Luo et al. Citation2022). In our study, phosphate, cytosine, and 5-methylcytosine abundance in the model group was increased, which is closely related to pyrimidine metabolism. After treatment with KGY, the phosphate, cytosine, and 5-methylcytosine contents decreased considerably, indicating that KGY may inhibit the adverse progression of cervicitis to cancer by regulating pyrimidine metabolism.
Phospholipids are essential components of biological membranes and participate in the exchange, secretion, and transport of substances, while maintaining the structure and function of cell membranes. Phospholipid metabolism plays a vital role in modulating immune responses (van der Veen et al. Citation2017). Common phospholipids include phosphatidylcholine (PC), phosphatidyl acetamide (PE), phosphatidylinositol (PI), phosphatidylserine (PS), phosphatidylglycerol (PG), etc. In this study, phospholipid metabolic pathways, including phosphatidylinositol phosphate metabolism, cardiolipin, phosphatidylethanolamine, and phosphatidylcholine biosynthesis were screened. Previous studies have shown that phospholipid metabolism disorders trigger inflammation in the body (Giron et al. Citation2021; Li X et al. Citation2021). Higher lysophospholipidcholine (LPC) content can be seen in an inflammatory state, cell damage, and other pathological states (Knuplez and Marsche Citation2020); Su et al. (Citation2022) found that glycerophospholipid metabolism can affect rheumatoid inflammation through the IL-6/JAK pathway. PC forms LPC vis phospholipase A2 and cholesterol acyltransferases. Studies have shown that LPC is a potential biomarker for cervicitis and other gynecological tumor diseases (Yang et al. Citation2017). In this experiment, lysopc (0:0/18:0), phosphate, and choline levels in the model group were increased, which may have led to a disorder in phospholipid metabolism, thus leading to the occurrence of cervicitis. KGY adjusts phospholipid metabolism in cervicitis rats by modulating the abundance of lysopc (0:0/18:0), phosphate, and choline to reduce the inflammatory response.
Human intestinal flora is important for health and regulates the inflammatory response, immune system, and metabolic function (Al Bander et al. Citation2020; Malard et al. Citation2021). To reveal changes in the intestinal microbiota in vivo, we performed a 16S rRNA study on rat feces. Lactobacillus, Rombousia, and Prevotella_9 increased at the genus level, whereas Bacteroides and Prevotellaceae_UCG-003 decreased after KGY treatment.
Lactobacillus plays an essential role in modulating the immune system, maintaining homeostasis of the microbiota, protecting the epithelial barrier, and inhibiting infection by pathogenic bacteria (Li Y et al. Citation2022). It is the most crucial probiotic in the human body, contributing to the secretion of mucus and antimicrobial peptides and changing the intestinal flora, thus protecting the intestinal mucosa. Lactobacillus is abundant in healthy mucosa and is considered a biomarker of healthy gut. Oral administration of probiotic preparations containing Lactobacillus can increase Prevotella and Oscillibacter, thus producing an anti-inflammatory effect (Zeng et al. Citation2021). Lactobacillus spp. are important for maintaining a healthy cervicovaginal environment. Lactobacillus protects the mucosal barrier and prevents infection by producing lactic acid to lower the pH and by secreting bacteriocins to prevent the colonization of harmful bacteria. Besides, Lactobacillus is crucial in treating diseases (neuroinflammation, liver injury, diabetes, etc.) by regulating inflammatory factors (TNF-α, IL-1β, IFN-ɣ, IL-6, IL-8, etc.) (Rastogi and Singh Citation2022; Das et al. Citation2023).
Rombousia is a common anaerobic bacterium in the intestine, which plays a crucial role in host health. Rombousia produces short-chain fatty acids, including acetate and formate, that can inhibit inflammation (Gerritsen et al. Citation2014; Mangifesta et al. Citation2018). Both Alistipes and Bacteroides belong to the Bacteroides phylum, which is closely related to chronic intestinal inflammation (Parker et al. Citation2020). Studies have shown that ileitis is related to enrichment of Alistipes (Rodriguez-Palacios et al. Citation2015, Citation2018). Bacteroides are important anaerobic bacteria in the human body and are clinically significant pathogens that spread when inflammation occurs or when mucosal surfaces are damaged, leading to anaerobic infections (Patrick Citation2022). Bacteroides are present at higher levels in patients with ulcerative colitis and exacerbate inflammation (Zhao et al. Citation2019). Prevotellaceae has both positive and negative effects on the body. This is beneficial for the hydrolysis of proteins and carbohydrates (Xu et al. Citation2022). In addition, Bacteroides fragilis ameliorated the intestinal flora disorder induced by C. malonaticus LPS by upregulating the abundance of Prevotella_9, thus alleviating intestinal damage (Ling et al. Citation2022). However, Prevotellaceae are closely related to human diseases by perturbing inflammasome pathways, such as NLRP6 and IL-18, and can disrupt mucosal barrier function by producing sulfatases, which actively degrade mucus oligosaccharides (Elinav et al. Citation2011). A previous study reported that an increased Prevotellaceae_UCG-003 might lead to inflammation (Wang B et al. Citation2022), such as IBD and periodontitis.
Our results suggest that KGY treatment alters the gut microbiota of cervicitis rats. Moreover, the cervical tissue and mucosal barrier of rats with cervicitis were significantly improved and pro-inflammatory factors were considerably reduced. Therefore, KGY may alleviate inflammatory responses by regulating gut microbiota disorders. The therapeutic effects of KGY may be most relevant to Lactobacillus, the dominant probiotic found in the KGYH group. In our study, the abundance of Lactobacillus in the intestinal flora of rats in the KGYH group was higher, and the levels of metabolites (L-phenylalanine, choline, phosphate, cholesterol, and chenodeoxycholic acid 3-sulfate) were significantly altered.
Numerous studies have proven that intestinal microbes and metabolites are closely related and thus influence the inflammatory response. In a rat model of alcoholic liver disease, phenylalanine levels and the expression of pro-inflammatory factors were positively correlated, and supplementation with Lactobacillus acidophilus affected phenylalanine levels and improved inflammation (Chen L et al. Citation2023). In addition, choline and phosphate participate in methionine and betaine metabolism. Methionine can inhibit oxidation and inflammation and contribute to the growth of Lactobacillus (Wu et al. Citation2019). Betaine modulates the intestinal flora in diet-induced obese mice and increases the abundance of Lactobacillus (Du et al. Citation2021). Lactobacillus can affect the expression of genes related to cholesterol, thereby lowering cholesterol levels. Our results showed that cholesterol level was downregulated after administration. The increase in chenodeoxycholic acid 3-sulfate level predicts that the level of chenodeoxycholic acid, which is a type of primary bile acid, might also be upregulated. Bile acids, produced by cholesterol catabolism, can indirectly reduce inflammation by inhibiting NF-κB pathway-dependent genes, thereby reducing the levels of inflammatory cytokines, including TNF-α, IL-6, and IL-1β (Fiorucci et al. Citation2018; Cao et al. Citation2021). Therefore, in our study, Lactobacillus might be closely associated with changes in these metabolites and related metabolic pathways and play a vital role in the KGY treatment of cervicitis, as shown in . This study only elucidated the mechanism of action of KGY through animal experiments, which was not sufficient to represent clinical efficacy. In future studies, we will explore and verify the mechanism of KGY in detail to provide more evidence for KGY treatment in cervicitis.
Conclusions
In this study, we conducted a qualitative analysis of KGY components and analyzed the mechanism of KGY in the treatment of cervicitis from pharmacological effects, plasma metabolomics, and changes in the intestinal flora, which allowed us to further understand the pathogenesis of cervicitis from a new perspective. Our study shows that the Chinese herbal prescription Kang-Gong-Yan can effectively treat cervicitis and provides a scientific basis for the clinical application of KGY.
Authors’ contributions
Conceptualization, ZYN, YMY, ZM and GXL; Data curation, ZYN and YMY; Formal analysis, ZYN, YMY, SXD and WPJ; Investigation, ZYN, YMY, SXD and WPJ; Methodology, ZYN, YMY, SXD, WPJ, MXX and ZS; Project administration, LW, ZM and GXL; Software, ZYN, YMY, SXD, WPJ, MXX, ZS and LW; Supervision, ZM and GXL; Writing - original draft, ZYN and YMY; Writing - review & editing, ZYN, YMY, SXD, WPJ, MXX, ZS, LW, ZM and GXL. The views and conclusions of this study were reviewed and approved by all the authors.
Acknowledgements
The authors sincerely thank all those who contributed to this study.
Disclosure statement
The authors declare no potential conflicts of interest regarding the research, authorship, or publication of this article.
Data availability statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Al Bander Z, Nitert MD, Mousa A, Naderpoor N. 2020. The gut microbiota and inflammation: an overview. Int J Environ Res Public Health. 17(20):7618. doi: 10.3390/ijerph17207618.
- Cao K, Zhang K, Ma M, Ma J, Tian J, Jin Y. 2021. Lactobacillus mediates the expression of NPC1L1, CYP7A1, and ABCG5 genes to regulate cholesterol. Food Sci Nutr. 9(12):6882–6891. doi: 10.1002/fsn3.2600.
- Chen L, Yang P, Hu L, Yang L, Chu H, Hou X. 2023. Modulating phenylalanine metabolism by L. acidophilus alleviates alcohol-related liver disease through enhancing intestinal barrier function. Cell Biosci. 13(1):24. doi: 10.1186/s13578-023-00974-z.
- Chen Q, Shao X, He Y, Lu E, Zhu L, Tang W. 2021. Norisoboldine attenuates sepsis-induced acute lung injury by modulating macrophage polarization via PKM2/HIF-1α/PGC-1α pathway. Biol Pharm Bull. 44(10):1536–1547. doi: 10.1248/bpb.b21-00457.
- Cheng X, Wang XN, Xin GZ, Liu LF. 2020. Comprehensive characterization of the chemical components of Kanggongyan by UPLC-QTOF MS. J Shenyang Pharm Univ. 37:1099–1110. Chinese.
- Das S, Bhattacharjee MJ, Mukherjee AK, Khan MR. 2023. Recent advances in understanding of multifaceted changes in the vaginal microenvironment: implications in vaginal health and therapeutics. Crit Rev Microbiol. 49(2):256–282. doi: 10.1080/1040841X.2022.2049696.
- Dludla PV, Nkambule BB, Jack B, Mkandla Z, Mutize T, Silvestri S, Orlando P, Tiano L, Louw J, Mazibuko-Mbeje SE. 2018. Inflammation and oxidative stress in an obese state and the protective effects of gallic acid. Nutrients. 11(1):23. doi: 10.3390/nu11010023.
- Dong X, Zhang Q, Zeng F, Cai M, Ding D. 2022. The protective effect of gentisic acid on rheumatoid arthritis via the RAF/ERK signaling pathway. J Orthop Surg Res. 17(1):109. doi: 10.1186/s13018-022-03006-7.
- Du J, Zhang P, Luo J, Shen L, Zhang S, Gu H, He J, Wang L, Zhao X, Gan M, et al. 2021. Dietary betaine prevents obesity through gut microbiota-drived microRNA-378a family. Gut Microbes. 13(1):1–19. doi: 10.1080/19490976.2020.1862612.
- Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, et al. 2011. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 145(5):745–757. doi: 10.1016/j.cell.2011.04.022.
- Fiorucci S, Biagioli M, Zampella A, Distrutti E. 2018. Bile acids activated receptors regulate innate immunity. Front Immunol. 9:1853. doi: 10.3389/fimmu.2018.01853.
- Gar C, Rottenkolber M, Prehn C, Adamski J, Seissler J, Lechner A. 2018. Serum and plasma amino acids as markers of prediabetes, insulin resistance, and incident diabetes. Crit Rev Clin Lab Sci. 55(1):21–32. doi: 10.1080/10408363.2017.1414143.
- Gerritsen J, Fuentes S, Grievink W, van Niftrik L, Tindall BJ, Timmerman HM, Rijkers GT, Smidt H. 2014. Characterization of Romboutsia ilealis gen. nov., sp. nov., isolated from the gastro-intestinal tract of a rat, and proposal for the reclassification of five closely related members of the genus Clostridium into the genera Romboutsia gen. nov., Intestinibacter gen. nov., Terrisporobacter gen. nov. and Asaccharospora gen. nov. Int J Syst Evol Microbiol. 64(Pt 5):1600–1616. doi: 10.1099/ijs.0.059543-0.
- Giron LB, Papasavvas E, Yin X, Goldman AR, Tang HY, Palmer CS, Landay AL, Li JZ, Koethe JR, Mounzer K, et al. 2021. Phospholipid metabolism is associated with time to HIV rebound upon treatment interruption. mBio. 12(1):e03444-20. doi: 10.1128/mBio.03444-20.
- Holubekova V, Kolkova Z, Kasubova I, Samec M, Mazurakova A, Koklesova L, Kubatka P, Rokos T, Kozubik E, Biringer K, et al. 2022. Interaction of cervical microbiome with epigenome of epithelial cells: significance of inflammation to primary healthcare. Biomol Concepts. 13(1):61–80. doi: 10.1515/bmc-2022-0005.
- Jayakumar NK. 2015. Cervicitis: how often is it non-specific! J Clin Diagn Res. 9(3):EC11–12.
- Johnson CH, Ivanisevic J, Siuzdak G. 2016. Metabolomics: beyond biomarkers and towards mechanisms. Nat Rev Mol Cell Biol. 17(7):451–459. doi: 10.1038/nrm.2016.25.
- Knuplez E, Marsche G. 2020. An updated review of pro- and anti-inflammatory properties of plasma lysophosphatidylcholines in the vascular system. Int J Mol Sci. 21(12):4501. doi: 10.3390/ijms21124501.
- Li JM. 2021. Study on the pharmacodynamic material basis and mechanism of action of compound apricot fragrance rabbit ear wind against cervicitis [dissertation]. Nanchang (Jiangxi): Jiangxi University of Traditional Chinese Medicine. Chinese.
- Li X, Nakayama K, Goto T, Akamatsu S, Kobayashi T, Shimizu K, Ogawa O, Inoue T. 2021. A narrative review of urinary phospholipids: from biochemical aspect towards clinical application. Transl Androl Urol. 10(4):1829–1849. doi: 10.21037/tau-20-1263.
- Li Y, Yao J, Han C, Yang J, Chaudhry MT, Wang S, Liu H, Yin Y. 2016. Quercetin, inflammation and immunity. Nutrients. 8(3):167. doi: 10.3390/nu8030167.
- Li Y, Zhao H, Sun G, Duan Y, Guo Y, Xie L, Ding X. 2022. Alterations in the gut microbiome and metabolome profiles of septic rats treated with aminophylline. J Translational Med. 20(1):69.
- Ling N, Zhang X, Forsythe S, Zhang D, Shen Y, Zhang J, Ding Y, Wang J, Wu Q, Ye Y. 2022. Bacteroides fragilis ameliorates Cronobacter malonaticus lipopolysaccharide-induced pathological injury through modulation of the intestinal microbiota. Front Immunol. 13:931871. doi: 10.3389/fimmu.2022.931871.
- Luo Y, Tian W, Lu X, Zhang C, Xie J, Deng X, Xie Y, Yang S, Du W, He R, et al. 2022. Prognosis stratification in breast cancer and characterization of immunosuppressive microenvironment through a pyrimidine metabolism-related signature. Front Immunol. 13:1056680. doi: 10.3389/fimmu.2022.1056680.
- Lv Y, Zou Y, Zhang X, Liu B, Peng X, Chu C. 2022. A review on the chemical constituents and pharmacological efficacies of Lindera aggregata (Sims) Kosterm. Front Nutr. 9:1071276. doi: 10.3389/fnut.2022.1071276.
- Malard F, Dore J, Gaugler B, Mohty M. 2021. Introduction to host microbiome symbiosis in health and disease. Mucosal Immunol. 14(3):547–554. doi: 10.1038/s41385-020-00365-4.
- Mangifesta M, Mancabelli L, Milani C, Gaiani F, de’Angelis N, de’Angelis GL, van Sinderen D, Ventura M, Turroni F. 2018. Mucosal microbiota of intestinal polyps reveals putative biomarkers of colorectal cancer. Sci Rep. 8(1):13974. doi: 10.1038/s41598-018-32413-2.
- Miao LL, Zhou QM, Peng C, Liu ZH, Xiong L. 2019. Leonurus japonicus (Chinese motherwort), an excellent traditional medicine for obstetrical and gynecological diseases: a comprehensive overview. Biomed Pharmacother. 117:109060. doi: 10.1016/j.biopha.2019.109060.
- Miranda AE, Silveira MFD, Pinto VM, Alves GC, Carvalho NS. 2021. Brazilian protocol for sexually transmitted infections 2020: infections that cause cervicitis. Epidemiol Serv Saude. 30(spe1):e2020587. doi: 10.1590/S1679-4974202100008.esp1.
- Ortiz-de la Tabla V, Gutiérrez F. 2019. Cervicitis: etiology, diagnosis and treatment. Enferm Infecc Microbiol Clin (Engl Ed). 37(10):661–667. doi: 10.1016/j.eimce.2018.12.011.
- Parker BJ, Wearsch PA, Veloo ACM, Rodriguez-Palacios A. 2020. The genus Alistipes: gut bacteria with emerging implications to inflammation, cancer, and mental health. Front Immunol. 11:906. doi: 10.3389/fimmu.2020.00906.
- Patrick S. 2022. A tale of two habitats: Bacteroides fragilis, a lethal pathogen and resident in the human gastrointestinal microbiome. Microbiology. 168(4):001156. doi: 10.1099/mic.0.001156.
- Qayed M, Michonneau D, Socié G, Waller EK. 2021. Indole derivatives, microbiome and graft versus host disease. Curr Opin Immunol. 70:40–47. doi: 10.1016/j.coi.2021.02.006.
- Rastogi S, Singh A. 2022. Gut microbiome and human health: exploring how the probiotic genus Lactobacillus modulate immune responses. Front Pharmacol. 13:1042189. doi: 10.3389/fphar.2022.1042189.
- Rodriguez-Palacios A, Harding A, Menghini P, Himmelman C, Retuerto M, Nickerson KP, Lam M, Croniger CM, McLean MH, Durum SK, et al. 2018. The artificial sweetener Splenda promotes gut Proteobacteria, dysbiosis, and myeloperoxidase reactivity in Crohn’s disease-like ileitis. Inflamm Bowel Dis. 24(5):1005–1020. doi: 10.1093/ibd/izy060.
- Rodriguez-Palacios A, Kodani T, Kaydo L, Pietropaoli D, Corridoni D, Howell S, Katz J, Xin W, Pizarro TT, Cominelli F. 2015. Stereomicroscopic 3D-pattern profiling of murine and human intestinal inflammation reveals unique structural phenotypes. Nat Commun. 6(1):7577. doi: 10.1038/ncomms8577.
- Rosas-Rodríguez JA, Valenzuela-Soto EM. 2021. The glycine betaine role in neurodegenerative, cardiovascular, hepatic, and renal diseases: insights into disease and dysfunction networks. Life Sci. 285:119943. doi: 10.1016/j.lfs.2021.119943.
- Shi A, Shi H, Wang Y, Liu X, Cheng Y, Li H, Zhao H, Wang S, Dong L. 2018. Activation of Nrf2 pathway and inhibition of NLRP3 inflammasome activation contribute to the protective effect of chlorogenic acid on acute liver injury. Int Immunopharmacol. 54:125–130. doi: 10.1016/j.intimp.2017.11.007.
- Shi H, Wang XL, Quan HF, Yan L, Pei XY, Wang R, Peng XD. 2019. Effects of betaine on LPS-stimulated activation of microglial M1/M2 phenotypes by suppressing TLR4/NF-κB pathways in N9 cells. Molecules. 24(2):367. doi: 10.3390/molecules24020367.
- Shi XD, Zhang JX, Hu XD, Zhuang T, Lu N, Ruan CC. 2022. Leonurine attenuates obesity-related vascular dysfunction and inflammation. Antioxidants. 11(7):1338. doi: 10.3390/antiox11071338.
- Strandwitz P. 2018. Neurotransmitter modulation by the gut microbiota. Brain Res. 1693(Pt B):128–133. doi: 10.1016/j.brainres.2018.03.015.
- Su J, Li S, Chen J, Jian C, Hu J, Du H, Hai H, Wu J, Zeng F, Zhu J, et al. 2022. Glycerophospholipid metabolism is involved in rheumatoid arthritis pathogenesis by regulating the IL-6/JAK signaling pathway. Biochem Biophys Res Commun. 600:130–135. doi: 10.1016/j.bbrc.2022.02.003.
- Sun X, Zhou M, Pu J, Wang T. 2022. Stachydrine exhibits a novel antiplatelet property and ameliorates platelet-mediated thrombo-inflammation. Biomed Pharmacother. 152:113184. doi: 10.1016/j.biopha.2022.113184.
- Sun XY, Li LJ, Dong QX, Zhu J, Huang YR, Hou SJ, Yu XL, Liu RT. 2021. Rutin prevents tau pathology and neuroinflammation in a mouse model of Alzheimer’s disease. J Neuroinflammation. 18(1):131. doi: 10.1186/s12974-021-02182-3.
- Tomé D. 2021. Amino acid metabolism and signalling pathways: potential targets in the control of infection and immunity. Eur J Clin Nutr. 75(9):1319–1327. doi: 10.1038/s41430-021-00943-0.
- Tu Y, Sun L, Guo M, Chen W. 2013. The medicinal uses of Callicarpa L. in traditional Chinese medicine: an ethnopharmacological, phytochemical and pharmacological review. J Ethnopharmacol. 146(2):465–481. doi: 10.1016/j.jep.2012.12.051.
- van der Veen JN, Kennelly JP, Wan S, Vance JE, Vance DE, Jacobs RL. 2017. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim Biophys Acta Biomembr. 1859(9 Pt B):1558–1572. doi: 10.1016/j.bbamem.2017.04.006.
- Wang B, Liu J, Lei R, Xue B, Li Y, Tian X, Zhang K, Luo B. 2022. Cold exposure, gut microbiota, and hypertension: a mechanistic study. Sci Total Environ. 833:155199. doi: 10.1016/j.scitotenv.2022.155199.
- Wang C, Ma C, Gong L, Dai S, Li Y. 2021. Preventive and therapeutic role of betaine in liver disease: a review on molecular mechanisms. Eur J Pharmacol. 912:174604. doi: 10.1016/j.ejphar.2021.174604.
- Wu CH, Ko JL, Liao JM, Huang SS, Lin MY, Lee LH, Chang LY, Ou CC. 2019. D-Methionine alleviates cisplatin-induced mucositis by restoring the gut microbiota structure and improving intestinal inflammation. Ther Adv Med Oncol. 11:1758835918821021. doi: 10.1177/1758835918821021.
- Wyse ATS, Dos Santos TM, Seminotti B, Leipnitz G. 2021. Insights from animal models on the pathophysiology of hyperphenylalaninemia: role of mitochondrial dysfunction, oxidative stress and inflammation. Mol Neurobiol. 58(6):2897–2909. doi: 10.1007/s12035-021-02304-1.
- Xu H, Liu L, Xu F, Liu M, Song Y, Chen J, Zhan H, Zhang Y, Xu D, Chen Y, et al. 2022. Microbiome-metabolome analysis reveals cervical lesion alterations. Acta Biochim Biophys Sin. 54(10):1552–1560. doi: 10.3724/abbs.2022149.
- Yang K, Xia B, Wang W, Cheng J, Yin M, Xie H, Li J, Ma L, Yang C, Li A, et al. 2017. A comprehensive analysis of metabolomics and transcriptomics in cervical cancer. Sci Rep. 7(1):43353. doi: 10.1038/srep43353.
- Ye N, Liu C, Shi P. 2015. Metabolomics analysis of cervical cancer, cervical intraepithelial neoplasia and chronic cervicitis by 1H NMR spectroscopy. Eur J Gynaecol Oncol. 36(2):174–180.
- Ye X, Liu Y, Hu J, Gao Y, Ma Y, Wen D. 2021. Chlorogenic acid-induced gut microbiota improves metabolic endotoxemia. Front Endocrinol. 12:762691. doi: 10.3389/fendo.2021.762691.
- Yuan MY, Zhang M, Zhang S, Cao SY, Ji JC, Wang PJ, Zhang RP, Gao XL. 2022. Q-marker analysis of Kanggongyan soft capsule. J China Pharmacy. 33:2082–2086 + 2092. Chinese.
- Zeng Z, Guo X, Zhang J, Yuan Q, Chen S. 2021. Lactobacillus paracasei modulates the gut microbiota and improves inflammation in type 2 diabetic rats. Food Funct. 12(15):6809–6820. doi: 10.1039/d1fo00515d.
- Zhang X, Li J, Xie B, Wu B, Lei S, Yao Y, He M, Ouyang H, Feng Y, Xu W, et al. 2018. Comparative metabolomics analysis of cervicitis in human patients and a phenol mucilage-induced rat model using liquid chromatography tandem mass spectrometry. Front Pharmacol. 9:282. doi: 10.3389/fphar.2018.00282.
- Zhao H, Cheng N, Zhou W, Chen S, Wang Q, Gao H, Xue X, Wu L, Cao W. 2019. Honey polyphenols ameliorate DSS-induced ulcerative colitis via modulating gut microbiota in rats. Mol Nutr Food Res. 63(23):e1900638.
- Zhao H, Raines LN, Huang SC. 2020. Carbohydrate and amino acid metabolism as hallmarks for innate immune cell activation and function. Cells. 9(3):562. doi: 10.3390/cells9030562.
- Zhu J, Thompson CB. 2019. Metabolic regulation of cell growth and proliferation. Nat Rev Mol Cell Biol. 20(7):436–450. doi: 10.1038/s41580-019-0123-5.
- Zuo T, Yue Y, Wang X, Li H, Yan S. 2021. Luteolin relieved DSS-induced colitis in mice via HMGB1-TLR-NF-κB signaling pathway. Inflammation. 44(2):570–579. doi: 10.1007/s10753-020-01354-2.