Abstract
Context
Rice bran arabinoxylan compound (RBAC) is a natural immunomodulator with anticancer properties.
Objective
This study critically evaluates the available evidence on the biological pathways of RBAC and its effects on cancer treatment.
Methods
This secondary analysis of a scoping review includes studies evaluating the mechanisms of RBAC on healthy or malignant cells, animal models, or humans for cancer prevention or treatment. Data from randomized controlled trials on survival and quality of life outcomes were subjectd to meta analysis.
Results
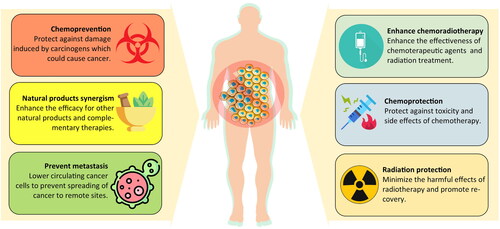
The evidence synthesis was based on 38 articles. RBAC exhibited antitumor properties by promoting apoptosis and restoring immune function in cancer patients to enhance inflammatory and cytotoxic responses to block tumorigenesis. RBAC works synergistically with chemotherapeutic agents by upregulating drug transport. In a clinical trial, combining RBAC with chemoembolization in treating liver cancer showed improved response, reduced recurrence rates, and prolonged survival. RBAC also augments the endogenous antioxidant system to prevent oxidative stress and protect against radiation side effects. In addition, RBAC has chemoprotective effects. Animals and humans have exhibited reduced toxicity and side effects from chemotherapy. Meta analysis indicates that RBAC treatment increases the survival odds by 4.02-times (95% CI: 1.67, 9.69) in the first year and 2.89-times (95% CI: 1.56, 5.35) in the second year.
Conclusion
RBAC is a natural product with immense potential in cancer treatment. Additional research is needed to characterize, quantify, and standardize the active ingredients in RBAC responsible for the anticancer effects. More well-designed, large-scale clinical trials are required to substantiate the treatment efficacies further.
Introduction
Cancer is a disease that often evokes an image of ‘dread and death’ in the minds of most people (Robb et al. Citation2014). According to the global mortality data estimates in 2019, cancer caused 3 out of 10 premature deaths due to non-communicable diseases (Bray et al. Citation2021). With an estimated 19.3 million new cancer cases and almost 10.0 million cancer deaths in 2020 worldwide, cancer is a global concern (Sung et al. Citation2021). Also called malignancy, cancer refers to any pathophysiological conditions resulting from the abnormal and uncontrolled growth of cells that can become invasive to other organs or parts of the body through the circulatory and lymphatic systems (National Cancer Institute 2023). From a philosophical perspective, such disordered growth signifies the breakdown of the natural selection within the host tissue that defines the order of life itself (Lemoine Citation2022). Cancer is thus not a disease introduced by some entity foreign to the body, but rather the host cells turning rogue to become agents of destruction (Hausman Citation2019).
Generally, cancer is named based on the primary site, and the most commonly diagnosed are breast, lung, colorectal, prostate, and stomach cancers (Sung et al. Citation2021). The aetiology of cancer can range from infectious agents (such as viruses, parasites, fungi, and bacteria) to environmental exposure (such as to pollutants, radiation, ultraviolet rays from sunlight, and chemical exposure) and lifestyle factors (such as cigarette smoking, an unhealthy diet with excessive fried foods and red meat, alcohol drinking, stress, obesity, and physical inactivity) (Blackadar Citation2016). Essentially, any endogenous or exogenous substance capable of inducing deoxyribonucleic acid (DNA) damage can lead to cancer, and these substances are termed carcinogens (Barnes et al. Citation2018). Moreover, hereditary genetic predispositions can also increase the relative risks of one or more types of cancer in some individuals (Knudson Citation2002).
At the cellular level, cancer develops from a single cell following genetic damage, possibly through exposure to a carcinogen, starting to grow and divide abnormally. This proliferation then leads to the selective clonal expansion of the initiated cells and gives rise to a small benign neoplasm. However, further selective and rapid cell mass growth increases the risk of genetic mutations in clonal cells to express the malignant phenotypes and become a cancerous tumour. Malignant cells acquire more aggressive characteristics through additional genetic and epigenetic changes, including the activation of protooncogenes and the functional loss of tumour suppressor genes (Wang et al. Citation2018). These changes lead to tumour progression and metastasis to other body parts (Weston and Harris Citation2003).
In terms of treatment, the conventional oncological options are surgical intervention to provide definitive locoregional control of the primary tumour (Dare et al. Citation2015), chemotherapy for inhibiting cell proliferation and tumour growth, thus avoiding invasion and metastasis (Amjad et al. Citation2023), and radiation therapy to deprive cancer cells of the multiplication potential (Baskar et al. Citation2012). Although modalities such as immunotherapy, targeted therapy, hormonal therapy, and gene therapy are existing systematic therapeutic alternatives, chemotherapy and radiotherapy remain the mainstays for cancer treatment in the foreseeable future. The global demand for first-course chemotherapy was projected to increase from 9.8 million patients annually in 2018 to 15.0 million in 2040 (Wilson et al. Citation2019). Furthermore, the optimal radiotherapy utilisation rate was estimated to account for almost half (48.3%) of all cancer patients indicated for irradiation treatment (Delaney and Barton Citation2015).
Conventional chemo and irradiation treatments are known for their undesirable side effects. Nausea, vomiting, fatigue, anorexia, dysgeusia, hair loss, dry mouth, and constipation are among the most common concomitant complaints against chemotherapy (Altun and Sonkaya Citation2018). Incidents of severe toxicity requiring medical intervention are not uncommon, and some can even be life-threatening. One study reported that 76.1% of participants with lung cancer from two clinical trials experienced severe toxicity during chemotherapy (SjØgren et al. Citation2020). Moreover, chemotherapy not only destroys malignant cells but also causes immunogenic cell death, making the host susceptible to opportunistic pathogenic infection that further weakens the immune system (Nesher and Rolston Citation2014). Cancer can also develop resistance to chemotherapy, reducing the administered drugs’ efficacy and causing treatment complications (Bukowski et al. Citation2020). Patients receiving radiotherapy also commonly experience fatigue and localized radiation-induced adverse events such as inflammation or ulceration (head and nose), dyspnoea and chronic lung fibrosis (thoracic), and gastrointestinal (GI) symptoms (pelvic) (Majeed and Gupta Citation2023). Furthermore, depression and anxiety are common among cancer patients during treatment and may linger for years in cancer survivors (Götze et al. Citation2020).
To improve the therapeutic efficacy of cancer treatment while reducing the potential toxicity, researchers often look to nature for ingredients and inspiration. Substances produced naturally from living organisms, such as plants, animals, and microbes, often possess pharmacological or biological properties worth harnessing for disease treatment. Unsurprisingly, natural products, especially biologically active compounds derived from plants, have been and continue to be invaluable in anticancer research and therapeutic discoveries (Muhammad et al. Citation2022). Among the better-known plant-based natural products with chemopreventive and anticancer properties include curcumin in Curcuma longa L. (Zingiberaceae) (turmeric), indole-3-carbinol from cruciferous vegetables, resveratrol in grapes and wine, epigallocatechin gallate from green tea, and genistein in soybeans (Muhammad et al. Citation2022). Another source of natural products that has gained much interest is rice bran, the hard outer layer of rice grain when removed during milling. Rice bran extracts, fermented rice bran products, and γ-oryzanol in rice bran have all been researched for their anticancer potentials (Yu et al. Citation2019).
The rice bran arabinoxylan compound (RBAC) is a heteropolysaccharide extract of defatted rice bran obtained through enzymatic treatment with Lentinus edodes (Berk.) Singer (Agaricomycetideae) mycelium (Ooi et al. Citation2021). The most studied RBAC is Biobran MGN-3 developed by Daiwa Pharmaceutical Co., Ltd. (Tokyo, Japan), which has been marketed as a dietary supplement for the immune system and used by cancer patients during and after treatment (Clark Citation1999). A previous review by the authors (SLO and SCP) has established RBAC as an effective immunomodulator for complementing conventional cancer treatment with favourable effects, including enhancing the immune profile, reducing side effects, improving treatment outcomes, and increasing survival rates (Ooi et al. Citation2018). However, the physiological process of RBAC wielding such synergistic anticancer effects has not been critically assessed. Furthermore, according to the guidelines of the American Society of Clinical Oncology (Citation1996), the primary outcomes of cancer treatment are survival, especially disease-free survival, and health-related quality-of-life (QoL), including overall QoL, as well as its physical, psychological, and social dimensions. Other outcome measures, such as toxicity, tumour response, and biomarkers, are means to assess or predict the survival or QoL of cancer patients. Hence, when considering a potential adjuvant therapeutic option for cancer, it is essential to consider the best available evidence based on the outcomes of survival and QoL.
Objective
This study critically evaluates the available evidence to answer the following two-part research questions to inform evidence-based clinical practice: (1) In cancer patients, what are the mechanisms and biological pathways that RBAC could exert synergistic effects on to prevent cancer development and support cancer treatment? (2) What are the survival and QoL outcome changes associated with RBAC as a complementary therapy compared to treatments without RBAC?
Materials and methods
Sources of evidence
This study is a secondary analysis of the evidence gathered from a previous scoping review that systematically identified all preclinical and clinical studies for RBAC published until the end of 2022. The characteristics of all included studies (n = 98) with bibliographic and network analyses were reported in an earlier manuscript (Ooi et al. Citation2023b). Two recent RBAC studies published after the scoping study completion were also considered in this review (Hajtó et al. Citation2022; Ghoneum et al. Citation2023).
Selection criteria
To answer the first research question, the reviewers screened and shortlisted the sources of evidence (n = 100) using the following concept-population-context criteria: (a) any studies of RBAC evaluating the mechanisms and biological pathways (concept); (b) on healthy or malignant cells, tissues, animal models or human participants including cancer patients (population); (c) concerning any synergistic effects to prevent cancer development or support cancer treatment (context). The reviewers excluded all case reports or series as they are not rigorous enough to investigate the effects and mechanisms of action of an intervention.
From the included studies, the reviewers further shortlisted the best available evidence for RBAC as an intervention for cancer to address the second research question based on the following patient-intervention-comparator-outcome criteria: (a) a randomized controlled trial (RCT); (b) includes patients of any malignancies; (c) uses RBAC as an intervention; (d) with any comparators; and (e) outcome measures include survival and/or QoL assessment. We included trials with outcome measures based on cancer treatment-related side effects as treatment-related side effects may predict QoL (Mazzotti et al. Citation2012).
Evidence synthesis, analysis, and presentation
Data and results from selected articles were extracted with specific details about the citation, study design, concept, context, methodology, outcome measures, and key findings relevant to the topic. The evidence synthesis is illustrated graphically, diagrammatically, or in tabular form, accompanying narrative summaries to demonstrate how the results relate to the first part of the research question.
For survival rate analysis, the sample sizes and survival events of RBAC and placebo groups of selected studies under similar time points were combined (published data only). The data from each study were weighted, such that studies with a smaller 95% confidence interval (CI) or a larger sample size contributed more heavily to the odds ratio (OR) estimate (Mantel-Haenszel) with a fixed effect model (Deeks et al. Citation2021). Review Manager 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) was used to calculate and display the meta-analysis results in a forest plot.
Due to the dissimilarity in the QoL assessment across studies, performing meta-analyses to estimate the effect sizes is not feasible. Instead, the visualization of the evidence is achieved on a bubble chart, with QoL outcome measures as the Y-axis and statistical significance (p-value) of the outcome as the X-axis. If the p-value of a continuous variable was not available, the reviewers used the standard deviation or 95% CI to estimate. Fisher’s exact test was used to calculate the p-value if not reported for dichotomous outcome variables, such as alopecia events. All charting and calculations were performed with Microsoft Excel 365 (Microsoft Corp, WA, USA).
Quality assessment
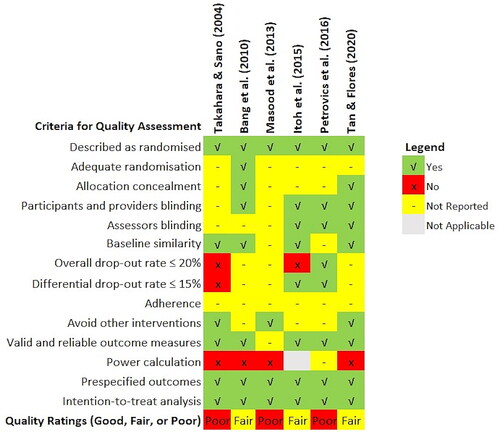
Assessment of the methodological quality of the evidence was based on the Quality Assessment Tool for Controlled Interventional Studies published by the National Heart Lung and Blood Institute (Citation2013). The assessment tool consists of 14 items covering all the essential quality criteria of an RCT, including randomization, allocation concealment, blinding, baseline similarity, dropout, adherence, concomitant avoidance, outcome validity, power, and intention-to-treat analysis. Two authors (PSM and SCP) and an independent assessor evaluated the study quality separately, with consensus achieved through the Delphi method (Nasa et al. Citation2021). A third author (SLO) was the facilitator, aggregating and sharing the responses to the checklist anonymously with the group after each assessment round.
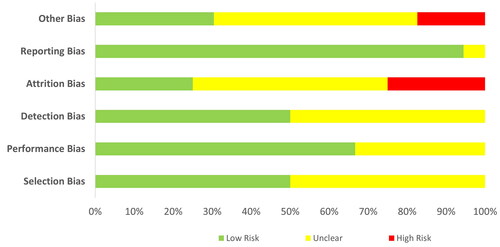
The assessors could adjust their answers at each iteration based on how they interpret the group response until the agreement is reached. The 14 quality assessment items can be further grouped for the detection of six types of bias, namely selection (items 1–3), performance (item 4), detection (item 5), attrition (items 7–8), reporting (items 11, 13–14) and other biases (items 6, 9–10, 12), summarized in a percentage-stacked bar chart. The clinical effects of the best available evidence and the assessed quality formed the basis for final recommendations.
Results
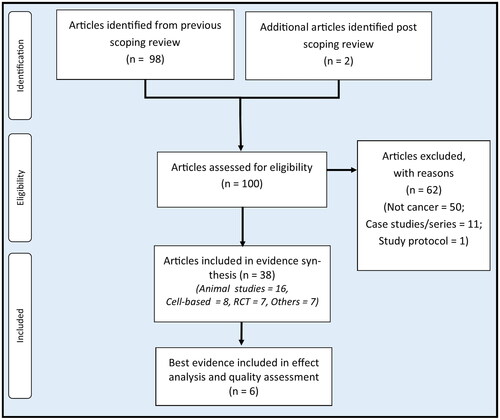
The flow of article selection is depicted in . Out of the 100 pre-identified RBAC sources of evidence, 50 non-cancer-related articles did not fulfil the inclusion criteria, and 11 case reports/series on cancer patients and one study protocol were excluded. Hence, 38 articles were included for evidence synthesis. Of these, 24 were preclinical studies (16 animal and eight in vitro), and 14 were human clinical trials (RCT = 7, non-RCT = 1, before and after = 5, cross-sectional = 1). Note that 89.5% (34/38) of all included studies are based on Biobran MGN-3, and the rest (10.5%, 4/38) are based on other RBAC products produced by Erom Co., Ltd. (Chuncheon, South Korea). The following sections synthesize the evidence on how RBAC exerts synergistic effects to prevent cancer development or support cancer treatment and the potential mechanisms. A preprint of this work has been deposited in an online platform for open access (Ooi et al. Citation2023a).
Immune restorative effects
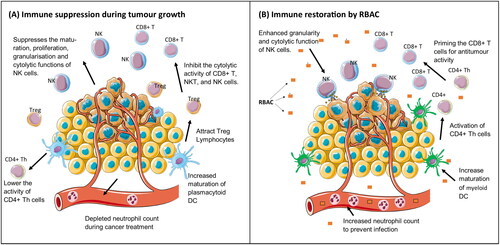
The immune system plays an essential role in suppressing cancer growth through immunosurveillance by cytotoxic lymphocytes, including the natural killer (NK) cells and cluster differentiation (CD) 8+ T cells. As such, developing a malignant tumour from initiation to proliferation requires cancer cells to evade the immune system attacks by avoiding recognition and instigating an immunosuppressive microenvironment conducive to tumour growth (Gonzalez et al. Citation2018). Restoring and harnessing the antitumor immune response of the body to control and eliminate tumours thus becomes a viable therapeutic option in cancer known as immunotherapy (Wu et al. Citation2021). RBAC was known as an immunomodulator, with the available evidence on the immune restorative effects in cancer listed in .
Table 1. Results from human and animal studies on the immune restorative capacity of RBAC in cancer.
Dysfunctional NK cells are often observed in the microenvironment of advanced solid tumours due to the production of soluble modulators, low nutrient levels, and hypoxic conditions that negatively affect the maturation, proliferation, activation, and cytolytic function of NK cells (Melaiu et al. Citation2019). This phenomenon has prompted the call for NK cell-based immunotherapy for cancer treatment (Riggan et al. Citation2021). Existing evidence has demonstrated RBAC to be a potent activator of NK cell cytolytic activity against malignant cells in cancer patients. Ghoneum and Brown (Citation1999) first reported this in a single-arm study of 32 patients with various malignancies (prostate, breast, multiple myeloma [MM], and leukemia) who had completed one or more conventional therapies (surgery, chemotherapy, and radiation). Low NK cell activity levels were prevalent among these patients. After taking RBAC (3 g/day) orally for one to two weeks, significant increases (p < 0.001) in NK cell activity of up to 10-fold compared to baseline were detected (Ghoneum and Brown Citation1999). In a separate but possibly related article, Ghoneum (Citation1999) reported that 86 out of 90 cancer patients (95.5%) with various malignancies who received 3 g/day of RBAC after completion of conventional therapies demonstrated 2- to 10-fold increases in NK cell cytolytic activity level at one to two weeks post-treatment. However, as the work of Ghoneum (Citation1999) is a conference abstract, insufficient detail was presented, and the data was not peer-reviewed.
Further examination of NK cell granularity by Ghoneum and Brown (Citation1999) with cytocentrifuge preparation of a patient’s peripheral blood lymphocytes (PBL) at baseline indicated low or absent granularity, indicating dysfunctional NK cell populations. Increased NK cell granularity was subsequently observed in the same patient after RBAC treatment for one week. These NK cells demonstrated enhanced capacity in binding and killing tumour cells (K562) in vitro compared to the low-granular NK cells isolated before treatment. Testing of T and B lymphocyte proliferation after one month of RBAC treatment in five selected patients also showed statistically significant (p < 0.001) increases in responses with phytohaemagglutinin (B cell mitogen), concanavalin A (T cell mitogen), and pokeweed (T and B cell mitogen) tests compared to baseline, all of which demonstrated signs of restoration of the adaptive immunity (Ghoneum and Brown Citation1999).
An in vivo experiment by Badr El-Din, et al. (Citation2016a) also showed that oral administration (p.o.) of RBAC at 40 mg/kg body weight (BW) every other day prevented lymphocyte depletion in male Wistar rats exposed to the carcinogen methylnitronitrosoguanidine (MNNG). After eight months, rats administered with MNNG alone had a significantly lower percentage of lymphocytes (↓23.3%, p < 0.01) compared to healthy controls. However, the group treated with RBAC after MNNG administration exhibited lymphocyte recovery, with the levels returning to normal, a significant difference from the untreated MNNG group (p < 0.05).
Takahara and Sano (Citation2004) analyzed the relationship between NK cell cytolytic activity and survival rate in an RCT with two groups of cancer patients. All participants had progressive cancer of late stages (III–IV) with recurrence, unresectable lesions, or metastasis after surgery. The intervention group (n = 96) received 3 g/day of RBAC oral supplement plus complementary therapies, whereas the control group (n = 109) received only the complementary therapies. Fifty patients in the control group could not complete the study due to cancer progression or pessimism in the treatment. After 18 months, a higher survival rate (p < 0.019) was observed in the RBAC group (54.2%, 52/96) compared to the control group (33.9%, 19/56). The difference between survival rates was more significant (p < 0.001) based on intention-to-treat analysis, which includes all dropouts (control = 53, RBAC = 0). The study found that all patients who dropped out did not survive at 18 months. Hence, the survival rate for the control group was only 17.4% (19/109) (Takahara and Sano Citation2004).
When categorizing the participants based on initial NK cell activity levels of low (< 20%), medium (20 to 40%), and high (> 40%), the study found that significantly higher rates of participants with low or medium NK cell activity levels in the RBAC group survived, compared to the control group (Low: 42.5% vs. 12.5%, p < 0.01; Medium: 51.4% vs. 28.0%, p < 0.05). Hence, RBAC upregulated the dysfunctional NK cells in late-stage cancer patients to prolong survival (Takahara and Sano Citation2004).
In contrast, an exploratory RCT by Itoh et al. (Citation2015) did not detect any significant differences in NK cell activities between the RBAC (n = 7) and the control (n = 7) groups in cervical cancer patients receiving chemoradiotherapy. The trial was conducted over three weeks of one treatment cycle, with the participants starting either oral RBAC (3 g/day) or placebo powder up to one week before treatment commenced. Both groups experienced a decline in NK cell activity levels after chemoradiotherapy compared to the baseline values. Hence, RBAC could not prevent the decline in NK cell activity levels during chemoradiotherapy in this trial. Nonetheless, with the small sample size and short duration, the study may not have sufficient statistical power to detect the treatment effects.
Cholujova et al. (Citation2013) studied the immunomodulatory effects of RBAC on the innate immunity of MM patients in a double-blind placebo-RCT. Admitted to this study were MM patients (n = 48) under observation and those receiving or completed chemotherapy. Participants were randomly assigned to take RBAC (2 g/day, n = 32) or a matching placebo (n = 16) orally for three months, and their blood samples were collected at baseline and monthly intervals. The study observed significant increases in the NK cell cytolytic activity of the RBAC group compared to the baseline (30.8 ± 7.4 lytic unit [LU]) in the first (47.0 ± 8.5 LU, p = 0.045) and second (56.6 ± 12.2 LU, p = 0.029) months but not the third month. No significant differences in NK cell cytolytic activity were observed in the placebo group throughout the trial. Additionally, Cholujova et al. (Citation2013) also detected a substantial increase in the percentage of circulating myeloid dendritic cells (DCs) after three months of RBAC treatment compared to baseline (25.8 ± 3.6% vs. 17.6 ± 2.6%, p = 0.036). The myeloid-to-plasmacytoid DC ratio in the RBAC group also significantly increased (p = 0.030). In contrast, no significant changes in either DC markers were detected in the placebo group over time.
The myeloid DCs capture and present antigens on their surface to T lymphocytes, thus bridging the innate immunity to adaptive immune responses (Chistiakov et al. Citation2015). Meanwhile, the plasmacytoid DCs are crucial to antiviral immunity, as they specialize in producing high levels of type I interferons (IFNs) (Ye et al. Citation2020). These DCs also play a role in immunosuppression by recruiting regulatory CD4 + CD25+ T lymphocytes (Treg) into the tumour microenvironment (Zhou et al. Citation2021). Treg lymphocytes are characterized by forkhead box protein p3 expression, a master transcription factor that suppresses anticancer immunity and thus promotes proliferation (Li et al. Citation2020). In MM patients, myeloid and plasmacytoid DC populations were inversely correlated with disease progression (Pasiarski et al. Citation2013). The increase in myeloid DC levels after the three-month RBAC supplementation coincided with the tapering of NK cell cytolytic activity levels. Such observations could signify a switch from innate immunity to more lasting adaptive immunity as part of the immune restorative process in MM patients.
Treg lymphocytes are immune regulatory cells that tightly regulate immune activation to prevent response to self-antigens, permit tolerance for weak antigens, and limit collateral damage in inflammation. Treg are essential to prevent autoimmune diseases, but they also suppress myeloid DC maturation and prevent T and B cell differentiation and proliferation, allowing cancer to escape detection (Sojka et al. Citation2008; Ohue and Nishikawa Citation2019; Togashi et al. Citation2019). Lissoni et al. (Citation2008) studied the changes in total NK cells, total T lymphocytes, and the T cell subpopulations (CD3+, CD4+ CD25+, CD4+, and CD8+) in 24 consecutive cancer patients who had received RBAC for two months (2 g/day for the first month and 1 g/day after). Among the participants, 18 did not respond to conventional treatment for solid metastatic tumours and had no other effective standard treatment. The remaining six had surgery only for locally limited neoplasms. Two participants died due to disease progression before the end of the study, leaving the results of 22 participants for evaluation.
The study by Lissoni et al. (Citation2008) observed no substantial changes in the mean number of lymphocytes, T lymphocytes (CD3+), T cytotoxic (CD8+) lymphocytes, and NK cells before and after RBAC intervention. The mean cell counts of T helper (Th, CD4+) and Treg increased and decreased, respectively, but without reaching statistical significance. Notwithstanding, a statistically significant change in the ratio of Th/Treg was detected (p = 0.025), and the increase in the Th/Treg ratio was more pronounced in participants with a low Th/Treg ratio at baseline (Lissoni et al. Citation2008). Hence, RBAC treatment inhibited the immunosuppressive Treg while restoring the adaptive immune responses facilitated by CD4+ Th in the fight against cancer.
Neutropenia is a common complication among cancer patients, especially those treated with chemotherapy, with almost one-third of patients developing low neutrophil count during treatment (Salako et al. Citation2021). The reduction of circulating neutrophils in the bloodstream increases the risk of infections. Neutropenia is even more common in patients with hematological malignancies, and the risk of bloodstream infection is more pronounced (Carvalho et al. Citation2020). The combination of fever and neutropenia (febrile neutropenia) is one of the most common causes of oncological emergencies, which can be fatal (Ba et al. Citation2020). The risks of further infections and mortality among patients with febrile neutropenia remained high for six months after the initial episode (Nordvig et al. Citation2018).
Golombick et al. (Citation2016) reported the potential restorative effects of RBAC on the depleted neutrophil count of patients with early B-cell lymphoid malignancies in a preliminary single-arm study. Recruiting patients with monoclonal gammopathy of undetermined significance (MGUS)/smoldering multiple myeloma (SMM) who had been on oral curcumin therapy (6 g/day) for six months or more, this study added RBAC (2 g/day). Inflammatory and immunologic markers were monitored every two months for six months. Half of the MGUS/SMM patients (n = 10) exhibited neutropenia at baseline. The study found an increased neutrophil count between 10 and 90% among eight participants after consuming RBAC. Such observations are encouraging but require validation through a larger controlled clinical trial.
As summarized in , RBAC appears to be a biological response modifier that could prevent or restore immune dysfunction in cancer patients by upregulating NK cell cytolytic activity, improving the maturation of myeloid DCs, inhibiting the immunosuppressive Treg, and reversing neutropenia. All these effects help to neutralize or eliminate immunity suppression triggered by tumour-associated inflammation, thus restoring the effectiveness of antitumor immune responses (Shalapour and Karin Citation2015).
Figure 2. The immune restorative effects of rice bran arabinoxylan compound (RBAC). (A) shows some immune functions that are affected by tumour growth resulting in the suppression of antitumor activity. (B) shows the biological response-modifying effects of RBAC restoring immune function in cancer patients.
Anticancer effects and pathways
Anticancer effects in vivo
RBAC arrests tumour growth and demonstrates anticancer activity directly. shows a list of murine models investigating the anticancer effects of RBAC in halting and reversing in vivo tumour growth and extending the survival rates of treated animals.
Table 2. The in vivo anticancer effects of RBAC from murine models with tumour growth inhibition and life prolongation as outcome measures.
Bae et al. (Citation2004) compared RBAC to polysaccharide peptide (PSP) extracted from the mycelium of basidiomycetes, a known natural anticancer product, in an experiment with ICR mice injected with S-180 squamous cells. The mice were orally fed with either RBAC or PSP (1.5 mg/day) as treatment or saline as a control for 23 days. RBAC was effective in inhibiting tumour growth by 66.2% based on tumour weight (TW) at the end of the study relative to untreated control mice (0.51 ± 0.34 g vs. 3.40 ± 1.46 g, p < 0.01). In contrast, TW reduction by PSP was less (↓49.0%, p < 0.05), albeit statistically significant relative to the untreated control. The mean BW of the RBAC and PSP groups was also significantly lower (p < 0.01) than that of the control mice from day eight onward.
Similarly, Badr El-Din et al. (Citation2008) observed that RBAC has in vivo anticancer effects in female Swiss albino mice inoculated with Ehrlich ascites carcinoma cells intramuscularly. After eight days, mice bearing a solid Ehrlich carcinoma (SEC) mass of ∼100 mm3 were randomly divided into receiving RBAC (40 mg/kg BW) either intraperitoneally (i.p., 3 ×/week from day 10) for three weeks or intratumorally (i.t., 3 ×/week from day 11) for five weeks. SEC-bearing mice receiving saline injections were used as controls. The delay in tumour development was apparent in RBAC-treated mice. In the i.p. group, the mean tumour volume (TV) became significantly lower than that in the control group starting from day 14 (p < 0.05), with between-group differences increasing throughout the study period. By day 35, the percentage difference in mean TV was 63.27% (p < 0.001) in favour of the i.p. group. The mean TW of the i.p. group at day 35 was also significantly lower (3.63 ± 0.45 g vs. 6.62 ± 0.38 g, p < 0.01) than that of the control. In parallel, the i.t. group also demonstrated a significant TV reduction trend starting from day 28, reaching a −44.83% (p < 0.01) difference on day 45. Through flow cytometry analysis of SEC, the study also observed a 1.8-fold increase in the percentage of apoptotic cells in RBAC-treated mice (74.68 ± 4.22%) compared to that in the control mice (42.61 ± 5.56%, p < 0.0001) with the enhanced apoptosis, further confirmed through histopathological examinations of the tumours.
The results of the anticancer effects of RBAC in SEC-bearing mice were also validated by Badr El-Din et al. (Citation2019) in a similar study with female Swiss albino mice. Treatment with RBAC at 40 mg/kg BW i.p. (3 ×/week from day 11) for three weeks significantly prevented BW loss (↓4.1% vs. 18%, p < 0.01) and reduced TW (↓46.3%, p < 0.01) in SEC-bearing mice compared to the control at day 30. Continuous suppression of TV throughout the study was detected: On day 14, the TV of RBAC-treated mice was 33.7% (p < 0.01) less than that of untreated mice, and the reduction reached 49.9% (p < 0.01) at the end of the study.
Noaman et al. (Citation2008) performed another study with SEC-bearing mice to compare the effect of low-dosage RBAC treatment (25 mg/kg BW i.p.) in two schedules on tumour growth. The early treatment schedule started from day four and continued to day 25 (19 injections, 6 ×/week), whereas the late treatment began from day 11 up to day 25 (13 injections). Early treatment significantly retarded TV by 54% relative to the control, compared to only 24% in the late treatment group (p < 0.01). Both treatment schedules also showed markedly reduced mean TW compared to the control, with −34% (p < 0.01) for the early group versus −12% (p < 0.05) for the late group.
Another RBAC product, Erom’s rice bran bio-exopolymer (RBEP), also shows anticancer effects in vivo. Kim et al. (Citation2007) conducted experiments on RBEP with two different models: (1) Survival time of ICR mice inoculated with S-180 sarcoma to induce malignant ascites, and (2) Solid tumour growth in C57/Bl6 mice transplanted with B16/Bl6 melanoma. In the first experiment, mice were treated with RBEP of different dosages (50 mg/kg BW i.p. or p.o., 250 mg/kg BW p.o.). RBEP prolonged the mean survival time of mice with malignant ascites, relative to the untreated mice (27.4 days), by 14.6% (31.4 days) and 30.3% (35.7 days) with 50 mg/kg and 250 mg/kg p.o. treatment, respectively. Further lifespan prolongation by 38.0% (37.8 days) was observed in mice treated with 50 mg/kg i.p., demonstrating that i.p. could be the preferred therapeutic route for RBEP (Kim et al. Citation2007). In the mice transplanted with B16/Bl6 solid tumours, RBEP significantly (p < 0.05) inhibited TW by 35.6% (2.38 g vs. 3.70 g of control mice) with the 50 mg/kg p.o. treatment, 41.7% (2.155 g) with 250 mg/kg, p.o., and 55.1% (1.66 g) with 50 mg/kg, i.p. For comparison, another group of mice was treated with fluorouracil, a pyrimidine antagonist (antimetabolite), and the TW was 0.851 g at the end of the study. Thus, the group of mice treated with fluorouracil exhibited inhibited tumour growth by 77% relative to the no-treatment group. Comparatively, RBEP was not as effective as fluorouracil in tumour growth inhibition.
An (Citation2011) also confirmed that treatment with 250 mg/kg BW of RBEP (p.o. and i.p.) daily for two weeks effectively extended survival and reduced cancer growth of sarcoma 180 (S-180)-inoculated ICR mice. The study observed higher survival rates in RBEP-treated mice than the controls, with a 5.3% higher survival rate in the p.o. group (19.9 vs. 18.9 days) and a 23.2% higher survival rate in the i.p. group (23 vs. 18.7 days). Notably, on day 23, all i.p. mice treated with RBEP remained alive, but none in the control group survived. Evaluating tumour growth by BW, the study observed a significantly (p < 0.05) lower BW than that of the control group in the p.o. group starting from day 13. For the p.o. group, a significantly lower BW was detected as early as day 10, and the difference continued to widen until the end of the study (p < 0.001).
RBAC derived from a specific black rice cultivar known as fermented SuperC3GHi bran (C3G-F) was also tested for its anticancer properties by Kim et al. (Citation2011) on mice models with malignant ascites (ICR mice + S-180 cells) and a solid tumour (C57BL/6 mice + B16/BI6 melanoma). The study observed that 250 mg/kg BW C3G-F administered orally reduced the BW gain of the ascites-bearing mice compared to the control mice. The between-group mean BW difference reached statistical significance (p < 0.05) from day eight onward. At day 15, the BW of the C3G-F group was about 60% lower than that of the control group (6.5 g vs. 11.8 g). In the second experiment, mice fed with 250 mg/kg BW C3G-F also exhibited solid tumours with 19.4% lower mass than untreated control mice three weeks after transplantation (0.514 ± 0.129 g vs. 0.635 ± 0.241 g, p < 0.05). Haematologic investigations observed that C3G-F-treated mice had a significantly higher white blood cell count than the control mice (4.24 ± 0.71 vs. 2.63 ± 1.26, p < 0.05). Accordingly, it was inferred that the in vivo antitumor effects of RBAC products involve strengthening the immune system.
To demonstrate that NK cells activated by RBAC could have a direct role in tumour suppression, Pérez-Martínez et al. (Citation2015) conducted an in vivo experiment with NOD-scid interleukin (IL)-2Rgnull mice inoculated with NB-1691luc neuroblastoma cells. Intravenous NK cellular therapy, with either fresh NK cells or NK cells activated with RBAC (100 mg/mL) overnight, began after seven days of tumour cell transplantation for four weeks (2 ×/week). Another group of cancer cell-inoculated mice received only saline injections as controls. Through bioluminescence imaging, the study observed that tumours in mice receiving RBAC-activated NK cell treatment had significantly lower TV (p < 0.05) than that of the two control groups at day 42. Furthermore, through Kaplan-Meier analysis, mice in the RBAC group survived significantly longer (p < 0.05) than the other two cohorts. RBAC, therefore, could activate NK cells to reduce TV and increase the chance of survival in cancer-bearing mice.
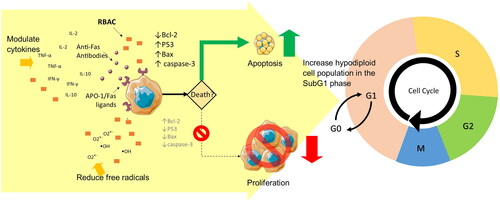
RBAC acts not only on the host immune system but also on cancer cells and arrests tumour growth directly. The potential mechanisms investigated in the literature, which include impacts on the proapoptotic pathway, oxidative stress, and cytokine signalling, are shown in .
Table 3. The direct anticancer effects of RBAC and the potential mechanisms.
Promotion of cancer cell apoptosis
Ghoneum et al. (Citation2000) reported that incubation of squamous cell carcinoma (SCC13) with RBAC showed a 30% decrease in cell numbers after 48 h and 50% at 72 h. In contrast, untreated SCC13 cells continued to grow over time. Coculturing of RBAC with human breast cancer cells (MCF-7) showed significant decreases in cell survival rates of 75, 70 and 63% after three days, at concentrations of 100, 500, and 1000 mg/mL, respectively (Gollapudi and Ghoneum Citation2008). The half maximal inhibitory concentration (IC50), a measure of the potency of RBAC against MCF-7 cells, was estimated to be approximately 800 μg/mL at 24 h and about 1000 μg/mL at 48 h. The effect of RBAC against murine breast cancer cells (4T1) was even more remarkable, with IC50 being 700 μg/mL at 24 h and 580 μg/mL at 48 h (Ghoneum et al. Citation2014). Likewise, Brush et al. (Citation2010) observed that RBAC significantly downregulated (p < 0.05) the proliferation of human prostate cancer cell lines (PC3 and LNCaP) in a dose-dependent manner after culturing the cells for 24, 48, and 72 h with different doses of RBAC (0–1000 µg/mL).
Because RBAC is non-cytotoxic to healthy cells with no direct effect on a healthy mouse fibroblast (L929) cell line (An Citation2011) and does not affect microbial cell viability in vitro (Ghoneum et al. Citation2008), the mechanism of how RBAC inhibits malignant cell growth is worth exploring. To this end, Ghoneum et al. (Citation2000) examined cytokine secretion by the SCC13 cells cultured with RBAC. There was an 8-fold increase in IL-10 levels and a 3-fold increase in IL-12 levels after 16 h, but no change in INF-γ content was detected. Thus, the reduction in the SCC13 cell count could be due to the enhanced secretion of IL-10 and IL-12 triggered by RBAC, as these are cytokines that induce programmed cell death via the CD95 (APO-1/Fas) receptor/ligand pathway (Schmidt et al. Citation2000; Fan et al. Citation2002).
To validate the proapoptotic mechanism, Ghoneum and Gollapudi (Citation2003) studied the effect of RBAC on CD95 death receptor-induced apoptosis in the human HUT 78 T lymphocyte cell line (leukemia). The study observed that HUT 78 cells treated with RBAC (100–1000 μg/mL) alone induced about 2.5–4.5% of specific apoptosis (over and above spontaneous programmed cell death) after 24 h. Meanwhile, anti-CD95 antibodies induced about 20% specific apoptosis. Most importantly, pre-treatment of HUT 78 cells with RBAC (for 3 h) before incubating with anti-CD95 antibodies increased the rate of specific apoptosis significantly (p < 0.01) by 35–42%, about double that in the treatment with anti-CD95 antibodies alone. Such an increase was not associated with the upregulation of death receptors on the HUT 78 cells, as the percentage of cells expressing CD95 and the density of CD95 on the cell surface did not differ between treated and untreated cells. Additional experiments by Ghoneum and Gollapudi (Citation2003) also observed that, compared to the untreated control, the activation of intracellular caspases 3, 8, and 9 was upregulated significantly (p < 0.001) in cells treated with RBAC and anti-CD95 antibodies. Moreover, a marked decrease in membrane potential and significant downregulation of the activity of the Bcl-2 antiapoptotic molecule in RBAC-treated HUT 78 cells compared to untreated cells were also detected. The results confirm that RBAC increases the susceptibility of cancer cells to undergo apoptosis mediated by the CD95 (APO-1/Fas) death ligands.
Badr El-Din, et al. (Citation2016a) performed cell-cycle analyses of the stomach tumour cells of male Wistar rats induced with the carcinogen MNNG to further understand the proapoptotic actions of RBAC. Significant differences were detected in cells in the G0/G1, SubG1, and S phases between rats fed with RBAC (40 mg/kg BW every other day) for eight months and those that were not. RBAC mitigated the carcinogenic effects of MNNG by causing cell-cycle arrest in the SubG1 phase with a 115.8% increase in the hypodiploid cell population (p < 0.01) compared to the MNNG group. Furthermore, comparing the ratio of the apoptotic index over the proliferation index (AI/PrI), the MNNG + RBAC group exhibited a 1.67-fold increase in AI/PrI relative to the MNNG group. AI/PrI is a prognostic marker for cancer proliferation, with a higher value indicating a much higher apoptotic rate of tumour cells, slowing down cancer proliferation (Liu et al. Citation2001). Quantification of apoptosis confirmed that the addition of RBAC increased the apoptotic cancer cell count in tumour tissues by 63.7% (p < 0.01) compared to MNNG treatment alone, most prominently during early apoptosis with a 230.1% (p < 0.01) increase to eliminate unwanted cells damaged by MNNG. In terms of the expression of apoptotic regulators in gastric tumour cells, RBAC induced apoptosis via mitochondria-dependent pathways through the downregulation of Bcl-2 (↓15.1%, p < 0.05) and the upregulation of p53 (↑37.3%, p < 0.05), Bax (↑49.3%, p < 0.01), the Bax/Bcl-2 ratio (↑75.7%, p < 0.01), and caspase-3 (↑34.8%, p < 0.01). The upregulation of p53 gene expression indicates that RBAC enhances tumour suppressor protein production to stop the division of mutated cells.
The effects of RBAC on N-nitrosodiethyamine (NDEA) + carbon tetrachloride (CCI4)-induced hepatocarcinogenesis based on cell cycle analysis of liver tissues were also reported by Badr El-Din et al. (Citation2020). Cell-cycle arrest rate in the SubG1 phase markedly increased by 126% and 99% (p < 0.01) through the pre- and post-treatment of RBAC, respectively, compared to that of the no-treatment group. Flow cytometric analysis of apoptosis also showed that RBAC treatment (pre-, post-) significantly reduced (p < 0.01) viable cell levels (↓74.51%, ↓72.54%) and the rate of necrosis (↑89%, ↑75.47%) while increasing the rates of early (↑316%, ↑309%) and late (↑255%, ↑237%) apoptosis, compared to rats that were not treated with a carcinogen. The analysis of apoptotic gene regulators also showed that RBAC treatment significantly (p < 0.01) upregulated p53, Bax, and caspase-3 expression while downregulating Bcl-2 gene expression relative to untreated rats. The study also observed a marked downregulation of nuclear factor kappa B/p65 inflammatory pathways in the liver of the RBAC-treated rats due to the reversal of the downregulation of nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha gene expression caused by NDEA + CCI4. Detection of DNA damage in liver tissues by gel electrophoresis also indicated fragmentation levels that increased 364.83% and 477.35% in the RBAC treatment groups (pre-, post-), respectively, compared to that in the untreated rats. Hence, RBAC inhibited hepatocarcinogenesis through induced apoptosis, suppressed inflammation, and downregulated tumour cell proliferation (Badr El-Din et al. Citation2020).
Similarly, in female Swiss albino mice inoculated with SEC, Badr El-Din et al. (Citation2019) showed that RBAC treatment (40 mg/kg BW i.p. 3 ×/week) for three weeks upregulated the apoptosis of tumour cells. A marked increase of 102% (p < 0.01) in the rate of cell cycle arrest in the SubG1 phase compared to that of the control group was detected in the RBAC group after 30 days of treatment. RBAC also increased the AI/PrI ratio by 2-fold (p < 0.01). Through quantitative histochemical analysis, the study also observed reduced levels of viable cells (28.2 ± 1.25% vs. 74.5 ± 2.25%) and increased levels of apoptotic cells (53.1 ± 1.21% vs. 18.2 ± 1.68%) in the tumour tissues of RBAC-treated mice compared to those of the control group. Additionally, RBAC also significantly (p < 0.01) upregulated p53 (↑113.78%), Bax (↑114.1%), and caspase-3 (↑123.22%), and downregulated Bcl-2 (↓53.32%) gene expression. The Bax/Bcl-2 ratio increased by 358.9% in the RBAC-treated mice relative to that in the no-treatment group.
Prevention of oxidative stress
Reactive oxygen species (ROS) such as superoxide radicals (O2•−), hydrogen peroxide (H2O2), and hydroxyl radicals (•OH) are genotoxins that can cause DNA damage leading to malignancy (Phillips and Arlt Citation2009). High levels of ROS accompany the hyperproliferation of cancer cells and deplete endogenous antioxidants, causing oxidative stress, which can harm the surrounding healthy tissues. Cancer cells, however, adapt and thrive under oxidative stress. As such, ROS greatly assist in initiating, promoting, and progressing tumour growth (Hayes et al. Citation2020). Antioxidants, such as endogenous glutathione (GSH), modulate DNA-repair activity to suppress tumour progression (Chatterjee Citation2013). Several plant-based antioxidants, including resveratrol, baicalein, and genistein, are genotoxic but not mutagenic and could selectively kill multidrug-resistant cancer cells (Fox et al. Citation2012). Hence, the ability to enhance the endogenous antioxidant system could be another mechanism by which RBAC impairs tumour growth.
Noaman et al. (Citation2008) evaluated the antioxidant status of the SEC-bearing mice and the corresponding effects of RBAC treatment. The study observed significant elevations in malondialdehyde (MDA), a measurement for lipid peroxidation, in the plasma (↑58.96%, p < 0.05) and liver (↑44.54%, p < 0.01) of SEC-bearing mice compared to those of healthy mice at day 25 after Ehrlich carcinoma cell inoculation. SEC-bearing mice also had significantly lower levels of GSH in the blood (↓25.96%, p < 0.05) and liver (↓59.31%, p < 0.01) than the control values. A marked depletion (p < 0.05) of endogenous antioxidant enzymes, including glutathione peroxidase (GPx), glutathione-S-transferase (GST), superoxide dismutase (SOD), and catalase (CAT) was also detected with a corresponding downregulation of gene expression. Such results confirmed the upregulated ROS attack and the presence of oxidative stress in mice with cancer.
For comparison, two groups of SEC-bearing mice were treated with RBAC (25 mg/kg BW starting from day 4 [early, E] or day 8 [late, L] after injection of Ehrlich ascites). These mice did not show signs of oxidative stress, with the MDA values in the blood (E ↑1.73% and L ↑7.52%) and liver (E ↓21.57% and L ↓9.03%) not significantly different from that of the control. Furthermore, when comparing the MDA levels within the tumour tissue, the early and late treatment group had significantly lower values than the untreated SEC-bearing mice, showing −39.34% (p < 0.01) and −36.43% (p < 0.05) reductions, respectively. The GSH levels of RBAC-treated SEC-bearing mice in the blood (E ↑39.0%, L ↑3.67%), liver (E ↑40.97%, L ↑14.04%), and tumour (E ↑74.41%, L ↑59.12%) were at normal or above-normal values, and significantly higher (p < 0.01) than those of the untreated SEC-bearing mice. Similarly, the levels of GPx, GST, SOD, and CAT and the related gene expression levels in both RBAC groups were significantly higher (p < 0.01) than those in the untreated mice and did not deviate much from the control values. Hence, RBAC attenuates oxidative stress to minimize tumour growth by instigating higher endogenous antioxidant production levels, thus averting collateral damage to healthy cells.
Modulating cytokine production
The anticancer effects of RBAC could also be linked to the ability to influence the cytokine production of immune cells. Badr El-Din et al. (Citation2008) reported that SEC-bearing mice treated with RBAC (40 mg/kg BW 3 ×/week) for three weeks had significantly higher levels (p < 0.01) of tumour necrosis factor (TNF)-α (↑15.63% over control) and IFN-γ (↑154.54% over control) compared to both untreated tumour-bearing mice (TNF-α ↑4.17%, IFN-γ ↓10.46%) and healthy control mice after 35 days. Additionally, untreated tumour-bearing mice exhibited elevated IL-10 levels compared to tumour-free mice by 111.71%, whereas only a minor change was detected in RBAC-treated mice (↑14.75%). The difference between the treated and untreated groups was statistically significant (p < 0.01). TNF-α and IFN-γ are secreted by Th1 cells and exert proinflammatory and anticancer activity, whereas IL-10 is a type of anti-inflammatory cytokine of Th2 cell response that mediates humoral immunity. High levels of Th2 response relative to low levels of Th1 response could favour tumour growth (Lin et al. Citation2019; Zhao et al. Citation2019).
Cholujova et al. (Citation2013) confirmed that a group of MM patients (n = 45) had a predominant Th2 response over Th1 by analyzing the ratios of the plasma concentrations of Th1 cytokines (IL-1β, IL-2, IL-12, IL-15, and IFN-γ) to Th2 cytokines (IL-4, IL-5, IL-6, IL-9, IL-10, and IL-13) compared to those of healthy donors (n = 30). Healthy donors had 20 Th1/Th2 ratios that were greater than 1.0 (Score: 20:10), whereas in MM patients, there were only 14 such ratios (Score: 14:16). RBAC significantly elevated (p < 0.05) the plasma concentration of several Th1 cytokines in MM patients over the placebo, especially IL-12, IL-17, and TNF-α, consistently when measured at one month and after three months. IL-1β levels were also elevated after one month (p = 0.047) but not after three months, whereas IFN-γ levels were significantly higher (p < 0.018) after three months. However, after three months of RBAC treatment, the levels of Th2 cytokines, including IL-4, IL-6, IL-9, IL-10, and IL-13, significantly increased (p < 0.05) compared to those in the placebo (Cholujova et al. Citation2013). Thus, RBAC supplementation affected both Th1 and Th2 cytokines, demonstrating immunomodulating effects, but how it could influence the disease progression of MM remained unclear.
In a non-randomized clinical trial, Kim et al. (Citation2020) found that cancer patients (n = 10, with various malignancies) consuming an oral nutritional supplement containing 0.4 g of RBEP for eight weeks exhibited significantly lower IL-1β, IL-6, and IL-8 levels (p < 0.05) compared to the control group (n = 24) receiving standard care in nutritional counselling only. The RBEP group, however, had a significantly higher IL-12p70 level (p < 0.05) than the control while no difference in TNF-α levels was detected. The authors also reported a marginally significant rise (p = 0.056) in the IL-10 level in the RBEP group at week eight compared to baseline, but the between-group difference was not significant. Notably, the cytokine levels in this study were measured from the PBL of patients after being stimulated by lipopolysaccharides to determine the levels of inflammatory responses. Again, supplementation of RBAC affected both Th1 (IL-12p70, IL-1β, and IL-8) and Th2 (IL-6 and IL-10) bi-directionally with no indication of whether how such cytokine modulation could influence the inflammatory responses of the body and its antitumor mechanisms and impact. More research in this area is needed.
Overall, RBAC exerts anticancer effects through multiple pathways, including selectively promoting apoptosis in cancer cells via both intrinsic and extrinsic pathways, acting as an antioxidant, and modulating antitumor cytokine secretion, as summarized in .
Figure 3. The anticancer effect of RBAC is achieved through both the intrinsic pathway via the increased susceptibility of CD95 (Fas/APO-1) ligands in the cancerous cells to promote apoptosis and the extrinsic pathway through the downregulation of the antiapoptotic Bcl-2 proteins to lower membrane potentials, leading to the upregulation of the tumour-suppressing P53 gene and the upregulated production of the apoptotic Bax and caspase-3 signalling proteins. Malignant cell proliferation is arrested with evidence of increased hypodiploid cell counts in the SubG1 phase in cell cycle analysis. The antioxidant and cytokine-modulating capacities of RBAC also augment proapoptotic activity.
Chemoprevention
Cancer chemoprevention is the use of natural, synthetic, or biological chemical agents to reverse, suppress, or prevent carcinogenic progression (Tsao et al. Citation2004). There has been a strong interest in leveraging natural products as a risk-modifying strategy to prevent, delay, or suppress tumour development or recurrence, especially in high-risk patients (Haque et al. Citation2021; Shankar et al. Citation2022). RBAC is a prophylactic agent against carcinogenesis in vivo, as summarized in .
Table 4. In vivo studies investigating the chemoprevention activities of RBAC.
Badr El-Din, et al. (Citation2016a) studied the chemoprevention activity of RBAC against chemical-induced glandular stomach carcinogenesis in rats. Male Wistar rats were given the carcinogen MNNG (200 mg/kg BW p.o. daily) for two weeks to instigate cancer growth. Along with chemical induction, the rats (n = 12) were given RBAC at 40 mg/kg BW every other day for eight months. Another group of rats (n = 10) were treated with MNNG alone. After eight months, histopathological examination of the gastric mucosa of the rats showed that 80% of the rats treated with MNNG only developed mild- and high-grade gastric glandular dysplasia (6/10, 60%) and invasive well-differentiated keratinizing cell carcinoma (2/10, 20%). The MNNG + RBAC group, however, showed significantly lower incidence (p < 0.01) of mild dysplasia, which was characterized by patchy and small growths (3.5/12, 29.2%), and carcinoma in situ only (1/12, 8.3%). In addition, the MNNG + RBAC group also had significantly lower (p < 0.01) Ki-67 tumour proliferation marker expression levels at 39.8% compared to 50.8% in the MNNG-only group. Hence, RBAC considerably lowered the risk of developing gastric dysplasia and adenocarcinoma while exposed to MNNG.
Another study by Badr El-Din, et al. (Citation2016c) explored the in vivo chemopreventive effects of RBAC (25 mg/kg BW i.p. 5×/week) on liver cancer under two treatment regimens. Male albino rats were administered NDEA (200 mg/kg BW, i.p.) at week 2 to induce hepatocarcinogenesis, followed by weekly subcutaneous injections of CCI4 (3 mL/kg BW for six weeks) as a promoter. Pre-treatment of RBAC for a group of mice (n = 20) commenced two weeks before the injections of NDEA + CCI4 and lasted for another 20 weeks. Conversely, the post-treatment group only received RBAC from week 10 to week 22. The study observed that NDEA + CCI4 induced significant BW loss (↓39.54%, p < 0.01) and increased liver mass (↑24.73%, p < 0.01) in untreated mice compared to the healthy control at the end of 22 weeks. Both RBAC treatment regimens kept the liver weight at the normal range and significantly reduced (p < 0.01) the percentage of BW loss caused by the carcinogens, with pre-treatment (↓17%) faring better than post-treatment (↓23.44%). Histopathological studies of the liver tissues of the NDEA + CCI4 mice showed signs of inflammation and hepatocarcinogenesis with fatty infiltration of hepatocytes, loss of architecture, necrosis, and fibrosis. As for rats pre-treated with RBAC, the liver tissues showed minimal changes in hepatocyte morphology and histology with no inflammation. Moderate liver damage was observed in the post-treatment group but with only a few degenerated hepatocytes. Testing of liver enzymes also showed similar findings between the two treatment regimes. Thus, RBAC treatment prevented carcinogenesis in the liver, even in the presence of known carcinogens.
Enhanced chemotherapy
Combining two or more therapeutic agents in oncological treatments is a common practice as it can reduce the risk of acquired resistance and enhance efficacy through the synergistic or additive effects of the agents (Palmer and Sorger Citation2017). For instance, combining immunotherapy and chemotherapy showed improvements in overall progression-free survival, response rates, and duration, as well as clinical benefits for MM, breast cancer, and lung cancer (Morse et al. Citation2023). With its immunomodulation and proapoptotic effects, RBAC could be a safe and effective addition to combination treatment, with evidence listed in .
Table 5. Results from in vitro, in vivo, and human studies on the synergistic effects of RBAC with chemotherapeutic agents.
Gollapudi and Ghoneum (Citation2008) explored the sensitizing activity of RBAC with daunorubicin, an anthracycline class of antibiotics, against human breast cancer cells (MCF-7 and HCC70) in vitro. Co-culturing RBAC with daunorubicin for three days lowered the IC50 values against MCF-7 cells by 3-, 5-, and 5.5-fold, at 100, 500, and 1000 µg/mL, respectively. The IC50 of daunorubicin for HCC70 cells also consistently decreased by 2.5-fold with RBAC of all concentrations. RBAC enhanced drug transport with evidence of increased accumulation of daunorubicin in both MCF-7 and HCC70 cells observed under flow cytometry. The administration of RBAC (500 mg/mL) enhanced drug accumulation in MCF-7 cells over time, with differences compared to daunorubicin-only uptake starting 45 min after culturing and becoming 26.21% higher at the hour.
RBAC was also tested for its synergistic effects with paclitaxel, a mitotic inhibiting taxane, on breast cancer cells (non-metastatic MCF-7 and metastatic 4T1) growth in vitro. Ghoneum et al. (Citation2014) showed that the IC50 values of paclitaxel against MCF-7 at 24 h were lowered by a factor of over 100 with the addition of 600, 750, and 1000 μg/mL of RBAC, compared to paclitaxel alone. Compared to paclitaxel alone against 4T1 cells, the IC50 value for paclitaxel at 24 h decreased by a factor of ∼3 at 600 μg/mL of RBAC and up to a factor of ∼100 at 1000 μg/mL. Additional in vitro experiments also showed that paclitaxel plus RBAC (500 and 600 μg/mL) significantly upregulated DNA damage, reduced proliferation, and induced apoptosis of 4T1 cells, compared to either agent alone.
Badr El-Din, et al. (Citation2016b) followed up with an in vivo study to examine the treatment effects of combining RBAC (40 mg/kg BW) and low-dose paclitaxel (2 mg/kg BW) in a murine model. They utilized female Swiss albino mice (n = 36) that were inoculated with Ehrlich ascites carcinoma. The mice received no treatment, RBAC only, paclitaxel only, or RBAC plus paclitaxel, every other day. At day 30 post-inoculation, the study observed that the combination therapy significantly reduced (p < 0.01) TV by 88.3% compared to that of the no-treatment group. The reduction in TV was more pronounced than the effects of either paclitaxel (↓58.9%) or RBAC (↓77.1%) alone. RBAC plus paclitaxel also inhibited tumour cell proliferation at a higher propensity (↓35.4%, p < 0.01 vs. untreated mice) compared to only 11.6 and 27.0% with paclitaxel or RBAC alone, respectively. RBAC plus paclitaxel also maximized the downregulation of Ki-67 expression by 85.7% (p < 0.01) compared to that of the no-treatment group; paclitaxel treatment alone and RBAC treatment alone downregulated Ki-67 expression by 51.7 and 80.6%, respectively. Significant increases (p < 0.01) in the percentage of cancer cell apoptosis were also detected in all treatment groups: 20.9% for paclitaxel only, 76.1% for RBAC only, and 93.2% for paclitaxel + RBAC. Analyses of DNA damage and cell cycle phases also showed a similar trend, with paclitaxel + RBAC being superior in causing more extensive DNA damage and maximizing the AI/PrI ratio compared to either agent alone.
The effectiveness of RBAC in improving the treatment outcomes of conventional antineoplastic drugs has been studied in an RCT by Bang et al. (Citation2010). Patients (n = 68) with hepatocellular carcinoma (stages I and II) participated in this study, with the intervention group (n = 38) receiving RBAC (1 g/day) as a dietary supplement for 12 months while receiving oncological treatment simultaneously. The control group (n = 30) received only the standard therapies. The oncological therapies were mainly transarterial oily chemoembolization (TOCE, n = 24) or TOCE in combination with percutaneous ethanol injection treatment (TOCE + PEIT, n = 34). A few participants received PEIT only (n = 6) or PEIT plus radiofrequency ablation (n = 4). Hence, all participants received antineoplastic drugs directly delivered to their tumour sites.
RBAC significantly improved (p < 0.01) the treatment response rate of standard therapies for liver cancer, with 89% of patients in the RBAC group responding to oncological treatment compared to only 80% in the control group. The mean post-treatment alpha-fetoprotein (AFP) tumour marker in the RBAC group significantly decreased by 38% compared to baseline (p < 0.001), a favourable contrast over the non-significant 7% increase in AFP in the control group. Furthermore, combining RBAC with standard therapies significantly decreased (p < 0.01) the average TV in patients by 36% compared to almost no change in the control group (↑0.2%). After the treatment, the patients were followed up every six months for up to three years, and the tumour recurrence rate in the RBAC was lower at 32% compared to 47% in the control group. In terms of survival, 63% of patients receiving only standard treatment survived the first year, only 6.7% lasted at least two years, and none survived after 30 months. In contrast, the RBAC group maintained a much higher survival rate at 76, 35, and 11% at the end of one, two, and three years, respectively. Patients receiving RBAC in addition to TOCE + PEIT survived, on average, ten months longer than those treated with TOCE + PEIT only. Hence, evidence from this RCT supported the synergistic anticancer effects of RBAC on the enhancement of the effectiveness of TOCE and/or PEIT in enhancing treatment response, reducing TV, lowering the AFP marker, and prolonging the survival of liver cancer patients (Bang et al. Citation2010).
Chemoprotection
Chemoprotection refers to protecting healthy cells and tissues from toxicity and side effects of chemotherapy. Several studies have demonstrated that RBAC could be a promising source to achieve such protection ().
Table 6. Results from animal and human studies on the protective effects of RBAC against the toxicity of chemotherapeutic agents.
Jacoby et al. (Citation2001) explored the in vivo effects of RBAC in reducing the toxicity of cisplatin (an alkylating agent) and doxorubicin (an anthracycline antibiotic like daunorubicin) with a murine model. Sprague-Dawley albino rats (n = 80) were orally fed with 0, 5, or 50 mg/kg BW of RBAC daily for 11 days. On day 3, rats were administered cisplatin (9 mg/kg BW), doxorubicin (10 mg/kg BW), or a vehicle control by a single i.p. injection. The study observed that RBAC prevented weight loss induced by chemotherapeutic agents. Rats administered with cisplatin alone showed weight loss at day 11 (98.5 ± 0.06% of initial BW). In contrast, weight gains were observed in both low and high-dose RBAC plus cisplatin groups (L: 111.5 ± 0.13%, H: 144.0 ± 0.15%) with significant differences compared to the cisplatin-only group (p < 0.05). The doxorubicin-only group also showed BW gain (132 ± 0.13%) but was significantly lower (p < 0.05) than the gains in the RBAC plus doxorubicin groups (L: 146.6 ± 0.08%, H: 143.5 ± 0.06%).
The toxicity of cisplatin was severe, with 50% deaths; 70% had gross GI mucosal pathology, and 100% showed signs of diarrhoea (Jacoby et al. Citation2001). The corresponding proportion in the low-dose RBAC plus cisplatin group was 10% death (p < 0.05), 40% GI pathology, and 50% diarrhoea (p < 0.05). The high-dose group exhibited 40% death, 50% GI pathology, and 40% diarrhoea (p < 0.05). Compared to cisplatin, doxorubicin had less toxicity, no death, and mostly non-significant differences in diarrhoea across all doxorubicin-treated groups. Notwithstanding, 50% of the doxorubicin-only group experienced GI pathology compared to only 10% in the low-dose RBAC plus doxorubicin group (p < 0.05) and 30% in the low-dose RBAC plus doxorubicin group (p > 0.05). Hence, RBAC at 5 mg/kg BW was more effective than at the higher dose of 50 mg/kg in preventing the toxicity and side effects of cisplatin and doxorubicin (Jacoby et al. Citation2001).
Endo and Kanbayashi (Citation2003) investigated the chemoprotective effects of RBAC (1 mg/day p.o. and i.p.) against BW loss due to cisplatin in BALB/c female mice over a longer duration. One shot of cisplatin (15 mg/kg i.p.) was administered after the mice had received RBAC for one week. The mice were weighed daily for 28 days. Control substances were either drinking water (p.o.) or phosphate saline (i.p.). Analysis of variance was conducted at weekly intervals corresponding to the (I) initial phase, (II) weight loss phase, (III) weight gain phase, and (IV) weight stabilizing phase. Statistically significant differences (p < 0.05) in BW were detected in phases II, III, and IV of both groups of RBAC (i.p. and p.o.) compared to their respective control groups, with the RBAC groups showing trends of reduced BW loss and faster BW recovery over time. When comparing the two groups of RBAC, there was no significant difference in the protective effect of the administration route on weight loss induced by cisplatin.
In humans, the chemoprotective effects of RBAC were validated by Masood et al. (Citation2013) in an RCT among breast cancer patients (n = 50) receiving chemotherapy. One group of patients (n = 25) were assigned to take RBAC (3 g/day) as a dietary supplement one week before and one week after chemotherapy. Another control group (n = 25) received only chemotherapy. The trial lasted for six cycles of chemotherapy, with the patients completing questionnaires before each treatment cycle to assess any chemotherapy-induced side effects. The study observed significant differences (p < 0.001) in the proportions of patients experiencing anorexia/tiredness (RBAC vs. control: 20% vs. 88%), nausea/vomiting (40% vs. 100%), hair loss (28% vs. 100%) between the two groups. Furthermore, the distribution of patients having weight gain or loss significantly differed with weight gain among 64% in the RBAC group but none in the control group. Instead, 84% of the control group experienced weight loss but no patient in the RBAC group experienced weight loss. Hence, RBAC mitigated the chemotherapy-induced side effects of anorexia/tiredness, nausea/vomiting, hair loss, and weight loss among breast cancer patients.
Radioprotection and radiotherapy enhancement
With antioxidant capacity, RBAC protects against the harmful effects of radiation treatment, as shown in .
Table 7. Results from animal and human studies on RBAC’s synergy with and protection against the adverse effects of radiation treatment.
Ghoneum et al. (Citation2013) explored how RBAC could protect mice against whole-body γ-irradiation. Female Swiss albino mice were irradiated with an acute single dose level of 5 Gy at a rate of 0.45 Gy/min. One group of mice (n = 6) received RBAC (40 mg/kg BW i.p.) every other day for two weeks before irradiation and continued receiving RBAC until four weeks after. Compared to irradiated mice that did not receive RBAC, the RBAC group showed less weight loss relative to control non-irradiated mice when measured at week 1 (↓1.41% vs. ↓20.03%, p < 0.01) and week 4 (↓0.54% vs. ↓7.79%, p < 0.05) after irradiation. RBAC prevented radiation-induced weight loss and helped maintain regular BW throughout the trial. Significant differences in the liver (RBAC vs. irradiation: ↓8.58% vs. ↓25.51%, p < 0.05) and kidney (↑5.04% vs. ↓23.19%, p < 0.05) weight were also observed between the two groups at week 1, although the organ weights for all groups returned to normal at week 4.
Exposure to γ-radiation also caused anaemia in the mice showing significantly lower (p < 0.05) than normal red blood cell (RBC) count and haemoglobin (Hb) levels measured after one and four weeks (Ghoneum et al. Citation2013). Moreover, irradiated mice also exhibited significant (p < 0.01) leukopoenia, lymphopenia, neutrophilia, and thrombocytopenia compared to the healthy control at week one before normalizing at week four except for platelet count, which remained significantly lower than normal (p < 0.5). Histopathological examination of the bone marrow revealed haematopoietic tissue damage with the absence of cellularity in irradiated mice and a significant decrease (p < 0.01) in spleen size (↓60%) and megakaryocyte density (↓75%) compared to control mice at week 1, which only partially recovered at week 4. In contrast, RBAC prevented anaemia from radiation exposure and maintained normal white blood cells, lymphocytes, neutrophils, and platelets in the treated mice. The histopathological examination showed the preservation of haematopoietic tissues by RBAC, with normal bone marrow cellularity, spleen size, and megakaryocyte density despite exposure to harmful irradiation.
The beneficial effects of RBAC against γ-irradiation could be due to its ability to protect against ROS by enhancing the endogenous antioxidant system discussed earlier. Oxidative stress was observed in irradiated mice, with the MDA level spiking at 106.34% (p < 0.01) above normal at week 1, accompanied by a significant decline in the GSH level (↓40%, p < 0.01). MDA remained high (43.44%) at week 4, while endogenous GSH content was restored over time. RBAC, however, showed only a slightly elevated MDA level at week 1, which did not significantly differ from that of the healthy control. The GSH content of RBAC-treated mice remained high throughout the trial.
The potential mechanisms for the radioprotective effect of RBAC were investigated by Zhao et al. (Citation2020) in an animal study with radiation-induced intestinal injury. One group of C57BL/6 mice was pre-treated with RBAC (40 mg/kg BW i.p.) every other day for two weeks before undergoing local high-dose abdominal precision irradiation at 2 Gy/min for 5 min (10 Gy single dose). RBAC treatment continued every other day for another four weeks. A separate group of mice received only irradiation. At the end of the study, the jejunal and colonic segments of the mice were collected for analysis. Irradiation disrupted cellular respiration with significant reductions (p < 0.05) in the activity level of mitochondrial respiratory chain complexes, resulting in the depletion of intercellular adenosine triphosphate (ATP) content in the jejunal and colonic mucosa compared to the healthy control. However, in mice treated with RBAC, the mitochondrial respiratory chain complex activity level and intercellular ATP content remained normal. Moreover, the abundance of mitochondria-encoded genes and mitochondrial copy numbers in the jejunal and colonic mucosa of irradiated mice treated with RBAC increased significantly (p < 0.05) compared to the reduction observed in the irradiation-only mouse group. Thus, RBAC preserved mitochondrial function from the harmful effects of radiation.
Zhao et al. (Citation2020) also evaluated the oxidative status of the intestinal epithelium after radiation by assessing the levels of ROS, reactive nitrogen species, MDA, and H2O2. As expected, all oxidative status markers were significantly elevated (p < 0.05) in the irradiation-only mouse group compared to the healthy control. Analysis of the antioxidative amplitude of SOD, GPx, and CAT, and the total antioxidant capacity in serum and intestinal mucosa also indicated significant depletion (p < 0.05) after irradiation in mice. In contrast, RBAC protected the intestinal epithelium from oxidative stress by enhancing the endogenous antioxidative activity and increasing the total antioxidant capacity to neutralize radiation-induced free radicals, thus maintaining oxidative status at normal levels. The study also observed a significant increase (p < 0.05) in intestinal permeability and disruption of the barrier function of mucosa after irradiation. However, RBAC restored these components to the levels of the control mice. As such, RBAC protects against irradiation-induced intestinal damage through its antioxidant capacity.
RBAC could not only protect against the adverse effects of radiation therapy but also enhance the efficacy of the treatment. Badr El-Din et al. (Citation2019) demonstrated the benefits of combining RBAC (40 mg/kg BW i.p. 5 ×/week for three weeks) and X-ray irradiation (3 × 2 Gy dose with a dose rate of 0.85 Gy/min) in female Swiss albino mice inoculated with SEC. At the experiment endpoint, the study observed that the combined treatment reduced the TV by 77.3% and TW by 56.9% compared to those in the no-treatment group. The reduction was significantly more than the effects of RBAC (TV↓66.4%, TW↓46.3%, p < 0.05) or radiation treatment (TV↓49.9%, TW↓30.7%, p < 0.01) alone, which serves as evidence for the synergistic effects of the two therapies. The enhanced efficacy was also accompanied by diminished adverse effects of irradiation as the addition of RBAC managed to significantly arrest BW loss in RBAC + radiation-treated mice compared to radiation-only mice (↓17.9% vs. 31.2%, p < 0.01). Badr El-Din et al. (Citation2019) also conducted quantitative histochemical analysis and reported that tumour tissues from RBAC + radiation-treated mice contained only 4.6 ± 0.93% viable cells, 64.0 ± 1.47% apoptotic cells, and 21.4 ± 1.7% necrotic cells. In comparison, the tumour tissues of RBAC-only (viable: 28.2 ± 1.25%, apoptotic: 53.1 ± 1.21%, necrotic: 18.8 ± 0.96%) and radiation-only (viable: 30.3 ± 1.23%, apoptotic: 41.3 ± 1.22%, necrotic: 28.4 ± 0.89%) groups contained more viable cells and less apoptotic cells. The increase in the rate of apoptosis of the tumour cells by RBAC + radiation treatment was also confirmed with the highest rate of cell-cycle arrest at the SubG1 phase at peak AI/PrI ratio, and maximized levels of apoptotic regulators (p53, Bax, caspase-3) and the corresponding apoptotic gene expression were observed (Badr El-Din et al. Citation2019).
Tan and Flores (Citation2020) confirmed the radioprotective effects of RBAC in a double-blind placebo-RCT with head and neck cancer patients (n = 65) undergoing radiotherapy and/or concurrent chemotherapy. The patients were mainly prescribed a total radiation dose of 70 Gy and randomly assigned to either the RBAC (n = 32) or placebo (n = 33) group. The oral supplementation dosage was 3 g/day and commenced two weeks before the start of oncological treatment, during chemoradiotherapy, and for two months after completion. The study observed reductions in the hematological parameters in both groups during chemoradiotherapy. Two months after treatment, significant between-group differences (p < 0.05) were detected in Hb, haematocrit, RBCs, platelets, neutrophils, and lymphocytes, with the RBAC group showing favourable recovery compared to the placebo group. However, the study did not detect any statistical differences in the radiation toxicity assessments between the two groups based on the Radiation Therapy Oncology Group severity grading. Notwithstanding, participants in the RBAC group reported significantly better mean scores in health-related QoL than those in the placebo group (1.53 ± 0.24 vs. 1.72 ± 0.33, p = 0.019). Clinical outcomes of the RBAC group were also significantly better than the placebo group in mortality (0% vs. 33.3%, p < 0.001), blood transfusion (51.5% vs. 3.1%, p < 0.001), hospitalization (63.6% vs. 6.2%), and metastasis (15.2% vs. 0%, p < 0.05). The placebo group also had marginally higher infection cases than the RBAC group (12.1% vs. 0%, p = 0.06). The results showed the superiority of RBAC over placebo in radiation protection, subjective QoL, and objective treatment outcomes.
Synergistic effects with other natural products and complementary therapies
RBAC works synergistically with other natural products or complementary therapies, including yeast, curcumin, mistletoe lectin, and oncothermia, as shown in .
Table 8. Results from animal and human studies on the synergistic effects of RBAC with other natural products.
Malignant cells may develop phagocytic behaviour against host cells or other microorganisms, especially in aggressive and invasive tumours (Lugini et al. Citation2003). Heat-killed Saccharomyces cerevisiae (Desm.) Meyen, commonly known as baker’s or brewer’s yeast, can cause apoptosis in breast cancer cells after being engulfed by phagocytic tumour cells (Ghoneum and Gollapudi Citation2004). S. cerevisiae has also been explored as a probiotic and natural product for antitumor action (Badr El-Din et al. Citation2018; Shamekhi et al. Citation2020).
Ghoneum and Gollapudi (Citation2005a) showed that RBAC synergistically enhanced the yeast-induced apoptosis of breast cancer cells in vitro. Tumour cells (MCF-7) were cocultured with yeast (1:10 ratio) in the presence (100, 500, 1000 µg/mL) or absence of RBAC. Treatment with RBAC showed a 2-fold increase (54% vs. 27%, p < 0.001) in the percentage of yeast attachment to the MCF-7 cells at 0.5 h post-incubation, which was accompanied by a significant increase (p < 0.01) in the rate of phagocytosis of MCF-7 cells cultured with yeast (72%), as compared to cells cultured with yeast without RBAC (23%). Upregulated apoptosis in RBAC-treated MCF-7 cells was also detected, with 32% undergoing apoptosis compared to only 18.7% in untreated cells with yeast alone at 0.5 h. The rate of apoptosis continued to rise for both cultures, reaching 31% for yeast alone and 39.3% in the presence of RBAC. However, with more cases of cell death, the yeast attachment percentage declined for RBAC, whereas untreated MCF-7 cells continued to attract yeasts. By 2 h, the attachment rates were reversed with a significant between-group difference (p < 0.001). Moreover, compared to the treatment with MCF-7 cells and yeast only, RBAC caused dose-dependent increases in the rate of phagocytosis-induced cell death of 35.4, 40.1, and 33.04 at 100, 500, and 1000 µg/mL, respectively. Further experiments with other cell lines (ZR-75 and HCC70) also showed consistent results of RBAC-enhanced yeast-induced apoptosis. The effect of RBAC was also associated with the upregulated activation of caspases 8 and 9 in MCF-7 cells and caspases 8, 9, and 3 in HCC70 cells (Ghoneum and Gollapudi Citation2005a).
Similar experiments were repeated by Ghoneum and Gollapudi (Citation2005b) using MCF-7 cells in a monolayer culture instead of a suspension culture. Non-cancerous breast epithelial cells (MCF-10A) were used as controls. Cells were cultured with heat-killed S. cerevisiae (1:10 ratio) in the presence or absence of RBAC (100 µg/mL). Monolayer MCF-7 cells also exhibited phagocytotic properties with enhanced attachment to yeast (↑13.4% at 1 h and ↑25% at 4 h) compared to the control MCF-10A cells, which exhibited no phagocytotic behaviours. RBAC increased the magnitude of the phagocytizing of yeast by MCF-7 cells by 2- to 3-fold after 1-4 h. The percentage of dead MCF-7 cells after treatment with RBAC, yeast, or yeast + RBAC for 4 h was 58, 85, and 92%, respectively, compared to only 9.5% in untreated MCF-7 cells. Hence, RBAC improves the effectiveness of yeast-included apoptosis in MCF-7 cells regardless of how the cell culture is maintained.
Ghoneum and Gollapudi (Citation2011) also reported that RBAC had a synergistic apoptotic effect with curcumin. The in vitro study found that treatment of the MM cell line U266 with curcumin alone (2.5 × 10 μM) and RBAC alone (50 and 100 μg/mL) resulted in a dose-dependent decreased cell survival rate. However, RBAC + curcumin caused a significant decrease (p < 0.0005) in the cell survival rate compared to either agent alone and achieved an 87% decrease in cell count at 100 μg/mL RBAC and 10 μM curcumin. Apoptosis determined by hypodiploid DNA showed that RBAC alone at 50 μg/mL caused about 10% of apoptosis cases in U266 cells, which was no different from background apoptosis. However, combining RBAC (50 μg/mL) with curcumin significantly increased (p < 0.05) the apoptotic rates to 20.0, 22.0, and 24.7% at 2.5, 5, and 10 μM, respectively. Cell cycle analysis showed that RBAC + curcumin caused a significant decrease in the G0 phase from 36 to 17% (p < 0.0005), a slight decline in the S phase (from 15.5 to 13%), and the G2-M phase (from 15 to 12%). Additionally, RBAC + curcumin treatment altered the Bax/Bcl-2 ratio to 2.5 compared to 0.9, 1.5, and 1.6 in the control, RBAC-alone, and curcumin-alone treatments. Hence, combining RBAC and curcumin could better promote cell apoptosis in malignant U266 cells.
RBAC has also been applied in cancer treatment with Viscum album Walter (Santalaceae) extract (mistletoe lectin), another natural product that possesses cytotoxic and immunostimulating effects (Majeed et al. Citation2021). Hajtó et al. (Citation2016b) surveyed the QoL of 35 patients (20 females, 15 males) with advanced (stage II–IV) cancer of various malignancies after being treated with RBAC (12 to 45 mg/kg BW 2 ×/week) as an oral supplement and a standardized mistletoe extract (Iscador M 5 mg 2 ×/week) as subcutaneous injections for six months or more. The patients were asked to complete a questionnaire about the perceived impacts of the combined therapy on anxiety, physical activity, appetite, sleep, digestion, side effects of cancer treatment, and disease progression. Improvement in physical activity (71%) and appetite (66%) were two of the most essential benefits reported by the patients. Among the 35 patients, 24 were concurrently treated with conventional cancer treatment, with 70.8% (17/24) also citing the reduction of side effects as a benefit of RBAC and mistletoe extract. Thus, RBAC works synergistically with mistletoe extract to improve cancer QoL according to this cross-sectional survey (Hajtó et al. Citation2016b). However, mistletoe extract alone improves the QoL of cancer patients, with a meta-analysis reporting a significant, medium-sized effect (Loef and Walach Citation2020). Hence, without a controlled study, the impact of RBAC on enhancing the effectiveness of mistletoe extract is unknown.
Oncothermia, also known as modulated electro-hyperthermia, is a complementary therapy that applies low-radiofrequency heat to the tumour site to direct energy absorption in the extracellular liquid and destroy the cell membrane of cancer (Andocs et al. Citation2009; Hegyi et al. Citation2013). By overheating the malignant tissues locally in a targeted and controlled manner, oncothermia is used to improve the efficacy of conventional cancer treatment while improving the QoL and survival rate of cancer patients (Alshaibi et al. Citation2020). The combined therapy of RBAC and oncothermia also works synergistically to reduce cancer patients’ symptoms with chronic fatigue syndrome (CFS) in an RCT.
Petrovics et al. (Citation2016) recruited cancer patients with various malignancies diagnosed with CFS (n = 50). One group of participants (n = 25) were randomized to consume RBAC (1 g/day for 24 weeks) and undergo oncothermia (60 min with 140 W energy weekly for 15 times) concurrently while being treated with chemo- or radiotherapy. Another group served only as controls receiving only conventional oncological treatment. The fatigue symptoms were measured with the Chalder Fatigue Scale (CFQ), and the subjective clinical outcome of the combined treatment was also assessed by the Patient Global Impression of Change Scale (PGIC). At 24 weeks, the RBAC + oncothermia group exhibited a significantly lower mean CFQ score compared to baseline (14.6 ± 2.3 vs. 23.9 ± 2.3, p < 0.01), while the control group exhibited no significant change in mean CFQ. The reduction in the degree of fatigue is also clinically significant as the RBAC + oncothermia group exhibited a mean PGIC score of 2.1 ± 0.5 at 24 weeks, indicating the patients’ perception of ‘much improved’ CFS symptoms after treatment. In contrast, the mean PGIC score of the control group was 4.3 ± 0.9, which indicates ‘no change’ (Petrovics et al. Citation2016). Notwithstanding, the study by Petrovics et al. (Citation2016) did not include oncothermia-only or RBAC-only treatment as a third and a fourth arm. Therefore, there was insufficient data to determine the effects of each therapy and any multiplicative impact on the combination.
Metastasis prevention
Metastasis refers to the development of a secondary tumour at a distance from the primary cancer site. Cancer metastasis is a major cause of disease morbidity and accounts for about 90% of cancer deaths (Guan Citation2015). Metastatic cascade is a complex process. Tumour cells must detach from the primary tumour and migrate through the microenvironment before entering the blood or lymph vessels (Schegoleva et al. Citation2022). Once in the bloodstream, these circulating tumour cells (CTCs) must survive immunosurveillance while being transported at a distance at which attachment to the endothelium in a target organ happens. The CTCs then invade the surrounding parenchyma and form new tumours (Lin et al. Citation2021). As such, CTCs play a major role in cancer metastasis and are suggested as a biomarker for cancer diagnosis and prognosis, and a therapeutic target for eradication (Deng et al. Citation2022; Schegoleva et al. Citation2022). Specifically, a high CTC count is clinically relevant in non-metastasized breast cancer for early detection of recurrence (Fridrichova et al. Citation2022). Meanwhile, in metastasized breast cancer, CTC and CTC cluster count are indicators of disease progression and therapy response (Fridrichova et al. Citation2022).
In a single-arm human study by Pescatore et al. (Citation2022), RBAC lowered the CTC count in cancer patients. The study evaluated the CTC counts of 12 participants (males to females = 1:1, aged 56–79) before and after taking RBAC (1 g/day for 10 to 19 weeks). The initial mean CTC count was 8.33 ± 8.89 for the group and was significantly reduced (p < 0.047) to 2.33 ± 3.50 at the end of the study. Of the 12 participants, two had a CTC count of zero throughout the study. The rest (n = 10) all exhibited lower CTC counts. In parallel, decreased tumour markers, including prostate-specific antigen (for prostate cancer, n = 6) and cancer antigens 125, 27.29, and 15-3 (CA125, CA27.29, and CA15-3 for breast [n = 2], ovarian [n = 1], and uterine [n = 1] cancer), were detected in nine participants, with one of them experiencing remission (Pescatore et al. Citation2022). Therefore, the evidence suggests that RBAC prevents disease progression and the potential metastasis of the primary tumours by reducing CTC levels. However, such observations require validation with a larger controlled study in future research.
To summarize, research evidence has shown that the potential beneficial effects of RBAC include chemoprotection against carcinogenesis, enhancement of chemotherapy and radiation treatment efficacies, protection against toxicity and side effects of oncological treatment, synergism with other natural and complementary cancer treatment, and prevention of metastasis. These effects are illustrated in .
Best available evidence of RBAC treatment in cancer patients from clinical trials
RCT is the best available evidence in clinical research to establish causal associations between interventions and outcomes (Zabor et al. Citation2020). As such, in evidence-based practice, RCT is considered the gold standard for evaluating the efficacy of a new treatment (Hariton and Locascio Citation2018). This review identified a total of seven RCTs of RBAC. However, only six fulfilled the inclusion criterion of having survival and/or QoL as outcome measures for evaluation (Takahara and Sano Citation2004; Bang et al. Citation2010; Masood et al. Citation2013; Itoh et al. Citation2015; Petrovics et al. Citation2016; Tan and Flores Citation2020). The excluded RCT by Cholujova et al. (Citation2013) investigated only the impact of RBAC based on changes in the immune profile of the participants without any treatment efficacy outcomes.
The characteristics of all the included RCTs are summarized in . Four RCTs recruited patients with a specific cancer type, including liver (Bang et al. Citation2010), breast (Masood et al. Citation2013), cervical (Itoh et al. Citation2015), and head and neck (Tan and Flores Citation2020). In contrast, Takahara and Sano (Citation2004) and Petrovics et al. (Citation2016) recruited patients with various cancer types. Additionally, Petrovics et al. (Citation2016) only included participants diagnosed with CFS. All these RCTs used Biobran MGN-3 as the RBAC intervention with a dose of either 1 g or 3 g per day. RBAC was used as an adjunct therapy for conventional cancer treatment except for the study of Takahara and Sano (Citation2004), where the participants were treated with complementary therapies plus anticancer drugs with fewer side effects. All studies assessed RBAC as a sole intervention except that of Petrovics et al. (Citation2016), which assessed the combination therapy of RBAC with oncorthemia. Comparator-wise, only two studies were placebo-controlled (Itoh et al. Citation2015; Tan and Flores Citation2020) while others used passive controls.
Table 9. A summary of all RBAC randomized controlled trials with survival and/or quality of life as outcome measures.
Survival rate analysis
Three RCTs reported the survival outcomes of participants (Takahara and Sano Citation2004; Bang et al. Citation2010; Tan and Flores Citation2020). shows a forest plot of the survival event analysis from these studies. Based on survival data (n = 133) from patients with lung cancer (n = 68) from the study of Bang et al. (Citation2010) and head and neck cancer (n = 65) from the study of Tan and Flores (Citation2020), the OR for RBAC treatment compared to that of the control for one year or less was 4.02 (95% CI: 1.67, 9.69) in favour of RBAC. Thus, cancer patients under RBAC treatment have 4.02 times better odds of surviving one year or less than those in the control group.
Figure 5. Forest Plot of survival events of RBAC-treated group compared to those of the control group.
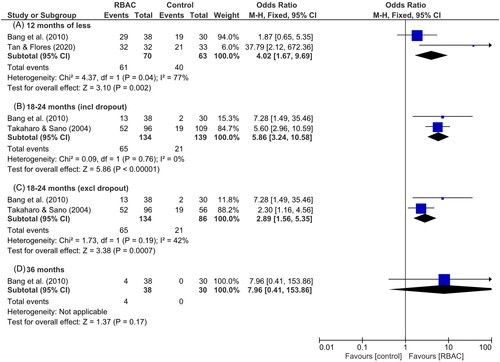
Meta-analysis of data with durations between 18 to 24 months shows that the OR of survival in the RBAC group is 5.86 (95% CI: 3.24, 10.58) over the control group based on intention-to-treat analysis that includes all dropouts (n = 273, RBAC = 134, control = 139). Excluding dropouts (n = 53 from the control group), the OR per protocol analysis is lower at 2.89 (95% CI: 1.56, 5.35) based on data from 223 participants (RBAC = 134, control = 86). The study by Takahara and Sano (Citation2004) is larger (n = 205) and included adult (mean age = 54.7) progress-cancer patients in the late III–IV stages of different malignancies (lung, liver, uterus, breast, prostate, rectum, stomach, lymph node, and others) who had completed conventional treatment. Hence, the meta-analysis shows that adult cancer patients, particularly those with lung cancer or in later stages (II–IV), are at least 2.89 times more likely to survive longer than 18 to 24 months after starting RBAC treatment than those not treated with RBAC.
Only Bang et al. (Citation2010) reported survival data up to 36 months, with the OR of RBAC over control being 7.96 (95% CI: 0.41, 153.86). However, these values are not statistically significant as the lower values of 95% CI are less than 1.0. With only a few lung cancer participants surviving up to 36 months, a large sample size is needed to confirm the life-prolonging effect of RBAC for the longer term.
Quality of life assessment
Participant QoL was assessed in five of the included RCTs (Takahara and Sano Citation2004; Masood et al. Citation2013; Itoh et al. Citation2015; Petrovics et al. Citation2016; Tan and Flores Citation2020), albeit with different outcome measures. A bubble chart for visualization of the available evidence is shown in . Each bubble in the plot represents an outcome assessed in one study, with the study sample size defining the diameter of the bubble. Furthermore, each study is assigned a different colour.
Figure 6. A Bubble chart visualizing the available evidence on the effect of RBAC on cancer patients’ quality of life (QoL). Each bubble represents an outcome assessed in one study, with the sample size defining the diameter of the bubble. Each study is assigned a different colour. X-axis: Statistical significance (p-value) of the outcome; Y-axis: QoL outcome measures.
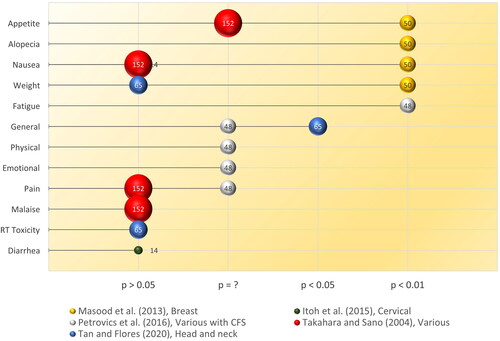
The most significant results were from the study of Masood et al. (Citation2013), with RBAC markedly (p < 0.01) improving anorexia, alopecia, nausea, and weight loss in breast cancer patients during chemotherapy compared to the control. Takahara and Sano (Citation2004) also reported improvement in appetite after RBAC treatment but reported no p-value and data for statistical significance estimation, which made the validity of the claim questionable. For nausea, unlike in the study of Masood et al. (Citation2013), both the studies of Takahara and Sano (Citation2004) and Itoh et al. (Citation2015) reported no significant difference between the RBAC and control groups post-treatment. Moreover, Tan and Flores (Citation2020) also observed no between-group difference in weight loss among head and neck cancer patients two months after chemoradiotherapy.
Among cancer patients with CFS (n = 48), Petrovics et al. (Citation2016) confirmed that the combination therapy of RBAC and oncothemia significantly lowered (p < 0.01) fatigue scores compared to those of the control group based on two validated instruments (CFQ and PGIC). However, other beneficial QoL outcomes stated by Petrovics et al. (Citation2016) include improvement in pain (based on a visual analog scale), general QoL, physical and emotional functioning (based on the European Organization for Research and Treatment of Cancer’s [EORTC] Quality of Life Questionnaire version 3.0 [QLQ-C3]). Although the outcomes were assessed with validated instruments, the lack of statistical analysis for these items in the published report rendered the assertion or benefits unconvincing.
Tan and Flores (Citation2020) also reported general QoL outcome improvement by RBAC treatment based on the EORTC QoL instrument specific for head and neck cancer (QLQ-H&N35). The mean QoL scores of patients treated with RBAC were significantly more favourable than those of patients in the control group two months after chemoradiotherapy (p < 0.05). Nevertheless, the study did not detect any between-group differences in radiation toxicities. Likewise, no between-group differences were reported for pain and malaise by Takahara and Sano (Citation2004) and diarrhoea by Itoh et al. (Citation2015).
Overall, QoL enhancement by RBAC was shown only in select types of cancer and for specific measures, most notably in the mitigation of anorexia, alopecia, nausea, and weight loss in breast cancer patients during chemotherapy (Masood et al. Citation2013), improvement of general QoL for head and neck cancer patients after radiation (Tan and Flores Citation2020), and amelioration of the fatigue syndrome in cancer patients with CFS during oncological treatment when used together with oncothermia (Petrovics et al. Citation2016). However, it remains unclear whether these QoL benefits apply to other cancer patients.
Quality assessment of the included studies
The quality assessment results for each study are summarized in . None of the studies were of good quality according to the assessors. Three studies, namely those of Bang et al. (Citation2010), Itoh et al. (Citation2015), and Tan and Flores (Citation2020), were rated as ‘fair’ in terms of quality as these studies adequately adhered to some of the required standards in reporting the RCT results to allow for a level of confidence on the internal validity of the study. The remaining three studies were considered to be of poor quality. The study of Takahara and Sano (Citation2004), in particular, suffered from high dropout rates and unclear baseline patient characteristics and allocation methods. For the study of Masood et al. (Citation2013), many required items were not reported, which placed the internal validity of the study in doubt. As for the study of Petrovics et al. (Citation2016), many of the findings, such as the QoL responses from the participants, were not presented correctly, making it difficult to compare outcomes between groups.
Based on the quality assessment outcomes, the risk of bias in all included studies is summarized in . Overall, these RCTs were assessed to have a low risk of reporting bias. The assessors reported that all studies used valid and reliable outcome measures, and the intention-to-treat analysis was adequately performed and documented. However, the risk of selection, performance, and detection biases were moderate. Although all studies were randomized trials, most did not report how random sequence generation was performed and whether there was any allocation concealment. There was some potential risk of attrition bias as there were high dropout rates in two studies (Takahara and Sano Citation2004; Itoh et al. Citation2015). The risk of other biases also exists with the lack of specific power calculations to detect a between-group difference in all these studies. Furthermore, all studies did not report any data on adherence to treatment.
Discussion and conclusions
This review identifies RBAC as an immunomodulating natural substance capable of restoring immune dysfunction due to tumour development. Specifically, by increasing the NK cell granularity and cytolytic activity levels, RBAC improved the immunosurveillance ability of the host to destroy malignant cells (Ghoneum and Brown Citation1999; Cholujova et al. Citation2013). These findings are consistent with the numerous pieces of in vivo and in vitro evidence that confirm the capacity of RBAC to enhance NK cell activity in healthy subjects (Ghoneum Citation1998; Ghoneum and Jewett Citation2000; Ghoneum and Abedi Citation2004; Kim et al. Citation2007; Badr El-Din et al. Citation2008; Giese et al. Citation2008; Pérez-Martínez et al. Citation2015). RBAC has also been shown to enhance the cytotoxic activity of NK cells via inducing the expression of CD107a in geriatric subjects (Elsaid et al. Citation2018), which led to enhanced innate defense and reduced cases of influenza-like illnesses in an RCT with elderly patients (Elsaid et al. Citation2021). Overall, the NK cell immunomodulatory effects of RBAC have also been thoroughly reviewed by Ghoneum (Citation2016); Ghoneum et al. (Citation2023); Yu et al. (Citation2019), and the lead author in another related publication (Ooi et al. Citation2023c). Additionally, arabinoxylan from rice bran extracted through other methods, such as acid hydrolysis and bioconversion, also exhibited potent immunomodulatory effects on the non-specific immune responses, including NK cell activity (Ji et al. Citation2020). Hence, RBAC could be a potential natural product candidate for NK cell immunotherapy, which seeks to alleviate the inhibited state of NK cells in the tumour microenvironment (Du et al. Citation2021). The existing NK cell therapy includes allogeneic NK cell infusion, cytokine treatment (IL-2 and IL-5), and immune checkpoint inhibitors (Du et al. Citation2021). Ghoneum et al (Citation2023), who has extensively investigated the NK cell augmentation capacity of RBAC, proclaims the superiority of RBAC compared to other NK cell therapies, in terms of the duration and extent of NK cell activation, as well as the lack of hyperresponsiveness over time. However, such an assertion requires further investigation as this study found no published human trials comparing the effects of RBAC to any existing NK cell therapy.
Other immune restorative effects of RBAC include promoting the increase of myeloid DC and CD4+ Th cell counts while lowering the level of the immunosuppressive Treg (Lissoni et al. Citation2008). As Treg promotes tumour cell proliferation by inhibiting antigen-presenting cells such as myeloid DCs (Koyama and Nishikawa Citation2021), reducing the abundance of Treg could enhance the activation of CD4+ Th cells by matured myeloid DCs, which in turn could prime the CD8+ T cells for antitumor activity in cancer patients. RBAC stimulates the maturation of DCs in healthy hosts by downregulating the immature surface markers (CD14, CD1a, and CD11c) and endocytic activities, while upregulating the maturation markers (CD12 and CD80) (Cholujova et al. Citation2009; Ghoneum and Agrawal Citation2011, Citation2014). Healthy CD8+ T cells cultured with RBAC-activated DCs also had increased cytotoxicity against tumour cell targets compared to those treated with unstimulated DCs (Ghoneum and Agrawal Citation2014). Hence, an alternative pathway that RBAC could exert the anticancer immune response on is via the indirect activation of cytotoxic CD8+ T cells – the most potent effectors among all the immune cells to be harnessed in immunotherapy (Raskov et al. Citation2021). CD8+ T cells and NK cells are parallel and complementary cytotoxic effectors crucial for the immunosurveillance of tumour cells (Rosenberg and Huang Citation2018). The depletion of CD8+ T cells and NK cells can lead to the escape of CTCs which, in turn, can lead to metastasis as CTC count negatively correlates with the lymphocyte ratios of CD4+/CD8+ and NK cells in late-stage cancer patients (Ye et al. Citation2017). Therefore, RBAC augments immunosurveillance to prevent cancer metastasis by lowering CTC levels (Pescatore et al. Citation2022). A comprehensive review of the immunomodulatory effects of RBAC on other immune cells, such as monocytes and macrophages, neutrophils, and T and B lymphocytes, in healthy participants, cells, and animals can be found in a separate publication of the lead author (Ooi et al. Citation2023c).
Beyond immunomodulation, RBAC also possesses direct anticancer effects achieved through the enhanced susceptibility of CD95 (Fas/APO-1) ligands in cancerous cells to extracellular apoptotic signals (the extrinsic pathway) and by downregulating anti-apoptotic Bcl-2 proteins (the intrinsic pathway) to lower mitochondrial membrane potentials while upregulating the tumour-suppressing P53 gene and increasing Bax and caspase-3 protein levels to initiate programmed cell death (Ghoneum and Gollapudi Citation2003; Badr El-Din, et al. Citation2016a; Badr El-Din et al. Citation2020). Hence, RBAC is not cytotoxic but rather proapoptotic to malignant cells.
Conventional antineoplastic agents can be classified into many families. The first group is the structural analogs of nucleobases, nucleosides, or folic analogs known as antimetabolites. Antimetabolites act as decoys to inhibit the synthesis of DNA components (Guichard et al. Citation2017). The second family includes DNA-interactive molecules, such as alkylating agents, which directly alter DNA replication and transcription processes. The third group consists of molecules acting on mitosis, such as antitubulin agents that cause cell death by blocking the division of the nucleus (Guichard et al. Citation2017). Other drug families include antitumor antibiotics, such as anthracyclines, that block DNA-to-RNA transcription (Martins-Teixeira and Carvalho et al. Citation2020), topoisomerase inhibitors that stop DNA unwinding (Kim and Khang Citation2020), and tyrosine kinase inhibitors which suppress growth factor receptors (Huang et al. Citation2020). However, RBAC does not belong to any of these classes of conventional antineoplastic agents but could be a member of a promising new class of plant-derived anticancer therapeutic agents that activate the apoptotic pathway (Pfeffer and Singh Citation2018).
Unlike most antineoplastic agents, which affect malignant and fast-growing normal cells, the proapoptotic effect of RBAC does not cause normal cell death unnecessarily, as it is non-cytotoxic (An Citation2011). As most antineoplastic drugs exploit the intact mitochondrial apoptotic signalling pathways to trigger cancer cell death, RBAC could improve the efficacy of these antineoplastic agents and thus prevent tumour resistance to therapies (Pistritto et al. Citation2016). It is not surprising that RBAC works synergistically with several commonly used antineoplastic agents, including daunorubicin and paclitaxel, to enhance the efficacy of chemotherapy (Gollapudi and Ghoneum Citation2008; Ghoneum et al. Citation2014; Badr El-din, et al. Citation2016b). RBAC may thus allow lower drug concentrations in chemotherapy, reducing cytotoxicity and unwanted side effects while achieving similar therapeutic objectives.
RBAC can also benefit cancer treatment through the antioxidant pathways to protect healthy tissues against increased oxidative stress from carcinogens, tumorigeneses, antineoplastic agents, and radiation treatment (Jacoby et al. Citation2001; Endo and Kanbayashi Citation2003; Ghoneum et al. Citation2013; Badr El-Din, et al. Citation2016a, Citation2016c; Badr El-Din et al. Citation2019; Zhao et al. Citation2020). This review reports that RBAC upregulates the endogenous GSH and antioxidant enzymes (GPx, GST, SOD, and CAT) in cancer and healthy tissues in vivo (Noaman et al. Citation2008). Other studies have also reported that RBAC has potent scavenging capacities towards ROS with an Oxygen Radical Absorption Capacity level higher than that of broccoli, a known high-antioxidant food (Tazawa et al. Citation2000; An Citation2011). The antioxidant effects of RBAC also protected against neurodegeneration due to sporadic Alzheimer’s disease in a mouse model (Ghoneum and El Sayed Citation2021). Interestingly, the protective effect of RBAC against brain tissue damage was made possible via the suppression of amyloid-beta-induced apoptosis through the upregulation of the activity of the antiapoptotic protein Bcl-2 and downregulation of the activity of the proapoptotic protein Bax and caspase-3 cleavage (Ghoneum and El Sayed Citation2021). Therefore, the effect of RBAC on the apoptotic pathway is selective and works against malignant cells but protects healthy cells, likely through its antioxidant activity which delays and inhibits cell damage (Redza-Dutordoir and Averill-Bates Citation2016).
With immune restorative, proapoptotic, and antioxidant effects, RBAC works synergistically with other complementary therapeutic agents, such as Baker’s yeast, curcumin, mistletoe lectin, and oncothermia, to improve treatment effects in cancer (Ghoneum and Gollapudi Citation2005a, Citation2005b, Citation2011; Hajtó et al. Citation2016b; Petrovics et al. Citation2016). Hence, RBAC could be a highly versatile plant-based therapeutic option in cancer treatment. An approach that combined RBAC with mistletoe lectin and wheat germ extract as plant-based immunomodulators to increase the sensitivity of tumour cells to low-dose anticancer drugs (such as gemcitabine or growth factor inhibitors) is being advocated by Hajtó et al. (Citation2013) as a novel oncological strategy. Favourable clinical responses of the combined treatment in different cancer metastases, including breast (Hajtó and Kirsch Citation2013; Hajtó Citation2018), colon (Hajtó and Kirsch Citation2013), ovarian (Hajtó et al. Citation2015; Hajtó Citation2018), lung (Hajtó et al. Citation2016a), and bile duct (Hajtó Citation2017) cancer, have been reported. However, good-quality research evidence remains lacking. Hajtó (Citation2023) reported experiencing difficulty in obtaining permission for clinical trials even though RBAC is considered ‘the most supported evidence-based and standardized plant immunomodulator without any side effects’ as the compound remains registered as a food supplement and not as a form of oncological therapy, which hindered clinical research.
The lack of RCTs severely restricts the application of RBAC in evidence-based practice. This review found only six RCTs that evaluated the survival and/or QoL of cancer patients with RBAC as an intervention. There is evidence that RBAC treatment could improve the survival rate of cancer patients (OR = 2.89, 95% CI: 1.56, 5.35) for the first two years, compared to treatment without RBAC. However, with only limited data (three studies, n = 338), the degree of confidence in this OR is low. Further research will likely change the estimate. Regarding QoL, there is evidence from small RCTs showing that RBAC reduces fatigue and mitigates the side effects of chemotherapy (anorexia, alopecia, nausea, and weight loss), and improves general QoL after radiation treatment. However, with results obtained from only a small number of trials conducted on patients with specific cancer types and conditions, to draw any conclusion on the effects of RBAC on cancer patients’ QoL, in general, is premature. Moreover, there could be risks of bias in many of these trials, with half of the included RCTs regarded as being of poor quality. Note that the risk of bias assessment of this review is consistent with that of a previous evaluation, which reported that all RBAC RCTs have either an unclear or a high risk of selection, performance, detection, and attrition biases, among other biases (Ooi et al. Citation2018). Hence, the current quality of evidence for RBAC as a credible oncological therapeutic option remains poor. Notwithstanding, RBAC is considered safe to consume at the typical dosage of 1–3 g/day, with no RBAC-related adverse events reported in any of the human studies.
Moving forward, research on RBAC in the context of cancer should focus on two areas. The first is to identify, quantify, and standardize the active ingredients of RBAC. Miura et al. (Citation2013) had previously identified the immune active compounds in RBAC, based on macrophage activation, to be complex heteropolysaccharides with arabinoxylan as its primary structure while also containing galactan and glucan. However, no follow-up research was conducted to ascertain the detailed molecular structure of the compound or the components that are most relevant in activating NK cells, promoting apoptosis in cancer cells, and scavenging free radicals. For example, could the antioxidant capacity of RBAC be due to the contents of ferulic acids or γ-oryzanol commonly found in rice bran? This is an intriguing hypothesis that merits investigation. Most importantly, clarifying the active ingredient in RBAC can also lead to an in-depth understanding of the molecular pathways in which RBAC induces cellular mechanisms such as proapoptotic cascade or myeloid DC maturation in the tumour microenvironment.
The second focus area in RBAC research should be clinical trials. There is a need for more well-designed RCTs to substantiate the therapeutic use of RBAC in cancer treatment for various cancer types with different therapeutic concomitants. These RCTs should have a sufficient sample size to detect the effects of outcomes in terms of treatment efficacies and side effects. Studies with longer-term follow-up periods of three years or more are also needed to confirm the long-term impact of RBAC on recurrence, QoL, and survival. Only with sufficient high-quality, favourable evidence from RCTs can RBAC become mainstream in oncological treatment.
In conclusion, current preclinical and clinical research evidence suggests that RBAC is a natural product with immense potential in cancer treatment. RBAC could serve as an immunomodulator that primes the cytotoxic immune response against tumorigenesis, promoting apoptosis in malignant cells. Working synergistically with chemoradiation therapies, RBAC could enhance treatment effectiveness while reducing side effects, improving patients’ QoL, and prolonging survival. Notwithstanding, further research is needed before RBAC can be considered a viable therapeutic option in cancer.
Author’s contributions
S.L.O.: Conceptualisation, methodology, software, formal analysis, investigation, data curation, writing—original draft, visualisation. P.S.M.: Conceptualisation, methodology, validation, writing—review and editing. J.K.: Resources, writing—review and editing. S.C.P: Conceptualisation, methodology, validation, resources, writing—review and editing, supervision, project administration.
Acknowledgments
The authors wish to thank Taylor & Francis Editing Services for their help in editing this paper.
Disclosure statement
JK is an employee of STR Biotech Co., Ltd., which develops and markets variants of RBAC products commercially. The other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability statement
All data used in this study will be available via email to the corresponding author upon request.
Additional information
Funding
References
- Alshaibi HF, Al-Shehri B, Hassan B, Al-Zahrani R, Assiss T. 2020. Modulated electrohyperthermia: a new hope for cancer patients. Biomed Res Int. 2020:8814878–8814813. doi: 10.1155/2020/8814878.
- Altun İ, Sonkaya A. 2018. The most common side effects experienced by patients were receiving first cycle of chemotherapy. Iran J Public Health. 47(8):1218–1219.
- American Society of Clinical Oncology. 1996. Outcomes of cancer treatment for technology assessment and cancer treatment guidelines. J Clin Oncol. 14:671–679.
- Amjad MT, Chidharla A, Kasi A. 2023. Cancer chemotherapy. Treasure Island. (FL): StatPearls Publishing; [accessed 2023 Nov 9]. https://www.ncbi.nlm.nih.gov/books/NBK564367/.
- An SY. 2011. Immune-enhance and anti-tumor effect of exo-biopolymer extract from submerged culture of Lentinus edodes with rice bran. [dissertation]. Seoul (Republic of Korea): Korea University.
- Andocs G, Szasz O, Szasz A. 2009. Oncothermia treatment of cancer: from the laboratory to clinic. Electromagn Biol Med. 28(2):148–165. doi: 10.1080/15368370902724633.
- Ba Y, Shi Y, Jiang W, Feng J, Cheng Y, Xiao L, Zhang Q, Qiu W, Xu B, Xu R, et al. 2020. Current management of chemotherapy-induced neuxtropenia in adults: key points and new challenges: committee of neoplastic supportive-care (CONS). China anti-cancer Association Committee of Clinical Chemotherapy, China anti-cancer Association. Cancer Biol Med. 17:896–909.)
- Badr El-Din NK, Abdel Fattah SM, Pan D, Tolentino L, Ghoneum M. 2016a. Chemopreventive activity of MGN-3/Biobran against chemical induction of glandular stomach carcinogenesis in rats and its apoptotic effect in gastric cancer cells. Integr Cancer Ther. 15(4):NP26–NP34. doi: 10.1177/1534735416642287.
- Badr El-Din NK, Ali DA, Alaa El-Dein M, Ghoneum M. 2016b. Enhancing the apoptotic effect of a low dose of paclitaxel on tumor cells in mice by arabinoxylan rice bran (MGN-3/Biobran). Nutr Cancer. 68(6):1010–1020. doi: 10.1080/01635581.2016.1192204.
- Badr El-Din NK, Ali DA, Othman RM. 2016c. Inhibition of experimental carcinogenesis by the bioactive natural product Biobran. J Plant Prot Pathol. 7(1):85–91.
- Badr El-Din NK, Ali DA, Othman R, French SW, Ghoneum M. 2020. Chemopreventive role of arabinoxylan rice bran, MGN-3/Biobran, on liver carcinogenesis in rats. Biomed Pharmacother. 126:110064. doi: 10.1016/j.biopha.2020.110064.
- Badr El-Din NK, Areida SK, Ahmed KO, Ghoneum M. 2019. Arabinoxylan rice bran (MGN-3/Biobran) enhances radiotherapy in animals bearing Ehrlich ascites carcinoma. J Radiat Res. 60(6):747–758. doi: 10.1093/jrr/rrz055.
- Badr El-Din NK, Mahmoud AZ, Hassan TA, Ghoneum M. 2018. Baker’s yeast sensitizes metastatic breast cancer cells to paclitaxel in vitro. Integr Cancer Ther. 17(2):542–550. doi: 10.1177/1534735417740630.
- Badr El-Din NK, Noaman E, Ghoneum M. 2008. In vivo tumor inhibitory effects of nutritional rice bran supplement MGN-3/Biobran on Ehrlich carcinoma-bearing mice. Nutrit Cancer. 60(2):235–244. doi: 10.1080/01635580701627285.
- Bae MJ, Lee ST, Sy C, Shin SH, Kwon SH, Park MH, Song MY, Hwang SJ. 2004. The effects of the arabinoxylane and the polysaccharide peptide (PSP) on the antiallergy, anticancer. J Korean Soc Food Sci Nutr. 33:469–474.
- Bang MH, Van Riep T, Thinh NT, Song LH, Dung TT, Van Truong L, Van Don L, Ky TD, Pan D, Shaheen M. 2010. Arabinoxylan rice bran (MGN-3) enhances the effects of interventional therapies for the treatment of hepatocellular carcinoma: a three-year randomized clinical trial. Anticancer Res. 30:5145–5151.
- Barnes JL, Zubair M, John K, Poirier MC, Martin FL. 2018. Carcinogens and DNA damage. Biochem Soc Trans. 46(5):1213–1224. doi: 10.1042/BST20180519.
- Baskar R, Lee KA, Yeo R, Yeoh K-W. 2012. Cancer and radiation therapy: current advances and future directions [Review]. Int J Med Sci. 9(3):193–199. doi: 10.7150/ijms.3635.
- Blackadar CB. 2016. Historical review of the causes of cancer. WJCO. 7(1):54–86. doi: 10.5306/wjco.v7.i1.54.
- Bray F, Laversanne M, Weiderpass E, Soerjomataram I. 2021. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 127(16):3029–3030. doi: 10.1002/cncr.33587.
- Brush TP, Trinh S, Brawner CM, Ali KH, Hauke RJ, Elkahwaji JE. 2010. RBAC, a modified form of arabinoxylan from rice bran, impairs prostate cancer cell line proliferation, adhesion, and invasion in vitro [Abstract]. Cancer Res. 70(8_Supplement):5662–5662. doi: 10.1158/1538-7445.AM10-5662.
- Bukowski K, Kciuk M, Kontek R. 2020. Mechanisms of multidrug resistance in cancer chemotherapy. Int J Mol Sci. 21(9):3233. doi: 10.3390/ijms21093233.
- Carvalho AS, Lagana D, Catford J, Shaw D, Bak N. 2020. Bloodstream infections in neutropenic patients with haematological malignancies. Infect Dis Health. 25(1):22–29. doi: 10.1016/j.idh.2019.08.006.
- Chatterjee A. 2013. Reduced glutathione: a radioprotector or a modulator of DNA-repair activity? Nutrients. 5(2):525–542. doi: 10.3390/nu5020525.
- Chistiakov DA, Sobenin IA, Orekhov AN, Bobryshev YV. 2015. Myeloid dendritic cells: development, functions, and role in atherosclerotic inflammation. Immunobiology. 220(6):833–844. doi: 10.1016/j.imbio.2014.12.010.
- Cholujova D, Jakubikova J, Czako B, Martisova M, Hunakova L, Duraj J, Mistrik M, Sedlak J. 2013. MGN-3 arabinoxylan rice bran modulates innate immunity in multiple myeloma patients. Cancer Immunol Immunother. 62(3):437–445. doi: 10.1007/s00262-012-1344-z.
- Cholujova D, Jakubikova J, Sedlak J. 2009. BioBran-augmented maturation of human monocyte-derived dendritic cells. neo. 56(2):89–95. doi: 10.4149/neo_2009_02_89.
- Clark HR. 1999. The natural pharmacy. Winning the war against cancer: MGN-3: mushroom ammunition. Alternative Medicine Magazine. Eagan (MN): InnoVision Communications Health Media. p. 26–28.
- Dare AJ, Anderson BO, Sullivan R, Pramesh CS, Andre I, Adewole IF, Badwe RA, Gauvreau CL., et al. 2015. Surgical services for cancer care. In: Gelband H, Jha P, Sankaranarayanan R editors. Cancer: disease control priorities. 3rd ed. Washington (DC): the International Bank for Reconstruction and Development/the World Bank; p. 223–238.
- Deeks JJ, Higgins JP, Altman DG., et al. 2021. Analysing data and undertaking meta-analyses. In: Higgins J, Thomas J, Chandler J editors. Cochrane handbook for systematic reviews of interventions version 62 (updated February 2021). London, UK: Cochrane Library.
- Delaney GP, Barton MB. 2015. Evidence-based estimates of the demand for radiotherapy. Clin Oncol. 27(2):70–76. doi: 10.1016/j.clon.2014.10.005.
- Deng Z, Wu S, Wang Y, Shi D. 2022. Circulating tumor cell isolation for cancer diagnosis and prognosis. eBioMedicine. 83:104237. doi: 10.1016/j.ebiom.2022.104237.
- Du N, Guo F, Wang Y, Cui J. 2021. NK cell therapy: a rising star in cancer treatment. Cancers. 13(16):4129. doi: 10.3390/cancers13164129.
- Elsaid AF, Agrawal S, Agrawal A, Ghoneum M. 2021. Dietary supplementation with Biobran/MGN-3 increases innate resistance and reduces the incidence of influenza-like illnesses in elderly subjects: a randomized, double-blind, placebo-controlled pilot clinical trial. Nutrients. 13(11):4133. doi: 10.3390/nu13114133.
- Elsaid AF, Shaheen M, Ghoneum M. 2018. Biobran/MGN-3, an arabinoxylan rice bran, enhances NK cell activity in geriatric subjects: a randomized, double-blind, placebo-controlled clinical trial. Exp Ther Med. 15(3):2313–2320. doi: 10.3892/etm.2018.5713.
- Endo Y, Kanbayashi H. 2003. Modified rice bran beneficial for weight loss of mice as a major and acute adverse effect of cisplatin. Pharmacol Toxicol. 92(6):300–303. doi: 10.1034/j.1600-0773.2003.920608.x.
- Fan H, Walters CS, Dunston GM, Tackey R. 2002. IL-12 plays a significant role in the apoptosis of human T cells in the absence of antigenic stimulation. Cytokine. 19(3):126–137. doi: 10.1006/cyto.2002.1958.
- Fox JT, Sakamuru S, Huang R, Teneva N, Simmons SO, Xia M, Tice RR, Austin CP, Myung K. 2012. High-throughput genotoxicity assay identifies antioxidants as inducers of DNA damage response and cell death. Proc Natl Acad Sci U S A. 109(14):5423–5428. doi: 10.1073/pnas.1114278109.
- Fridrichova I, Kalinkova L, Ciernikova S. 2022. Clinical relevancy of circulating tumor cells in breast cancer: epithelial or mesenchymal characteristics, single cells or clusters? IJMS. 23(20):12141. doi: 10.3390/ijms232012141.
- Ghoneum M. 1998. Enhancement of human natural killer cell activity by modified arabinoxylane from rice bran (MGN-3). Int J Immunother. 14(2):89–99.
- Ghoneum M. 1999. Immunostimulation and cancer prevention. Abstract presented at: 7th International Congress on Anti-Aging & Biomedical Technologies. Dec 11-13; Las Vegas (NV).
- Ghoneum M. 2016. From bench to bedside: the growing use of arabinoxylan rice bran (MGN-3/Biobran) in cancer immunotherapy. Austin Immunol. 1(2):1006.
- Ghoneum M., et al. 2023. Enhancing natural killer cell activity. In: Pak SC, Ooi SL, Micalos PS editors. Modified rice bran arabinoxylan: therapeutic applications in cancer and other diseases. Singapore: Springer Nature. p. 15–25.
- Ghoneum M, Abdulmalek S, Fadel HH. 2023. Biobran/MGN-3, an arabinoxylan rice bran, protects against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): an in vitro and in silico study. Nutrients. 15(2):453. doi: 10.3390/nu15020453.
- Ghoneum M, Abedi S. 2004. Enhancement of natural killer cell activity of aged mice by modified arabinoxylan rice bran (MGN-3/Biobran). J Pharm Pharmacol. 56(12):1581–1588. doi: 10.1211/0022357044922.
- Ghoneum M, Agrawal S. 2011. Activation of human monocyte-derived dendritic cells in vitro by the biological response modifier arabinoxylan rice bran (MGN-3/Biobran). Int J Immunopathol Pharmacol. 24(4):941–948. doi: 10.1177/039463201102400412.
- Ghoneum M, Agrawal S. 2014. MGN-3/biobran enhances generation of cytotoxic CD8+ T cells via upregulation of DEC-205 expression on dendritic cells. Int J Immunopathol Pharmacol. 27(4):523–530. doi: 10.1177/039463201402700408.
- Ghoneum M, Badr El-Din NK, Abdel Fattah SM, Tolentino L. 2013. Arabinoxylan rice bran (MGN-3/Biobran) provides protection against whole-body γ-irradiation in mice via restoration of hematopoietic tissues. J Radiat Res. 54(3):419–429. doi: 10.1093/jrr/rrs119.
- Ghoneum M, Badr El-Din NK, Ali DA, El-Dein MA. 2014. Modified arabinoxylan from rice bran, MGN-3/Biobran, sensitizes metastatic breast cancer cells to paclitaxel in vitro. Anticancer Res. 34(1):81–87.
- Ghoneum M, Brown J. 1999. NK Immunorestoration and cancer patients by MGN-3, a modified arabinoxylan rice bran (Study of 32 patients followed for up to 4 years). In: Klatz RM, Goldman R, editors. Anti-aging medical therapeutics. Marina del Rey (CA): Health Quest Publications; p. 217–226.
- Ghoneum M, El Sayed NS. 2021. Protective effect of Biobran/MGN-3 against sporadic Alzheimer’s disease mouse model: possible role of oxidative stress and apoptotic pathways. Oxid Med Cell Longev. 2021:8845064–8845015. doi: 10.1155/2021/8845064.
- Ghoneum M, Gollapudi S. 2003. Modified arabinoxylan rice bran (MGN-3/Biobran) sensitizes human T cell leukemia cells to death receptor (CD95)-induced apoptosis. Cancer Lett. 201(1):41–49. doi: 10.1016/S0304-3835(03)00458-0.
- Ghoneum M, Gollapudi S. 2004. Induction of apoptosis in breast cancer cells by Saccharomyces cerevisiae the baker’s yeast, in vitro. Anticancer Res. 24(3A):1455–1463.
- Ghoneum M, Gollapudi S. 2005a. Modified arabinoxylan rice bran (MGN-3/Biobran) enhances yeast-induced apoptosis in human breast cancer cells in vitro. Anticancer Res. 25(2A):859–870.
- Ghoneum M, Gollapudi S. 2005b. Synergistic role of arabinoxylan rice bran (MGN-3/Biobran) in S. cerevisiae-induced apoptosis of monolayer breast cancer MCF-7 cells. Anticancer Res. 25(6B):4187–4196.
- Ghoneum M, Gollapudi S. 2011. Synergistic apoptotic effect of arabinoxylan rice bran (MGN-3/Biobran) and curcumin (turmeric) on human multiple myeloma cell line U266 in vitro. Neoplasma. 58(2):118–123.
- Ghoneum M, Jewett A. 2000. Production of tumor necrosis factor-alpha and interferon-gamma from human peripheral blood lymphocytes by MGN-3, a modified arabinoxylan from rice bran, and its synergy with interleukin-2 in vitro. Cancer Detect Prev. 24(4):314–324.
- Ghoneum M, Matsuura M, Gollapudp S. 2008. Modified arabinoxylan rice bran (MGN-3/Biobran) enhances intracellular killing of microbes by human phagocytic cells in vitro. Int J Immunopathol Pharmacol. 21(1):87–95. doi: 10.1177/039463200802100110.
- Ghoneum M, Tachiki KH, Ueyama K, Makinodan T, Makhijani N, Yamaguchi D. 2000. Natural biological response modifier (MGN-3) shown to be ‑effective against tumor cell growth. Abstract presented at: 8th International Congress on Anti-Aging & Biomedical Technologies. Dec 14-17; Las Vegas (NV).
- Giese S, Sabell GR, Coussons-Read M. 2008. Impact of ingestion of rice bran and shitake mushroom extract on lymphocyte function and cytokine production in healthy rats. J Diet Suppl. 5(1):47–61. doi: 10.1080/19390210802329196.
- Gollapudi S, Ghoneum M. 2008. MGN-3/Biobran, modified arabinoxylan from rice bran, sensitizes human breast cancer cells to chemotherapeutic agent, daunorubicin. Cancer Detect Prev. 32(1):1–6. doi: 10.1016/j.cdp.2008.02.006.
- Golombick T, Diamond TH, Manoharan A, Ramakrishna R. 2016. Addition of rice bran arabinoxylan to curcumin therapy may be of benefit to patients with early-stage B-cell lymphoid malignancies (monoclonal gammopathy of undetermined significance, smoldering multiple myeloma, or stage 0/1 chronic lymphocytic leukemia): a preliminary clinical study. Integr Cancer Ther. 15(2):183–189. doi: 10.1177/1534735416635742.
- Gonzalez H, Hagerling C, Werb Z. 2018. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 32(19–20):1267–1284. doi: 10.1101/gad.314617.118.
- Götze H, Friedrich M, Taubenheim S, Dietz A, Lordick F, Mehnert A. 2020. Depression and anxiety in long-term survivors 5 and 10 years after cancer diagnosis. Support Care Cancer. 28(1):211–220. doi: 10.1007/s00520-019-04805-1.
- Guan X. 2015. Cancer metastases: challenges and opportunities. Acta Pharm Sin B. 5(5):402–418. doi: 10.1016/j.apsb.2015.07.005.
- Guichard N, Guillarme D, Bonnabry P, Fleury-Souverain S. 2017. Antineoplastic drugs and their analysis: a state of the art review. Analyst. 142(13):2273–2321. doi: 10.1039/c7an00367f.
- Hajtó T. 2017. Can a standardized plant immunomodulator (rice bran arabinoxylan concentrate/MGN-3) increase the effects of MEK and BRAF inhibitors with clinical benefit? Case report of a patient with carcinoma in biliary duct. Res Rev Insights. 1(3):1–4.
- Hajtó T. 2018. New perspectives to improve the MHC-I unrestricted immune mechanisms against malignant tumors. Adv Clin Transl Res. 2(3):100014.
- Hajtó T. 2023. The therapeutic application of RBAC in cancer. In: Pak SC, Ooi SL, Micalos PS., editors. Modified rice bran arabinoxylan: therapeutic applications in cancer and other diseases. Singapore: Springer Nature. p. 67–78.
- Hajtó T, Adámy A, Langmár Z, Kirsch A, Ábrahám L, Perjési P, Németh P. 2013. Enhanced effectiveness of conventional oncotherapy with plant immunomodulators: overview of recent advances. Adv Med Plant Res. 1(3):56–65.
- Hajtó T, Baranyai L, Kirsch A, Kuzma M, Perjési P. 2015. Can a synergistic activation of pattern recognition receptors by plant immunomodulators enhance the effect of oncologic therapy? Case Report of a patient with uterus and ovary sarcoma. Clin Case Rep Rev. 1(10):235–238. doi: 10.15761/CCRR.1000176.
- Hajtó T, Horváth A, Baranyai L, Kuzma M, Perjési P. 2016a. Can the EGFR inhibitors increase the immunomodulatory effects of standardized plant extracts (mistletoe lectin and arabonoxylan) with clinical benefit? Case report of a patient with lung adenocarcinoma. Clin Case Rep Rev. 2(6):456–459. doi: 10.15761/CCRR.1000244.
- Hajtó T, Horváth A, Papp S. 2016b. Improvement of quality of life in tumor patients after an immunomodulatory treatment with standardized mistletoe lectin and arabinoxylan plant extracts. Int J Neurorehabil. 3(2):2–4.
- Hajtó T, Kirsch A. 2013. Case reports of cancer patients with hepatic metastases treated by standardized plant immunomodulatory preparations. J Can Res Updates. 2(1):1–9.
- Hajtó T, Péczely L, Kuzma M, Hormay E, Ollmann T, Jaksó P, Baranyai L, Karádi Z. 2022. Enhancing effect of streptozotocin-induced insulin deficit on antitumor innate immune defense in rats. Res Rev Insights. 6(1):1000170.
- Haque A, Brazeau D, Amin AR. 2021. Perspectives on natural compounds in chemoprevention and treatment of cancer: an update with new promising compounds. Eur J Cancer. 149:165–183. doi: 10.1016/j.ejca.2021.03.009.
- Hariton E, Locascio JJ. 2018. Randomised controlled trials – the gold standard for effectiveness research. BJOG. 125(13):1716–1716. doi: 10.1111/1471-0528.15199.
- Hausman DM. 2019. What is cancer? Perspect Biol Med. 62(4):778–784. doi: 10.1353/pbm.2019.0046.
- Hayes JD, Dinkova-Kostova AT, Tew KD. 2020. Oxidative stress in cancer. Cancer Cell. 38(2):167–197. doi: 10.1016/j.ccell.2020.06.001.
- Hegyi G, Szasz O, Szasz A. 2013. Oncothermia: a new paradigm and promising method in cancer therapies. Acupunct Electrother Res. 38(3):161–197. doi: 10.3727/036012913X13831832269243.
- Huang L, Jiang S, Shi Y. 2020. Tyrosine kinase inhibitors for solid tumors in the past 20 years (2001–2020). J Hematol Oncol. 13(1):143. doi: 10.1186/s13045-020-00977-0.
- Itoh Y, Mizuno M, Ikeda M, Nakahara R, Kubota S, Ito J, Okada T, Kawamura M, Kikkawa F, Naganawa S. 2015. A randomized, double-blind pilot trial of hydrolyzed rice bran versus placebo for radioprotective effect on acute gastroenteritis secondary to chemoradiotherapy in patients with cervical cancer. Evid Based Complement Alternat Med. 2015:974390–974396. doi: 10.1155/2015/974390.
- Jacoby HI, Wnorowski G, Sakata K, Maeda H. 2001. The effect of MGN-3 on cisplatin and doxorubicin induced toxicity in the rat. J Nutraceuticals Funct Med Foods. 3(4):3–11. doi: 10.1300/J133v03n04_02.
- Ji MG, Lee YR, Nam YH, Castañeda R, Hong BN, Kang TH. 2020. Immunostimulatory action of high-content active arabinoxylan in rice bran. ACS Omega. 5(41):26374–26381. doi: 10.1021/acsomega.0c02472.
- Kim DJ, Ryu S-N, Han SJ, Kim HY, Kim JH, Hong SG. 2011. In vivo immunological activity in fermentation with black rice bran. Korean J Food Nutr. 24:p. 273–281. Korean: The Korean Society of Food and Nutrition.
- Kim HY, Kim JH, Yang SB, Hong SG, Lee SA, Hwang SJ, Shin KS, Suh HJ, Park MH. 2007. A polysaccharide extracted from rice bran fermented with Lentinus edodes enhances natural killer cell activity and exhibits anticancer effects. J Med Food. 10(1):25–31. doi: 10.1089/jmf.2006.116.
- Kim JM, Hong SG, Song BS, Sohn HJ, Baik H, Sung MK. 2020. Efficacy of cereal-based oral nutrition supplement on nutritional status, inflammatory cytokine secretion and quality of life in cancer patients under cancer therapy. J Cancer Prev. 25(1):55–63. doi: 10.15430/JCP.2020.25.1.55.
- Kim K, Khang D. 2020. Past, present, and future of anticancer nanomedicine. Int J Nanomedicine. 15:5719–5743. doi: 10.2147/IJN.S254774.
- Knudson AG. 2002. Cancer genetics. Am J Med Genet. 111(1):96–102. doi: 10.1002/ajmg.10320.
- Koyama S, Nishikawa H. 2021. Mechanisms of regulatory T cell infiltration in tumors: implications for innovative immune precision therapies. J Immunother Cancer. 9(7):e002591. doi: 10.1136/jitc-2021-002591.
- Lemoine M. 2022. Anya Plutynski’s explaining cancer: finding order in disorder. Philos Med. 3(1):1–8.
- Li C, Jiang P, Wei S, Xu X, Wang J. 2020. Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects. Mol Cancer. 19(1):116. doi: 10.1186/s12943-020-01234-1.
- Lin D, Shen L, Luo M, Zhang K, Li J, Yang Q, Zhu F, Zhou D, Zheng S, Chen Y. 2021. Circulating tumor cells: biology and clinical significance. Signal Transduct Target Ther. 6(1):404.
- Lin W, Niu Z, Zhang H, Kong Y, Wang Z, Yang X, Yuan F. 2019. Imbalance of Th1/Th2 and Th17/Treg during the development of uterine cervical cancer. Int J Clin Exp Pathol. 12:3604–3612.
- Lissoni P, Messina G, Brivio F, Fumagalli L, Rovelli F, Maruelli L, Miceli M, Marchiori P, Porro G, Held M. 2008. Modulation of the anticancer immunity by natural agents: inhibition of T regulatory lymphocyte generation by arabinoxylan in patients with locally limited or metastatic solid tumors. Cancer Ther. 6:1011–1016.
- Liu S, Edgerton SM, Moore DH, Ii, T, Ad. 2001. Measures of cell turnover (proliferation and apoptosis) and their association with survival in breast cancer. Clin Cancer Res. 7:1716–1723.
- Loef M, Walach H. 2020. Quality of life in cancer patients treated with mistletoe: a systematic review and meta-analysis. BMC Complement Med Ther. 20(1):227. doi: 10.1186/s12906-020-03013-3.
- Lugini L, Lozupone F, Matarrese P, Funaro C, Luciani F, Malorni W, Rivoltini L, Castelli C, Tinari A, Piris A, et al. 2003. Potent phagocytic activity discriminates metastatic and primary human malignant melanomas: a key role of ezrin. Lab Invest. 83(11):1555–1567. doi: 10.1097/01.lab.0000098425.03006.42.
- Majeed H, Gupta V. 2023. Adverse effects of radiation therapy. Treasure Island (FL): StatPearls Publishing; [accessed 2023 Nov 13]. https://www.ncbi.nlm.nih.gov/books/NBK563259/.
- Majeed M, Hakeem KR, Rehman RU. 2021. Mistletoe lectins: from interconnecting proteins to potential tumour inhibiting agents. Phytomed Plus. 1(3):100039. doi: 10.1016/j.phyplu.2021.100039.
- Martins-Teixeira MB, Carvalho I. 2020. Antitumour anthracyclines: progress and perspectives. ChemMedChem. 15(11):933–948. doi: 10.1002/cmdc.202000131.
- Masood AI, Sheikh R, Anwer RA. 2013. BIOBRAN MGN-3"; Effect of reducing side effects of chemotherapy in breast cancer patients. Prof Med J. 20(1):13–16.
- Mazzotti E, Antonini Cappellini GC, Buconovo S, Morese R, Scoppola A, Sebastiani C, Marchetti P. 2012. Treatment-related side effects and quality of life in cancer patients. Support Care Cancer. 20(10):2553–2557. doi: 10.1007/s00520-011-1354-y.
- Melaiu O, Lucarini V, Cifaldi L, Fruci D. 2019. Influence of the tumor microenvironment on NK cell function in solid tumors. Front Immunol. 10:3038. doi: 10.3389/fimmu.2019.03038.
- Miura T, Chiba M, Miyazaki Y, Kato Y, Maeda H. 2013. Chemical structure of the component involved in immunoregulation. BioBran/MGN-3 (Rice Bran Arabinoxylan Coumpound): basic and clinical application to integrative medicine. 2nd ed. Tokyo (Japan): BioBran Research Foundation; p. 14–22.
- Morse W, Nawaz H, Choudhry AA. 2023. Combination of chemotherapeutic agents and biological response modifiers (immunotherapy) in triple-negative/Her2( +) breast cancer, multiple myeloma, and non-small-cell lung cancer. J Egypt Natl Canc Inst. 34(1):58. doi: 10.1186/s43046-022-00159-8.
- Muhammad N, Usmani D, Tarique M, Naz H, Ashraf M, Raliya R, Tabrez S, Zughaibi TA, Alsaieedi A, Hakeem IJ, et al. 2022. The role of natural products and their multitargeted approach to treat solid cancer. Cells. 11(14):2209. doi: 10.3390/cells11142209.
- Nasa P, Jain R, Juneja D. 2021. Delphi methodology in healthcare research: how to decide its appropriateness. World J Methodol. 11(4):116–129. doi: 10.5662/wjm.v11.i4.116.
- National Cancer Institute. 2023. Cancer. [accessed 2023 Oct 20]. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/cancer.
- National Heart Lung and Blood Institute. 2013. Study quality assessment tools. [accessed 2023 Oct 20]. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- Nesher L, Rolston KVI. 2014. The current spectrum of infection in cancer patients with chemotherapy related neutropenia. Infection. 42(1):5–13. doi: 10.1007/s15010-013-0525-9.
- Noaman E, Badr El-Din NK, Bibars MA, Abou Mossallam AA, Ghoneum M. 2008. Antioxidant potential by arabinoxylan rice bran, MGN-3/biobran, represents a mechanism for its oncostatic effect against murine solid Ehrlich carcinoma. Cancer Lett. 268(2):348–359. doi: 10.1016/j.canlet.2008.04.012.
- Nordvig J, Aagaard T, Daugaard G, Brown P, Sengeløv H, Lundgren J, Helleberg M. 2018. Febrile neutropenia and long-term risk of infection among patients treated with chemotherapy for malignant diseases. Open Forum Infect Dis. 5(10):ofy255.
- Ohue Y, Nishikawa H. 2019. Regulatory T (Treg) cells in cancer: can Treg cells be a new therapeutic target? Cancer Sci. 110(7):2080–2089. doi: 10.1111/cas.14069.
- Ooi SL, McMullen D, Golombick T, Nut D, Pak SC. 2018. Evidence-based review of BioBran/MGN-3 arabinoxylan compound as a complementary therapy for conventional cancer treatment. Integr Cancer Ther. 17(2):165–178. doi: 10.1177/1534735417735379.
- Ooi SL, Micalos PS, Kim J, Pak SC. 2023a. Rice bran arabinoxylan compound as a natural product for cancer treatment – an evidence-based assessment of the effects and mechanisms. Preprints. 2023: 2023101057. doi: 10.20944/preprints202310.1057.v1.
- Ooi SL, Micalos PS, Pak SC. 2023b. Modified rice bran arabinoxylan as a nutraceutical in health and disease – a scoping review with bibliometric analysis. PLOS One. 18(8):e0290314. doi: 10.1371/journal.pone.0290314.
- Ooi SL, Micalos PS, Pak SC. 2023c. Modified rice bran arabinoxylan by Lentinus edodes mycelial enzyme as an immunoceutical for health and aging – a comprehensive literature review. Molecules. 28(17):6313. doi: 10.3390/molecules28176313.
- Ooi SL, Pak SC, Micalos PS, Schupfer E, Lockley C, Park MH, Hwang SJ. 2021. The health-promoting properties and clinical applications of rice bran arabinoxylan modified with shiitake mushroom enzyme – a narrative review. Molecules. 26(9):2539. doi: 10.3390/molecules26092539.
- Palmer AC, Sorger PK. 2017. Combination cancer therapy can confer benefit via patient-to-patient variability without drug additivity or synergy. Cell. 171(7):1678–1691.e13. doi: 10.1016/j.cell.2017.11.009.
- Pasiarski M, Grywalska E, Kosmaczewska A, Góźdź S, Roliński J. 2013. The frequency of myeloid and lymphoid dendritic cells in multiple myeloma patients is inversely correlated with disease progression. Postepy Hig Med Dosw (Online). 67:926–932. doi: 10.5604/17322693.1065871.
- Pérez-Martínez A, Valentín J, Fernández L, Hernández-Jiménez E, López-Collazo E, Zerbes P, Schwörer E, Nuñéz F, Martín IG, Sallis H, et al. 2015. Arabinoxylan rice bran (MGN-3/Biobran) enhances natural killer cell-mediated cytotoxicity against neuroblastoma in vitro and in vivo. Cytotherapy. 17(5):601–612. doi: 10.1016/j.jcyt.2014.11.001.
- Pescatore FM, Přestáno C, Kichikova M. 2022. RBAC and its role with the immune system. Altern Ther Health Med. 28(1):8–10.
- Petrovics G, Szigeti G, Hamvas S, Máté Á, Betlehem J, Hegyi G. 2016. Controlled pilot study for cancer patients suffering from chronic fatigue syndrome due to chemotherapy treated with BioBran (MGN-3Arabinoxylane) and targeted radiofrequency heat therapy. Eur J Integr Med. 8:29–35. doi: 10.1016/j.eujim.2016.10.004.
- Pfeffer CM, Singh ATK. 2018. Apoptosis: a target for anticancer therapy. IJMS. 19(2):448. doi: 10.3390/ijms19020448.
- Phillips DH, Arlt VM. 2009. Genotoxicity: damage to DNA and its consequences. In: Luch A, editor. Molecular, clinical and environmental toxicology. Basel (Switzerland): Birkhäuser Basel; p. 87–110.
- Pistritto G, Trisciuoglio D, Ceci C, Garufi A, D’Orazi G. 2016. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging. 8(4):603–619. doi: 10.18632/aging.100934.
- Raskov H, Orhan A, Christensen JP, Gögenur I. 2021. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br J Cancer. 124(2):359–367. doi: 10.1038/s41416-020-01048-4.
- Redza-Dutordoir M, Averill-Bates DA. 2016. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta Mol Cell Res. 1863(12):2977–2992. doi: 10.1016/j.bbamcr.2016.09.012.
- Riggan L, Shah S, O’Sullivan TE. 2021. Arrested development: suppression of NK cell function in the tumor microenvironment. Clin Transl Immunology. 10(1):e1238.
- Robb KA, Simon AE, Miles A, Wardle J. 2014. Public perceptions of cancer: a qualitative study of the balance of positive and negative beliefs. BMJ Open. 4(7):e005434–e005434. doi: 10.1136/bmjopen-2014-005434.
- Rosenberg J, Huang J. 2018. CD8+ T cells and NK cells: parallel and complementary soldiers of immunotherapy. Curr Opin Chem Eng. 19:9–20. doi: 10.1016/j.coche.2017.11.006.
- Salako O, Okunade KS, Adeniji AA, Fagbenro GT, Afolaranmi OJ. 2021. Chemotherapy induced neutropenia and febrile neutropenia among breast cancer patients in a tertiary hospital in Nigeria. ecancer. 15:1188. doi: 10.3332/ecancer.2021.1188.
- Schegoleva AA, Khozyainova AA, Gerashchenko TS, Zhuikova LD, Denisov EV. 2022. Metastasis prevention: targeting causes and roots. Clin Exp Metastasis. 39(4):505–519. doi: 10.1007/s10585-022-10162-x.
- Schmidt M, Lügering N, Pauels H-G, Schulze-Osthoff K, Domschke W, Kucharzik T. 2000. IL-10 induces apoptosis in human monocytes involving the CD95 receptor/ligand pathway. Eur J Immunol. 30(6):1769–1777. doi: 10.1002/1521-4141(200006)30:6<1769::AID-IMMU1769>3.0.CO;2-9.
- Shalapour S, Karin M. 2015. Immunity, inflammation, and cancer: an eternal fight between good and evil. J Clin Invest. 125(9):3347–3355. doi: 10.1172/JCI80007.
- Shamekhi S, Abdolalizadeh J, Ostadrahimi A, Mohammadi SA, Barzegari A, Lotfi H, Bonabi E, Zarghami N. 2020. Apoptotic effect of Saccharomyces cerevisiae on human colon cancer sw480 cells by regulation of Akt/NF-ĸB signaling pathway. Probiotics & Antimicro Prot. 12(1):311–319. doi: 10.1007/s12602-019-09528-7.
- Shankar MG, Swetha M, Keerthana CK, Rayginia TP, Anto RJ. 2022. Cancer chemoprevention: a strategic approach using phytochemicals. Front Pharmacol. 12:809308.
- SjØgren K, Jacobsen KA, GrØnberg BH, Halvorsen TO. 2020. Timing of severe toxicity from chemotherapy in patients with lung cancer. Anticancer Res. 40(11):6399–6406. doi: 10.21873/anticanres.14661.
- Sojka DK, Huang Y-H, Fowell DJ. 2008. Mechanisms of regulatory T-cell suppression – a diverse arsenal for a moving target. Immunology. 124(1):13–22. doi: 10.1111/j.1365-2567.2008.02813.x.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. 2021. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71(3):209–249. doi: 10.3322/caac.21660.
- Takahara K, Sano K. 2004. The life prolongation and QOL improvement effect of rice bran arabinoxylan derivative (MGN-3, Biobran) for progressive cancer. Clin Pharmacol Ther . 14:267–271.
- Tan DFS, Flores JAS. 2020. The immunomodulating effects of arabinoxylan rice bran (Lentin) on hematologic profile, nutritional status and quality of life among head and neck carcinoma patients undergoing radiation therapy: a double blind randomized control trial. Radiol J. 12:11–16.
- Tazawa K, Namikawa H, Oida N, Itoh K, Yatsuzuka M, Koike J, Masada M, Maeda H. 2000. Scavenging activity of MGN-3 (arabinoxylane from rice bran) with natural killer cell activity on free radicals. Biotherapy. 14:493–495.
- Togashi Y, Shitara K, Nishikawa H. 2019. Regulatory T cells in cancer immunosuppression—implications for anticancer therapy. Nat Rev Clin Oncol. 16(6):356–371. doi: 10.1038/s41571-019-0175-7.
- Tsao AS, Kim ES, Hong WK. 2004. Chemoprevention of cancer. CA Cancer J Clin. 54(3):150–180. doi: 10.3322/canjclin.54.3.150.
- Wang LH, Wu CF, Rajasekaran N, Shin YK. 2018. Loss of tumor suppressor gene function in human cancer: an overview. Cell Physiol Biochem. 51(6):2647–2693. doi: 10.1159/000495956.
- Weston A, Harris CC. 2003. Multistage carcinogenesis. In Kufe DW, Pollock RE, Weichselbaum RR., editors. Holland-Frei cancer medicine. 6th ed. Hamilton (ON):B.C. Decker.
- Wilson BE, Jacob S, Yap ML, Ferlay J, Bray F, Barton MB. 2019. Estimates of global chemotherapy demands and corresponding physician workforce requirements for 2018 and 2040: a population-based study. Lancet Oncol. 20(6):769–780. doi: 10.1016/S1470-2045(19)30163-9.
- Wu Z, Li S, Zhu X. 2021. The mechanism of stimulating and mobilizing the immune system enhancing the anti-tumor immunity. Front Immunol. 12:682435. doi: 10.3389/fimmu.2021.682435.
- Ye L, Zhang F, Li H, Yang L, Lv T, Gu W, Song Y. 2017. Circulating tumor cells were associated with the number of T lymphocyte subsets and NK cells in peripheral blood in advanced non-small-cell lung cancer. Dis Markers. 2017:1–6. doi: 10.1155/2017/5727815.
- Ye Y, Gaugler B, Mohty M, Malard F. 2020. Plasmacytoid dendritic cell biology and its role in immune-mediated diseases. Clin Transl Immunology. 9(5):e1139.
- Yu Y, Zhang J, Wang J, Sun B. 2019. The anti-cancer activity and potential clinical application of rice bran extracts and fermentation products. RSC Adv. 9(31):18060–18069. doi: 10.1039/C9RA02439E.
- Zabor EC, Kaizer AM, Hobbs BP. 2020. Randomized controlled trials. Chest. 158(1):S79–S87. doi: 10.1016/j.chest.2020.03.013.
- Zhao X, Liu J, Ge S, Chen C, Li S, Wu X, Feng X, Wang Y, Cai D. 2019. Saikosaponin A inhibits breast cancer by regulating Th1/Th2 balance. Front Pharmacol. 10:624. doi: 10.3389/fphar.2019.00624.
- Zhao Z, Cheng W, Qu W, Wang K. 2020. Arabinoxylan rice bran (MGN-3/Biobran) alleviates radiation-induced intestinal barrier dysfunction of mice in a mitochondrion-dependent manner. Biomed Pharmacother. 124:109855. doi: 10.1016/j.biopha.2020.109855.
- Zhou B, Lawrence T, Liang Y. 2021. The role of plasmacytoid dendritic cells in cancers. Front Immunol. 12:749190. doi: 10.3389/fimmu.2021.749190.