 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Context
Menhaden fish oil (FO) is widely recognized for inhibiting neuroinflammatory responses and preserving brain function. Nevertheless, the mechanisms of FO influencing brain cognitive function in diabetic states remain unclear.
Objective
This study examines the potential role of FO in suppressing LPS-induced neuroinflammation and cognitive impairment in diabetic animals (DA).
Materials and methods
Thirty male Wistar rats were divided into 5 groups: i) DA received LPS induction (DA-LPS); ii) DA received LPS induction and 1 g/kg FO (DA-LPS-1FO); iii) DA received LPS induction and 3 g/kg FO (DA-LPS-3FO); iv) animals received normal saline and 3 g/kg FO (NS-3FO) and v) control animals received normal saline (CTRL). Y-maze test was used to measure cognitive performance, while brain samples were collected for inflammatory markers and morphological analysis.
Results
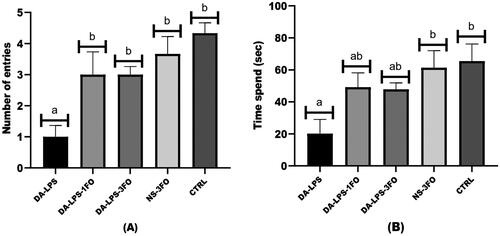
DA received LPS induction, and 1 or 3 g/kg FO significantly inhibited hyperglycaemia and brain inflammation, as evidenced by lowered levels of pro-inflammatory mediators. Additionally, both DA-LPS-1FO and DA-LPS-3FO groups exhibited a notable reduction in neuronal damage and glial cell migration compared to the other groups. These results were correlated with the increasing number of entries and time spent in the novel arm of the Y-maze test.
Discussion and conclusion
This study indicates that supplementation of menhaden FO inhibits the LPS signaling pathway and protects against neuroinflammation, consequently maintaining cognitive performance in diabetic animals. Thus, the current study suggested that fish oil may be effective as a supporting therapy option for diabetes to avoid diabetes-cognitive impairment.
Introduction
Long-term high glucose concentrations in the blood, referred to as hyperglycaemia, have been linked to a variety of organ and tissue damage, including the brain tissue (Berbudi et al. Citation2020). The necessity of glucose as the principal energy source for the normal functioning of the mammalian brain is indisputable (Takada et al. Citation2021). Glucose regulation is extremely strict, and if blood sugar levels are not treated or maintained, it can cause various physiological and pathophysiological changes leading to neurological disorders (Luna et al. Citation2021). In fact, a study claimed that neurodegenerative diseases and neuroinflammation could be controlled by managing blood glucose levels (Hung et al. Citation2022). As several theories have pointed out, there is a strong link between diabetes, brain inflammation, and the risk of developing neurodegenerative disorders (De Felice and Ferreira Citation2014; Pugazhenthi et al. Citation2017; Rom et al. Citation2019; De Sousa et al. Citation2020).
It is widely recognized that diabetic encephalopathy, a condition characterized by brain injury caused by diabetes mellitus, has the potential to impact the central nervous system, leading to cognitive impairment and motor dysfunctions (McCrimmon et al. Citation2012; Muramatsu Citation2020). The exact mechanism underlying this condition is complex and still not completely comprehended. However, a previous study reported that diabetic encephalopathy is associated with the presence of glucolipotoxicity, chronic inflammation, and oxidative stress burden (Díaz-Gerevini et al. Citation2019). Meanwhile, brain cells are vulnerable to oxidative stress, and the reactive oxygen species (ROS) generated in the brain may contribute to various neurodegenerative disorders (Anwar Citation2022). Therefore, it is essential to maintain blood sugar levels to avoid brain inflammation to prevent or slow down diabetic encephalopathy progression.
Generally speaking, insulin has served as the primary treatment for regulating glucose levels in the bloodstream (Mesa Citation2015). Regrettably, insulin has unavoidable side effects, including the destruction of pancreatic cells (Harrison Citation2021), the occurrence of severe hypoglycemia (Karges et al. Citation2017), and the disruption of brain insulin signaling (Crabtree et al. Citation2016). Furthermore, it has been recognized that consuming an adequate amount of fish oil, which is high in omega-3 fatty acids, helps to preserve the brain’s insulin signaling pathways (Bhatia et al. Citation2011), prevent insulin resistance (Agrawal and Gomez-Pinilla Citation2012), inhibit neuroinflammation and cognitive dysfunction (Lu et al. Citation2010; Devassy et al. Citation2016). While numerous studies have demonstrated the evidence of omega-3 fatty acid’s ability to successfully decrease brain inflammation and disturbance, its effectiveness in restoring blood glucose levels in individuals with diabetes is still uncertain and inconsistent. Several studies have suggested that omega-3 fatty acids have antidiabetic properties (Iwase et al. Citation2015; Laubertová et al. Citation2017). However, other studies have reported either less or no impact (Brown et al. Citation2019; Gao et al. Citation2020), or even an elevated risk of developing diabetes (Hu et al. Citation2022). Additionally, prolonged hyperglycaemia will promote abnormal expression of apoptosis-associated genes and inflammatory cytokines expression, leading to the initiation of neuronal death (Wang et al. Citation2021). This implies that keeping normal blood sugar levels is beneficial for maintaining good brain function.
To the best of our knowledge, the anti-inflammatory and neuroprotective potential of dietary menhaden fish oil supplementation associated with diabetes has not yet been explored. Thus, our objective was to examine the impact of fish oil supplementation on cellular and molecular changes in the brain, as well as cognitive performance in diabetic rats with LPS-induced brain inflammation. It has been established that the LPS induces systemic neuroinflammation, resulting in cognitive impairment (Batista et al. Citation2019). As a result, the systemic inflammatory response increases the synthesis and release of cytokines into the circulatory system, which may impact brain function (Perry Citation2004).
Materials and methods
Animals
Wistar rats (n = 30, male, 8 weeks) were purchased from Prima Nexus company, Malaysia and Anilab company (Indonesia). Rats were acclimatized for seven days and were given feed and water ad libitum. Rats were maintained in group cages (n = 3/cage) with ambient temperatures of around 20-26 °C. All animal procedures and experiments handling were performed according to the protocol of the Institutional for Animal Care and Use Committee (IACUC), Universiti Putra Malaysia, Selangor, Malaysia (UPM/IACUC/AUP-R017/2022) and also approved by the Research Ethics Committee of Universitas Brawijaya, Malang, Indonesia (070 KEP-UB-2022).
Experimental induction of diabetes
Streptozotocin (STZ) (Cat no: SC-200719) was purchased from Santa Cruz Biotechnology (Aspire Biosains PLT, Malaysia). In the fasting condition, the animals were injected with STZ at 45 mg/kg/day (i.p) in 0.5 mL of 10 mM citrate buffer (pH 5.5) for three days continuously. The blood glucose was measured using a blood glucometer (Gluco Dr, South Korea) from the withdrawn tail blood vein. Animals were considered as hyperglycaemia or diabetic state if the blood glucose level at or more than 250 mg/dL (Gholamhosseinian et al. Citation2020).
Experimental induction of neuroinflammation
Lipopolysaccharide (LPS) (Cat no: L2630) was purchased from Sigma-Aldrich Company (Aspire Biosains PLT, Malaysia). The animals were injected with a dilution of LPS (250 µg/kg) and normal saline by intraperitoneal (i.p) injection for seven days (Mahdi et al. Citation2019).
Fish oil treatment
Menhaden fish oil (Cat no: F8020) was purchased from Sigma-Aldrich Company (Aspire Biosains PLT, Malaysia). The fatty acid composition of menhaden fish oil is shown in .
Table 1. Fatty acid composition of menhaden fish oil (sigma aldrich, USA).
Experimental design
A total of eighteen (n = 18) adult Wistar rats were injected with STZ to develop hyperglycaemia for three days. Next, these animals were induced with LPS for brain inflammation development for seven days. The animals were then randomly divided into three groups; i) diabetic animals with LPS induction (n = 6; DA-LPS); ii) diabetic animals with LPS induction and treated with 1 g/kg fish oil (n = 6; DA-LPS-1FO); iii) diabetic animals with LPS induction and treated with 3 g/kg fish oil (n = 6; DA-LPS-3FO). Another two groups of animals were selected as control; iv) animals received normal saline and treated with 3 g/kg FO (n = 6; NS-3FO) and v) control animals received normal saline (n = 6; CTRL). After 30 days of fish oil supplementation by oral gavage, all animals were assessed for cognitive performance using a Y-maze test. At the end of the test, all animals were euthanized by injection of sodium pentobarbital at 100 mg/kg and brain organs were collected for histological examination and analysis of inflammation markers.
Cognitive performance by Y-maze test
The Y-maze apparatus was constructed from acrylic plastic and consisted of three arms: start arm, familiar arm, and novel arm, with an angle of 120° between each two arms. Each arm has a similar measurement: 60 cm in length, 15 cm in width, and 23 cm in heigh. The Y-maze test had two trials separated by an interval of 60 min. In the first trial, the animal was placed individually in the start arm for a duration of 10 min, with the novel arm being blocked. Subsequently, the animal was extracted from the Y-maze equipment and allowed to rest for a duration of 60 min. In the second trial, the animal had unrestricted access to all three arms and spent 5 min exploring the novel arm, which was not obstructed. Trials were documented through the utilization of a camera mounted on the ceiling, and subsequently, the recordings were reviewed to determine the quantity of entries and the duration spent in the novel arm. The higher cognitive performance of animals is demonstrated by the greater number of entries and increased time spent in the novel arm (Hafandi et al. Citation2014; Sopian et al. Citation2015).
Relative cerebrum weight
The rat brain organ was isolated from the skull and washed with saline solution to remove excess blood. Later on, the weight of the cerebrum part was recorded using digital weight scale. The relative organ weights of the animals were calculated as:
Brain histology analysis
The left hemisphere of the cerebrum was thereafter preserved in a solution of neutral buffered formalin (NBF) at a concentration of 10% for a minimum duration of 24 h. Following fixation, the tissue samples were adjusted to the appropriate size and orientation and then inserted into the organ cassette. The samples were further dehydrated by subjecting it to a sequence of alcohol concentrations (70%, 80%, 85%, 90%, 95%, 100% ethanol). Samples were then treated with xylol to remove any remaining impurities, followed by paraffinization with paraffin. Finally, the samples were embedded and sectioned. The paraffin sections, which had a thickness of 4 μm, were subsequently treated to remove the paraffin and then stained using the hematoxylin-eosin (HE) method. The Optilab Camera Microscope (Software Image Raster) was used to capture photomicrograph images at magnifications of 10Ó¿ and 40Ó¿ objective lens magnification.
Brain inflammation markers analysis
The flow cytometry approach was used to evaluate the expression of tumour necrosis factor-α (TNF-α) and interleukin-6 (IL-6) cytokines in the cerebrum homogenates. The right hemisphere of the brain was crushed in 1 mL phosphate-buffered saline (PBS) solution. The specimen mixture was inserted into the microtube and underwent centrifugation at a speed of 2500 revolutions per min (rpm) for 5 min at a temperature of 10 °C. Following the removal of the supernatant, 50 µL of fixation buffer (Cat no: 42001, Biolegend) was introduced and left to incubate at a temperature of 4 °C in a dark environment for a duration of 20 min. Subsequently, 500 µL of intracellular staining permeabilization wash solution (Catalogue number: 421002, Biolegend) was introduced, followed by centrifugation at 2500 rpm for 5 min at 10 °C. The supernatant was discarded, and the pellet was treated with 50 μL of antibodies TNF-α (Cat no: TN3-19.12, BioLegend) or IL-6 (Cat no: bs-0379R, Bioss antibodies). The mixture was then kept at a temperature of 4 °C in a dark environment for 20 min. Following the incubation period, 400 μL of PBS was added to each sample, which was subsequently placed into a flow cytometry cuvette for analysis.
Statistical analysis
The collected data were analyzed using SPPS 20.0 software. The results were provided as mean ± SEM, except for the study of brain histology. A one-way ANOVA was used to compare all groups, followed by the Tukey post hoc test. The P value at p < 0.05 was considered to be a significant difference between groups.
Results
Effects of fish oil on blood glucose level of diabetic animals with LPS induction
displays the blood glucose levels of different groups of animals. All diabetic rats that were induced with LPS (DA-LPS; DA-LPS-1FO and DA-LPS-3FO) expressed high blood glucose levels (>250 mg/dL) on day 3. Further, on the 30 days following the injection of STZ, all diabetic animals showed a reduction in blood glucose levels. Still, the blood glucose levels of the DA-LPS group remained above 250 mg/dL (259 ± 54.40), showing that the animals were still in a diabetic state. While the blood glucose levels of CTRL and NS-3FO (88.83 ± 8.73 and 68.67 ± 4.29, respectively) are not relatively similar to those of DA-LPS-1FO (135.17 ± 30.91). Nevertheless, both DA-LPS-1FO and DA-LPS-3FO groups showed a significant decrease in comparison to the DA-LPS group.
Table 2. The blood glucose levels (mg/dL) of different groups of animals after 3 and 30 days.
Effects of fish oil on cognitive performance of diabetic animals with LPS induction
displays the outcome of cognitive performance as assessed by the Y-maze test. Diabetic animals with LPS induction (DA-LPS group) had the lowest number of novel arm entries and time spent in the novel arm compared to the NS-3FO and CTRL groups. On the other hand, the groups DA-LPS-1FO and DA-LPS-3FO exhibited a significant rise in the number of entries into the novel arm and the duration of time spent in the novel arm after three weeks of administering fish oil (at doses of 1 and 3 g/kg) in comparison to the DA-LPS group (as seen in -A and 1-B).
Figure 1. The Y-maze performance of diabetic animals with LPS and fish oil supplementation; (A) the number of entries and (B) The time spent in the novel arm. The result is expressed as means ± SEM. *significant difference (p < 0.001) compared to the CTRL group, using one-way ANOVA followed by the tukey test.
Effects of fish oil on brain relative weight and histopathology of diabetic animals with LPS induction
The cerebrum weight did not differ among the treatments, while the relative cerebrum weight of the DA-LPS group was significantly higher (p < 0.05) compared with the CTRL and NS-3FO groups (). Likewise, the DA-LPS-1FO showed significant differences in relative cerebrum weight when compared to both the normal and fish oil control rats (p < 0.05). Meanwhile, the DA-LPS-3FO group did not exhibit any statistically significant differences (p > 0.05) in terms of the relative weight of the cerebrum compared to the NS-3FO and CTRL groups.
Table 3. The weight and relative weight of the cerebrum between different groups.
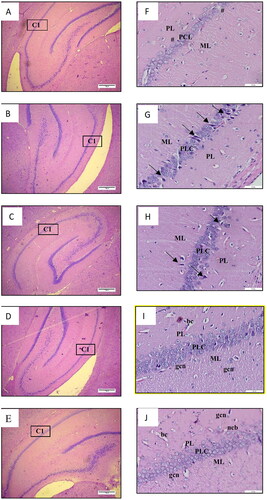
displays the histological analysis of the cerebral cortex, while presents the observations of the hippocampal region referred to as cornu ammonis 1 (CA 1). The results of the histological examination revealed normal brain structure in both the NS-3FO and CTRL groups. The cerebral cortex exhibited dispersed nuclei consisting of both neurons and glial cells, whereas the CA 1 region of the hippocampus displayed 5-8 densely packed layers of pyramidal neurons in pyramidal cell layers (PCL). By contrast, the DA-LPS group exhibited noticeable neuronal necrosis in the cerebral cortex, characterized by the presence of dispersed degenerative neurons with pycnotic nuclei. This was followed by an increase in glial cells and dilatation of blood vessels. In a comparable manner, the CA 1 area of the hippocampus of DA-LPS group exhibited a reduction in the thickness of the PCL. Interestingly, the DA-LPS-1FO and DA-LPS-3FO exhibited a reduction in the number of deteriorated neurons and glial cells in the cortex. Additionally, in the CA 1 region, almost all pyramidal cell bodies were distributed normally, and the number of layers in the PCL approached the normal range.
Figure 2. A-E Low magnification image of the cerebral cortex (10×, scale bar 200 µm), and F-J high magnification image of the cerebral cortex (40×, scale bar 20 µm) of HE staining from rat cerebrum. The DA-LPS group (F) shows scattered degenerating neurons with pyknotic nuclei, a large number of glial cells and dilated blood capillaries. The DA-LPS-1FO (G), and DA-LPS-3FO (H) showed fewer degenerating neurons with pyknotic nuclei (long arrow), a smaller number of glial cells, and slightly dilated blood capillaries. Meanwhile, the NS-3FO group (I) and CTRL group (J) showed no histopathological changes. ncb: neuron cell bodies; gcl: glial cell nuclei; bc: blood capilaries.
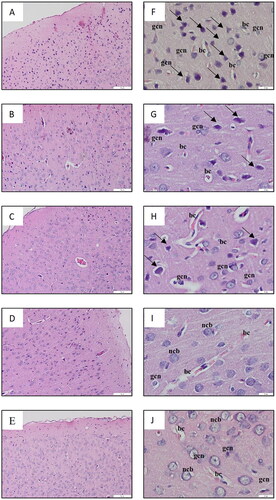
Figure 3. A-E Low magnification image of the hippocampus (4×, scale bar 200 µm) and F-J high magnification image of the hippocampus (20×, scale bar 50 µm) of HE staining from rat cerebrum. DA-LPS group (F) showed decreased of the PCL thickness (#). DA-LPS groups treated with 1 g/kg fish oil (G) and 3 g/kg fish oil (H) showed near normal thickness of the PCL with few degenerating neurons (black arrow). Meanwhile, the pyramidal neurons on the PCL formed a compact layer in the NA-3FO group (I) and the CTRL (J). ML: molecular layer, PCL: pyramidal cell layer, PL: Polymorphic layer, ncb: neuron cell bodies; gcl: glial cell nuclei; bc: blood capilaries.
Effects of fish oil on the inflammatory mediators of diabetic animals with LPS induction
The inflammatory markers expression of TNF-α and IL-6 in different groups are presented in . The result showed that the inflammatory mediators of TNF-α and IL-6 in DA-LPS group was significantly higher (p < 0.001) compared to animals treated with 1 or 3 g/kg of fish oil supplementation (e.g., NS-3FO, DA-LPS-1FO and DA-LPS-3FO) and control group (CTRL).
Table 4. The Inflammatory markers expression of TNF-α and IL-6 in different groups.
Discussion
The current study shows that the combinatorial effects of STZ-induced hyperglycaemia and LPS-induced systemic inflammation in aggravating such effects. Diabetic animals with LPS-induced inflammation and treated with fish oil at either 1 or 3 g/kg for duration of 21 days had notably lower blood glucose levels. It has been established that fish oil administration showed an anti-hyperglycemic effect in both in vivo (Jangale et al. Citation2013; Parveen et al. Citation2019; Kobyliak et al. Citation2020) and in vitro (Laubertová et al. Citation2017; Das et al. Citation2022) studies. Indeed, fish oil has been found to possess antioxidant and anti-inflammatory properties (Erdogan et al. Citation2004; Purdel et al. Citation2021), which may help in preventing pancreatic histological alterations (Bellenger et al. Citation2011; Habib Citation2013), improving insulin production (Soltan Citation2012) and enhancing insulin sensitivity (Keapai et al. Citation2016). In addition, the present study demonstrated that diabetic rats receiving LPS-induced inflammation had a higher relative brain weight in comparison to the control groups. Presumably, the justification for this is that the hyperglycaemia generated by STZ may lead to significant weight loss as a result of insufficient insulin (Ventura-Sobrevilla et al. Citation2011; Hikmah et al. Citation2015; Samsulrizal et al. Citation2021), which triggers the breakdown of body fat and protein for energy production (Ghule et al. Citation2010). Previous study demonstrated that weight loss does not directly impact brain weight, but it does lead to a higher brain-to-body weight ratio (Sellers et al. Citation2007).
The development of hyperglycaemia and systemic brain inflammation complications in this study were evident from increasing cytokine pro-inflammation, altered brain tissue, and influence the cognitive performance in diabetic animals with LPS-induced inflammation. The current study discovered that diabetic animals with LPS-induced brain inflammation had higher concentrations of TNF-α and IL-6. This chronic inflammation in the brain ultimately impaired the animal’s cognitive function, as indicated by a reduced number of entries and time spent in the novel arm of the Y-maze test. Research has confirmed that prolonged inflammation in the brain caused by diabetes can activate many metabolic pathways, leading to impairments in cognitive abilities including as learning, memory, and spatial navigation (Gupta et al. Citation2023). Meanwhile, according to several studies, this memory impairment can also be caused by LPS injection (Zhu et al. Citation2014; Anaeigoudari et al. Citation2015; Zakaria et al. Citation2017). A study conducted on Sprague-Dawley diabetic rats revealed that the introduction of LPS caused a disturbance in insulin signaling, leading to an immediate inflammatory response and decreased spatial learning and memory (Murtishaw Citation2014).
Nevertheless, the present study found that diabetic animals receiving LPS induction and treated with fish oil supplementation had significantly lower levels of inflammation markers (e.g., TNF-α and IL-6) and improved cognitive performance in comparison to diabetic animals given LPS only. The cognitive performance was enhanced, as indicated by the increased number of entries in the novel arms relative to the other arms of the Y-maze test. Indeed, previous studies have mentioned that fish oil supplementation improves learning and memory performance in diabetic mice (Yang et al. Citation2012; Wang et al. Citation2020). This could indicate that cognitive impairment was closely related to brain inflammation due to the induction of LPS in diabetic rats. In addition, a study conducted on animal models utilizing diabetic and obese rats discovered a correlation between neuroinflammation and memory decline. The study observed a decrease in spatial memory and an increase in levels of pro-inflammatory cytokines (Dinel et al. Citation2011).
Physiologically, hyperglycaemia will activate the PKC pathway, which in turn activates the NF-kB pathway, resulting in neuroinflammation (Gupta et al. Citation2023). The findings indicated that fish oil, which contains higher levels of omega-3 fatty acids, has anti-inflammatory properties that can effectively suppress brain inflammation (Asari et al. Citation2019; Wang et al. Citation2020). Previous studies reported that omega-3 fatty acids can effectively suppress brain inflammatory responses by inhibiting microglial cell reactivity (Inoue et al. Citation2017; Gholamhosseinian et al. Citation2020). It is widely known that the overactivation of microglia and astrocytes will stimulate NF-ĸB molecules within the cells. This, in turn, results in the production of several cytotoxic substances, including superoxide radicals and pro-inflammatory mediators such as TNF-α, IL-1, and IL-6 (Kwon and Koh Citation2020; Rauf et al. Citation2022). Yet, the omega-3 fatty acids modulate microglial cells by inhibiting the activation of p38 MAPK (Lu et al. Citation2013), reducing the production of inflammatory molecules, and activating peroxisome proliferator-activated receptor γ (PPARγ) (Ajmone-Cat et al. Citation2012).
Finally, our research confirmed that the administration of fish oil at doses of 1 and/or 3 g/kg in diabetic animals with LPS-induced inflammation effectively decreased the migration of glial cells and the degeneration of neurons in the cerebral cortex of the brain. The same outcomes were also observed in the hippocampus, a region of the brain that has been extensively studied for its role in memory and learning (Anand and Dhikav Citation2012). The CA 1, which is the biggest region in the hippocampus, is commonly employed as a model system for investigating plasticity, pharmacological effects, and intracellular characteristics (Mizuseki et al. Citation2011). The histological examination of the CA 1 region revealed less severe neuronal damage in diabetic animals that received fish oil supplementation together with LPS, compared to diabetic animals which received LPS without fish oil supplementation. This study believed that the Y-maze outcome, which demonstrated an improvement in the rat’s spatial learning and memory, is directly related to the ameliorative role of fish oil in preventing brain tissue damage. Moreover, this study assumed that the histological findings were strongly correlated with the increasing levels of pro-inflammatory mediators in diabetic animals receiving LPS induction but without fish oil supplementation. Diabetes has the ability to release pro-inflammatory mediators that can modify the brain’s structure (Gaspar et al. Citation2016). Furthermore, the increase in pro-inflammatory mediators may provoke and intensify neurodegeneration, resulting in tissue damage (Glass et al. Citation2010; Konsman Citation2022). Taken together, the histopathological findings in this study support previous research (Afshordel et al. Citation2015) that claims fish oil rich in omega-3 fatty acids provides neuroprotection. Indeed, beyond its well-known anti-inflammatory and neuroprotective effects, omega-3 fatty acids also prolong neuronal cell longevity, reduce neurodegeneration, and prevent cognitive impairment (Kim Citation2014).
Conclusions
This study highlights the potential of menhaden fish oil in mitigating hyperglycaemia, neuroinflammation, brain morphological changes, and cognitive dysfunction in diabetic rats induced with LPS. Thus, our findings promote the regular intake of fish oil as an attempt to preserve brain health and function to avoid the cognitive impairment associated with diabetes. Nonetheless further clinical trial research is required to provide additional evidence for the beneficial effects of fish oil in preventing diabetic brain disruption.
Authors’ contributions
Nurina Titisari: conceptualization, performing experiment, data curation, methodology, formal analysis, writing-original draft. Ahmad Fauzi: analysis, editing. Intan Shameha Abdul Razak: conceptualization, methodology, supervision. Mohd Hezmee Mohd Noor: methodology, supervision. Nurdiana Samsulrizal: conceptualization, methodology, supervision. Hafandi Ahmad: conceptualization, data curation, methodology, validation, project administration, supervision, review, and editing. Authors agree to be accountable for all aspects of work, ensuring integrity and accuracy.
Acknowledgments
The authors would like to thank the Universiti Putra Malaysia. The authors also would like to express gratitude to the Faculty of Veterinary Medicine, Universitas Brawijaya, for supporting this study.
Disclosure statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Funding
References
- Afshordel S, Hagl S, Werner D, Röhner N, Kögel D, Bazan NG, Eckert GP. 2015. Omega-3 polyunsaturated fatty acids improve mitochondrial dysfunction in brain aging - impact of Bcl-2 and NPD-1 like metabolites. Prostaglandins Leukot Essent Fatty Acids. 92:23–31. doi: 10.1016/j.plefa.2014.05.008.
- Agrawal R, Gomez-Pinilla F. 2012. ‘Metabolic syndrome’ in the brain: deficiency in omega-3 fatty acid exacerbates dysfunctions in insulin receptor signalling and cognition. J Physiol. 590(10):2485–2499. doi: 10.1113/jphysiol.2012.230078.
- Ajmone-Cat MA, Salvatori ML, De Simone R, Mancini M, Biagioni S, Bernardo A, Cacci E, Minghetti L. 2012. Docosahexaenoic acid modulates inflammatory and antineurogenic -functions of activated microglial cells. J Neurosci Res. 90(3):575–587. doi: 10.1002/jnr.22783.
- Anaeigoudari A, Shafei MN, Soukhtanloo M, Sadeghnia HR, Reisi P, Beheshti F, Mohebbati R, Mousavi SM, Hosseini M. 2015. Lipopolysaccharide-induced memory impairment in rats is preventable using 7-nitroindazole. Arq Neuropsiquiatr. 73(9):784–790. doi: 10.1590/0004-282X20150121.
- Anand K, Dhikav V. 2012. Hippocampus in health and disease: an overview. Ann Indian Acad Neurol. 15(4):239–246. doi: 10.4103/0972-2327.104323.
- Anwar MM. 2022. Oxidative stress-A direct bridge to central nervous system homeostatic dysfunction and Alzheimer’s disease. Cell Biochem Funct. 40(1):17–27. doi: 10.1002/cbf.3673.
- Asari MA, Zulkaflee MH, Sirajudeen KNS, Mohd Yusof NA, Mohd Sairazi NS. 2019. Tualang honey and DHA-rich fish oil reduce the production of pro-inflammatory cytokines in the rat brain following exposure to chronic stress. J Taibah Univ Med Sci. 14(4):317–323. doi: 10.1016/j.jtumed.2019.06.004.
- Batista CRA, Gomes GF, Candelario-Jalil E, Fiebich BL, de Oliveira ACP. 2019. Lipopolysaccharide-induced neuroinflammation as a bridge to understand neurodegeneration. IJMS. 20(9):2293. doi: 10.3390/ijms20092293.
- Bellenger J, Bellenger S, Bataille A, Massey KA, Nicolaou A, Rialland M, Tessier C, Kang JX, Narce M. 2011. High pancreatic n-3 fatty acids prevent stz-induced diabetes in fat-1 mice: inflammatory pathway inhibition. Diabetes. 60(4):1090–1099. doi: 10.2337/db10-0901.
- Berbudi A, Rahmadika N, Tjahjadi AI, Ruslami R. 2020. Type 2 diabetes and its impact on the immune system. Curr Diabetes Rev. 16(5):442–449. doi: 10.2174/1573399815666191024085838.
- Bhatia HS, Agrawal R, Sharma S, Huo YX, Ying Z, Gomez-Pinilla F. 2011. Omega-3 fatty acid deficiency during brain maturation reduces neuronal and behavioral plasticity in adulthood. PLOS One. 6(12):e28451. doi: 10.1371/journal.pone.0028451.
- Brown TJ, Brainard J, Song F, Wang X, Abdelhamid A, Hooper L,. 2019. Omega-3, omega-6, and total dietary polyunsaturated fat for prevention and treatment of type 2 diabetes mellitus: systematic review and meta-analysis of randomised controlled trials. BMJ. 366:l4697. doi: 10.1136/bmj.l4697.
- Crabtree T, Altaan S, Tarik A. 2016. Early-onset dementia in type 1 diabetes mellitus. Pract Diab. 33(4):133–134. doi: 10.1002/pdi.2019.
- Das M, Banerjee A, Roy R. 2022. A novel in vitro approach to test the effectiveness of fish oil in ameliorating type 1 diabetes. Mol Cell Biochem. 477(8):2121–2132. doi: 10.1007/s11010-022-04424-1.
- De Felice FG, Ferreira ST. 2014. Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes. 63(7):2262–2272. doi: 10.2337/db13-1954.
- De Sousa RAL, Harmer AR, Freitas DA, Mendonça VA, Lacerda ACR, Leite HR. 2020. An update on potential links between type 2 diabetes mellitus and Alzheimer’s disease. Mol Biol Rep. 47(8):6347–6356. doi: 10.1007/s11033-020-05693-z.
- Devassy JG, Leng S, Gabbs M, Monirujjaman M, Aukema HM. 2016. Omega-3 polyunsaturated fatty acids and oxylipins in neuroinflammation and management of Alzheimer disease. Adv Nutr. 7(5):905–916. doi: 10.3945/an.116.012187.
- Díaz-Gerevini GT, Daín A, Pasqualini ME, López CB, Eynard AR, Repossi G. 2019. Diabetic encephalopathy: beneficial effects of supplementation with fatty acids ω3 and nordihydroguaiaretic acid in a spontaneous diabetes rat model. Lipids Health Dis. 18(1):43. doi: 10.1186/s12944-018-0938-7.
- Dinel AL, André C, Aubert A, Ferreira G, Layé S, Castanon N. 2011. Cognitive and emotional alterations are related to hippocampal inflammation in a mouse model of metabolic syndrome. PLoS One. 6(9):e24325. doi: 10.1371/journal.pone.0024325.
- Erdogan H, Fadillioglu E, Ozgocmen S, Sogut S, Ozyurt B, Akyol O, Ardicoglu O. 2004. Effect of fish oil supplementation on plasma oxidant/antioxidant status in rats. Prostaglandins Leukot Essent Fatty Acids. 71(3):149–152. doi: 10.1016/j.plefa.2004.02.001.
- Gao C, Liu Y, Gan Y, Bao W, Peng X, Xing Q, Gao H, Lai J, Liu L, Wang Z, et al. 2020. Effects of fish oil supplementation on glucose control and lipid levels among patients with type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Lipids Health Dis. 19(1):87. doi: 10.1186/s12944-020-01214-w.
- Gaspar JM, Baptista FI, MacEdo MP, Ambrósio AF. 2016. Inside the diabetic brain: role of different players involved in cognitive decline. ACS Chem Neurosci. 7(2):131–142. doi: 10.1021/acschemneuro.5b00240.
- Gholamhosseinian A, Abbasalipourkabir R, Ziamajidi N, Sayadi M, Sayadi K. 2020. The anti-inflammatory effect of omega-3 polyunsaturated fatty acids dramatically decreases by iron in the hippocampus of diabetic rats. Life Sci. 245:117393. doi: 10.1016/j.lfs.2020.117393.
- Ghule S, Prakash T, Kotresha D, Karki R, Surendra V, Goli D. 2010. Anti-diabetic activity of Celosia argentea root in streptozotocin-induced diabetic rats. Int J Green Pharm. 4(3):206–211. doi: 10.4103/0973-8258.69183.
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. 2010. Mechanisms underlying inflammation in neurodegeneration. Cell. 140(6):918–934. doi: 10.1016/j.cell.2010.02.016.
- Gupta M, Pandey S, Rumman M, Singh B, Mahdi AA. 2023. Molecular mechanisms underlying hyperglycaemia associated cognitive decline. IBRO Neurosci Rep. 14:57–63. doi: 10.1016/j.ibneur.2022.12.006.
- Habib EK. 2013. Possible role of Omega-3 on the pancreas of streptozotocin-induced diabetes in adult albino rats: histological and immunohistochemical study. Egypt J Histol. 36(3):579–591. doi: 10.1097/01.EHX.0000431956.27366.8f.
- Hafandi A, Begg DP, Premaratna SD, Sinclair AJ, Jois M, Weisinger RS. 2014. Dietary repletion with ω3 fatty acid or with COX inhibition reverses cognitive effects in F3 ω3 fatty-acid-deficient mice. Comp Med. 64(2):106–109.
- Harrison LC. 2021. The dark side of insulin: a primary autoantigen and instrument of self-destruction in type 1 diabetes. Mol Metab. 52:101288. doi: 10.1016/j.molmet.2021.101288.
- Hikmah N, Dewi A, Shita P, Maulana H. 2015. Diabetic blood glucose level profile with stratified dose streptozotocin (sd-stz) and multi low dose streptozotocin (mld-stz) induction methods. J Trop Life Sci. 5:30–34.
- Hu M, Fang Z, Zhang T, Chen Y. 2022. Polyunsaturated fatty acid intake and incidence of type 2 diabetes in adults: a dose response meta-analysis of cohort studies. Diabetol Metab Syndr. 14(1):34. doi: 10.1186/s13098-022-00804-1.
- Hung HC, Tsai SF, Sie SR, Kuo YM. 2022. High glucose enhances lipopolysaccharide-induced inflammation in cultured BV2 microglial cell line. Immunity, Inflamm Dis. 10:1–9.
- Inoue T, Tanaka M, Masuda S, Ohue-Kitano R, Yamakage H, Muranaka K, Wada H, Kusakabe T, Shimatsu A, Hasegawa K, et al. 2017. Omega-3 polyunsaturated fatty acids suppress the inflammatory responses of lipopolysaccharide-stimulated mouse microglia by activating SIRT1 pathways. Biochim Biophys Acta Mol Cell Biol Lipids. 1862(5):552–560. doi: 10.1016/j.bbalip.2017.02.010.
- Iwase Y, Kamei N, Takeda-Morishita M. 2015. Antidiabetic effects of omega-3 polyunsaturated fatty acids: from mechanism to therapeutic possibilities. PP. 06(03):190–200. doi: 10.4236/pp.2015.63020.
- Jangale NM, Devarshi PP, Dubal AA, Ghule AE, Koppikar SJ, Bodhankar SL, Chougale AD, Kulkarni MJ, Harsulkar AM. 2013. Dietary flaxseed oil and fish oil modulates expression of antioxidant and inflammatory genes with alleviation of protein glycation status and inflammation in liver of streptozotocin-nicotinamide induced diabetic rats. Food Chem. 141(1):187–195. doi: 10.1016/j.foodchem.2013.03.001.
- Karges B, Schwandt A, Heidtmann B, Kordonouri O, Binder E, Schierloh U, Boettcher C, Kapellen T, Rosenbauer J, Holl RW. 2017. Association of insulin pump therapy vs. insulin injection therapy with severe hypoglycemia, ketoacidosis, and glycemic control among children, adolescents, and young adults with type 1 diabetes.J Am Med Assoc. 318(14):1358–1366. doi: 10.1001/jama.2017.13994.
- Keapai W, Apichai S, Amornlerdpison D, Lailerd N. 2016. Evaluation of fish oil-rich in MUFAs for anti-diabetic and antiinflammation potential in experimental type 2 diabetic rats. Korean J Physiol Pharmacol. 20(6):581–593. doi: 10.4196/kjpp.2016.20.6.581.
- Kim HY. 2014. Neuroprotection by docosahexaenoic acid in brain injury. Mil Med. 179(11 Suppl):106–111. doi: 10.7205/MILMED-D-14-00162.
- Kobyliak N, Falalyeyeva T, Mykhalchyshyn G, Molochek N, Savchuk O, Kyriienko D, Komisarenko I. 2020. Probiotic and omega-3 polyunsaturated fatty acids supplementation reduces insulin resistance, improves glycemia and obesity parameters in individuals with type 2 diabetes: a randomised controlled trial. Obes Med. 19:100248. doi: 10.1016/j.obmed.2020.100248.
- Konsman JP. 2022. Cytokines in the brain and neuroinflammation: we didn’t starve the fire!. Pharmaceuticals . 15(2):140. doi: 10.3390/ph15020140.
- Kwon HS, Koh SH. 2020. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener. 9(1):42. doi: 10.1186/s40035-020-00221-2.
- Laubertová L, Koňariková K, Gbelcová H, Ďuračková Z, Muchová J, Garaiova I, Žitňanová I. 2017. Fish oil emulsion supplementation might improve quality of life of diabetic patients due to its antioxidant and anti-inflammatory properties. Nutr Res. 46:49–58. doi: 10.1016/j.nutres.2017.07.012.
- Lu DY, Tsao YY, Leung YM, Su KP. 2010. Docosahexaenoic acid suppresses neuroinflammatory responses and induces heme oxygenase-1 expression in BV-2 microglia: implications of antidepressant effects for omega-3 fatty acids. Neuropsychopharmacology. 35(11):2238–2248. doi: 10.1038/npp.2010.98.
- Lu Y, Zhao LX, Cao DL, Gao YJ. 2013. Spinal injection of docosahexaenoic acid attenuates carrageenan-induced inflammatory pain through inhibition of microglia-mediated neuroinflammation in the spinal cord. Neuroscience. 241:22–31. doi: 10.1016/j.neuroscience.2013.03.003.
- Luna R, Talanki Manjunatha R, Bollu B, Jhaveri S, Avanthika C, Reddy N, Saha T, Gandhi F. 2021. A comprehensive review of neuronal changes in diabetics. Cureus. 13(10):e19142. doi: 10.7759/cureus.19142.
- Mahdi O, Baharuldin MTH, Nor NHM, Chiroma SM, Jagadeesan S, Moklas MAM. 2019. Chemicals used for the induction of Alzheimer’s disease-like cognitive dysfunctions in rodents. Biomed Res Ther. 6(11):3460–3484. doi: 10.15419/bmrat.v6i11.575.
- McCrimmon RJ, Ryan CM, Frier BM. 2012. Diabetes and cognitive dysfunction. Lancet. 379(9833):2291–2299. doi: 10.1016/S0140-6736(12)60360-2.
- Mesa J. 2015. New insulin types in type 1 diabetes mellitus. Med Clin . 145(2):70–75. doi: 10.1016/j.medcle.2014.04.005.
- Mizuseki K, Diba K, Pastalkova E, Buzsáki G. 2011. Hippocampal CA1 pyramidal cells form functionally distinct sublayers. Nat Neurosci. 14(9):1174–1181. doi: 10.1038/nn.2894.
- Muramatsu K. 2020. Diabetes mellitus-related dysfunction of the motor system. Int J Mol Sci. 21(20):1–26.
- Murtishaw AS. 2014. [The effect of acute LPS-induced immune activation and brain insulin signaling disruption in a diabetic model of Alzheimer’s disease]. [UNLV Theses, Dissertations, Professional Papers, and Capstones]. p. 2199.
- Parveen K, Siddiqui WA, Arif JM, Kuddus M, Shahid SMA, Kausar MA. 2019. Evaluation of vegetables and fish oils for the attenuation of diabetes complications. Cell Mol Biol (Noisy-le-Grand). 65(7):38–45. doi: 10.14715/cmb/2019.65.7.8.
- Perry VH. 2004. The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain Behav Immun. 18(5):407–413. doi: 10.1016/j.bbi.2004.01.004.
- Pugazhenthi S, Qin L, Reddy PH. 2017. Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochim Biophys Acta Mol Basis Dis. 1863(5):1037–1045. doi: 10.1016/j.bbadis.2016.04.017.
- Purdel C, Ungurianu A, Margina D. 2021. Metabolic and metabolomic insights regarding the omega-3 PUFAs intake in type 1 diabetes mellitus. Front Mol Biosci. 8:783065. doi: 10.3389/fmolb.2021.783065.
- Rauf A, Badoni H, Abu-Izneid T, Olatunde A, Rahman MM, Painuli S, Semwal P, Wilairatana P, Mubarak MS. 2022. Neuroinflammatory markers: key indicators in the pathology of neurodegenerative diseases. Molecules. 27(10):3194. doi: 10.3390/molecules27103194.
- Rom S, Zuluaga-Ramirez V, Gajghate S, Seliga A, Winfield M, Heldt NA, Kolpakov MA, Bashkirova YV, Sabri AK, Persidsky Y. 2019. Hyperglycaemia-driven neuroinflammation compromises bbb leading to memory loss in both diabetes mellitus (dm) type 1 and type 2 mouse models. Mol Neurobiol. 56(3):1883–1896. doi: 10.1007/s12035-018-1195-5.
- Samsulrizal N, Goh YM, Ahmad H, Md Dom S, Azmi NS, NoorMohamad Zin NS, Ebrahimi M. 2021. Ficus deltoidea promotes bone formation in streptozotocin-induced diabetic rats. Pharm Biol. 59(1):66–73. doi: 10.1080/13880209.2020.1865411.
- Sellers RS, Morton D, Michael B, Roome N, Johnson JK, Yano BL, Perry R, Schafer K. 2007. Society of Toxicologic Pathology position paper: organ weight recommendations for toxicology studies. Toxicol Pathol. 35(5):751–755. doi: 10.1080/01926230701595300.
- Soltan SSAM. 2012. The effects of varieties sources of omega-3 fatty acids on diabetes in rats. FNS. 03(10):1404–1412. doi: 10.4236/fns.2012.310184.
- Sopian NFA, Ajat M, Shafie NI, Noor MHM, Ebrahimi M, Rajion MA, Meng GY, Ahmad H. 2015. Does short-term dietary omega-3 fatty acid supplementation influence brain hippocampus gene expression of zinc transporter-3? Int J Mol Sci. 16(7):15800–15810. doi: 10.3390/ijms160715800.
- Takada A, Shimizu F, Ishii Y, Ogawa M, Takao T. 2021. Roles of glucose and sucrose intakes on the brain functions measured by the working ability and Morris maze. In: Takada A and Hubertus Himmerich H, ed. Psychology and pathophysiological outcomes of eating. London, United Kingdom: IntechOpen. doi: 10.5772/intechopen.99203.
- Ventura-Sobrevilla J, Boone-Villa VD, Aguilar CN, Román-Ramos R, Vega-Ávila E, Campos-Sepúlveda E, Alarcón-Aguilar F. 2011. Effect of varying dose and administration of streptozotocin on blood sugar in male CD1 mice. Proc West Pharmacol Soc. 54:5–9.
- Wang G, Zhang X, Lu X, Liu J, Zhang Z, Wei Z, Wu Z, Wang J. 2020. Fish oil supplementation attenuates cognitive impairment by inhibiting neuroinflammation in STZ-induced diabetic rats. Aging. 12(15):15281–15289. doi: 10.18632/aging.103426.
- Wang H, Deng JL, Chen L, Ding K, Wang Y. 2021. Acute glucose fluctuation induces inflammation and neurons apoptosis in hippocampal tissues of diabetic rats. J Cell Biochem. 122(9):1239–1247. doi: 10.1002/jcb.29523.
- Yang RH, Wang F, Hou XH, Cao ZP, Wang B, Xu XN, Hu SJ. 2012. Dietary omega-3 polyunsaturated fatty acids improves learning performance of diabetic rats by regulating the neuron excitability. Neuroscience. 212:93–103. doi: 10.1016/j.neuroscience.2012.04.005.
- Zakaria R, Wan Yaacob WMH, Othman Z, Long I, Ahmad AH, Al-Rahbi B. 2017. Lipopolysaccharide-induced memory impairment in rats: a model of Alzheimer’s disease. Physiol Res. 66(4):553–565. doi: 10.33549/physiolres.933480.
- Zhu B, Wang ZG, Ding J, Liu N, Wang DM, Ding LC, Yang C. 2014. Chronic lipopolysaccharide exposure induces cognitive dysfunction without affecting BDNF expression in the rat hippocampus. Exp Ther Med. 7(3):750–754. doi: 10.3892/etm.2014.1479.
