Abstract
Context
Glioma, the most common primary malignant brain tumour, is a grave health concern associated with high morbidity and mortality. Current treatments, while effective to some extent, are often hindered by factors such as the blood–brain barrier and tumour microenvironment. This underscores the pressing need for exploring new pharmacologically active anti-glioma compounds.
Methods
This review synthesizes information from major databases, including Chemical Abstracts, Medicinal and Aromatic Plants Abstracts, ScienceDirect, SciFinder, Google Scholar, Scopus, PubMed, Springer Link and relevant books. Publications were selected without date restrictions, using terms such as ‘Hymenocrater spp.,’ ‘phytochemical,’ ‘pharmacological,’ ‘extract,’ ‘essential oil’ and ‘traditional uses.’ General web searches using Google and Yahoo were also performed. Articles related to agriculture, ecology, synthetic work or published in languages other than English or Chinese were excluded.
Results
The marine environment has been identified as a rich source of diverse natural products with potent antitumour properties.
Conclusions
This paper not only provides a comprehensive review of marine-derived compounds but also unveils their potential in treating glioblastoma multiforme (GBM) based on functional classifications. It encapsulates the latest research progress on the regulatory biological functions and mechanisms of these marine substances in GBM, offering invaluable insights for the development of new glioma treatments.
Introduction
Glioma, especially glioblastoma multiforme (GBM), is the predominant primary intracranial tumour, accounting for approximately 50.1% of all malignant brain tumours (Nguyen et al. Citation2021). An average annual mortality rate of 4.41 per 100,000 population represents a significant health challenge (Ostrom et al. Citation2022). The gold standard for GBM treatment involves surgical resection, radiotherapy and chemotherapy (Lang et al. Citation2021). However, despite these efforts, the primary chemotherapeutic agent for GBM, temozolomide (TMZ), only produces a median overall survival (OS) of 14.6 months (Sun et al. Citation2021). Several factors contribute to the limited effectiveness of standard therapies. The activation of DNA repair pathways, the presence of the blood–brain barrier (BBB), and the complex tumour microenvironment collectively hinder treatment outcomes, resulting in a bleak prognosis for patients with GBM (Delello Di Filippo et al. Citation2021; Tomar et al. Citation2021; Cai et al. Citation2023). Consequently, the exploration of new, safe chemical compounds exhibiting antitumour pharmacological activity, along with the development of innovative therapeutic approaches, remains crucial. These efforts are essential in the ongoing quest to create more effective drugs capable of significantly improving the survival rates of individuals diagnosed with GBM.
With the advancement of human technology in ocean exploration, an increasing number of marine source materials are being studied in-depth worldwide. The marine environment has become a rich reservoir of various natural products, which exhibit significant biological activities, including cytotoxic, antibacterial, antifungal, antiviral, antiparasitic, antitumour and immunomodulatory properties (Zhou X et al. Citation2013; Norris and Perkins Citation2016; Abdelmohsen et al. Citation2017; Guo K et al. Citation2021; Bohringer et al. Citation2023). Approximately 49 marine-derived active substances or their derivatives have been approved or entered clinical trials (Zhang QT et al. Citation2021). Many of these bioactive molecules are extracted from marine sponges, mollusks and algae. Currently, 11 types of marine drugs have been approved for clinical use by European and US Drug Authorities, five demonstrating anticancer efficacy: Cytosar-U, Yondelis, Halaven, Adcetris and Aplidin (Jimenez et al. Citation2020). Marine-derived compounds have exhibited potent antitumour effects against various cancers, including ovarian, breast, liver, lung and colorectal cancer (Terasaki et al. Citation2019; van Rixel et al. Citation2019; Binnewerg et al. Citation2020; Li W et al. Citation2020; Zhou B et al. Citation2021). These compounds exert anticancer activities through multiple mechanisms, such as inhibiting tumour growth, impeding cancer cell migration, inducing apoptosis and causing cell cycle arrest (Liberio et al. Citation2014; Oliveira et al. Citation2019; Piazzini et al. Citation2019; Xie Q et al. Citation2019). Several in vitro experiments have demonstrated the inhibitory effects of different doses of marine-derived organic compounds on the biological functions of glioma cells, offering promising avenues for treating GBM (Alves et al. Citation2021; Khotimchenko et al. Citation2021). However, despite the demonstrated potential of many marine-derived substances in GBM, none has yet been approved as a drug specifically for the treatment of this formidable brain cancer.
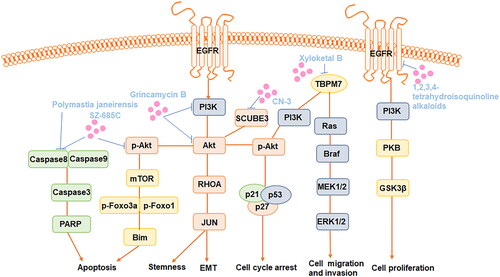
Numerous studies have highlighted the inhibitory effects of marine-origin compounds and their derivatives on glioma cell growth, apoptosis induction, autophagy stimulation and cell cycle arrest (Jarry et al. Citation2014). Abnormalities in multiple signalling pathways, including the epidermal growth factor receptor (EGFR) signalling pathway, the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) pathway and the extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) signalling pathways, are involved in the anti-glioma effects of marine-derived natural products ().
Among them, the PI3K/AKT/mTOR pathway is the crucial pathway highly involved in cell proliferation, growth, migration and cell cycle arrest in glioma. The interaction of EGFR with tyrosine kinase activates PI3K, leading to the phosphorylation of phosphatidylinositol 4,5-bisphosphate (PIP2) and its conversion into phosphatidylinositol 3,4,5-trisphosphate (PIP3). PIP3 activation reduces AKT activation while increasing the expression of mTOR complexes 1 and 2 (Ramar et al. Citation2023). Activation of mTORC1 increases cellular development by enhancing the anabolic process of other macromolecule formation while decreasing the catabolic response. In contrast, activation of mTOR complex 2 improves cell survival and proliferation (Obrador et al. Citation2024). The Ras/RAF/MEK/ERK pathway is hyperactive in glioma cells (Schreck et al. Citation2019). Activation of the Ras protein occurs through the replacement of GDP by GTP, initiating the activation of the MAP kinases. These kinases phosphorylate and activate downstream ERK proteins, which promote glioma cell proliferation and migration (Obrador et al. Citation2024). For example, Xyloketal B, a novel marine compound isolated from the mangrove fungus Xylaria sp. (No. 2508), exerts its effects by overexpressing the TRPM7 and suppressing the PI3K/Akt and MEK/ERK signalling pathways, thus contributing to antiproliferation and migration effects in GBM (Chen WL et al. Citation2015).
Marine-derived natural compounds have higher toxicity against GBM than TMZ, making them promising candidates for chemotherapy (Alves et al. Citation2021; Peng et al. Citation2023). However, it is essential to recognize that research on marine-derived compounds is still in the early stages of drug development. Most of their bioactivity is evaluated based on the half-maximal inhibitory concentration (IC50) of the cells.
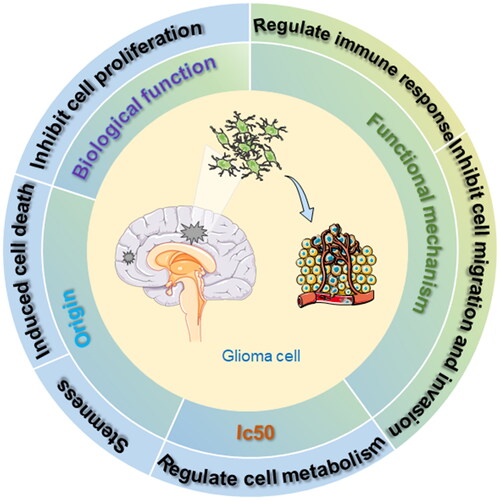
This review systematically and comprehensively examines the effects of marine-sourced materials on GBM, discussing their advantages and disadvantages based on existing research findings. This analysis provides a valuable reference for future research. It details the physiological impacts of marine-derived substances on glioma over the past two decades, highlighting their roles in inhibiting cell proliferation, inducing apoptosis and autophagy, and triggering cell cycle arrest (). Subsequent sections elaborate on these biological functions, providing practical insights into potential therapeutic applications.
The marine-derived natural products that affect the proliferation of glioma cells
In various pathological conditions, specific agents, such as environmental and chemical factors, have the potential to initiate the carcinogenesis process by inducing successive rounds of cell proliferation and clonal selection (Kasprzak Citation2023). Numerous natural compounds exhibit biological activities that inhibit cancer cell proliferation (Mohammadi et al. Citation2022), making them viable candidates for chemotherapeutic strategies.
Natural anti-glioma products from marine-associated actinomycete Streptomyces
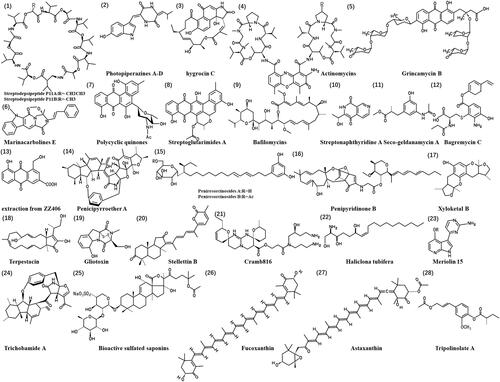
The primary biological function of effective anticancer compounds lies in their ability to hinder tumour cell viability and impact cell proliferation. Given the diverse marine sources and their derivatives, we discuss these compounds according to their origin. Marine-derived actinomycete Streptomyces are crucial reservoirs for discovering novel bioactive natural products. One such extraction was derived from the marine actinomycete Streptomyces sp. culture. P11-23B produced the streptodepsipeptides P11A and P11B (1). The streptodepsipeptide P11A exhibited significant activity against proliferation, demonstrating inhibitions of 87.17% for U87-MG glioma cells and 86.84% for U251 cells. Both streptodepsipeptides, P11A and P11B, showed potent activity with IC50 values ranging from 0.3 to 0.4 μM and 0.1 to 1.4 μM, respectively (Ye et al. Citation2017).
Marine-derived actinomycetes from the Streptomycetaceae family, such as photopiperazines A–D (2) (Kim et al. Citation2019), hygrocins C (3) (Yi, Newaz, et al. Citation2022), actinomycins (4) (Zhang X et al. Citation2016), grincamycin B (5) (Yao et al. Citation2020), marinacarbolines E (6) (Qin et al. Citation2020), exhibited inhibitory activities against glioma cell proliferation. Photopiperazines showed an IC50 value of 0.12 μM in U87 cells, while hygrocins C showed the most potent activity against U87MG and U251 glioma cells with IC50 values of 0.16 μM and 0.35 μM, respectively. The IC50 values for actinomycins in U251, SHG44 and C6 glioma cells range as follows: 1.01–10.06 nM for actinomycin D, 0.42–1.80 nM for actinomycin V and 3.26–25.18 nM for actinomycin X0β. The control drug doxorubicin exhibited activity with IC50 values ranging from 0.70 to 9.61 μM. Marinacarbolines E showed cytotoxic activity against U87MG cells with IC50 values of 2.3–8.9 μM.
Several bioactive compounds isolated from marine Streptomyces, for example, polycyclic quinones (7) (Liang et al. Citation2016), Streptoglutarimides A–J (8) (Zhang D et al. Citation2019) and bafilomycins (9) (Zhang X et al. Citation2017; Zhang Z et al. Citation2017), streptonaphthyridine A (10) (Qin et al. Citation2023), seco-geldanamycin A (11) (Yi, Lian, et al. Citation2022), bagremycin C (12) (Chen L et al. Citation2017) and Streptomyces sp. ZZ406 (13) (Chen M et al. Citation2018) showed potent efficacy in suppressing proliferation of tested glioma cell lines. Anthraquinones exhibited a notable inhibition of proliferation in four different glioma cell lines, with IC50 values ranging from 0.5 to 7.3 μM, and induced apoptosis in these glioma cells. ZZ741 streptoglutarimide H and streptovitacin A demonstrated a potent antiproliferative activity against human glioma U87MG and U251 cells, with IC50 values ranging from 1.5 to 3.8 μM and 0.05 to 0.22 μM, respectively. The four bafilomycins exhibited potent activity in suppressing the proliferation of U87-MG, U251 and SHG-44 cells and C6 cells from rat glioma, with IC50 values ranging from 0.35 to 2.95 µM. The positive control doxorubicin showed similar activity with IC50 values of 0.48–1.76 µM. Streptonaphthyridine A suppressed proliferation in U87MG and U251 cells with IC50 values of 7.9 ± 1.3 and 13.4 ± 2.7 µM, respectively. Bagremycin C was active against four glioma cell lines, with IC50 values ranging from 2.2 to 6.4 μM. The IC50 values for ZZ406 in different glioma cells range from 4.7 μM to 8.1 μM. These results underscore the robust activity of these natural product molecules isolated from marine-derived Streptomyces in inhibiting glioma cell proliferation.
A cytotoxic peptide, newly isolated from the marine-derived bacterium Brevibacillus sp. S-1, demonstrated cytotoxicity against U251 human glioma cells (Zheng et al. Citation2014). This discovery implies that various strains of marine origin may possess anti-glioma growth effects, opening avenues for further exploration and investigation.
Natural anti-glioma products from marine-associated fungi
Marine-derived fungi serve as abundant reservoirs for natural products characterized by their uniqueness in structure and bioactivity. They play a pivotal role in exploring new bioactive compounds and identifying potential drug-lead compounds. Fungi belonging to Penicillium species have emerged as prolific producers of new bioactive compounds. Penicipyrroether A (14) identified from the marine-derived Penicillium sp. ZZ380 represents a promising chemical compound with antitumour pharmacological activity. This compound exhibits potent antiproliferative effects against human glioma U87MG and U251 cells, with IC50 values ranging from 1.64 to 5.50 μM (Song et al. Citation2019). A culture of the marine-associated fungus Penicillium sp. ZZ1750 in rice medium produced seven new compounds, peniresorcinosides A–E, penidifarnesylin A, penipyridinone A and penipyridinone B. Peniresorcinosides A and B (15), classified as rare glycosylated alkylresorcinols, demonstrated potent antiglioma activity. They exhibited IC50 values of 4.0 and 5.6 µM against U87MG cells and 14.1 and 9.8 µM against U251 cells, respectively (Yong et al. Citation2021). Penipyridinone B (16) had a potent anti-glioma activity, with IC50 values of 2.45 μM for U87MG cells and 11.40 μM for U251 cells (Yong et al. Citation2022).
Beyond Penicillium species fungi, various marine fungi compounds have shown cytotoxic effects against glioma. Xyloketal B, a novel marine compound isolated from the mangrove fungus Xylaria sp. (No. 2508) in the South China Sea, has been identified as one such compound (Lin et al. Citation2001). Xyloketal B (17) exerts its effects by suppressing the PI3K/Akt and MEK/ERK signalling pathways, contributing to antiproliferation and migration effects in GBM (Chen WL et al. Citation2015). Its IC50 was measured at 287.1 ± 1.0 μM. Terpestacin (18), isolated from the fermentation broth of the coral endophytic fungus Arthrinium sp. SCSIO41221 has also shown promising effects. Specific compounds (5, 11, 13 and 15) showed a strong ability to inhibit proliferation (IC50 2.8–6.9 μM) and the formation of tumour spheres of glioma stem cells (GSCs). Compounds 13 and 15 effectively induced apoptosis and significantly inhibited GSC invasion (95% and 97% inhibition, respectively, at 2.5 μM) (Liao et al. Citation2023).
Gliotoxin (19), a hydrophobic fungal metabolite from the ocean, binds directly to pyruvate kinase M2 (PKM2) in the human glioma cell line U87. This interaction reduces glucose metabolism and induces cell death, with an IC50 value of 0.54 mM in U87 cells, indicating antitumour activity (Tang W et al. Citation2020). In summary, numerous compounds extracted from marine fungi inhibit glioma cell proliferation, suggesting their potential for prevention and treatment (Li Q et al. Citation2018; Zhang D et al. Citation2021).
Natural anti-glioma products from marine-associated animals
Extracts from marine animals, including sponges, ascidians, jellyfish and others, have shown significant inhibitory effects on glioma growth and progression, offering a new avenue for treatment. Four decades ago, the isolation of C-nucleoside from a Caribbean sponge laid the foundation for the synthesis of cytarabine. Cytarabine is the first marine-derived anticancer agent developed for clinical use and is currently a routine treatment for leukaemia and lymphoma (Schwartsmann et al. Citation2001). Sponge metabolites, known for their diverse pharmaceutical potential, exhibit various activities, including anticancer, anti-inflammatory, antiviral and antibacterial properties (http://marinepharmacology.midwestern.edu/clinPipeline.htm). Stellettin B (20), isolated from the marine sponge Jaspis stellifera, Carter 1879 (Ancorinidae), has shown remarkable inhibition against human glioblastoma cell line SF295 growth, with in vitro IC50 values of 0.01 μM. Its cytotoxic effects on human cancer cells exhibit relative selectivity compared to normal cell lines. The antiproliferative effects of stellettin B may involve inhibition of the PI3K/Akt pathway.
Stellettin B promotes PI3Kα degradation through the ubiquitination-proteasome pathway, reducing the expression of key components of the homologous recombination (HR) repair pathway, such as BRCA1, BRCA2 and RAD51. This mechanism improves the sensitivity of GBM cells to ionizing radiation by delaying DNA damage repair, ultimately resulting in GBM cell death. Stellettin B is a potential lead compound to discover promising drug candidates in treating certain glioblastomas (Tang SA et al. Citation2014; Peng et al. Citation2023).
Calcium channels are common targets for natural toxins, with two notable families of guanidine alkaloids, crambescins and crambescidins, originating from the marine sponge Crambe crambe (Crambeidae). Cramb816 (21) has been identified as a partial blocker of the voltage-gated calcium channels (CaV) and voltage-gated sodium channels (NaV) in neurons. This action reduces neurotransmitter release and synaptic transmission in the central nervous system, which may influence cell growth (Martin et al. Citation2013). Furthermore, the marine sponge species Haliclona tubifera George & Wilson (Chalinidae) (22), abundant along the Brazilian coastline, has shown significant cytotoxic effects and the ability to inhibit the production of peroxyl radicals (Biegelmeyer et al. Citation2015). Numerous sponge-derived compounds exhibit varying degrees of inhibition, ranging from moderate to potent, against the growth of human glioma cells (Neupane et al. Citation2019).
Ascidians, marine invertebrates with high bioactivity, have found extensive applications in cancer treatment (Watters Citation2018). Meridianins represent a group of marine natural compounds that exhibit various levels of kinase inhibition extracted from ascidian Aplidium meridianum, Sluiter (Polyclinidae). Meriolin 15 (23) has antiproliferative and pro-apoptotic activities against glioma and intratumoural endothelial cells. Meriolin 15 demonstrates IC50 values of 46 nM for SW1088 cells and 5.1 nM for U87 cells, respectively. Its impact includes inhibition of cell proliferation, reduction of undifferentiated cells and vascular components, and induction of cell death, in part through a caspase-3-dependent apoptotic pathway (Jarry et al. Citation2014).
Vitilevuamide, a bicyclic 13-amino acid peptide, was isolated from two marine ascidians, Didemnum cuculiferum Sluiter (Didemnidae) and Polysyncraton lithostrotum Brewin (Didemnidae). This peptide inhibited purified tubulin polymerization and reduced cell viability in vitro, with IC50 values of approximately 2 μM (Edler et al. Citation2002). Additionally, trichobamide A (24), isolated from the ascidian-derived fungus Trichobotrys effuse 4729, significantly inhibited proliferation in two glioma cell lines, U251 and SNB19 (Chen S et al. Citation2019). These findings lay a promising foundation for developing ocean-derived compounds into effective targeted drugs.
The jellyfish extract has shown inhibitory effects on glioma cell growth. The crude venom extract of the Mediterranean jellyfish Pelagia noctiluca (Pelagiidae) was fractionated into four fractions (F1, F2, F3 and F4) using Sephadex G-75 chromatography. U87 cells cultured with 10 μg/mL of venom or its fractions (F1–F4) for specified periods were assessed for cell proliferation activity using the MTT assay. Among these, fraction F3 showed a slight inhibitory effect on glioma cell growth, reducing it by approximately 15%. F4 did not show an effect, while F1 and F2 demonstrated greater effectiveness. These fractions could inhibit U87 cell viability in a time- and dose-dependent manner, with IC50 values of 125 and 179 μg/mL (Ayed et al. Citation2012). The bioactive sulphated saponins (25) of the sea cucumber Holothuria moebii Ludwig (Holothuriidae) had an activity that suppressed the proliferation of four different glioma cells with IC50 values ranging from 0.99 to 8.64 µM (Yu et al. Citation2015).
Natural anti-glioma products from marine-associated plants
Marine plants have long served as vital sources of drugs to combat human and animal diseases. Polyphenols derived from marine halophytes have shown activity in inhibiting glioma cell proliferation through a caspase-dependent pathway mediated by reactive oxygen species (ROS). These polyphenols have been found to impact proteins involved in the MAPK signalling pathway, such as the MMK3, p53, p70 S6 kinase and RSK1 (Murugesan et al. Citation2023). Two notable xanthophylls, astaxanthin (27) and fucoxanthin (26), commonly found in Haematococcus pluvialis (Haematococcaceae), Phaffia rhodozyma (Mrakiaceae), Phaeophyceae and Bacillariophyta, demonstrated toxicity at doses greater than 200 µM and 8 µM, respectively. Astaxanthin and fucoxanthin were found to limit considerable DNA damage (about 18 times) caused by 3 mJ/cm2 UV radiation in C6 cells. UV radiation led to down-regulation of the MRN complex, checkpoint kinase (Chk)1/2 activation, heterochromatin protein 1 (HP1)γ and mortalin, and up-regulation of the DNA damage markers 53BP1 and phosphorylated ATR. However, cells pretreated with astaxanthin and fucoxanthin showed a significant recovery in MRE11 expression. Non-toxic doses of these compounds prevented protein aggregation and protein misfolding and induced differentiation in glioma cells (Afzal et al. Citation2019). Furthermore, the PI3K inhibitor LY-294002 and fucoxanthin were found to modulate several common pathways, including inhibition of cell proliferation, DNA damage, DNA replication and cell cycle pathways (Pruteanu et al. Citation2020). Tripolinolate A (28), isolated from a Tripolium pannonicum (Jacq.) Dobrocz. (Asteraceae) halophyte, inhibited the proliferation of different glioma cells with IC50 values of 7.97–14.02 µM and had a significant inhibitory effect on the glioma growth in U87MG xenograft nude mice (Chai et al. Citation2018). In conclusion, a growing body of research demonstrates that marine-derived substances inhibit glioma cell proliferation and exhibit antitumour effects. shows the chemical structures of natural products (1-28).
The marine-derived natural products that affect the migration and invasion of glioma cell
Migration and invasion are critical cellular mechanisms involved in tumour metastasis. Astaxanthin, a natural carotenoid derivative found in microalgae and marine organisms, has been investigated for its impact on glioblastoma cells (A172). MTT assay results indicate that astaxanthin is not toxic to A172 cells. In wound scratch and Boyden chamber assays, astaxanthin inhibited in vitro migration and invasion of A172 cells in a time- and concentration-dependent manner. These effects were associated with regulating matrix metalloproteinases (MMPs), with significant reductions in MMP-2 and MMP-9 observed after astaxanthin treatments. This suggests that astaxanthin may protect against glioblastoma metastasis (Siangcham et al. Citation2020).
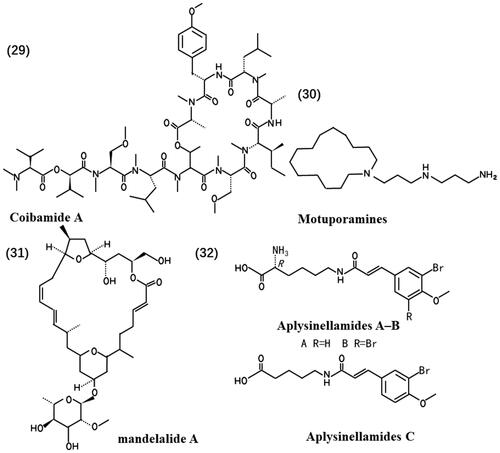
Coibamide A (29), isolated from a marine cyanobacterial assemblage collected within the Coiba National Park in Panama (Medina et al. Citation2008), has shown significant anti-glioblastoma activity. U87-MG cells exhibited a high invasive capacity at the beginning of the assay, reaching a plateau in cell index after 12–15 h, while SF-295 cells invaded slowly and continuously for 48 h. Coibamide A exhibited inhibitory effects on cell migration and demonstrated antiangiogenic properties. It effectively suppressed tumour growth in a subcutaneous mouse model of glioblastoma. In summary, Coibamide A showed potent antitumour activity in vitro and in vivo (Serrill et al. Citation2016).
Motuporamines (30), extracted from marine sponges, have been shown to inhibit invasion in U-87 and U-251 glioma cells (Lin et al. Citation2001). Another marine compound, xyloketal B, is derived from the mangrove fungus Xylaria sp. 2508 and has been found to exert antiproliferative and migration effects on U251 cells by modulating the PI3K/Akt and MEK/ERK signalling pathways (Chen WL et al. Citation2015). In summary, compounds sourced from the marine environment inhibit the migration and invasion of glioma cells, ultimately contributing to their antitumour effects.
The marine-derived natural products that affect the metabolism of glioma cells
Cell metabolism encompasses a series of organized chemical reactions within cells that are crucial to maintaining life. The three key metabolic pathways are glycolysis, the tricarboxylic acid cycle (TCA) and oxidative phosphorylation (Martínez-Reyes and Chandel Citation2021). Regulation of tumour cell metabolism significantly influences glioma cell survival (Guo D et al. Citation2022). Studies have indicated that certain marine-derived compounds also modulate glioma cell metabolism.
The marine-derived natural products that affect the ATP consumption of the glioma cell
ATP is the energy exchange factor that bridges anabolism and catabolism, which plays a crucial role in motile contraction, phosphorylations and active transport (Bonora et al. Citation2012). Mandelalides (A to L) (31), originally isolated from a newly discovered species of Lissoclinum tunicate Monniot F. & Monniot C (Didemnidae) in and around Algoa Bay in the Eastern Cape of South Africa, constitute a family of polyketide macrolactones. Treatment with mandelalide A induces time- and concentration-dependent increases in AMPKα (Thr172) and ACC (Ser79) phosphorylation states compared to control U87MG cells treated with drug-loaded (0.1% DMSO) alone. This suggests that mandelalide induces an indirect AMPK activation mode to maintain cellular ATP and nucleotide balance in response to energy stress. As a result, cytotoxic mandelalides represent a class of potentially valuable inhibitors of ATP synthase (Mattos et al. Citation2022), which affect cell metabolism.
The marine-derived natural products that affect the lipid metabolism of the glioma cell
Lipids play essential biological roles in the body, including energy storage and acting as signalling molecules and structural components of membranes (Martin-Perez et al. Citation2022). Regulation of lipid metabolism can affect tumour cell viability. Aplysinellamides A–C (32), isolated from an Australian Aplysinella sp. (Aplysinellidae) marine sponge, exhibit notable effects. Among these compounds, Aplysamine-1 significantly modulates apolipoprotein E (ApoE) and doubles its secretion from human CCF-STTG1 astrocytoma cells at a concentration of 30 μM. ApoE is a major apolipoprotein in the central nervous system and plays a central role in cholesterol transport.
Aplysinellamide A restricts the binding of ApoE to lipids, such as cholesterol, thus regulating lipid metabolism (Tian LW et al. Citation2014). Maitotoxin, a potent marine toxin, induces the accumulation of inositol 1,4,5-trisphosphate in a time-dependent manner. Maitotoxin stimulates phosphoinositide hydrolysis in an extracellular Ca2+-dependent manner but not intracellular Ca2+ in 1321N1 human astrocytoma cells (Nakahata et al. Citation1999). Phosphoinositides, lipid signalling molecules derived from phosphatidylinositol, act as master regulators of cell signalling (Posor et al. Citation2022). Maitotoxin regulates the processes of signalling and lipid transport. These findings collectively demonstrate that marine-derived compounds can influence glioma cell lipid metabolism, thus exerting antitumour effects. Streptodepsipeptide P11A and P11B induced glioma cell death with down-regulation of key metabolic regulators, including hexokinase 2 (HK2), phosphofructokinase-2/fructose-2,6-bisphosphatase 3 (PFKFB3), PKM2, glutaminase (GLS) and fatty acid synthase (FASN), which govern glycolysis, glutaminolysis and lipogenesis (Ye et al. Citation2017). Therefore, marine-derived natural products affect the lipid metabolism of the glioma cell. shows the chemical structures of natural products (29-32).
The marine-derived natural products that affect the death of glioma cells
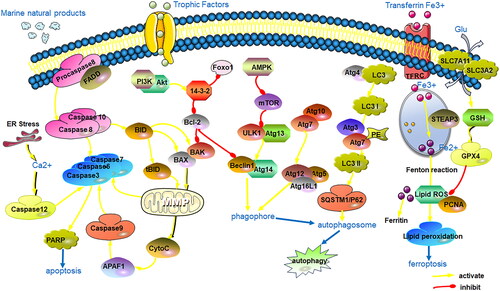
The discovery of regulated cell death processes has significantly advanced cancer treatment. Two main types of cell death are recognized: regulatory cell death (RCD) and accidental cell death (ACD) (Galluzzi et al. Citation2018). ACD is an uncontrolled process resulting from unintentional injury, where damage stimulation exceeds the cell’s regulatory capacity, leading to cell death. RCD, also known as programmed cell death (PCD), involves the spontaneous and orderly death of cells controlled by genes to maintain the stability of the internal environment. Various known types of RCD include autophagy-dependent cell death, apoptosis, necroptosis, pyroptosis, ferroptosis, parthanatos, entosis, NETosis, lysosome-dependent cell death (LCD), alkaliptosis and oxeiptosis (Christgen et al. Citation2022). Marine-derived compounds have shown the ability to induce apoptosis, autophagy and ferroptosis in glioma cells, thus limiting tumour growth. This opens possibilities for rationally developing marine-derived compounds as potential antitumour drugs ().
The marine-derived natural products that affect glioma cell apoptosis
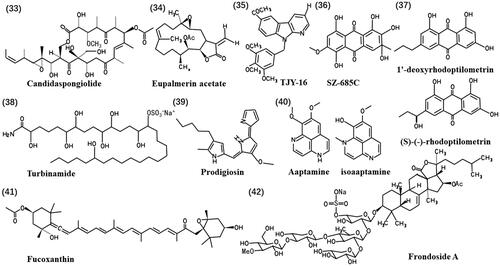
Apoptosis is a controlled cell death independent, organized and orchestrated by multiple genes. It can occur through one of three main pathways, including an endogenous pathway that involves the release of Bcl-2-mediated mitochondrial cytochrome C, an exogenous pathway mediated by the expression of the death receptor ligand, and the dependent cytotoxic T lymphocyte/natural killer cell (CTL/NK) pathway (Wu et al. Citation2023). Compounds such as Polymastia janeirensis (da Frota et al. Citation2009), candidaspongiolide (33) (Trisciuoglio et al. Citation2008) and eupalmerin acetate (34) (Iwamaru et al. Citation2007), sourced from marine sponges or Caribbean gorgonian octocorals, induce caspase-dependent apoptosis through the endogenous pathway in human glioma cell lines.
Crude venom from the Persian Gulf Snail (Conus textile) has been found to combat U87MG human glioma cells by activating intrinsic apoptosis pathways (Salimi et al. Citation2021). Additionally, α-carboline derivatives TJY-16 (35) (Huang et al. Citation2016) and SZ-685C (36) (Xie G et al. Citation2010), isolated from marine animals or marine-derived mangrove endophytic fungi, induce arrest and apoptosis of the G2/M cell cycle in glioma cells through extrinsic and intrinsic apoptotic pathways. Marine-derived compounds can also induce glioma cell apoptosis through alternative pathways. For example, 1′-deoxyrhodoptilometrin and (S)-(−)-rhodoptilometrin (37), isolated from the marine echinoderm Comanthus sp. (Aplysinellidae), induce apoptotic and necrotic cell death by inhibiting the EGF receptor/MAPK pathway (Wätjen et al. Citation2017). Furthermore, 1,2,3,4-tetrahydroisoquinoline alkaloids from Thai marine invertebrates induce apoptosis in U373MG cells through the ErbB (EGFR) signalling pathway, which involves focal adhesion kinase (FAK)/PTK2, AKT3 and glycogen synthase kinase 3 Beta (GSK3B) (Tabunoki et al. Citation2012). Turbinamide (38), isolated from the marine ascidian Sydnium turbinatum (Polyclinidae), induces apoptosis in C6 cells (Esposito et al. Citation2002). Saponins, natural glycosides, have significant anti-tumour activity by inducing apoptosis (Tian X et al. Citation2013). These diverse marine-derived compounds have the potential for therapeutic applications in glioma treatment.
The marine-derived natural products that affect glioma cell autophagy
Autophagy is a process that directs damaged organelles to the lysosome through double-membraned vesicles for degradation (Mizushima and Komatsu Citation2011). Studies indicate that autophagy plays a bidirectional role in cancer (Kocaturk et al. Citation2019). Under certain conditions, excess or deregulated autophagy activity may lead to glioma cell death. Prodigiosin (39), a secondary metabolite isolated from marine Vibrio sp. (Vibrionaceae), has decreased cell viability, and can form neurospheres in glioblastoma cells. LC3 puncta formation and acridine orange staining in U87MG and GBM8401 human glioblastoma cells indicate that prodigiosin induces excessive levels of autophagy. These results suggest a potential mechanism through which prodigiosin may exert its anti-cancer effects. The autophagy inhibitor 3-methyladenine reverses prodigiosin-induced autophagic cell death.
The c-Jun N-terminal kinase (JNK) pathway and the AKT/mTOR pathway were implicated in prodigiosin-induced cell death, triggering autophagic cell death and apoptosis in glioblastoma cells (Cheng SY et al. Citation2018). Autophagy is regulated by autophagy-related genes such as UNC-51-like kinase 1 (ULK1), Beclin-1, light chain 3 (LC3), p62 and FoxO, each playing different roles in autophagy processes. ULK1 promotes autophagy by regulating its initiation (Mokarram et al. Citation2017). Similarly, aaptamine and its isoform isoaaptamine (40), alkaloids extracted from the tropical sponge Aaptos sp. (Suberitidae), have been shown to induce cell death in GBM 8401, U87 MG, U138 MG and T98G cells in a concentration- and time-dependent manner, with IC50 values ranging from 8 to 60 µM. Isoaaptamine specifically induces ROS overproduction, up-regulates autophagy-associated proteins p62 and LC3, and forms acid organelle vesicles related to autophagy, leading to autophagic cell death (Wen et al. Citation2023). These findings underscore the potential of marine-derived compounds to induce autophagic cell death in glioma cells, highlighting their importance in cancer therapy.
The marine-derived natural products that affect glioma cell ferroptosis
Ferroptosis is an iron-dependent form of regulated cell death triggered by the toxic accumulation of lipid peroxides in cell membranes (Lei et al. Citation2022). Fucoxanthin (41), extracted from seaweed, exhibits antitumour effects by inhibiting the survival of GBM cells through the induction of ferroptosis. Fucoxanthin administration can block the transferrin receptor (TFRC) degradation and down-regulate the expression of proliferating cell nuclear antigen (PCNA), restricting cell growth in vivo and in vitro (Zhu et al. Citation2023). These findings highlight the significant inhibitory effects of marine-derived compounds on glioma by activating ferroptosis, providing a novel treatment option for patients with glioma.
The marine-derived natural products that affect the immune cell response of glioma cells
The brain tumour microenvironment is highly immunosuppressive and differs from other malignancies due to the presence of glial, neural and immune cell populations (Andersen et al. Citation2021). Immunotherapy has shown effective therapeutic responses in various tumours, including brain tumours. Frondoside A (FA) (42), a triterpenoid glycoside isolated from sea cucumber, has emerged as a potential therapeutic agent. It suppresses the expression of MYC and its downstream gene targets, including cyclin-dependent kinases and other oncogenic regulators. FA induces brain tumour cell death with an IC50 value of 0.37 μM. Administration of FA potently induces cytotoxicity in tumour xenografts, significantly extending the survival of tumour-bearing animals.
FA-treated xenografts exhibited a higher abundance of Iba1 microglia/macrophages and CD8 cytotoxic T lymphocytes (CTLs) in the periphery than mock-treated tumours. This region also contained more perforin-labelled cells, a key component of cytotoxic granules released by active CTLs and natural killer cells. These findings suggest a potential role for FA in modulating tumour-associated immune responses (Xue et al. Citation2021). shows the chemical structures of natural products (33-42).
The marine-derived natural products that affect the stemness of glioma cells
Glioblastoma stem cells, a subpopulation of glioma cells, exhibit characteristics of stem cell. They can self-renew, proliferate and differentiate into various cell types, which underlies cellular heterogeneity and plays a pivotal role in the initiation, progression, resistance to treatment, and relapse of GBM (Sharifzad et al. Citation2019). Marine natural products have been shown to exhibit strong inhibitory effects on GSCs, providing a new avenue to explore novel drugs for GBM treatment. Grincamycin B inhibits the formation of GBM spheres and the expression of the GBM stem cell marker in a dose-dependent manner (Yao et al. Citation2020). The most common pathways involved in maintaining GSCs include the Notch signalling pathway, the Sonic hedgehog (SHH)/glioma-associated oncogene (GLI) signalling pathway and Wnt/β-catenin signalling pathway. However, these pathways are also shared by normal stem cells (Sharifzad et al. Citation2019). The Notch signalling pathway maintains the stemness of GSCs and promotes cell migration. The SHH/GLI signalling pathway and the Wnt/β-catenin signalling pathway are crucial for CNS self-renewal, differentiation and neural stem cell development. The NF-κB pathway regulates the self-renewal capacity of CSCs, the JAK-STAT pathway, the tumour growth factor (TGF)/SMAD pathway, the PI3K/AKT/mTOR pathway and the EGFR pathways (Yang et al. Citation2020; Nasrolahi et al. Citation2023). Grincamycin B decreased PI3K/AKT activation and targeted RHOA in GSCs. RHOA can affect cancer stem cell (CSC) phenotypes through WNT/β-catenin signalling. GCN B is a potential RHOA and PI3K/AKT pathway inhibitor, which might be a dual inhibitor to treat GBM (Yao et al. Citation2020). Meanwhile, the principal pathway in which marine-derived compounds regulate CSCs has not been explored.
Discussion and conclusions
This review summarizes the sources, structures, in vitro pharmacological activities and mechanisms of marine-derived compounds to explore their potential in treating GBM. Compounds of marine origin and their derivatives exhibit inhibitory effects on cell proliferation, migration and invasion. They also regulate cell metabolism and stem, induce cell death in glioma, and modulate the immune response in GBM ().
Table 1. Activities of marine sources and their derivatives against glioma cells.
Glioma is a highly malignant cancer in the brain. First-line therapy for GBM is surgery followed by radiation and the alkylating chemotherapeutic agent TMZ (Schaff and Mellinghoff Citation2023). TMZ was approved by the FDA in 2005 based on a marginal increase (∼2 months) in OS (Karve et al. Citation2024). TMZ is converted to an alkylating methyldiazonium cation, leading to cell death by breaking the DNA double-strand. However, TMZ treatment faces challenges due to factors such as the BBB, tumour heterogeneity, GSCs, drug efflux pumps, DNA damage repair mechanisms, and high expression of the MGMT gene, all of which contribute to resistance to TMZ therapy (Angom et al. Citation2023; Fang Citation2024). Compounds derived from marine sources exhibit various origins and mechanisms, inhibiting glioma cell growth and migration through various means, such as inducing multiple modes of cell death and regulating immune cell activity. In recent years, immunotherapy and targeted therapy have been extensively studied and clinically tested in various tumours, demonstrating specific therapeutic effects. Both in vitro and in vivo experiments have shown that many compounds exhibit lower IC50 values compared to TMZ treatment. Furthermore, combining these compounds with TMZ results in a more effective inhibition than using TMZ alone. Therefore, marine-derived compounds provide a multi-modal approach, combining different therapies or therapeutic agents with distinct molecular mechanisms, to enhance the efficacy of glioma cell eradication.
Recent studies have highlighted that in addition to TMZ, the standard medication for glioma treatment, numerous natural compounds show promise as treatment options. These include betulinic acid (Fernandes et al. Citation2024), perillyl alcohol (Alharbi et al. Citation2024), matrine (Qiu et al. Citation2024), 9′-lithospermic acid methyl ester (Tzitiridou et al. Citation2024), cannabinoid compounds (Pires et al. Citation2024), eugenol (Padhy et al. Citation2022), curcumin and flavonoids (Persano et al. Citation2022). For example, magnolol, a hydroxylated biphenyl compound isolated from the bark of Magnolia officinalis Rehder & Wilson (Magnoliaceae), suppresses cell proliferation and induces cell cycle arrest and apoptosis at a concentration of 50 µM by inhibiting DNA synthesis in glioma cells (Chen and Lee Citation2013). This concentration is significantly higher than the IC50 value for compounds of marine origin. Furthermore, magnolol induces cytotoxic autophagy in glioma by inhibiting the PI3K/AKT/mTOR signalling pathway (Cheng YC et al. Citation2018). It can potentiate the apoptotic effects of TMZ by inhibiting MGMT activation mediated by the NF-κB pathway (Kundu et al. Citation2023).
Although marine-derived compounds offer unique advantages in glioma treatment, such as their ability to cross the BBB and influence cell metabolism, immune cell response, and the stemness of glioma cells, there are still significant gaps in our understanding. The mechanisms underlying resistance to TMZ, a key challenge in glioma treatment, require further investigation. Most studies are limited to reporting IC50 values and using bioinformatics for predictive analysis. Comprehensive in-depth mechanistic studies and in vivo experiments are crucial in advancing these marine compounds into viable therapeutic options for GBM.
In conclusion, exploring ocean-derived drugs for treating GBM is still in its early stages and requires more preclinical and clinical research. This review provides a comprehensive overview of marine natural products with potential applications in GBM treatment, facilitating ongoing scientific research and serving as a valuable resource for researchers selecting drug candidates for further exploration.
Author contributions
Liu Y, Zhou Z and Sun S: study conception. Liu Y, Zhou Z and Sun S: literature search, data synthesis and manuscript writing and editing.
Ethical approval
This study does not involve human participants, human data or human tissues. No ethics approval is required.
Consent form
This study does not involve human participants, human data or human tissues. No consent to participate is required.
Disclosure statement
Shusen Sun is an Associate Editor of this journal but was not involved in the peer review process, in line with journal protocol, COPE guidelines and current best practice. No other authors reported a conflict of interest.
Additional information
Funding
References
- Abdelmohsen UR, Balasubramanian S, Oelschlaeger TA, Grkovic T, Pham NB, Quinn RJ, Hentschel U. 2017. Potential of marine natural products against drug-resistant fungal, viral, and parasitic infections. Lancet Infect Dis. 17(2):e30–e41.
- Afzal S, Garg S, Ishida Y, Terao K, Kaul SC, Wadhwa R. 2019. Rat glioma cell-based functional characterization of anti-stress and protein deaggregation activities in the marine carotenoids, astaxanthin and fucoxanthin. Mar Drugs. 17(3):189.
- Alharbi WS, Alshehri AA, Ahmed TA, Md S, Almehmady AM, Alshabibi MA, Altamimi RM, El-Say KM. 2024. Enhancing the antiproliferative activity of perillyl alcohol against glioblastoma cell lines through synergistic formulation with natural oils. Curr Pharm Des. 30(14):1075–1084. doi: 10.2174/0113816128293758240318080527.
- Alves A, Costa P, Pinto M, Ferreira D, Correia-da-Silva M. 2021. Small molecules of marine origin as potential anti-glioma agents. Molecules. 26(9):2707. doi: 10.3390/molecules26092707.
- Andersen BM, Faust Akl C, Wheeler MA, Chiocca EA, Reardon DA, Quintana FJ. 2021. Glial and myeloid heterogeneity in the brain tumour microenvironment. Nat Rev Cancer. 21(12):786–802. doi: 10.1038/s41568-021-00397-3.
- Angom RS, Nakka NMR, Bhattacharya S. 2023. Advances in glioblastoma therapy: an update on current approaches. Brain Sci. 13(11):1536. doi: 10.3390/brainsci13111536.
- Ayed Y, Bousabbeh M, Mabrouk HB, Morjen M, Marrakchi N, Bacha H. 2012. Impairment of the cell-to-matrix adhesion and cytotoxicity induced by the Mediterranean jellyfish Pelagia noctiluca venom and its fractions in cultured glioblastoma cells. Lipids Health Dis. 11(1):84. doi: 10.1186/1476-511X-11-84.
- Biegelmeyer R, Schröder R, Rambo DF, Dresch RR, Carraro JLF, Mothes B, Moreira JCF, Junior MLCdF, Henriques AT. 2015. Sphingosines derived from marine sponge as potential multi-target drug related to disorders in cancer development. Mar Drugs. 13(9):5552–5563. doi: 10.3390/md13095552.
- Binnewerg B, Schubert M, Voronkina A, Muzychka L, Wysokowski M, Petrenko I, Djurovic M, Kovalchuk V, Tsurkan M, Martinovic R, et al. 2020. Marine biomaterials: biomimetic and pharmacological potential of cultivated Aplysina aerophoba marine demosponge. Mater Sci Eng C Mater Biol Appl. 109:110566.
- Bohringer N, Kramer JC, de la Mora E, Padva L, Wuisan ZG, Liu Y, Kurz M, Marner M, Nguyen H, Amara P, et al. 2023. Genome- and metabolome-guided discovery of marine BamA inhibitors revealed a dedicated darobactin halogenase. Cell Chem Biol. 30:943–952.
- Bonora M, Patergnani S, Rimessi A, De Marchi E, Suski JM, Bononi A, Giorgi C, Marchi S, Missiroli S, Poletti F, et al. 2012. ATP synthesis and storage. Purinergic Signal. 8:343–357.
- Cai Q, Li X, Xiong H, Fan H, Gao X, Vemireddy V, Margolis R, Li J, Ge X, Giannotta M, et al. 2023. Optical blood–brain–tumor barrier modulation expands therapeutic options for glioblastoma treatment. Nat Commun. 14(1):4934. doi: 10.1038/s41467-023-40579-1.
- Chai W, Chen L, Lian XY, Zhang Z. 2018. Anti-glioma efficacy and mechanism of action of tripolinolate A from Tripolium pannonicum. Planta Med. 84(11):786–794. doi: 10.1055/s-0044-101038.
- Chen L, Chai W, Wang W, Song T, Lian XY, Zhang Z. 2017. Cytotoxic bagremycins from mangrove-derived Streptomyces sp. Q22. J Nat Prod. 80(5):1450–1456. doi: 10.1021/acs.jnatprod.6b01136.
- Chen LC, Lee WS. 2013. P27/Kip1 is responsible for magnolol-induced U373 apoptosis in vitro and in vivo. J Agric Food Chem. 61(11):2811–2819. doi: 10.1021/jf400542m.
- Chen M, Chai W, Song T, Ma M, Lian XY, Zhang Z. 2018. Anti-glioma natural products downregulating tumor glycolytic enzymes from marine actinomycete Streptomyces sp. ZZ406. Sci Rep. 8(1):72. doi: 10.1038/s41598-017-18484-7.
- Chen S, Shen H, Zhang P, Cheng H, Dai X, Liu L. 2019. Anti-glioma trichobamide A with an unprecedented tetrahydro-5H-furo[2,3-b]pyrrol-5-one functionality from ascidian-derived fungus Trichobotrys effuse 4729. Chem Commun. 55(10):1438–1441.
- Chen WL, Turlova E, Sun CL, Kim JS, Huang S, Zhong X, Guan YY, Wang GL, Rutka JT, Feng ZP, et al. 2015. Xyloketal B suppresses glioblastoma cell proliferation and migration in vitro through inhibiting TRPM7-regulated PI3K/Akt and MEK/ERK signaling pathways. Mar Drugs. 13(4):2505–2525.
- Cheng SY, Chen NF, Kuo HM, Yang SN, Sung CS, Sung PJ, Wen ZH, Chen WF. 2018. Prodigiosin stimulates endoplasmic reticulum stress and induces autophagic cell death in glioblastoma cells. Apoptosis. 23(5–6):314–328. doi: 10.1007/s10495-018-1456-9.
- Cheng YC, Tsao MJ, Chiu CY, Kan PC, Chen Y. 2018. Magnolol inhibits human glioblastoma cell migration by regulating N-cadherin. J Neuropathol Exp Neurol. 77(6):426–436. doi: 10.1093/jnen/nly021.
- Christgen S, Tweedell RE, Kanneganti TD. 2022. Programming inflammatory cell death for therapy. Pharmacol Ther. 232:108010. doi: 10.1016/j.pharmthera.2021.108010.
- da Frota MLJr, Braganhol E, Canedo AD, Klamt F, Apel MA, Mothes B, Lerner C, Battastini AM, Henriques AT, Moreira JC. 2009. Extracts of marine sponge Polymastia janeirensis induce oxidative cell death through a caspase-9 apoptotic pathway in human U138MG glioma cell line. Invest New Drugs. 27(5):440–446. doi: 10.1007/s10637-008-9198-0.
- da Frota MLJr, Braganhol E, Canedo AD, Klamt F, Apel MA, Mothes B, Lerner C, Battastini AM, Henriques AT, Moreira JC. 2009. Brazilian marine sponge Polymastia janeirensis induces apoptotic cell death in human U138MG glioma cell line, but not in a normal cell culture. Invest New Drugs. 27(1):13–20. doi: 10.1007/s10637-008-9134-3.
- Delello Di Filippo L, Hofstätter Azambuja J, Paes Dutra JA, Tavares Luiz M, Lobato Duarte J, Nicoleti LR, Olalla Saad ST, Chorilli M. 2021. Improving temozolomide biopharmaceutical properties in glioblastoma multiforme (GBM) treatment using GBM-targeting nanocarriers. Eur J Pharm Biopharm. 168:76–89. doi: 10.1016/j.ejpb.2021.08.011.
- Edler MC, Fernandez AM, Lassota P, Ireland CM, Barrows LR. 2002. Inhibition of tubulin polymerization by vitilevuamide, a bicyclic marine peptide, at a site distinct from colchicine, the Vinca alkaloids, and dolastatin 10. Biochem Pharmacol. 63(4):707–715.
- Esposito G, Aiello A, Carbonelli S, Menna M, Fattorusso E, Iuvone T. 2002. Mechanism of cytotoxicity of turbinamide in vitro. Anticancer Res. 22(5):2827–2831.
- Fang Q. 2024. The versatile attributes of MGMT: its repair mechanism, crosstalk with other DNA repair pathways, and its role in cancer. Cancers. 16(2):331. doi: 10.3390/cancers16020331.
- Fernandes S, Vieira M, Prudêncio C, Ferraz R. 2024. Betulinic acid for glioblastoma treatment: reality, challenges and perspectives. Int J Mol Sci. 25(4):2108. doi: 10.3390/ijms25042108.
- Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, et al. 2018. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 25(3):486–541. doi: 10.1038/s41418-017-0012-4.
- Guo D, Tong Y, Jiang X, Meng Y, Jiang H, Du L, Wu Q, Li S, Luo S, Li M, et al. 2022. Aerobic glycolysis promotes tumor immune evasion by hexokinase2-mediated phosphorylation of I-kappa-B-alpha. Cell Metab. 34:1312–1324.
- Guo K, Liu Y, Li SH. 2021. The untapped potential of plant sesterterpenoids: chemistry, biological activities and biosynthesis. Nat Prod Rep. 38:2293–2314.
- Huang HC, Liu WT, Hua KS, Hung HC, Tsai JY, Kuo SC, Huang LJ, Gean PW. 2016. alpha-Carboline derivative TJY-16 inhibits tumor growth by inducing G2/M cell cycle arrest in glioma cells. J Biomed Sci. 23(1):10. doi: 10.1186/s12929-016-0222-y.
- Iwamaru A, Iwado E, Kondo S, Newman RA, Vera B, Rodríguez AD, Kondo Y. 2007. Eupalmerin acetate, a novel anticancer agent from Caribbean gorgonian octocorals, induces apoptosis in malignant glioma cells via the c-Jun NH2-terminal kinase pathway. Mol Cancer Ther. 6(1):184–192. doi: 10.1158/1535-7163.MCT-06-0422.
- Jarry M, Lecointre C, Malleval C, Desrues L, Schouft MT, Lejoncour V, Liger F, Lyvinec G, Joseph B, Loaec N, et al. 2014. Impact of meriolins, a new class of cyclin-dependent kinase inhibitors, on malignant glioma proliferation and neo-angiogenesis. Neuro Oncol. 16(11):1484–1498.
- Jimenez PC, Wilke DV, Branco PC, Bauermeister A, Rezende-Teixeira P, Gaudencio SP, Costa-Lotufo LV. 2020. Enriching cancer pharmacology with drugs of marine origin. Br J Pharmacol. 177(1):3–27.
- Karve AS, Desai JM, Gadgil SN, Dave N, Wise-Draper TM, Gudelsky GA, Phoenix TN, DasGupta B, Yogendran L, Sengupta S, et al. 2024. A review of approaches to potentiate the activity of temozolomide against glioblastoma to overcome resistance. Int J Mol Sci. 25:3217.
- Kasprzak A. 2023. Prognostic biomarkers of cell proliferation in colorectal cancer (CRC): from immunohistochemistry to molecular biology techniques. Cancers. 15(18):4570. doi: 10.3390/cancers15184570.
- Khotimchenko R, Bryukhovetskiy I, Khotimchenko M, Khotimchenko Y. 2021. Bioactive compounds with antiglioma activity from marine species. Biomedicines. 9(8):886. doi: 10.3390/biomedicines9080886.
- Kim MC, Cullum R, Machado H, Smith AJ, Yang I, Rodvold JJ, Fenical W. 2019. Photopiperazines A–D, photosensitive interconverting diketopiperazines with significant and selective activity against U87 glioblastoma cells, from a rare, marine-derived actinomycete of the family Streptomycetaceae. J Nat Prod. 82(8):2262–2267.
- Kocaturk NM, Akkoc Y, Kig C, Bayraktar O, Gozuacik D, Kutlu O. 2019. Autophagy as a molecular target for cancer treatment. Eur J Pharm Sci. 134:116–137. doi: 10.1016/j.ejps.2019.04.011.
- Kundu M, Das S, Nandi S, Dhara D, Mandal M. 2023. Magnolol and temozolomide exhibit a synergistic anti-glioma activity through MGMT inhibition. Biochim Biophys Acta Mol Basis Dis. 1869(7):166782. doi: 10.1016/j.bbadis.2023.166782.
- Lang F, Liu Y, Chou FJ, Yang C. 2021. Genotoxic therapy and resistance mechanism in gliomas. Pharmacol Ther. 228:107922. doi: 10.1016/j.pharmthera.2021.107922.
- Lei G, Zhuang L, Gan B. 2022. Targeting ferroptosis as a vulnerability in cancer. Nat Rev Cancer. 22(7):381–396. doi: 10.1038/s41568-022-00459-0.
- Li Q, Zhu R, Yi W, Chai W, Zhang Z, Lian XY. 2018. Peniciphenalenins A–F from the culture of a marine-associated fungus Penicillium sp. ZZ901. Phytochemistry. 152:53–60. doi: 10.1016/j.phytochem.2018.04.021.
- Li W, Xie X, Wu T, Yang H, Peng Y, Luo L, Chen Y. 2020. Targeted delivery of auristatin PE to Hep G2 cells using folate-conjugated boron nitride nanotubes. Mater Sci Eng C Mater Biol Appl. 109:110509.
- Liang Y, Xie X, Chen L, Yan S, Ye X, Anjum K, Huang H, Lian X, Zhang Z. 2016. Bioactive polycyclic quinones from marine Streptomyces sp. 182SMLY. Mar Drugs. 14(1):10.
- Liao S, Yuk N, Kim YJ, Xu H, Li X, Wang L, Liu Y, Jung HJ. 2023. Novel terpestacin derivatives with l-amino acid residue as anticancer agents against U87MG-derived glioblastoma stem cells. Bioorg Chem. 132:106392.
- Liberio MS, Sadowski MC, Nelson CC, Davis RA. 2014. Identification of eusynstyelamide B as a potent cell cycle inhibitor following the generation and screening of an ascidian-derived extract library using a real time cell analyzer. Mar Drugs. 12(10):5222–5239. doi: 10.3390/md12105222.
- Lin Y, Wu X, Feng S, Jiang G, Luo J, Zhou S, Vrijmoed LL, Jones EB, Krohn K, Steingrover K, et al. 2001. Five unique compounds: xyloketals from mangrove fungus Xylaria sp. from the South China Sea coast. J Org Chem. 66:6252–6256.
- Martin V, Vale C, Bondu S, Thomas OP, Vieytes MR, Botana LM. 2013. Differential effects of crambescins and crambescidin 816 in voltage-gated sodium, potassium and calcium channels in neurons. Chem Res Toxicol. 26(1):169–178.
- Martínez-Reyes I, Chandel NS. 2021. Cancer metabolism: looking forward. Nat Rev Cancer. 21(10):669–680. doi: 10.1038/s41568-021-00378-6.
- Martin-Perez M, Urdiroz-Urricelqui U, Bigas C, Benitah SA. 2022. The role of lipids in cancer progression and metastasis. Cell Metab. 34(11):1675–1699. doi: 10.1016/j.cmet.2022.09.023.
- Mattos DR, Wan X, Serrill JD, Nguyen MH, Humphreys IR, Viollet B, Smith AB3rd, McPhail KL, Ishmael JE. 2022. The marine-derived macrolactone mandelalide A is an indirect activator of AMPK. Mar Drugs. 20(7):418.
- Medina RA, Goeger DE, Hills P, Mooberry SL, Huang N, Romero LI, Ortega-Barria E, Gerwick WH, McPhail KL. 2008. Coibamide A, a potent antiproliferative cyclic depsipeptide from the Panamanian marine cyanobacterium Leptolyngbya sp. J Am Chem Soc. 130:6324–6325.
- Mizushima N, Komatsu M. 2011. Autophagy: renovation of cells and tissues. Cell. 147(4):728–741. doi: 10.1016/j.cell.2011.10.026.
- Mohammadi M, Bagheri L, Badreldin A, Fatehi P, Pakzad L, Suntres Z, van Wijnen AJ. 2022. Biological effects of gyrophoric acid and other lichen derived metabolites, on cell proliferation, apoptosis and cell signaling pathways. Chem Biol Interact. 351:109768. doi: 10.1016/j.cbi.2021.109768.
- Mokarram P, Albokashy M, Zarghooni M, Moosavi MA, Sepehri Z, Chen QM, Hudecki A, Sargazi A, Alizadeh J, Moghadam AR, et al. 2017. New frontiers in the treatment of colorectal cancer: autophagy and the unfolded protein response as promising targets. Autophagy. 13(5):781–819. doi: 10.1080/15548627.2017.1290751.
- Murugesan M, Kandhavelu M, Thiyagarajan R, Natesan S, Rajendran P, Murugesan A. 2023. Marine halophyte derived polyphenols inhibit glioma cell growth through mitogen-activated protein kinase signaling pathway. Biomed Pharmacother. 159:114288. doi: 10.1016/j.biopha.2023.114288.
- Nakahata N, Yaginuma T, Ohizumi Y. 1999. Maitotoxin-induced phosphoinositide hydrolysis is dependent on extracellular but not intracellular Ca2+ in human astrocytoma cells. Jpn J Pharmacol. 81(2):240–243. doi: 10.1254/jjp.81.240.
- Nasrolahi A, Azizidoost S, Radoszkiewicz K, Najafi S, Ghaedrahmati F, Anbiyaee O, Khoshnam SE, Farzaneh M, Uddin S. 2023. Signaling pathways governing glioma cancer stem cells behavior. Cell Signal. 101:110493. doi: 10.1016/j.cellsig.2022.110493.
- Neupane RP, Parrish SM, Neupane JB, Yoshida WY, Yip MLR, Turkson J, Harper MK, Head JD, Williams PG. 2019. Cytotoxic sesquiterpenoid quinones and quinols, and an 11-membered heterocycle, kauamide, from the Hawaiian marine sponge Dactylospongia elegans. Mar Drugs. 17(7):423.
- Nguyen HM, Guz-Montgomery K, Lowe DB, Saha D. 2021. Pathogenetic features and current management of glioblastoma. Cancers. 13(4):856. doi: 10.3390/cancers13040856.
- Norris MD, Perkins MV. 2016. Structural diversity and chemical synthesis of peroxide and peroxide-derived polyketide metabolites from marine sponges. Nat Prod Rep. 33(7):861–880. doi: 10.1039/c5np00142k.
- Obrador E, Moreno-Murciano P, Oriol-Caballo M, Lopez-Blanch R, Pineda B, Gutierrez-Arroyo JL, Loras A, Gonzalez-Bonet LG, Martinez-Cadenas C, Estrela JM, et al. 2024. Glioblastoma therapy: past, present and future. Int J Mol Sci. 25:2529.
- Oliveira C, Granja S, Neves NM, Reis RL, Baltazar F, Silva TH, Martins A. 2019. Fucoidan from Fucus vesiculosus inhibits new blood vessel formation and breast tumor growth in vivo. Carbohydr Polym. 223:115034. doi: 10.1016/j.carbpol.2019.115034.
- Ostrom QT, Price M, Neff C, Cioffi G, Waite KA, Kruchko C, Barnholtz-Sloan JS. 2022. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2015–2019. Neuro Oncol. 24(Suppl. 5):v1–v95. doi: 10.1093/neuonc/noac202.
- Padhy I, Paul P, Sharma T, Banerjee S, Mondal A. 2022. Molecular mechanisms of action of eugenol in cancer: recent trends and advancement. Life. 12(11):1795. doi: 10.3390/life12111795.
- Peng X, Zhang S, Wang Y, Zhou Z, Yu Z, Zhong Z, Zhang L, Chen ZS, Claret FX, Elkabets M, et al. 2023. Stellettin B sensitizes glioblastoma to DNA-damaging treatments by suppressing PI3K-mediated homologous recombination repair. Adv Sci. 10(3):e2205529.
- Persano F, Gigli G, Leporatti S. 2022. Natural compounds as promising adjuvant agents in the treatment of gliomas. Int J Mol Sci. 23(6):3360. doi: 10.3390/ijms23063360.
- Piazzini V, Vasarri M, Degl’Innocenti D, Guastini A, Barletta E, Salvatici MC, Bergonzi MC. 2019. Comparison of chitosan nanoparticles and soluplus micelles to optimize the bioactivity of Posidonia oceanica extract on human neuroblastoma cell migration. Pharmaceutics. 11(12):655. doi: 10.3390/pharmaceutics11120655.
- Pires HFO, da Silva PR, Dias AL, de Sousa Gomes C, de Sousa NF, Dos Santos AMF, Souza LRP, de Figueiredo Lima JL, Oliveira MCN, Felipe CFB, et al. 2024. Mechanisms involved in the therapeutic effect of cannabinoid compounds on gliomas: a review with experimental approach. Curr Protein Pept Sci. 25(1):27–43.
- Posor Y, Jang W, Haucke V. 2022. Phosphoinositides as membrane organizers. Nat Rev Mol Cell Biol. 23(12):797–816. doi: 10.1038/s41580-022-00490-x.
- Pruteanu L-L, Kopanitsa L, Módos D, Kletnieks E, Samarova E, Bender A, Gomez LD, Bailey DS. 2020. Transcriptomics predicts compound synergy in drug and natural product treated glioblastoma cells. PLOS One. 15(9):e0239551. doi: 10.1371/journal.pone.0239551.
- Qin L, Yi W, Lian XY, Zhang Z. 2020. Bioactive alkaloids from the Actinomycete Actinoalloteichus sp. ZZ1866. J Nat Prod. 83(9):2686–2695. doi: 10.1021/acs.jnatprod.0c00588.
- Qin L, Yong K, Lian XY, Zhang Z. 2023. Streptonaphthyridine A, a new naphthyridine analogue with antiproliferative activity against human glioma cells from Mariana trench-associated actinomycete Streptomyces sp. SY2111. Nat Prod Res. 37(3):478–483.
- Qiu G, Li F, Kowah JAH, Xie J, Long Q, Wang L, Liu X. 2024. Novel chiral matrine derivatives as potential antitumor agents: design, synthesis and biological evaluation. Bioorg Chem. 146:107276. doi: 10.1016/j.bioorg.2024.107276.
- Ramar V, Guo S, Hudson B, Liu M. 2023. Progress in glioma stem cell research. Cancers. 16(1):102. doi: 10.3390/cancers16010102.
- Salimi A, Rahimitabar N, Vazirizadeh A, Adhami V, Pourahmad J. 2021. Persian Gulf snail crude venom (Conus textile): a potential source of anti-cancer therapeutic agents for glioblastoma through mitochondrial-mediated apoptosis. Asian Pac J Cancer Prev. 22(S1):49–57. doi: 10.31557/APJCP.2021.22.S1.49.
- Schaff LR, Mellinghoff IK. 2023. Glioblastoma and other primary brain malignancies in adults: a review. JAMA. 329(7):574–587. doi: 10.1001/jama.2023.0023.
- Schreck KC, Grossman SA, Pratilas CA. 2019. BRAF mutations and the utility of RAF and MEK inhibitors in primary brain tumors. Cancers. 11(9):1262. doi: 10.3390/cancers11091262.
- Schwartsmann G, Brondani da Rocha A, Berlinck RG, Jimeno J. 2001. Marine organisms as a source of new anticancer agents. Lancet Oncol. 2(4):221–225. doi: 10.1016/s1470-2045(00)00292-8.
- Serrill JD, Wan X, Hau AM, Jang HS, Coleman DJ, Indra AK, Alani AW, McPhail KL, Ishmael JE. 2016. Coibamide A, a natural lariat depsipeptide, inhibits VEGFA/VEGFR2 expression and suppresses tumor growth in glioblastoma xenografts. Invest New Drugs. 34(1):24–40. doi: 10.1007/s10637-015-0303-x.
- Sharifzad F, Ghavami S, Verdi J, Mardpour S, Mollapour Sisakht M, Azizi Z, Taghikhani A, Los MJ, Fakharian E, Ebrahimi M, et al. 2019. Glioblastoma cancer stem cell biology: potential theranostic targets. Drug Resist Updat. 42:35–45.
- Siangcham T, Vivithanaporn P, Sangpairoj K. 2020. Anti-migration and invasion effects of astaxanthin against A172 human glioblastoma cell line. Asian Pac J Cancer Prev. 21(7):2029–2033. doi: 10.31557/APJCP.2020.21.7.2029.
- Song T, Tang M, Ge H, Chen M, Lian X, Zhang Z. 2019. Novel bioactive penicipyrroether A and pyrrospirone J from the marine-derived Penicillium sp. ZZ380. Mar Drugs. 17(5):292.
- Sun W, Zhang W, Yu J, Lu Z, Yu J. 2021. Inhibition of Nrf2 might enhance the anti-tumor effect of temozolomide in glioma cells via inhibition of Ras/Raf/MEK signaling pathway. Int J Neurosci. 131:975–983.
- Tabunoki H, Saito N, Suwanborirux K, Charupant K, Satoh J. 2012. Molecular network profiling of U373MG human glioblastoma cells following induction of apoptosis by novel marine-derived anti-cancer 1,2,3,4-tetrahydroisoquinoline alkaloids. Cancer Cell Int. 12(1):14. doi: 10.1186/1475-2867-12-14.
- Tang SA, Zhou Q, Guo WZ, Qiu Y, Wang R, Jin M, Zhang W, Li K, Yamori T, Dan S, et al. 2014. In vitro antitumor activity of stellettin B, a triterpene from marine sponge Jaspis stellifera, on human glioblastoma cancer SF295 cells. Mar Drugs. 12(7):4200–4213.
- Tang W, Liu ZL, Mai XY, Qi X, Li DH, Gu QQ, Li J. 2020. Identification of gliotoxin isolated from marine fungus as a new pyruvate kinase M2 inhibitor. Biochem Biophys Res Commun. 528(3):594–600. doi: 10.1016/j.bbrc.2020.05.139.
- Terasaki M, Ikuta M, Kojima H, Tanaka T, Maeda H, Miyashita K, Mutoh M. 2019. Dietary fucoxanthin induces anoikis in colorectal adenocarcinoma by suppressing integrin signaling in a murine colorectal cancer model. J Clin Med. 9(1):90.
- Tian LW, Feng Y, Shimizu Y, Pfeifer T, Wellington C, Hooper JN, Quinn RJ. 2014. Aplysinellamides A–C, bromotyrosine-derived metabolites from an Australian Aplysinella sp. marine sponge. J Nat Prod. 77(5):1210–1214. doi: 10.1021/np500119e.
- Tian X, Tang H, Lin H, Cheng G, Wang S, Zhang X. 2013. Saponins: the potential chemotherapeutic agents in pursuing new anti-glioblastoma drugs. Mini Rev Med Chem. 13:1709–1724.
- Tomar MS, Kumar A, Srivastava C, Shrivastava A. 2021. Elucidating the mechanisms of temozolomide resistance in gliomas and the strategies to overcome the resistance. Biochim Biophys Acta Rev Cancer. 1876(2):188616. doi: 10.1016/j.bbcan.2021.188616.
- Trisciuoglio D, Uranchimeg B, Cardellina JH, Meragelman TL, Matsunaga S, Fusetani N, Del Bufalo D, Shoemaker RH, Melillo G. 2008. Induction of apoptosis in human cancer cells by candidaspongiolide, a novel sponge polyketide. J Natl Cancer Inst. 100(17):1233–1246. doi: 10.1093/jnci/djn239.
- Tzitiridou P, Zoi V, Papagrigoriou T, Lazari D, Sioka C, Alexiou GA, Kyritsis AP. 2024. Antineoplastic activity of 9″-lithospermic acid methyl ester in glioblastoma cells. Int J Mol Sci. 25(4):2094. doi: 10.3390/ijms25042094.
- van Rixel VHS, Ramu V, Auyeung AB, Beztsinna N, Leger DY, Lameijer LN, Hilt ST, Le Devedec SE, Yildiz T, Betancourt T, et al. 2019. Photo-uncaging of a microtubule-targeted rigidin analogue in hypoxic cancer cells and in a xenograft mouse model. J Am Chem Soc. 141:18444–18454.
- Wätjen W, Ebada SS, Bergermann A, Chovolou Y, Totzke F, Kubbutat MHG, Lin W, Proksch P. 2017. Cytotoxic effects of the anthraquinone derivatives 1′-deoxyrhodoptilometrin and (S)-(−)-rhodoptilometrin isolated from the marine echinoderm Comanthus sp. Arch Toxicol. 91(3):1485–1495. doi: 10.1007/s00204-016-1787-7.
- Watters DJ. 2018. Ascidian toxins with potential for drug development. Mar Drugs. 16(5):162.
- Wen ZH, Kuo HM, Shih PC, Hsu LC, Chuang JM, Chen NF, Sun HW, Liu HT, Sung CS, Chen WF. 2023. Isoaaptamine increases ROS levels causing autophagy and mitochondria-mediated apoptosis in glioblastoma multiforme cells. Biomed Pharmacother. 160:114359. doi: 10.1016/j.biopha.2023.114359.
- Wu Y, Song Y, Wang R, Wang T. 2023. Molecular mechanisms of tumor resistance to radiotherapy. Mol Cancer. 22(1):96. doi: 10.1186/s12943-023-01801-2.
- Xie G, Zhu X, Li Q, Gu M, He Z, Wu J, Li J, Lin Y, Li M, She Z, et al. 2010. SZ-685C, a marine anthraquinone, is a potent inducer of apoptosis with anticancer activity by suppression of the Akt/FOXO pathway. Br J Pharmacol. 159(3):689–697.
- Xie Q, Yang Z, Huang X, Zhang Z, Li J, Ju J, Zhang H, Ma J. 2019. Ilamycin C induces apoptosis and inhibits migration and invasion in triple-negative breast cancer by suppressing IL-6/STAT3 pathway. J Hematol Oncol. 12(1):60. doi: 10.1186/s13045-019-0744-3.
- Xue Y, Fu Y, Zhao F, Gui G, Li Y, Rivero-Hinojosa S, Liu G, Li Y, Xia S, Eberhart CG, et al. 2021. Frondoside A inhibits an MYC-driven medulloblastoma model derived from human-induced pluripotent stem cells. Mol Cancer Ther. 20(6):1199–1209. doi: 10.1158/1535-7163.MCT-20-0603.
- Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang J, Zhang G, Wang X, Dong Z, Chen F, et al. 2020. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 5(1):8.
- Yao Y, Sun S, Cao M, Mao M, He J, Gai Q, Qin Y, Yao X, Lu H, Chen F, et al. 2020. Grincamycin B functions as a potent inhibitor for glioblastoma stem cell via targeting RHOA and PI3K/AKT. ACS Chem Neurosci. 11(15):2256–2265.
- Ye X, Anjum K, Song T, Wang W, Liang Y, Chen M, Huang H, Lian XY, Zhang Z. 2017. Antiproliferative cyclodepsipeptides from the marine actinomycete Streptomyces sp. P11-23B downregulating the tumor metabolic enzymes of glycolysis, glutaminolysis, and lipogenesis. Phytochemistry. 135:151–159. doi: 10.1016/j.phytochem.2016.12.010.
- Yi W, Lian XY, Zhang Z. 2022. Cytotoxic metabolites from the marine-associated Streptomyces sp. ZZ1944. Phytochemistry. 201:113292. doi: 10.1016/j.phytochem.2022.113292.
- Yi W, Newaz AW, Yong K, Ma M, Lian XY, Zhang Z. 2022. New hygrocins K-U and streptophenylpropanamide A and bioactive compounds from the marine-associated Streptomyces sp. ZZ1956. Antibiotics. 11(11):1455. doi: 10.3390/antibiotics11111455.
- Yong K, Kaleem S, Ma M, Lian X, Zhang Z. 2022. Antiglioma natural products from the marine-associated fungus Penicillium sp. ZZ1750. Molecules. 27(20):7099. doi: 10.3390/molecules27207099.
- Yong K, Kaleem S, Wu B, Zhang Z. 2021. New antiproliferative compounds against glioma cells from the marine-sourced fungus Penicillium sp. ZZ1750. Mar Drugs. 19(19):483.
- Yu S, Ye X, Huang H, Peng R, Su Z, Lian XY, Zhang Z. 2015. Bioactive sulfated saponins from sea cucumber Holothuria moebii. Planta Med. 81(2):152–159. doi: 10.1055/s-0034-1383404.
- Zhang D, Yi W, Ge H, Zhang Z, Wu B. 2019. Bioactive streptoglutarimides A–J from the marine-derived Streptomyces sp. ZZ741. J Nat Prod. 82(10):2800–2808.
- Zhang D, Yi W, Ge H, Zhang Z, Wu B. 2021. A new antimicrobial indoloditerpene from a marine-sourced fungus aspergillus versicolor ZZ761. Nat Prod Res. 35:3114–3119.
- Zhang QT, Liu ZD, Wang Z, Wang T, Wang N, Wang N, Zhang B, Zhao YF. 2021. Recent advances in small peptides of marine origin in cancer therapy. Mar Drugs. 19:115.
- Zhang X, Chen L, Chai W, Lian XY, Zhang Z. 2017. A unique indolizinium alkaloid streptopertusacin A and bioactive bafilomycins from marine-derived Streptomyces sp. HZP-2216E. Phytochemistry. 144:119–126. doi: 10.1016/j.phytochem.2017.09.010.
- Zhang X, Ye X, Chai W, Lian XY, Zhang Z. 2016. New metabolites and bioactive actinomycins from marine-derived Streptomyces sp. ZZ338. Mar Drugs. 14(10):181.
- Zhang Z, Chen L, Zhang X, Liang Y, Anjum K, Chen L, Lian XY. 2017. Bioactive bafilomycins and a new N-arylpyrazinone derivative from marine-derived Streptomyces sp. HZP-2216E. Planta Med. 83(18):1405–1411. doi: 10.1055/s-0043-111897.
- Zheng L, Yi Y, Liu J, Lin X, Yang K, Lv M, Zhou X, Hao J, Liu J, Zheng Y, et al. 2014. Isolation and characterization of marine Brevibacillus sp. S-1 collected from South China Sea and a novel antitumor peptide produced by the strain. PLOS One. 9(11):e111270. doi: 10.1371/journal.pone.0111270.
- Zhou B, Ji YY, Zhang HJ, Shen L. 2021. Gephyyamycin and cysrabelomycin, two new angucyclinone derivatives from the Streptomyces sp. HN-A124. Nat Prod Res. 35(13):2117–2122. doi: 10.1080/14786419.2019.1660336.
- Zhou X, Liu J, Yang B, Lin X, Yang XW, Liu Y. 2013. Marine natural products with anti-HIV activities in the last decade. Curr Med Chem. 20:953–973.
- Zhu Q, Zhou Y, Wang H, Cao T, Wang X, Liu R, Wu H, Lin B. 2023. Fucoxanthin triggers ferroptosis in glioblastoma cells by stabilizing the transferrin receptor. Med Oncol. 40(8):230. doi: 10.1007/s12032-023-02095-6.