Abstract
A synopsis of the genus Philosina is provided. Larvae of the two known species, P. alba and P. buchi are described for the first time. The distribution of both species is discussed and information on behaviour and habitat is summarized. The specialized larvae of Philosina show a strong resemblance to those of Rhinagrion, suggesting that they are sister genera. The unique characters of the larva, especially the arrangement and structure of the caudal lamellae, mean that neither genus fits into any of the currently recognized families of Zygoptera. It is noted that these genera could be placed in their own family. However, caution is exercised pending a better understanding of the family Megapodagrionidae based on DNA work, and they are therefore retained in Megapodagrionidae.
Introduction
The genus Philosina was established for Philosina buchi Ris, Citation1917, which was described in the same paper from Fujian, China. Philosina was placed in Megapodagrionidae based on wing venation, but was considered sufficiently distinct from other genera in this family to be placed in its own subfamily Philosininae by Kennedy Citation(1925) and later authors (e.g. Davies & Tobin, Citation1984). In 1999 a second species of the genus, P. alba Wilson, Citation1999, was described from Guangdong. Only a handful of records have been published for either species, information on their distribution, habitat and behaviour is scarce, and no larva has been described for the genus. Based on resemblance of the adults and preliminary DNA results Kalkman, Choong, Orr and Schütte Citation(2010) suggested that Philosina and Rhinagrion Calvert, 1913 might be closely related. The larva of Rhinagrion is structurally unique among the Zygoptera and it was suggested that larval characters of Philosina, once discovered, would either confirm or falsify the hypothesis of a close relationship between Philosina and Rhinagrion. HZ was able to collect larvae of both Philosina alba and P. buchi which are here described for the first time.
Material and methods
Terminology largely follows Watson and O'Farrell Citation(1991).
Acronyms for collections are as follows:
SCAU South China Agricultural University, Guangzhou, China
CUMZ Cambridge University Museum of Zoology, Cambridge, UK
NMSF Forschungsinstitut und Naturmuseum Senckenberg, Frankfurt a.M, Germany
RMNH Nationaal Natuurhistorisch Museum Naturalis, Leiden, the Netherlands
Recognition of Philosina
Adults of Philosina can be distinguished from all zygopteran genera except Rhinagrion by the combination of the following characters: (1) two antenodal crossveins; (2) Pt more than twice as long as broad; (3) R4 and IR3 originating well beyond midpoint between the arculus and the level of the subnodus; (4) median cleft of labium deep, about half the length of the prementum itself.
Philosina alba Wilson, 1999; (Figures 1–4, 5a–d)
Specimens studied
Adults: 2 ♂, China, Hainan Province, Lishui County, Mt. Diaoluoshan, 900 m, 24 April 2009, leg: HZ, SCAU. — 4 ♂, 1 ♀, China, Guangdong Province, Conghua City, Liuxihe National Forest Park (23°43′51 N, 113°48′24
E), 15 May 2009, leg. HZ, SCAU. — 1 ♀, China, Hainan Province, Lishui County, Mt. Diaoluoshan, 23 April 2009, leg: HZ, RMNH. — 1 ♂, China, Hainan Province, Lishui County, Mt. Diaoluoshan, 15 May 2009, leg: HZ, RMNH.
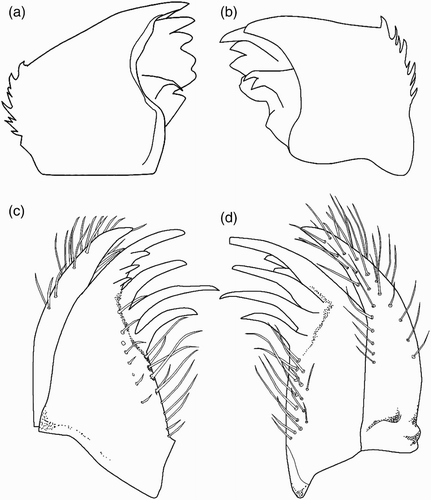
Figure 3. Larval structure of Philosina alba: (a) labium, dorsal view; (b) ventral view of female abdomen showing gonapophyses; (c) right lateral palp of labium, dorsal view; (d) antenna (setae not shown); (e) pattern on median lamella, lateral view; (f) pattern on lateral lamella, lateral view.
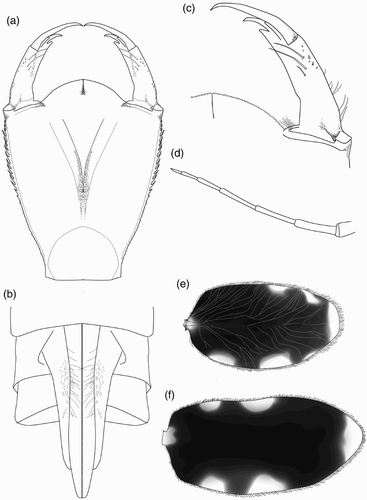
Figure 4. Larval structure of Philosina alba: (a) right mandible, ventral view; (b) left mandible, ventral view; (c) right maxilla, ventral view; (d) same, dorsal view.
Larvae (final instar): 1 ♂, 1 ♀, China, Guangdong Province, Conghua City, Liuxihe National Forest Park (23°43′51 N, 113°48′24
E), 12 March 2009, leg. HZ, SCAU. — 1 ♂, same location, 25 November 2008, RMNH.
Differential diagnosis of adults
Males are easily recognised in the field by their habit of perching with their wings held flat, their medium size (Hw 28–31 mm), moderately heavy build and the whitish-blue pruinosity covering all segments of the abdomen (a–c). The thorax does not become as pruinose as in P. buchi and the yellow antehumeral stripes are clearly visible even in fully mature males. Females are less striking than males but are still easy to identify by their medium size and black abdomen with broad lateral yellow stripes and a thin median stripe on S1-7 (d). The only species with a very similar pattern is P. buchi, but in this species the antehumeral stripe runs the full length of the mesepimeron (only for four-fifths in P. alba), and there is an additional thin yellow line below the mesopleural suture which is absent or only present along the posterior half of the suture in P. alba.
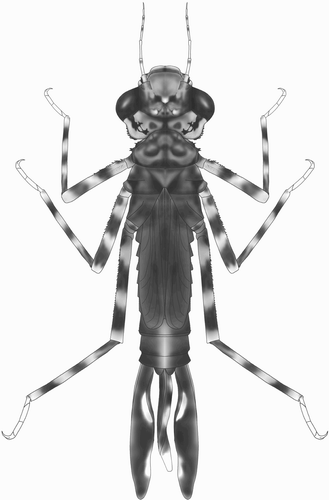
Description of the larva
A stout, brownish larva with depressed head and short abdomen (), surface of body covered with minute pyramidal nodules.
Head
Head flattened above, widest across the eyes, rear of head square but hind corner rounded and bearing irregular stout spines, hind margin of occiput concave. Row of six short spines along the eye-margin on the underside of the head. The labrum, clypeus and frons are entirely dark brown; anterior margin of anteclypeus with a row of fine setae. Ocellar triangle pale brown, ocelli large and distinct. Antenna with seven segments, long and filiform with fine setae, pale brown (d). Antennal segments 1, 2, 4 and 5 each with a black subapical ring. Length of each antennal segment (mm): 0.40; 0.60; 0.68; 0.64; 0.40; 0.28; 0.20. Mandibles biramous, incisor of the right mandible with six teeth, identical to the left one, the molar surface with one tooth, less developed, hook shaped and not bifid apically (a). Incisor of the left mandible with six teeth, the molar surface with one large tooth, apically bifid (b). Maxilla with four long, stout dorsal hooks and three smaller ventral hooks, sharply pointed (c, d). Basally with long setae. Maxillary palp curved and tapering to a blunt point, bearing dense, long setae. Labium broad and flat (a). Length to width ratio of prementum 1.25:1. Median lobe strongly developed, anterior margin strongly convex with regular short, crenulations and an apically closed median cleft (c). Outer margin of prementum with strong, sharp, forward pointing denticles. Premental setae absent. Labial palp narrow, bearing three inner, subapical setae and apically with three strong and sharply pointed hooks, the median one largest; movable hook long and sharply pointed (c).
Thorax
Thorax brown, sides of prothorax with two prominences, one beneath pronotum with the outer margin slightly concave, the lower one pyramid-shaped, approaching the front coxa. Distal margins of these prominences with pyramidal spines. Pronotum well developed and shield-shaped; brown with a pair of black spots near the hind margin. Anterior margin more or less straight, anterior corner forming an angle of 135°, posterior corner rounded. Synthorax dark brown and robust, wing buds narrow, reaching the hind margin of abdominal S8. Legs with hind femora reaching S9, covered with fine setae and with rows of short spines. All femora and tibiae brown with three pale bands. Anterior margin of each femur with a row of short pyramidal spines. Tarsi with three segments, claws simple.
Abdomen
Brown and densely covered with fine setae. Cylindrical in shape and slightly tapering backwards, with a pale middorsal line on each segment. Lateral spines and dorsal hooks absent. Caudal lamellae arranged vertically, broad and oblong, largely black with marginal pale spots, the margin bearing with long, fine setae. The median lamella (e) is shorter than the lateral ones (f) and has on both sides a slightly expanded midrib, which is free of spines. The lateral lamellae have basally an expanded midrib on the exterior, bearing dense, short spines. The median lamella is thinner than the fleshy, undulating lateral lamellae. Female gonapophyses long, almost twice as long as S9-10 (b).
Measurements (mm)
Body length (including lamellae): 14.1; maximum width of head: 4.4 hind femur: 5.0; hind wing buds: 4.8; median lamella: 3.8; lateral lamellae: 5.5.
Distribution, habitat and behaviour
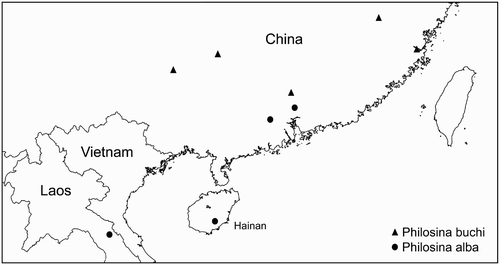
Philosina alba was described from Dinghushan, Zhaoqing City, Guangdong, China (Wilson, Citation1999; Wilson & Xu, Citation2007) and has since been found in the Chinese province of Hainan (Wilson & Reels, Citation2001) and in the Lak Sao area in Laos (Karube, Citation2002) ().
The habitats at Diaoluoshan and at Liuxihe National Forest Park are largely shaded, large, rocky brooks in forest (). The species has been recorded from 350–500 m a.s.l. (Liuxihe National Forest Park) and between 850–1000 m a.s.l. (Diaoluoshan).
The earliest seasonal record is that of an immature male on 25 March 2008 at Diaoluoshan National Nature Reserve, Hainan, observed by HZ and Shanlian Mo. In late April of that year more than 10 mature males were observed in three days. At Liuxihe National Forest Park (Guangdong Province) more than 50 males were seen on a single day in mid May. The species was still common there in June but by mid July only two old males could be found. Based on these records the flight period extends from the end of March well into July, with the highest densities found from late April to June.
Wilson and Reels Citation(2001) stated that males hold “territory on horizontal surfaces of logs and tree roots, which overhang small fast running hill streams”. At Diaoluoshan and at Liuxihe National Forest Park males usually occupied territories on the slower flowing parts of the stream at places bordered by dense vegetation. They perched on trees or branches along the shady streams at about 0.5–2 m height and established territories but seldom patrolled. Males with a territory were seen to drive other males away, during which activity they were hovering face to face mid air. Following a chase the male was seen to return to his territory. When disturbed, they quickly moved away with a sudden and rapid flight. No courtship was observed; a single copulation was observed in the afternoon.
Philosina buchi Ris, 1917 (Figures 1, 5e–f, 6–9)
Specimens studied
Adults: 15♂, 1♀ (syntypes), Fujian Province, 1916, leg. P.A. Buch, NMSF (numbered as 7192, 7193, 7194, 7200, 7205, 7210–7212, 7214–7217, 7219, 7220, 7222, 7223 (female)). — 17♂, Fujian Province, 1917, leg. P.A. Buch, NMSF (numbered as 7191, 7195–7199, 7201–7204, 7206–7209, 7213, 7218, 7221). — 2 ♂, 2 ♀, China, Guangxi Province, Guilin City, Longsheng, 11–15 July 2005, leg. VJK, RMNH. — 1 ♂, 4 ♀, China, Guangxi Province, between Longsheng and hot springs, 13 July 2005, leg. VJK, RMNH. — 2 ♂, China, Guizhou Province, Libo County, Maolan Nature Reserve, Zhangjiang River, 7 May 2007, leg. HZ, SCAU. —1 ♂, China, same locality, 27 July 2008, leg. HZ, SCAU.
Additional records: China, Guangxi Province, Guilin City, Longsheng, 11–15 July 2000, M. Wasscher (picture of male published on www.asia-dragonfly).
Larva: 1 ♀, China, Guangdong Province, Yingde City, Qiaotou, Wengjiang River (24°16′36 N, 113°46′38
E), 27 February 2009, leg. Yufeng Wang, SCAU.
Differential diagnosis of adults
Males are very striking and are easily recognized by their large size (Hw 39–41 mm), blue pruinosity on head, thorax, S1-6 and S10 and bright red S7-9 of the abdomen (e). With their size and strong flight they can be mistaken for Anisoptera, especially on the wing, but no Chinese Anisoptera species has S7-9 red with the remainder of the abdomen blue. The genital ligula is illustrated in a, b. Females are less striking than males but are still easy to identify by their large size and black abdomen with broad lateral stripes and a thin median stripe from S1-7. Differences from the female of P. alba are given in the diagnosis of that species.
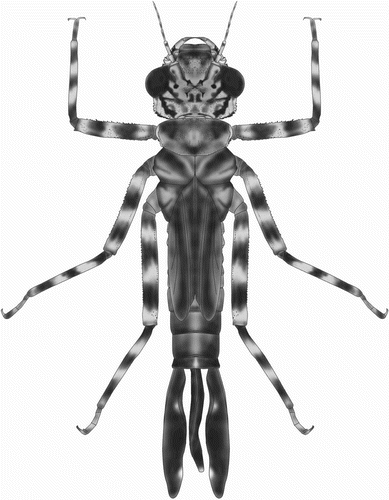
Description of the larva
A stout, brownish larva with depressed head and short abdomen (), surface of body covered with minute pyramidal and rounded nodules.
Head
Head flattened above, widest across the eyes, rear of head square but hind corner rounded, with a broad black stripe on each side, and bearing stout denticles; hind margin of occiput concave. Six spines are present along the eye-margin on the underside of the head. Labrum and clypeus entirely dark brown. Frons largely brown with a pale central spot. Ocellar triangle pale brown; ocelli large and distinct. Antenna with seven segments, long and filiform with fine setae; pale brown, darkening toward the apex of each segment except the basal one (e). Length of each antennal segment (mm): 0.52; 0.88; 0.72; 0.44; 0.56; 0.56; 0.48. Mandibles biramous, incisor of the right mandible with six teeth, similar to the left one, the molar surface with one, less developed, tooth (a). Incisor of the left mandible with six teeth, the molar surface with one large tooth, apically bifid and pointed (b). Outer margin of each mandible with two rows of well developed large spines. The ventral row contains six sharply pointed dentate spines. Maxilla with four long, stout dorsal hooks and three smaller ventral hooks, sharply pointed. Basally with long setae. Maxillary palp curved and tapered to a blunt point, bearing dense, long setae. Labium broad and flat (a). Ratio of length to width of prementum 1.3:1. Median lobe strongly developed, anterior margin strongly projecting with regular short crenulations and an apically closed median cleft (d). Outer margin of prementum with strong, sharp, forward pointing denticles. Premental setae absent. Labial palpus narrow, with three subapical setae and three strong and sharply pointed hooks apically, the median one largest; movable hook long and sharply pointed (c).
Figure 7. Larval structure of Philosina buchi: (a) labium, dorsal view; (b) ventral view of female abdomen showing gonapophyses; (c) right lateral palpus of labium, dorsal view; (d) median cleft, dorsal view; (e) antenna (setae not shown); (f) pattern on median lamella, lateral view; (g) pattern on lateral lamella, lateral view.
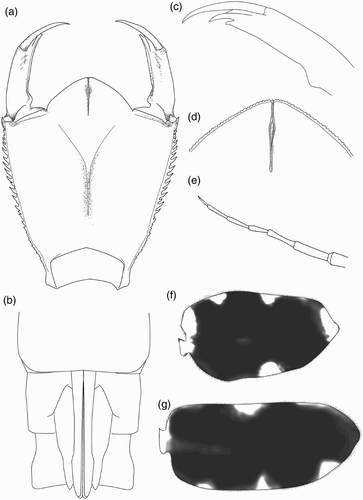
Figure 8. Larval structure of Philosina buchi: (a) right mandible, ventral view; (b) left mandible, ventral view; (c) left maxilla, ventral view; (d) same, dorsal view.
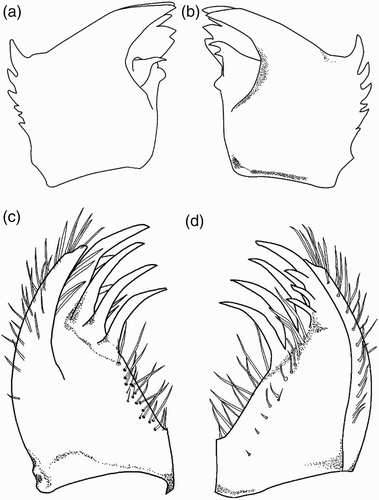
Figure 9. SEM photographs of the genital ligula of: (a) Philosina buchi, China, Gaungxi, near Longsheng, 2005, ventral view; (b) same lateral view. Photos: Dirk Gassmann.
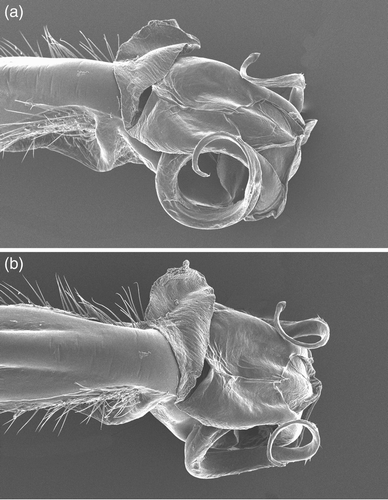
Figure 10. Habitat of the species of the Philosina. Habitat of P. alba: (a) Mt. Diaoluoshan, Hainan Province, China; (b) Liuxihe Forest Park, Guangdong Province, China. Habitat of P. buchi: (c) Longsheng, Guangxi Province, China; (d) Zhangjiang River, Guizhou Province, China. Photos by HZ (a, b, d), and VJK (c).

Thorax
Thorax brown, sides of prothorax with two prominences, one beneath pronotum bifid apically; the lower one pyramidal, approaching the front coxa. Distal margins of these prominences with pyramidal spines. Pronotum shield-shaped, its anterior margin more or less straight, anterior corner forming an angle of 135°, posterior corner rounded. Synthorax dark brown and robust, wing buds narrow, reaching the hind margin of S7. Legs with hind femora reaching halfway along S8, covered with fine setae and rows of short spines. All femora and tibiae brown with three pale bands. Anterior margin of each femur and tibia with a row of short spines. Tarsi with three segments, claws simple.
Abdomen
Cylindrical, slightly tapering backwards, with a pale middorsal line on each segment. Lateral spines and dorsal hooks absent. Caudal lamellae arranged vertically, broad and oblong, black with marginal pale spots, the margin bearing long, fine setae. The median lamella (f) is shorter than the lateral ones (g) and has on both sides a slightly expanded midrib, which is free of spines. The lateral lamellae have a basally expanded midrib on the exterior only, bearing dense, short spines. The median lamella is thinner than the fleshy undulating lateral lamellae. Female gonapophyses a little longer than S10 (b).
Measurements (mm)
Body length (including lamellae): 20.0; maximum width of head: 5.8; hind femur: 5.8; hind wing buds: 6.6; median lamella: 6.0; lateral lamellae: 7.2.
Distribution, habitat and behaviour
Philosina buchi is endemic to China and has been recorded from the provinces of Fujian (Asahina, Citation1979; Ris, Citation1917), Guangdong (Asahina, Citation1979; Wilson & Reels, Citation2003; Wilson & Xu, Citation2007) and Guangxi (Needham, Citation1930; Wilson & Reels, Citation2003) (). The locality Lo-chen-hsien in Guangxi given by Needham Citation(1930) and later also mentioned by Asahina Citation(1979) probably refers to Lo-ch'eng Hsien and is shown as such on the distribution map. The species is found along larger rivers. At Longsheng it is found on a river more than 100 m wide with steep and largely unshaded banks (). The whole area is strongly influenced by human activity with the vegetation on the riverbank consisting of agricultural fields or secondary regrowth. The water of the river is presumably strongly affected by run-off from the nearby towns and agricultural fields. Zhangjiang River is about 30 m wide. Here also the surroundings are strongly influenced by human activity and the vegetation on the river banks consists mainly of bamboo. Based on its preferred habitats and the locations were it has been found to date the species is likely confined to lower altitudes.
Many immature individuals and some mature individuals were found in early May at the Zhangjiang River (Maolan Nature Reserve). All individuals seen in July at Longsheng were adults, indicating that emergence is largely finished before July. Only a single old male was found in late July at the Zhangjiang River. Based on these records the flight period extends at least from the beginning of May to the end of July with the highest densities probably present from May to the start of July.
At Longsheng P. buchi was reasonably abundant and 10 males and four females were found along a stretch of 1 km. At Longsheng and the Zhangjiang River both males and females were found in the bushes close to the river perching on branches or the surface of leaves. A female was observed laying eggs along the bank of the river while a male hovered nearby, guarding her. Some males sat close to the water along the bank presumably in order to defend territories. The flight of the males is very strong, resembling that of anisopterans such as an Orthetrum species.
Discussion
The larvae of P. alba and P. buchi closely resemble each other but can be recognized by the characters given in . The larvae of both species of Philosina also are very similar to those of Rhinagrion viridatum Fraser, 1938, R. borneense Selys, 1886 and R. philippinum Selys, 1882 (Lieftinck, Citation1956; Needham & Gyger, Citation1939; Orr, Citation2003). gives the characters separating the larvae of Philosina from that of Rhinagrion viridatum, the species for which the best larval description is available. The resemblance between larvae of Philosina and Rhinagrion includes the general build of the larva, the strong pattern on the legs and the broad, oblong vertically arranged lamellae which are dark with distinct pale spots. In addition, several unique characters are shared only by these two genera: (1) prementum with strongly developed median lobe with deep, apically closed, median cleft; (2) median caudal lamella shorter and thinner than the lateral caudal lamellae; (3) lateral lamellae fleshy and undulating; and (4) lateral lamellae each with basally expanded midrib armed with dense spines only on exterior side. In Rhinagrion, in life the lateral lamellae form a tube shielding the median lamellae (Kalkman et al., Citation2010). This is probably also the case in Philosina, but no living larvae were studied. The tracheation of the gills in Philosina resembles that of Rhinagrion as illustrated by Lieftinck Citation(1956), which was remarked by him as “being quite unlike anything found in other larvae that I know of”. The four long, stout dorsal hooks of the maxilla in the larva of Philosina are unlike those described in other Zygoptera. However we are unsure if this character is the same in Rhinagrion and do not know enough of this character state in other genera to understand its taxonomic importance.
Table 1. Differences between larvae of Philosina alba and P. buchi.
Table 2. Differences between larvae of Rhinagrion and Philosina.
The morphology of the adults of these two genera also shows a strong resemblance. Species of both genera are thick bodied and colourful with the same type of simple pincer shaped appendages. P. buchi shows some differences in venation from that of Rhinagrion. It has, for instance, three rows of cells between the anal vein and the wing border instead of one in Rhinagrion. These differences are, however, related to its large size and P. alba, which falls within the size range of the average Rhinagrion, has the venation very similar to that genus. The only adult character separating the two genera is the pruinosity of the males of Philosina, absent in Rhinagrion. The adults of the two genera share a deep labial cleft and corresponding long mandibles that give the face a drawn out appearance, a character state not known in other megapodagrionids and in other Zygoptera found only in species of Philoganga Kirby 1890, a species generally placed in the Amphipterygidae. The relatively short spines on the femora, about as long as the distance between the spines, set both genera apart from most other genera presently included in Megapodagrionidae. The general shape of the genital ligula of P. alba is like that of R. mima (see Kalkman & Villanueva, 2010) and both have strong and very dense setae on the shaft. The genital ligula of P. buchi is of somewhat different appearance but this is mainly due to the grossly expanded and probably soft part of the ligula head.
The larval and adult characters leave no doubt that these two genera are sister groups. The unique characters of the genera, especially those of the caudal lamellae of the larva, indicate that neither of the two genera fits securely within any of the currently recognized families and that they might warrant their own family. The family-group name Philosininae, based on Philosina established by Kennedy Citation(1925) would be available for this family. It is however considered prudent to retain these genera in the Megapodagrionidae pending a better understanding of the family based on DNA work.
No genera are obvious candidates to be the closest relatives of Philosina and Rhinagrion. The two Asian genera Mesopodagrion MacLachlan, 1896 and Philoganga show some resemblance to Philosina and Rhinagrion and might be their closest, albeit distant, relatives. Aside from general build they share the relatively short spines on the femora, the simple pincer-shaped upper appendages and Philoganga also shares the long prementum with the deep medial cleft. However the larvae of Philoganga have balloon-shaped caudal appendages.
In this paper we have made one small step toward unravelling the megapod mystery. Many more questions remain.
Acknowledgements
The project was funded by the Kadoorie Farm and Botanic Garden, Hong Kong Special Administrative Region, China, and the International Dragonfly Fund. We thank Bert Orr for his helpful comments on an early version of this paper and Matti Hämäläinen, Bert Orr, and Gunther Theischinger for reviewing the paper. Marcel Wasscher provided VJK with information that Philosina buchi was found at Longsheng, Guangxi Province and Shanlian Mo provided HZ with photos and kindly offered assistance during the field surveys.
References
- Asahina , S. 1979 . Notes on Chinese Odonata, 8: three small collections in the U.S. National Museum of Natural History . Kontyû , 47 : 328 – 334 .
- Davies , D. A.L. and Tobin , P. 1984 . The dragonflies of the world: a systematic list of the extant species of Odonata. Vol. 1 Zygoptera, Anisozygoptera , Societas Internationalis Odonatologica Rapid Communications (Supplements) 3
- Kalkman , V. J. and Villanueva , R. J.T. 2011 . A synopsis of the genus Rhinagrion with description of two new species from the Philippines (Odonata: Megapodagrionidae) . International Journals of Odonatology , 14 : 11 – 31 .
- Kalkman , V. J. , Choong , C. Y. , Orr , A. G. and Schütte , K. 2010 . Remarks on the taxonomy of Megapodagrionidae with emphasis on the larval gills (Odonata) . International Journal of Odonatology , 13 : 119 – 135 .
- Karube , H. 2002 . A new record of Philosina alba Wilson from Laos . Tombo , 44 : 5 – 6 .
- Kennedy , C. H. 1925 . New genera of Megapodagrioninae with notes on the subfamily . Bulletin of Museum of the Comparative Zoology at Harvard College , 67 : 291 – 311 . pl. I excl
- Lieftinck , M. A. 1956 . Revision of the genus Argiolestes Selys (Odonata) in New Guinea and the Moluccas, with notes on the larval forms of the family Megapodagrionidae . Nova Guinea (New Series) , 7 : 59 – 121 .
- Needham , J. G. 1930 . A manual of the dragonflies of China , A monographic study of the Chinese Odonata. [Zoologia Sinica, Series A. Invertebrates of China, Volume XI, Fascicle 1.] Peiping: The Fan Memorial Institute of Biology
- Needham , J. G. and Gyger , M. K. 1939 . The Odonata of the Philippines, II. Suborder Zygoptera. Philippine . Journal of Science , 70 : 239 – 314 . pls 11–22 excl
- Orr , A. G. 2003 . A guide to the Dragonflies of Borneo: their identification and biology , Kota Kinabalu: Natural History Publications (Borneo)
- Ris , F. 1917 . [Eine neue Agrioniden-Gattung der “Légion Podagrion” (Odonata) aus China.] . Tijdschrift voor Entomologie , 60 : 185 – 191 .
- Watson , J. A.L. and O'Farrell , A. F. 1991 . “ Odonata (Dragonflies and damselflies) ” . In The insects of Australia. A textbook for students and research workers , 2 , Edited by: Naumann , I. D. , Carne , P. B. , Lawrence , J. F. , Nielsen , E. S. , Spradbery , J. P. , Taylor , R. W. , Whitten , M. J. and Littlejohn , M. J. 294 – 310 . Melbourne : Melbourne University Press . (Vol. 1)
- Wilson , K. D.P. 1999 . Dragonflies (Odonata) of Dinghu Shan Biosphere Reserve, Guangdong province, China . International Journal of Odonatology , 2 : 23 – 53 .
- Wilson , K. D.P. and Reels , G. T. 2001 . Odonata of Hainan, China . Odonatologica , 30 : 145 – 208 .
- Wilson , K. D.P. and Reels , G. T. 2003 . Odonata of Guangxi Zhuang Autonomous Region, China, part 1: Zygoptera . Odonatologica , 32 : 237 – 279 .
- Wilson , K. D.P. and Xu , Z. 2007 . Odonata of Guangdong, Hong Kong and Macau, South China, part 1: Zygoptera . International Journal of Odonatology , 10 : 87 – 128 . pls I–VIII excl