Abstract
This study examines the effects of an intensive voice treatment focusing on increasing voice intensity, LSVT LOUD® Lee Silverman Voice Treatment, on voice use in daily life in a participant with Parkinson’s disease, using a portable voice accumulator, the VoxLog. A secondary aim was to compare voice use between the participant and a matched healthy control. Participants were an individual with Parkinson’s disease and his healthy monozygotic twin. Voice use was registered with the VoxLog during 9 weeks for the individual with Parkinson’s disease and 2 weeks for the control. This included baseline registrations for both participants, 4 weeks during LSVT LOUD for the individual with Parkinson’s disease and 1 week after treatment for both participants. For the participant with Parkinson’s disease, follow-up registrations at 3, 6, and 12 months post-treatment were made. The individual with Parkinson’s disease increased voice intensity during registrations in daily life with 4.1 dB post-treatment and 1.4 dB at 1-year follow-up compared to before treatment. When monitored during laboratory recordings an increase of 5.6 dB was seen post-treatment and 3.8 dB at 1-year follow-up. Changes in voice intensity were interpreted as a treatment effect as no significant correlations between changes in voice intensity and background noise were found for the individual with Parkinson’s disease. The increase in voice intensity in a laboratory setting was comparable to findings previously reported following LSVT LOUD. The increase registered using ambulatory monitoring in daily life was lower but still reflecting a clinically relevant change.
Introduction
Dysarthria, defined as motor speech disorders of a neurological etiology, is common in Parkinson’s disease (PD). One of the most commonly occurring voice symptoms and one of the primary targets in intervention in PD is reduced voice intensity. Other symptoms include imprecise articulation, monopitch and monoloudness and variable speech rate (Citation1). Together these symptoms are commonly referred to as hypokinetic dysarthria. Historically, it has been suggested that effects of behavioral intervention in PD is limited (Citation2). More recent research have shown that there are complex sensory mechanisms that play a role in the speech and voice symptoms in addition to the more well-known motor deficits. Changes in sensory perception have been reported for individuals with PD which could lead to an underestimation of required effort when speaking (Citation3). An impaired ability to regulate and scale intensity and range of motor functions has also been described (Citation4,Citation5) as well as difficulties adjusting voice intensity in response to implicit cues (Citation6).
Even though there are diverse pharmacological and surgical treatment options available to alleviate motor symptoms in PD, their effect on the dysarthria is limited and variable (Citation7–14). The leading treatment option for speech and voice symptoms in PD today is the LSVT LOUD® Lee Silverman Voice Treatment (LSVT Global, Tucson, AZ) (Citation15). It is an intensive treatment program focusing on increased effort to increase voice intensity with the goal to achieve normal levels. It includes 16 h-long individual treatment sessions four times per week over 4 weeks together with a speech and language pathologist as well as daily exercises performed by the patient at home. Each session includes daily tasks that are consistent during the program as well hierarchically structured exercises that increases in difficulty from session to session. Tasks solely focus on increasing the voice intensity as the single motor control parameter trained during treatment to promote activity-driven neural plasticity (Citation16). The treatment program also aims to ‘recalibrate’ the patient’s perception of speech production enabling them to compensate for deficits in internal cueing and self-regulation of vocal effort during speech. Positive outcomes lasting for up to 2 years after treatment have been reported in a clinical setting (Citation17).
Some individuals with PD struggle with the carry-over of changes obtained in treatment to spontaneous speech during daily activities. This could be attributed to the complex sensory deficits described (Citation3–6). Attempts have been made to enhance the long-term maintenance and generalization of treatment effects following LSVT LOUD®. Several studies have shown positive outcomes following use of different technology such as telemedicine (Citation18–21) and other software solutions (Citation22).
Evaluation of speech and voice function is commonly based on recordings made in controlled environments, such as a sound-treated recording studio in a laboratory or a treatment room in the clinic, to provide a setting that is structured and replicable. For the same reason, the tasks speakers are asked to perform often consist of reading a paragraph or connected speech in a monologue, and specific tasks such as sustained phonation. Voice use in such structured tasks may differ from voice use in daily life where many other factors can affect a speaker, such as environmental noise, stress, physical movements as well as the cognitive load of participating in conversations.
It is however possible to study voice use with a more ecologically valid approach through the use of wearable devices such as portable voice accumulators (PVAs). With a PVA, such as the VoxLog (Sonvox AB, Umeå, Sweden), it is possible to study voice use in habitual speech outside a clinical setting. The VoxLog allows for long-term monitoring of voice use in settings outside the clinic during the speakers’ regular activities. A unique feature of the VoxLog is its ability to monitor environmental noise in addition to the voice parameters.
It has been shown that speakers generally have an involuntary tendency to increase vocal effort to improve intelligibility when speaking in loud environmental noise. This phenomenon is commonly referred to as the Lombard effect (Citation23). The Swedish Work Environment Authority’s guidelines for speech in noise describe that a normal voice sound level can be used to make oneself heard at a distance of 1 m when the environmental noise level is 55 dB. When the environmental noise level reaches 70 dB a loud voice is needed to be intelligible at distance of 1 m (Citation24). Several studies have looked at how variations in environmental noise effect speech regulation in PD (Citation6,Citation25–27). In most studies, individuals with PD have been shown to react to increased environmental noise in a similar way as healthy speakers do on a group level. There are however exceptions where individuals with PD fail to regulate voice sound level in response to increased environmental noise (Citation26,Citation27). In a study by Ho et al. (Citation6), it was shown that individuals with PD did not react to implicit cues following increased environmental noise, but they were able to increase their voice intensity in response to explicit cues, such as verbal instructions to increase effort and intensity. The variations in findings may be a result of individual differences in the ability to regulate intensity, or a result of the fact that the different levels of environmental noise imposed on the speakers varied greatly between studies.
The goal of the present study was to examine the treatment outcome following LSVT LOUD® on voice use in daily life for a participant with PD, using a PVA. A further aim was to compare voice use in daily life in different environmental noise ranges and a controlled studio recording in a laboratory setting, for a participant with PD and a healthy monozygotic twin control participant.
Method
This study was a prospective pseudo-single case, experimental design with one participant with PD and a healthy control. Voice use of the participant with PD was studied through the use of a PVA during 9 weeks, including treatment, pre-, post- and follow-up periods up to 1 year post-treatment. The healthy control was followed during 2 weeks, mirroring the participants pre- and post-treatment periods.
Participants
One individual with PD, 4 years post-onset and diagnosis (made by a neurologist specialized in movement disorders), and one healthy control participated in the study. The patient/control dyad were monozygotic twins, 51 years old at the start of the study, with similar working and living conditions. Both participants had manager positions at work where both described themselves as highly dependent on their voices as they often held meetings or interacted with people in other contexts. The individual with PD worked at a more physically active workplace where meetings were often held standing or on the go while the control worked at an office where meetings often where held in conference rooms with fewer participants. They had similar family situations, living with their spouse and teenage children.
The individual with PD described subjective voice and speech symptoms like increased speech rate, slurred speech, decreased voice intensity and decreased fluency with frequent stops. He also described that his voice use varied to a great degree depending on general fatigue. He was usually able to function properly at work but when he got home in the afternoon he needed to sleep for an hour or two to be able to enjoy spending time with his family in the evening. He felt that the general fatigue mainly had a negative impact on his fluency and voice intensity. The individual with PD and the control both reported that they had a high speech rate since childhood, sometimes leading to articulatory difficulties. The individual with PD’s speech rate had increased since PD onset, while the control described his speech rate as unchanged since childhood; although he felt he had become better at controlling it and compensating for it over the years. Neither the individual with PD nor the control had received any voice or speech treatment prior to participating in the study. The individual with PD was medicated with Levodopa; the dose was however unchanged during the course of this study.
During speech and voice assessment with the Swedish Clinical Dysarthria Assessment (Citation28), performed by the first author (J.K.G.), it was found that the individual with PD’s voice intensity was generally adequate at the beginning of utterances, but in many cases his voice intensity decreased to soft levels during continuous speech. His speech rate was high with rushes of increased rate leading to imprecise articulation. Speech rushes were more common at the end of utterances which in combination with the decaying voice intensity could lead to decreased intelligibility. Interruptions of speech fluency were frequent. During alternating motion rate assessment he produced repeated syllables with a rapid, accelerated rate but with clear restrictions in range of movement. The combined dysarthria score from the clinical assessment tool (described below (Citation28)) was 0.9, indicating a mild symptom severity. The control neither reported nor showed signs of pre-clinical PD.
Materials
The VoxLog (firmware 2.2.3) is a PVA that enables long-term ambulatory monitoring of voice use during daily life in a variety of settings outside the clinic. Voice sound level [Lvoice (dB)], phonation frequency (Hz), phonation time (percent time spent phonating during the registration period) and level of environmental noise [Lnoise (dB)] can be registered. Registrations are done using an accelerometer and a microphone placed in a collar worn around the neck. The accelerometer registers phonation frequency through a fast Fourier transform-based method and phonation time through an energy-based method for voice activity detection using the vibrations in the neck tissue created by the vocal folds during phonation (Citation29). The microphone registers the Lvoice when the accelerometer detects phonation and it registers the Lnoise when no phonation is detected. The neck collar is connected to the VoxLog device, a box that can be worn in a belt clip or a pocket, where data is stored. The device has to be charged nightly. The VoxLog can easily be put on and taken off if the wearer needs to. Continuous monitoring of voice use can be performed for up to 1 week before the device has to be connected to a computer to transfer the data to the accompanying VoxLog Discovery software (version 1.0.14) for analysis. The VoxLog Discovery software provides different analysis options including means, medians and standard deviations as well as histograms and speech phonetograms. Time periods can be labeled, for example to indicate activities, such that separate means can be calculated for speech during different activities or in different settings. The VoxLog registrations has been shown to have good agreement with other PVAs regarding measures of voice sound level, phonation frequency and phonation time. This was examined in a study where all PVAs available on the market were compared through simultaneous registrations of a speaker in a controlled setting (Citation30).
Clinical Dysarthria Assessment (in Swedish: Dysartritestet) is a standardized clinical assessment tool for evaluation of respiration, phonation, oral motor function, articulation, prosody and intelligibility. Function is rated on a five-point scale from 0 to 4 (0: normal function to 4: severe deviation or no function) for each item. A mean score is calculated which indicates dysarthria severity (Citation28).
Questionnaire on Acquired Speech Disorders (QASD, in Swedish: Självsvarsformulär om Förvärvade Talsvårigheter, SOFT) is a self-report questionnaire regarding subjective experience of living with an acquired speech disorder. It is divided into three parts covering (i) my speech and language, (ii) speech and language in social interaction, and (iii) personal and environmental factors. Ratings are done on a four-point scale (0: definitely false, 1: partly false, 2: mostly true and 3: definitely true) where a higher score indicates a subjective rating of more severe symptoms. QASD is a tool developed for clinical use in Sweden and results have been shown to have a moderate-to-strong correlation with similar but more extensive instruments (Citation31).
Procedure
Voice use was registered with the VoxLog for a total of 9 weeks for the individual with PD and 2 weeks for the control. This included 1 week of baseline registrations for both participants, 4 weeks during LSVT LOUD® treatment (administered by a LSVT LOUD® certified clinician) for the individual with PD, 1 week post-treatment period for both participants. The individual with PD’s voice use was monitored during additional follow-up weeks at 3, 6 and 12 months after the treatment period to study retention of the treatment effect.
The participants visited the laboratory for controlled recordings with the VoxLog of monologue speech two times per recording period with exception of the pre-treatment baseline period. During the baseline registration week three controlled recordings were performed to obtain reliable baseline data and to verify that the participants followed instructions properly. The monologue recordings were handled by an audio technician who assists with all voice recordings in the lab. During each recording session the participant was asked to produce a monologue on any topic for a minimum of 3 min. Topic suggestions were given if asked for. Examples included work experience, hobbies, activities during the last week, family, pets and travel experiences. If needed, the audio technician asked a simple follow-up question to help the participant continue. Data from the long-term registrations were downloaded from the VoxLog during each visit to the laboratory.
The participants were instructed to put on the VoxLog and begin registration when they woke up in the morning, and to turn it off before they went to bed in the evening. If needed, the VoxLog could be taken off and turned off during the day and registration could be resumed when possible. The participants were given verbal and written instructions on how to put on and place the collar. The participants were asked to show how they put on the collar at each laboratory or clinic visit to make sure that the sensor placement would be consistent and accurate between registrations.
The length of each registration period was chosen to be 1 week to be representative for all facets of the participants’ daily life and activities, including work and leisure time. A study by Mehta et al. (Citation32) reported, using a cumulative average technique, that an accelerometer-based method of estimating fundamental frequency and SPL reached an error of <5% after just 1 h of registration. After 20 h of registration the average error had decreased to ∼1% for both parameters. Phonation time estimates reached an average error of ∼5% after 26 h. The week-long registration periods were expected to yield sufficient data with a low margin of error.
Prior to enrollment in the study, both participants underwent assessment including background history and assessment with the self-rating questionnaire QASD. The individual with PD was also assessed with a clinical dysarthria test and a pre-treatment laryngeal exam. Assessment with the self-report questionnaire QASD was repeated after the treatment period and during the follow-up periods at 6 and 12 months follow-up.
During the long-term registrations, both participants logged their hourly activities in a voice diary. Participants were asked to describe roughly in text what activities they had performed during that time including time spent at work.
Data analysis
Mean values of Lvoice and Lnoise for the different week-long registration periods were calculated with the analysis options available in VoxLog Discovery. Both means for the whole periods and separate means for work and leisure activities during these periods were calculated and presented separately. The analyses of the work and leisure periods were based on the participants’ notes in their voice diaries. The individual with PD’s notes during the treatment period were not sufficiently detailed, so means for work and leisure activities are only shown for the pre-treatment, post-treatment and follow-up periods for which the notes were consistent and detailed enough to permit the analysis. Data regarding pitch and phonation time which the VoxLog registers were not included in the study to focus the analyses on the changes in Lvoice following treatment and the regulation of Lvoice following changes in Lnoise.
The data from the VoxLog Discovery program was exported to Microsoft Excel spreadsheets, where means of Lvoice were calculated for voice use in different ranges of Lnoise. The cut-off for different Lnoise ranges was based on the Swedish Work Environment Authority guidelines for speech in noise. Mean Lvoice were compared between VoxLog registrations during monologue speech in a sound-treated laboratory environment with no environmental noise, and spontaneous speech in environments outside the clinic for noise levels below 55 dB SPL, between 55 and 70 dB SPL as well as above 70 dB SPL.
Correlation analysis was performed to assess the impact of the mean environmental noise on the variability of the participants’ mean Lvoice. Correlation coefficients were calculated between mean Lvoice and mean Lnoise across all of the full week-long registration periods to assess whether changes in voice intensity after treatment followed changes in environmental noise. Furthermore, mean Lvoice and mean Lnoise were calculated for each 30-min segment within each registration period and correlation coefficients were calculated for each registration period, based on the 30-min segments, to assess the participants’ ability to regulate voice intensity in response to changes in environmental noise.
Statistical methods
Statistical analysis was performed in SPSS (IBM SPSS Statistics 23, IBM Corporation, Armonk, NY). Descriptive statistics were used to calculate means of the different voice parameters registered by the VoxLog. Spearman’s ρ was used for all correlation analyses, as some variables were not normally distributed. Shapiro–Wilks test was performed to assess the normality of the data.
Ethical considerations
The study was approved by the Regional Ethics Committee in Stockholm, Sweden (Dnr 2010/1023-31/1) and was thus performed in accordance with the ethical standards laid down in the 1964 Declaration at Helsinki.
Results
Subjectively reported outcome after LSVT LOUD®
During interviews, the individual with PD reported that he was able to use an increased vocal loudness after treatment, with less negative impact from general fatigue. He felt he had more energy to speak in the evenings compared to before. His fluency was improved with fewer stops which only appeared in very stressful situations following treatment. He no longer had problems making himself heard at meetings or in groups of people. At follow-up the individual with PD reported a continuous progression of his general symptoms, especially regarding general limb motor function and balance, but he reported his voice function being stable since treatment ended. below shows results from self-ratings with QASD before and after treatment, as well as at follow-up 6 and 12 months post-treatment. Before treatment the individual with PD rated a mean score of 1.1 indicating mild self-perceived symptoms. Ratings related to personal and environmental factors were most prominent. A decrease was seen post-treatment with a mean score of 0.4 indicating an improved status. At one year follow-up the symptoms had increased again reaching a mean score of 1.1 although the distribution of points on the subscales differed compared to before treatment.
Table 1. Results from individual with PD’s ratings with QASD pre-treatment, post-treatment and at follow-up (FU) 6 and 12 months post-treatment. Total and separate subscale scores are shown. A higher score indicates more severe symptoms.
Duration of registrations
The duration of each registration period was 1 week. below shows the duration of the registrations for each period reported in hours and minutes for the individual with PD and the control participant. A total of 378 h and 9 min was registered for both participants across all periods. The maximum duration of a registration period was 72 h and 5 min and the minimum was 15 h and 18 min.
Table 2. Duration of the different registration periods pre-treatment, during treatment (Treat 1–4), post-treatment and at follow-up (FU) 3, 6 and 12 months post-treatment.
Changes in Lvoice and Lnoise
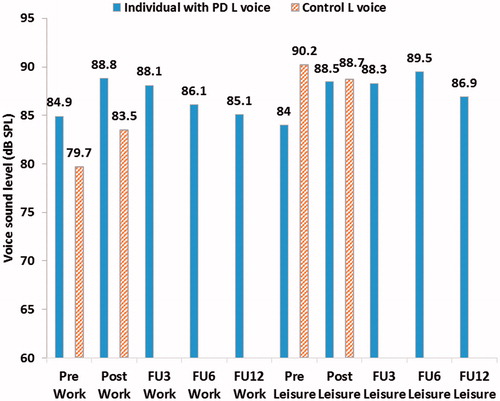
The mean Lvoice for all full monitoring periods for both participants can be seen in . When comparing the individual with PD’s Lvoice pre- and post-treatment an increase of 4.1 dB can be seen after treatment. The change in Lnoise for the same period is 0.1 dB. The difference in Lvoice for the control while comparing pre- and post-treatment is 0.5 dB. A small continuous decay in Lvoice can be seen for the individual with PD post-treatment. At 12 months follow-up Lvoice is 1.4 dB higher than compared to pre-treatment. shows the Lvoice presented for work and leisure activities respectively in the pre-, post- and follow-up periods. An increase in Lvoice of 4.5 dB can be seen post-treatment when compared to pre-treatment for the individual with PD during leisure activities. The corresponding difference for work activities is 3.9 dB. The remaining increase at 12 months follow-up for the individual with PD is 2.9 dB during leisure activities and 0.2 dB during work activities.
Figure 1. Mean voice sound level (Lvoice) and mean environmental noise sound level (Lnoise) during the whole week-long monitoring periods for both participants (vertical mouth-to-microphone distance of approximately 10 cm).
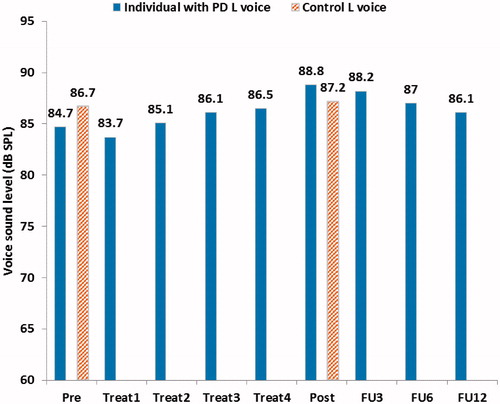
Figure 2. Mean voice sound level (Lvoice) and mean environmental noise sound level (Lnoise) during the different monitoring periods with data presented for leisure and work activities respectively for both participants (vertical mouth-to-microphone distance of approximately 10 cm).
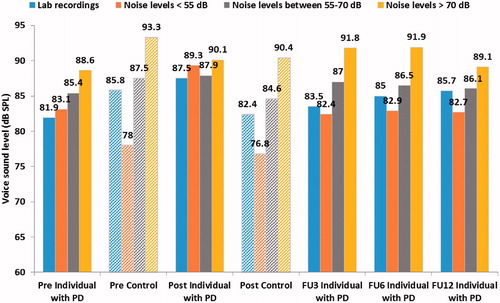
The individual with PD increased his Lvoice during speech in daily life with a mean of 4.1 dB in the week directly post-treatment when compared to directly before treatment. At the 1-year follow-up, the mean increase was 1.4 dB during speech in daily life compared to before treatment. When monitored during laboratory recordings of monologue speech an increase of 5.6 dB was seen in the recording the week post-treatment (seen in below). During laboratory recordings the mean increase was 3.8 dB at the 1-year follow-up.
Figure 3. Mean voice sound levels (Lvoice) from laboratory recordings and for different ranges of environmental noise outside the clinic for both the individual with PD and the control (vertical mouth-to-microphone distance of approximately 10 cm).
Before treatment the individual with PD’s mean Lvoice was lower compared to that of the control, whereas after treatment the opposite was found. The individual with PD used a similar Lvoice during both work and leisure time, despite varying Lnoise. The control used a higher Lvoice during leisure activities compared to work, with corresponding changes in Lnoise.
The impact of variations in Lnoise on treatment outcome between registration periods
There were no significant correlations between the individual with PD’s mean Lvoice and mean Lnoise across all full periods of registration (data registered for approximately a whole week per period; data shown in ), Spearman’s ρ = 0.25 (p = .515). Furthermore, there were no significant correlations between Lvoice and Lnoise for the individual with PD for all periods with means calculated for work and leisure activities separately (data shown in ), Spearman’s ρ = 0.25 (p = .483). There was however a significant correlation between the control’s Lvoice and Lnoise, Spearman’s ρ = 0.94 (p = .005).
Regulation of Lvoice following variations in Lnoise within registration periods
shows the correlation between both participants’ mean Lvoice and the mean Lnoise within the different registration periods based on paired 30-min long segments. The generally higher correlation coefficient for the control shows that he regulated his Lvoice following the Lnoise to a higher degree compared to the individual with PD.
Table 3. Correlations between the participants’ Lvoice and the Lnoise within respective registration period including pre-treatment, post-treatment and at follow-up (FU) 3, 6 and 12 months post-treatment.
Changes in Lvoice in different ranges of Lnoise
shows the Lvoice for both participants in different ranges of Lnoise during ambulatory monitoring in daily life and during monologue speech from laboratory recordings. The individual with PD’s Lvoice was generally louder during daily activities compared to sessions in the lab. During the post-treatment period, the individual with PD used a less varied Lvoice compared to the other periods regardless of the range of Lnoise. The variation in Lvoice in different ranges of Lnoise was greater for the control compared to the individual with PD.
Discussion
This study is novel in that it aims to examine the long-term effects of LSVT LOUD® on voice use in daily life, studied with a PVA. The goal was also to study how voice use in daily life is affected by PD by comparing voice use before treatment with a healthy control. Furthermore, a secondary aim was to examine the difference in voice use in a controlled laboratory setting compared to registrations with a PVA outside the clinic. These aims were met finding that the overall increase in Lvoice from recordings made in a laboratory setting was comparable to findings reported in previous studies on LSVT LOUD® up to 1-year post-treatment. The increase during ambulatory registrations outside a clinical setting where more distracting factors are present was lower but still at a clinically relevant level.
Effect of LSVT LOUD® on voice intensity in daily life
An increase of 4.1 dB was seen during ambulatory registration of Lvoice after treatment compared to before treatment. The increase during monologue speech in a laboratory setting was 5.6 dB. The increase in the more controlled environment where the individual with PD is primed to perform at his or her best was greater. This was expected as there are fewer factors, such as noise and stress, distracting the speaker and the individual with PD only has to focus on speaking with adequate voice intensity for a short period of time. The increase in voice intensity is comparable to earlier studies where a mean increase of 4.7 dB [standard deviation (SD): 2.6] was seen during monologue speech during laboratory recordings (Citation17). There was a continuing drop-off in Lvoice during the three follow-up periods. At one year post-treatment, the increase compared to before treatment in Lvoice registered with the VoxLog outside the clinic was 1.4 dB. The corresponding increase was 3.8 dB for monologue speech in laboratory recordings. In Ramig et al. (Citation17) a follow-up was performed 2 years after treatment. The reported mean increase in Lvoice during laboratory recordings was 2.3 dB (SD: 1.9) at that point. In the current study, the mean increase post-treatment in Lvoice during laboratory recordings was 3.8 dB at the 1-year follow-up. This corresponds to a decrease of voice intensity in laboratory recordings at the 1-year follow-up of 1.8 dB. The decay at two years follow-up compared to post-treatment in the study by Ramig et al. (Citation17) was 2.3 dB. The loss of treatment effect, or progression of disease symptoms, seems to be following a similar pattern as in the study by Ramig et al. (Citation17).
It is difficult to define what a clinically significant increase in Lvoice is. At the 1-year follow-up the individual with PD had retained an increase of 1.4 dB during ambulatory monitoring of voice intensity in daily life. A mean increase of 1.4 dB generally does not impact intelligibility greatly, even though many factors are at play, such as signal-to-noise ratio. It is important, however, to note that a mean increase for the whole period does not necessarily imply an even increase across all utterances from the week-long registration period. Also, an improved ability to sustain voice intensity without loudness decay at the end of utterances; or, to be able to increase intensity in situations where it is needed, as in noisy surroundings, would both be clinically important outcomes; but they might not alter the mean Lvoice very much. It is also possible that the Lvoice sometimes dropped below the Lnoise before treatment so that it could not be registered by the VoxLog. The individual with PD’s subjective experience of his voice at the 1-year follow-up would point to the change still being clinically relevant. He described his voice as one of the few things that had not changed markedly as a result of the disease progression during the year that had passed. However, a gradual increase of subjective voice symptoms can be seen in the results from QASD during the follow-up periods.
To assess whether changes in Lvoice after treatment was an effect of treatment or a response to changes in environmental noise, the correlation of the mean Lvoice and mean Lnoise was computed between the full registration periods. There were no significant correlations for the individual with PD’s Lvoice and Lnoise across the different registration periods. This supports the notion that the changes in voice intensity for the individual with PD was a result of the treatment, and was not due to changes in environmental noise.
Comparison of voice use between the individual with PD and the control
Before treatment, the individual with PD’s mean Lvoice was lower than the control’s, and after treatment, it was higher. The control varied his Lvoice between work and leisure activities following the changes in environmental noise, and a strong correlation was found for the control’s Lvoice and Lnoise across the different registration periods. The individual with PD, on the other hand, used a similar Lvoice during both work and leisure activities despite varying Lnoise resulting in weaker correlations as seen in . This could be a result of difficulties regulating voice intensity in response to the environmental noise as previously shown by Ho et al. (Citation6). It is also possible that the variations in environmental noise were not big enough to elicit a response. During all periods both participants used a mean Lvoice with a signal-to-noise ratio higher than 15 dB, which is needed to ensure intelligibility (Citation33). The lack of variation could then be an effect of monoloudness which is commonly described as a symptom in PD.
Voice use during laboratory recordings compared to daily life
Both the individual with PD and the control generally spoke with a louder Lvoice during ambulatory registrations compared to during laboratory recordings. When comparing laboratory recordings to ambulatory monitoring in low environmental noise (<55 dB) the control used a higher Lvoice in the laboratory than outside. Interestingly, the individual with PD used a higher Lvoice in all noise levels during ambulatory registrations compared to during laboratory recordings directly before and after treatment. This could also be attributed to the monoloudness common in PD (Citation1). During the period directly post-treatment, the individual with PD had very little variation in his mean Lvoice between the different ranges of Lnoise. This might be a sign that he has incorporated an increased voice intensity from the treatment, but he has not yet become able to regulate it effectively based on the environmental noise he is in which could be related to the difficulties scaling and regulating intensity in PD (Citation4,Citation5). During the follow-up periods, an increased variability can be seen for the individual with PD based on what environment he is in, and it follows the same pattern as the healthy control more closely. The pattern that is interpreted as monoloudness seen pre-treatment and directly post-treatment, before a more natural generalization seems to have taken place, is no longer as prominent. This possible pattern can also be seen in the correlation between Lvoice and Lnoise within each registration period, shown in . The correlation coefficient decreases directly post-treatment but reaches slightly increased levels during the follow-up periods, compared to before treatment, showing that the individual with PD regulates his Lvoice more following Lnoise than before. This change in the ability to regulate Lvoice following Lnoise over time could be interpreted as a successful ‘recalibration’ of his perception of his own speech production which is an integral part of the LSVT LOUD® (Citation16).
Limitations and future direction for research
The duration of the registration periods was chosen to be 1 week to provide a sufficient amount of data to be representative of the participants daily voice use. Even though the number of days of registrations was the same, for both participants and for all registration periods, the length of the usable data differed, as seen in . The reason for this was mainly participant compliance. Data was missing from parts of days, or in some cases from whole days. Other reasons were technical difficulties such as insufficient battery power if the participant had forgotten to charge the VoxLog, or the collar losing its electrical connection without the wearer noticing. This was particularly the case during the individual with PD’s first treatment week but it was not possible to prolong the registration period as treatment had already begun. The length of the recordings still generally exceeded the duration needed for reliable data as recommended by Mehta et al. (Citation32). Data collection and analysis were non-blinded to the first author which is a possible confound. Analyses of results were made together with all authors to in an attempt to minimize the possibility of personal bias.
Even though the VoxLog has been shown to be in good agreement with other PVAs when registering voice parameters, the published results regarding validity and reliability are limited. Further assessment of the validity and reliability of the VoxLog is recommended to strengthen findings using this method.
No standard deviations were reported for the mean Lvoice and mean Lnoise in this study. Standard deviations are typically used to show the speakers variability in for example voice intensity during a speech task. In this study, voice use in the form of spontaneous speech has been monitored for long periods during a variety of activities in different levels of environmental noise. The long registration periods result in large standard deviations, simply because voice use is registered during different conditions in a variety of settings. The large standard deviations don’t necessarily reflect how the voice is regulated, or not, in response to changes in environmental noise. Attempts to show important variation in voice use in different environmental noise have instead been made through the correlation analysis performed.
Wearing a device for registration of voice use creates a concern that the speaker may alter his/her way of using their voice because of awareness of being monitored. The fact that the VoxLog does not record speech, registering only the given voice parameters, alleviates some of that concern. Still, carrying a visible device for long periods of time still may affect one’s way of living. Both participants in this study reported that they often forgot that they were wearing the device until they were reminded of it, for example by someone else noticing it or during a change of clothes.
The study was limited to one case, one individual with PD with a matched healthy twin control. The use of a twin control in a study about PD could be seen as problematic, as hereditary factors have been shown in PD (Citation34). In a cross-sectional study by Wirdefeldt et al. (Citation35), all individuals above 50 years of age in the Swedish Twin Registry (n = 49,813) were screened for PD. Only one monozygotic pair was found with concordant PD in both siblings in the entire cohort. In a longitudinal follow-up study of the Swedish Twin Registry (n = 46,436) by Wirdefeldt et al. (Citation36), the cohort was studied from the 1960s and onwards to 2005, which increased sensitivity for cases where the PD onset differed greatly in time. In the later study, nine monozygotic twin pairs with concordant PD were found, leading to the authors’ conclusion that PD is modestly heritable. In this particular case, the strengths of how well-matched the pair was regarding occupation and home conditions was considered to outweigh the risk of the control having prodromal PD. The control reported no pre-clinical signs of PD or voice symptoms, so in the eventuality that he suffers prodromal PD, the impact on his voice use should be minimal during the study period.
Future research could increase the strength of the findings by using blinded data collection and analysis as well as studying larger groups including both treated and untreated individual with PD and controls. Additional methods could be used such as naïve listener ratings and communication partner ratings to increase the knowledge about treatment efficacy and provide more ecologically valid outcome measures. The effect of approaches to help individual with PD maintain and generalize treatment effects in daily life after treatment could also be studied by monitoring voice use in groups who continue with post-treatment practice in different forms and groups who do not. Following this, future studies are under way where voice use in PD and effects of LSVT LOUD® are studied on larger groups.
Conclusion
Ambulatory monitoring of voice use with a PVA has not been used previously to study treatment effects and carry-over of results after LSVT LOUD®. This study is a first step to support that the results previously seen in controlled environments also is reflected in habitual voice use in daily life. The study is however limited in scope and further research is required.
The fact that voice use during laboratory recordings differed from voice use in daily life outside a clinical setting supports the notion that there is a need for methods to objectively study voice use outside a clinical setting. The use of PVAs could be a valuable complement to laboratory recordings of voice use.
Acknowledgements
The authors would like to thank the participants for taking part in the study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Duffy JR. Motor speech disorders: substrates, differential diagnosis and management. 3rd ed. St Louis, Missouri: Elsevier Mosby; 2013.
- Weiner WJ, Singer C. Parkinson’s disease and nonpharmacologic treatment programs. J Am Geriatr Soc 1989;37:359–63.
- Ho AK, Bradshaw JL, Iansek R. Volume perception in parkinsonian speech. Mov Disord 2000;15:1125–31.
- Klockgether T, Borutta M, Rapp H, et al. A defect of kinesthesia in Parkinson’s disease. Mov Disord 1995;10:460–5.
- Demirci M, Grill S, McShane L, Hallet M. A mismatch between kinesthetic and visual perception in Parkinson’s disease. Ann Neurol 1997;41:781–8.
- Ho AK, Bradshaw JL, Iansek R, Alfredson R. Speech volume regulation in Parkinson’s disease: effects of implicit cues and explicit instructions. Neuropsychologia 1999;37:1453–60.
- Ramig LO, Fox C, Sapir S. Speech treatment for Parkinson’s disease. Expert Rev Neurother 2008;8:297–311.
- Ho AK, Bradshaw JL, Iansek R. For better or worse: the effect of levodopa on speech in Parkinson’s disease. Mov Disord 2008;23:574–80.
- Plowman-Prine EK, Okun MS, Sapienza CM, et al. Perceptual characteristics of Parkinsonian speech: a comparison of the pharmacological effects of levodopa across speech and non-speech motor systems. NeuroRehabilitation 2009;24:131–44.
- Schulz GM, Hosey LA, Bradberry TJ, et al. Selective left, right and bilateral stimulation of subthalamic nuclei in Parkinson’s disease: differential effects on motor, speech and language function. J Parkinsons Dis 2012;2:29–40.
- Karlsson F, Olofsson K, Blomstedt P, et al. Pitch variability in patients with Parkinson’s disease: effects of deep brain stimulation of caudal zona incerta and subthalamic nucleus. J Speech Lang Hear Res 2013;56:1–9.
- Karlsson F, Olofsson K, Blomstedt P, et al. Articulatory closure proficiency in patients with Parkinson's disease following deep brain stimulation of the subthalamic nucleus and caudal zona incerta. J Speech Lang Hear Res 2014;57:1178–90.
- Sandström L, Hägglund P, Johansson L, et al. Speech intelligibility in Parkinson’s disease patients with zona incerta deep brain stimulation. Brain Behav 2016;5:e00394.
- Atkinson-Clement C, Sadat J, Pinto S. Behavioral treatments for speech in Parkinson’s disaease: meta-analyses and review of the literature. Neurodegener Dis Manag 2015;5:233–48.
- Fox C, Ebersbach G, Ramig L, Sapir S. LSVT LOUD and LSVT BIG: Behavioral treatment programs for speech and body movement in Parkinson disease. Parkinson’s Disease 2012;2012:391946.
- Ramig LO, Fox C, Sapir S, Speech and voice disorders in Parkinson’s disease. In: Olanow W, et al., eds. Parkinson’s disease: non-motor and non-dopaminergic features. Oxford, UK: Blackwell Publishing Ltd; 2011.
- Ramig LO, Sapir S, Countryman S, et al. Intensive voice treatment (LSVT) for patients with Parkinson’s disease: a 2 year follow up. J Neurol Neurosurg Psychiatry 2001;71:493–8.
- Constantinescu GA, Theodoros DG, Russel TG, et al. Home-based speech treatment for Parkinson’s disease delivered remotely: a case report. J Telemed Telecare 2010;16:100–4.
- Constantinescu GA, Theodoros DG, Russel TG, et al. Treating disordered speech and voice in Parkinson’s disease online: a randomized controlled non-inferiority trial. Int J Lang Com Dis 2011;46:1–16.
- Theodoros DG, Ramig LO. Telepractice supported delivery of LSVT LOUD. Sem Speech Lang Pat 2011;3:107–19.
- Theodoros DG, Hill AJ, Russel RG. Clinical and quality of life outcomes of speech treatment for Parkinson’s disease to the home via telerehabilitation: a non-inferiority randomized controlled trial. Am J Speech Lang Pat 2016;25:214–32.
- Halpern A, Ramig LO, Matos C. Innovative technology for the assisted delivery of intensive voice treatment (LSVT®LOUD) for Parkinson disease. Am J Speech Lang Pathol 2012;21:354–67.
- Lane H, Tranel B. The Lombard sign and the role of hearing in speech. J Speech Lang Hear Res 1971;14:677–709.
- Arlinger S, Störande buller: Kunskapsöversikt för kriteriedokumentation [Disturbing noise: systematic review for criteria documentation]. Stockholm, Sweden: Arbetslivsinstitutet, Förlagstjänst; 1999.
- Adams SG, Lang AE. Can the Lombard effect be used to improve low voice intensity in Parkinson’s disease. Eur J Disord Com 1992;27:121–7.
- Sadagopan N, Huber JE. Effects of loudness cues on respiration in individuals with Parkinson’s disease. Mov Disord 2007;22:651–9.
- Stathopoulos ET, Huber JE, Richardson K, et al. Increased vocal intensity due to the Lombard effect in speakers with Parkinson’s disease: simultaneous laryngeal and respiratory strategies. J Com Disord 2014;48:1–17.
- Hartelius L, Svensson P, Dysartritestet. Stockholm, Sweden: Psykologiförlaget AB; 1990.
- Virebrand M, Real-time monitoring of voice characteristics using accelerometer and microphone measurements [unpublished master’s thesis]. Linköping, Sweden: Linköping University; 2011.
- Van Stan JH, Gustafsson J, Schalling E, Hillman RE. Direct comparison of three commercially available devices for voice ambulatory monitoring and biofeedback. SIG3 Perspect 2014;24:80–6.
- Hartelius L, Elmberg M, Holm R, et al. Living with dysarthria: evaluation of a self-report questionnaire. Folia Phoniatr Logop 2008;60:11–19.
- Mehta DD, Woodbury Listfield R, Cheyne HA, et al. Duration of ambulatory monitoring needed to accurately estimate voice use. Portland, OR: Proceedings of InterSpeech: Annual Conference of the International Speech Communication Association; 2012.
- Södersten M, Lindhe C, Kunskapsöversikt: Yrkesrelaterade röststörningar och röstergonomi [Systematic review: work-related voice disorders and voice ergonomics]. Stockholm, Sweden: Arbetsmiljöverket; 2011.
- De Lau LML, Breteler MMB. Epidemiology of Parkinson’s disease. Lancet Neurol 2006;5:525–35.
- Wirdefeldt K, Gatz M, Bakaysa SL, et al. Complete ascertainment of Parkinson disease in the Swedish Twin Registry. Neurobiol Aging 2008;29:1765–73.
- Wirdefeldt K, Gatz M, Reyhnolds RA, et al. Heritability of Parkinson disease in Swedish twins: a longitudinal study. Neurobiol Aging 2011;32:1923.e1–e8.
