Abstract
Objectives. To assess the influence of concomitant hypertension on left ventricular hypertrophy regression and exercise capacity in patients operated for aortic stenosis. Design. We performed echocardiography 1 week, 6- and 18-month postoperatively in 78 patients, aged 70 (28–86) years, who received Medtronic Hall (33), Biocor (8), Carpentier-Edwards S.A.V. (14) and Freestyle (23) prosthetic valves for severe aortic stenosis. Forty nine patients participated in treadmill tests with ergospirometry at the 6- and 18-month visits. Results. Left ventricular mass index was comparably reduced in normotensive and hypertensive patients (34 vs. 40 g/m2 after 6 months, and 43 vs. 46 g/m2 after 18 months, ns). In multiple regression analysis, adjusting for baseline left ventricular mass index, larger reduction in left ventricular mass index was associated with younger age and having a Freestyle prosthesis, but not with gender or history of hypertension (multiple R2=0.68, p < 0.05). Exercise capacity assessed as peak oxygen uptake increased from early to late evaluation in normotensive patients (VO2max 24.27 vs. 27.08 ml/kg/min, p < 0.05) while remained unchanged in hypertensive patients (VO2max 22.2 vs. 21.1 ml/kg/min). In multiple regression analysis, higher improvement in exercise capacity was predicted by male gender, younger age and absence of hypertension, while no independent association was found with Freestyle prosthesis (multiple R2 = 0.37, p < 0.05). Conclusions. In patients operated for aortic stenosis, concomitant hypertension is associated with lack of improvement in exercise capacity in spite of early left ventricular hypertrophy reduction comparable to what is found in normotensive patients.
In patients operated for severe symptomatic aortic stenosis, postoperative left ventricular (LV) hypertrophy regression is suggested as the underlying determinant of LV function, survival and quality of life Citation1, Citation2. By echocardiography, some degree of LV hypertrophy regression is observed in most patients operated for aortic stenosis Citation1. However, the majority of these patients continue to have LV hypertrophy during long-term follow-up Citation1, Citation2.
Hypertension is common among patients with aortic stenosis and associated with higher postoperative LV mass, morbidity and mortality Citation3. Hypertension is also associated with reduced exercise capacity in patients with essential hypertension and LV hypertrophy Citation4. Thus, less postoperative improvement in exercise capacity might be expected in hypertensive patients operated for aortic stenosis. However, this has not been previously evaluated. Therefore, the aim of this study was to assess the influence of concomitant hypertension on LV hypertrophy regression and improvement in exercise capacity in patients operated for aortic stenosis.
Material and methods
Patients
A total of 96 patients operated for severe symptomatic aortic valve stenosis and living in the local hospital community were consecutively invited during two time periods (in 1991 and 1995, respectively) to participate in this study which included postoperative echocardiography after 1 week, 6 and 18 months, respectively. Five patients were excluded due to poor acoustic windows. Of the remaining 91 patients, 78 patients completed the echocardiographic follow-up: 33 patients with Medtronic-Hall single-disc mechanical prosthesis, eight with Biocor stented porcine bioprosthesis, 14 with Carpentier-Edwards S.A.V. stented porcine bioprosthesis (all included during the first recruitment period) and 23 with Freestyle stentless porcine bioprosthesis, implanted subcoronary (included during the last recruitment period).
Only 49 of the 78 patients accepted to participate in additional exercise testing at the 6- and 18-month follow-up visits. Refusal of vigorous exercise testing was mostly due to hip or knee problems. Forty five of the 49 patients participating in the exercise protocol completed both exercise tests. Failure of completing the second exercise test was due to: femoral neck fracture in two patients, recent implantation of a hip prosthesis in one patient and recent implantation of a knee prosthesis in one patient.
All patients had a preoperative aortic valve area < 0.5 cm2/m2 body surface area determined by cardiac catheterization. Twenty four patients (five with Medtronic-Hall, seven Biocor, five Carpentier-Edwards S.A.V. and seven Freestyle prosthetic valve) had additional coronary artery bypass grafting (on average: 2±1 graft). All patients were fully revascularized. Concomitant hypertension was defined as history of hypertension documented in the hospital medical records. Treatment of hypertension was left to the discretion of the cardiac surgeon and general practitioner managing the individual patient. All patients gave written informed consent to participate in the study, which was approved by the Regional Ethics Committee.
Echocardiography
All patients underwent a standard Doppler echocardiographic examination at rest, including M-mode and 2D echocardiography, continuous wave, pulsed wave and colour Doppler, run by one experienced echocardiographer (EG). All echocardiograms were done using a Vingmed 800 ultrasound system and a 3.5 MHz transducer. LV chamber dimensions and wall thicknesses were measured in the parasternal long-axis view using M-mode or 2D echocardiography as appropriate, following the American Society of Echocardiography standards Citation5, Citation6.
LV ejection fraction was calculated by the Teichholtz method Citation7. Fractional shortening was calculated as (LV end-diastolic diameter – LV end-systolic diameter)/LV end-diastolic diameter. The relative wall thickness was calculated as: posterior wall thickness/ internal LV radius at end-diastole Citation8. LV mass was calculated from LV end-diastolic diameter and wall thicknesses using Devereux's anatomically validated formula and was indexed for body surface area Citation9.
Pulsed wave Doppler was used to measure peak velocity in the LV outflow tract and continuous wave Doppler to measure transvalvular aortic flow taking the mean of Doppler measurements from three consecutive beats. Diameter of LV outflow tract was measured in 2D parasternal long-axis view, parallel and adjacent to the aortic prosthesis plane, by an inner-edge-to-inner-edge method Citation10. Effective prosthetic valve area was calculated using the continuity Citationequation 10. LV diastolic function was assessed by pulsed Doppler recording of peak early (E) and atrial (A) transmitral velocities and E/A ratio of the diastolic transmitral flow between the tips of the mitral leaflets. The isovolumic relaxation time was measured from pulsed Doppler recordings in LV outflow tract catching both the inflow and outflow profiles simultaneously.
Aortic regurgitation was assessed by standard colour Doppler technique Citation11.
At the end of the echocardiographic examination, blood pressure was measured by cuff using a mercury sphygmomanometer. Heart rate was taken from the resting echocardiogram.
Exercise testing
Peak exercise capacity was evaluated by treadmill test using the Chronotropic Assessment Exercise Protocol (CAEP) at 6 and 18 months postoperatively Citation12. Before starting, 12-lead ECG, heart rate and blood pressure were recorded with the patient in the sitting position. ECG was continuously monitored during exercise. Systolic blood pressure was measured at the end of each stage by cuff using a mercury sphygmomanometer. All patients were exercised until exhaustion unless the following stop criteria occurred: 1) ST segment depression ≥ 5 mm on ECG, 2) chest pain, or 3) complex ventricular arrhythmia on ECG.
Ventilatory gas exchange was measured using a Quinton ergospirometer (Q-Plex I, Quinton Model 645; Quinton Instruments, Seattle, Washington, USA) by a breath-to-breath technique. Oxygen uptake and carbon dioxide production (in ml/kg/min) and their ratio (the respiratory quotient) were recorded, together with total exercise time, total exercise distance and the metabolic equivalents. The peak oxygen uptake (VO2max) was defined as the highest oxygen uptake measured during exercise. Exercise capacity was assessed as VO2max.
Statistical analysis
The SPSS statistical computing program version 13.0 (SPSS Inc., Chicago, Illinois, USA) was used for statistical analysis. Continuous variables are expressed as mean±standard deviation and categorical variables as percentages. Unpaired and paired samples T-tests, ANOVA and χ2 tests were used for comparison of groups, as appropriate. Univariate correlations between variables were assessed using the Pearson's correlation coefficients. The variables with p < 0.1 in univariate analyses were entered in multivariate analyses. Multivariate regression analyses, performed with an enter procedure, identified the independent covariates of LV hypertrophy reduction and improvement in exercise capacity from early to late evaluation. A two-tailed p < 0.05 was considered statistically significant.
Results
Patients and blood pressure
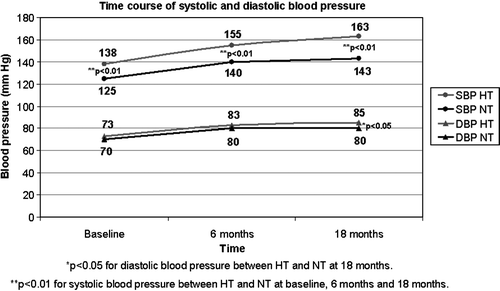
Of the 78 patients completing the echocardiographic follow-up, 43 were known hypertensive (HT) and 35 normotensive (NT). The HT and NT groups of patients did not differ in gender or prosthetic valve size, but the HT group was older and included more patients with Freestyle prostethic valves (). Four HT and two NT patients had a history of myocardial infarction (ns). A grade 1 transprosthetic aortic regurgitation was present in 32 patients (16 HT and 16 NT, ns). None of the patients had any paravalvular leak.
Table I. Baseline characteristics of the study population.
Postoperatively, the systolic and diastolic blood pressure values rose in all patients (p < 0.01), but significantly more in HT patients (25/12±31/15 mm Hg versus 18/10±21/10 mm Hg in NT patients, respectively, p < 0.05) from baseline to 18-month follow-up ().
Only 27 of the 43 HT patients received medical antihypertensive treatment. Nine patients received β-blockers, ten patients diuretics, 16 patients angiotensin converting enzyme inhibitors, two patient α-blockers, and six patients used calcium-channel blockers. The average number of antihypertensive drugs in an individual HT patient was 1.74±0.94. After 18 months, only eight of the HT had controlled hypertension (clinic blood pressure < 140/90 mmHg). Furthermore, 16 patients in the NT group had elevated clinic blood pressure (≥140/90 mmHg) indicating that some of the NT patients might have developed hypertension during follow-up.
Reduction in LV hypertrophy
Echocardiographic findings at baseline and during follow-up are presented in . LV mass index decreased significantly in all the patients during the 18-month follow-up (p < 0.01), in particular during the first 6 months after surgery (37 g/m2 from a total reduction of 45 g/m2 during follow-up) (). The reduction in LV mass index was mainly due to reduction in septal and posterior wall thicknesses (). The total reduction in LV mass index from baseline to late evaluation did not significantly differ between the NT and HT groups of patients. However, the reduction in LV mass index between 6- and 18-month follow-up was 45% less in the HT group (). The prevalence of LV hypertrophy was 85% at baseline evaluation, 64% after 6 months and 62% after 18 months. LV mass index was slightly higher in HT patients at all visits, but the difference was not statistically significant (). However, at late evaluation, patients with elevated blood pressure values (HT or NT with late clinic blood pressure ≥ 140/90 mmHg) tended to have higher LV mass index (127±38g/m2 versus 102±34g/m2, p = 0.08).
Table II. Echocardiographic characteristics of hypertensive versus normotensive patients
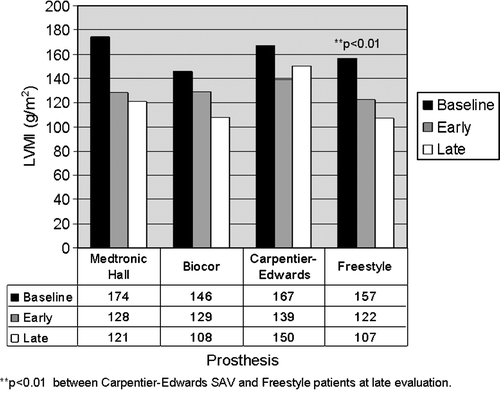
LV mass index regression during the first six months was parallel in all patients, irrespective of prosthetic valve type (). However, while further reduction in LV mass index was found in patients with Medtronic-Hall, Biocor and Freestyle prostheses also between the 6- and 18-month visits, LV mass index increased during this period in patients having Carpentier-Edwards S.A.V. prostheses. At 18-month follow-up, LV mass index was significantly higher in the Carpentier-Edwards S.A.V. group compared to the Freestyle group (p < 0.01) (). Within the HT group, total reduction in LV mass index was 45 + 50 g/m2 in patients with Freestyle prostheses and 47 + 55 g/m2 in those with other valves (ns).
Reduction in LV mass correlated negatively with patient's age (r = − 0.24) and increase in systolic blood pressure during follow-up (r = − 0.21, both p < 0.05). LV mass index reduction did not differ between genders, but LV mass index was significantly larger in men compared to women throughout the study (135 + 32 vs. 107 + 39 g/m2 at final follow-up, p < 0.01). A multiple linear regression model was constructed using reduction in LV mass index from baseline to 18-month follow-up as dependent variable, and including age, gender, prosthetic valve Doppler effective area, baseline ejection fraction and indicator variables for history of HT and Freestyle prosthetic valve as independent variables. Adjusting for baseline LV mass index, larger reduction in LV mass index was found in younger patients, patients with larger initial LV mass index and in patients who had received Freestyle prosthetic valves (multiple R2=0.68, p < 0.05) (), while no independent association was found with gender, prosthetic valve Doppler effective area, baseline systolic function or history of hypertension. In subsequent models, adding an indicator variable for HT treatment or coronary artery bypass grafting and replacing the indicator variable for history of hypertension with actual change in systolic blood pressure during follow-up, the results did not change (data not shown).
Table III. Predictors of LV hypertrophy regression during 18-month follow-up. Multiple regression analysis (multiple R2=0.68, p < 0.05).
LV systolic function did not differ between HT and NT groups at baseline, and improved in both groups during follow-up (). HT patients had longer isovolumic relaxation time and higher peak atrial velocity at all evaluations (p < 0.05), suggesting a more impaired diastolic relaxation in spite of similar degree of LV hypertrophy ().
Exercise capacity
Forty five patients (26 HT and 19 NT), aged 49–86 (mean age 72±8 years) completed both exercise tests. The predefined stop criteria did not occur in any patients. All patients were exercised until exhaustion, and the level of exercise, reflected by the respiratory quotient, was similar in HT and NT patients (). Peak oxygen uptake during exercise testing improved significantly in NT patients during follow-up, while remained virtually unchanged in hypertensive patients (). Peak oxygen uptake at 18-month follow-up correlated positively with prosthetic valve size (r = 0.30) and negatively with systolic blood pressure at 18-month follow-up (r = − 0.38, both p < 0.05). In multiple regression analysis, including age, gender, concomitant hypertension and prosthetic valve size as independent variables, higher peak oxygen uptake at 18-month follow-up was associated with absence of hypertension, younger age and male gender (multiple R2=0.43, p < 0.05). In additional models, adding LV mass index at late evaluation or an index variable for Freestyle prosthetic valve, the result was not changed. In multiple linear regression, adjusting for initial peak oxygen uptake and including patient's age, gender and indicator variables for history of hypertension and Freestyle prosthetic valve as independent variables, increase in peak oxygen uptake during follow-up was associated with absence of hypertension, younger age and male gender (multiple R2=0.37, p < 0.05) ().
Table IV. Exercise capacity in hypertensive and normotensive patients.
Table V. Predictors of improvement in exercise capacity in multiple regression analysis (multiple R2=0.37, p < 0.05).
Discussion
This study shows that in patients operated for aortic stenosis, concomitant hypertension is associated with lack of improvement in postoperative exercise capacity in spite of an early LV hypertrophy regression that is comparable to what is observed in normotensive patients. Our results add to previous reports that focus on the implication of concomitant hypertension on long-term morbidity and mortality in patients operated for aortic stenosis Citation2, Citation3.
The prevalence of hypertension increases with age, and our finding that 55% of the study population had concomitant hypertension is in accordance with expected prevalence of hypertension in this age group from epidemiological surveys, as well as previous reports in patients with aortic stenosis Citation13–15. Thus having concomitant hypertension, which impairs improvement in exercise capacity, is common in patients operated for aortic stenosis.
The finding that LV hypertrophy regression mainly took place during the first 6 postoperative months is in accordance with previous reports Citation1, Citation3, Citation16. Our HT and NT patients experienced a similar LV mass index reduction, but 16 patients (45%) in the NT group developed new-onset hypertension during follow up, possibly obscuring a difference in LV hypertrophy reduction between true NT and HT patients. However, we chose not to redefine the groups retrospectively.
Few published studies have reported the prevalence of late hypertrophy among HT and NT patients operated for aortic stenosis. In our study, 62% of the total study population had residual LV hypertrophy 18 months post aortic valve replacement, compared to 83% reported by Lund et al. in a series of 41 patients operated for severe aortic stenosis Citation2. Differences in patient characteristics, including age, gender, degree of preoperative LV hypertrophy and prosthetic valve type probably explain the higher prevalence of residual LV hypertrophy in their study. In particular, while no information on hypertension was given, the study by Lund et al. included 19% patients with preoperative renal failure and 47% of the study population had reduced systolic LV function, both factors associated with persistent LV hypertrophy. In another study from the same group, 91 patients operated for aortic stenosis were followed up with echocardiography for ten years, including ten patients with hypertension Citation1. LV mass index was reduced by 33 g/m2 during the first 18 months in this study, rather similar to the hypertrophy reduction seen in the present study. However, LV mass index increased significantly in HT patients from 18 month to ten years follow up, in particular in patients who developed mitral regurgitation, representing volume overload of the LV. Recently the same investigators published the ten years data on 37 patients with St. Jude bileaflet disc valve, selected from the 91 patients series Citation17. In this report, the 11 patients with hypertension had significantly more hypertrophic and dilated ventricles ten years postoperatively, in spite of comparable LV mass index reduction to NT during the first 1.5 years of the study follow-up, as also found in the present study.
In a study evaluating the impact of LV hypertrophy on survival in 260 patients after aortic valve replacement for aortic stenosis, Gaudino et al. found that LV hypertrophy reduction over 28 months was only seen in patients without history of hypertension, on average 32 g/m2 Citation3. Their finding that LV mass index was not reduced postoperatively in HT patients is surprising, in particular as all HT patients were reported to be treated medically with angiotensin converting enzyme inhibitors, a drug class proven to be superior in inducing LV hypertrophy reduction in HT hearts Citation18. However, their study population also differs significantly from ours both in age, prosthetic valve type and prevalence of women Citation3.
Previous studies focusing on the influence of hypertension on LV hypertrophy regression in patients operated for aortic stenosis were conducted mainly on patients that received bileaflet mechanical valves (90% and 72% of the patients in Citation1 and Citation3, respectively) Citation1, Citation3. In the present study, only 42% of patients received mechanical valves. Early LV hypertrophy regression did not differ between prosthetic valve types in our study. However, in the group of patients with Carpentier-Edwards S.A.V. prosthesis, LV mass index increased from 6 to 18 months. At 18-month follow-up, LV mass index was significantly higher in the Carpentier-Edwards S.A.V. group compared to the Freestyle group, despite similar age, valve diameter and prevalence of hypertension. In fact, having a Freestyle stentless prosthesis was identified as one of the predictors of larger hypertrophy regression in our study.
Inclusion in our study was done during two different time periods. By chance, more HT patients had received a Freestyle prosthetic valve, but LV hypertrophy regression was similar within the HT group in patients with or without Freestyle prosthesis.
Peak oxygen uptake during exercise, reflecting cardiac output, is a reliable and reproducible measure of fitness and cardiopulmonary function in patients with valvular heart disease Citation19. Clinically, improvement in exercise capacity in parallel with LV hypertrophy reduction is to be expected. In the present study, increase in peak oxygen uptake from 6- to 18-month follow-up was associated with younger age, male gender and absence of hypertension. This is in accordance with previous knowledge Citation4, Citation20. As suggested by others, the late LV hypertrophy reduction is probably more related to reduction in interstitial tissue, while early hypertrophy regression reflects reduction in cardiomyocyte hypertrophy mainly caused by abruptly reduced pressure overload of the left ventricle as a consequence of aortic valve replacement Citation2, Citation21. It has previously been reported that diastolic reserve in patients operated for aortic stenosis improves significantly one year post aortic valve replacement Citation22. On the other hand, it is well known that hypertension leads to increased interstitial collagen content which impairs LV filling, in particular during exercise, leading to reduced exercise capacity Citation4, Citation23. Increased interstitial collagen content was probably also present in the HT patients in our study population, as reflected by the persistently more impaired diastolic relaxation and the somewhat attenuated late LV hypertrophy regression in the HT group.
In conclusion, our study shows that in patients operated for aortic stenosis concomitant hypertension is associated with lack of improvement in postoperative exercise capacity in spite of an early LV hypertrophy regression that is comparable to what is observed in normotensive patients. Interpreting our results in the context of previous publications, better management of hypertension is suggested to improve both LV hypertrophy regression and exercise capacity in patients operated for aortic stenosis. However, the clinical effect of optimized antihypertensive treatment in this patient group remains to be tested in a prospectively designed study.
Study limitations
Study limitations include the relatively low number of patients that completed all the follow-up examinations, and the limited study duration of 18 months. Furthermore, different types of prosthetic valve were included during the two inclusion periods; therefore an undetected bias can not be excluded. As antihypertensive treatment and control was not standardized, but was left to the discretion of the cardiac surgeon and general practitioner managing the individual patient, impact of antihypertensive treatment on hypertrophy regression and exercise capacity could not be assessed in this study.
References
- Lund O, Emmertsen K, Dørup I, Jensen FT, Flø C. Regression of left ventricular hypertrophy during 10 years after valve replacement for aortic stenosis is related to the preoperative risk profile. Eur Heart J. 2003; 24: 1437–46
- Lund O, Kristensen LH, Baandrup U, Hansen OK, Nielsen TT, Emmertsen K, et al. Myocardial structure as a determinant of pre- and postoperative ventricular function and long-term prognosis after valve replacement for aortic stenosis. Eur Heart J. 1998; 19: 1099–108
- Gaudino M, Alessandrini F, Glieca F, Luciani N, Cellini C, Pragliola C, et al. Survival after aortic valve replacement for aortic stenosis: Does left ventricular mass regression have a clinical correlate?. Eur Heart J. 2004; 26: 51–7
- Gerdts E, Bjørnstad H, Toft S, Devereux RB, Omvik P. Impact of diastolic Doppler indices on exercise capacity in hypertensive patients with electrocardiographic left ventricular hypertrophy (a LIFE substudy). J Hypertens. 2002; 20: 1223–9
- Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: Results from a survey of echocardiographic measurements. Circulation. 1978; 58: 1072–83
- Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux RB, Feigenbaum H, et al. Recommendations for quantification of the left ventricle by two-dimensional echocardiography: American Society of Echocardiography Committee on Standards. Subcommittee on Quantification of two-dimensional echocardiograms. J Am Soc Echocardiogr. 1989; 2: 358–67
- Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: Echocardiographic/angiographic correlations in the presence of asynergy. Am J Cardiol. 1986; 57: 450–8
- Reichek N, Devereux RB. Reliable estimation of peak left ventricular systolic pressure by M-mode echocardiographic-determined end-diastolic relative wall thickness: Identification of severe valvular aortic stenosis in adult patients. Am Heart J. 1982; 103: 202–9
- Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am J Cardiol. 1986; 57: 450–8
- Chafizadeh ER, Zoghbi WA. Doppler echocardiographic assessment of the St Jude Medical prosthetic valve in the aortic position using the continuity equation. Circulation. 1991; 83: 213–23
- Lebowitz NE, Bella JN, Roman MJ, Liu JE, Fishman DP, Paranicas M, et al. Prevalence and correlates of aortic regurgitation in American Indians: The Strong Heart Study. J Am Coll Cardiol. 2000; 36: 461–7
- Wilkoff BL, Corey J, Blackburn G. A mathematical model of the cardiac chronotropic response to exercise. J Electrophysiol. 1989; 3: 176–80
- Franklin SS, Guston W IV, Wong ND, Larson MG, Weber MA, Kannel WB, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997; 96: 308–15
- Ikram H, Marshall DE, Moore SM, Bones PJ. Hypertension in valvular aortic stenosis. N Z Med J. 1979; 89: 204–7
- Vánky FB, Håkanson E, Maros T, Svedjeholm R. Different characteristics of postoperative heart failure after surgery for aortic stenosis and coronary disease. Scand Cardiovasc J. 2004; 38: 152–8
- Jin X, Zhang ZM, Gibson DG, Yacoub M, Pepper JR. Effects of valve substitute on changes in left ventricular function and hypertrophy after aortic valve replacement. Ann Thorac Surg. 1996; 62: 683–90
- Lund O, Dørup I, Emmertsen K, Jensen FT, Flø C. Hemodynamic function of the standard St. Jude bileaflet disc valve has no clinical impact 10 years after aortic valve replacement. Scand Cardiovasc J. 2005; 39: 237–43
- Dahlof B, Pennert K, Hansson L. Regression of left ventricular hypertrophy–a meta-analysis. Clin Exp Hypertens A. 1992; 14(1-2)173–80
- Lehmann G, Kolling K. Reproducibility of cardiopulmonary exercise parameters in patients with valvular heart disease. Chest. 1996; 110: 685–92
- Pardaens K, Vanhaecke J, Fagard RH. Impact of age and gender on peak oxygen uptake in chronic heart failure. Med Sci Sports Exerc 1997; 29(6)733–7
- Krayenbuehl HP, Hess OM, Monrad ES, Schneider J, Mall G, Turina M. Left ventricular myocardial structure in aortic valve disease before, intermediate, and late after aortic valve replacement. Circulation. 1989; 79: 744–55
- Gonzales-Juanatey JR, Vega FM, Gude F, Duran Munoz DD, Roman V, Iglesias Carreno C. Influence of prosthesis size and left ventricular diastolic reserve in patients with aortic valve prosthesis. J Heart Valve Dis. 2001; 10: 611–8
- Omvik P, Lund-Johansen P. Hemodynamic response to exercise in hypertension and its modulation by anti-hypertensive therapy. The heart in hypertension, ME Safar, FM Fouad-Tarazi. Kluwer Academic Publishers, Dordrecht 1989; 370–94