Abstract
Objective. To define the mechanisms of ischemic mitral regurgitation (MR) and its correlation with left ventricular (LV) function prior to and 1 year following mitral valve (MV) repair. Design. Fifty-three patients (pts) underwent echocardiographic evaluation of the MR mechanism according to Carpentier's classification; quantification of MR and LV function. Results. Forty-one, 5% of pts had Type I (annulus dilation), 20, 5% had Type II (commissural prolapse) and 38% had Type IIIb MR (predominant posterior leaflet restriction). Preoperative LV function was slightly better preserved in pts with Type II and IIIb MR. Despite similar MV repair efficiency intraoperatively, after 1 year Type I MR progressed vs the remaining types. LV function, including dimensions, ejection fraction and pulmonary artery pressure had a tendency to worsen in pts with Type I and markedly improved in Type II and IIIb MR. Conclusions. Ischemic MR of Type I is associated with more marked LV dysfunction preoperatively, its further deterioration and MR progression after MV repair. Type II and IIIb MR correlates with better preserved LV function preoperatively and its incremental improvement late after surgery.
Introduction
Mitral regurgitation (MR) is a frequently observed complication of coronary artery disease (CAD) and myocardial infarction (MI). Recent studies strongly support the strategy of correcting even moderate ischemic MR at the time of coronary artery bypass grafting (CABG) as there is increasing evidence of the negative impact of even a moderate MR on medium- and long-term survival in patients (pts) with CAD Citation1–3.
However, pts with ischemic MR still represent a challenge to surgeons. Hospital mortality after combined operations is substantially high (7–18%) Citation4–6 and different methods of mitral valve (MV) repair are under discussion Citation7.
One of the determinants of suboptimal clinical outcome after surgical correction of MR may be a complex mechanism of ischemic MR. Ischemic MR occurs despite a structurally normal MV as a consequence of left ventricular (LV) dilation and remodeling – distortion of ventricular geometry, displacing the papillary muscles apically and posteriorally, excessive valvular tenting or tethering with or without annular dilation and restricting the ability of mitral leaflets to close effectively at the annular level Citation8–11.
According to Carpentier's functional classification, Type IIIb valve dysfunction, which is characterized by leaflet restriction related to ventricular dilatation and papillary muscle displacement with or without associated annular dilation, is the most common mechanism of ischemic MR Citation12. In certain cases Type I (annular dilation) is the predominant mechanism of ischemic MR, while Type II valve dysfunction resulting in leaflet prolapse is regarded as less commonly associated with ischemic MR Citation12,13.
Owing to the complex mechanism of ischemic MR, annuloplasty techniques only incompletely address tethering at the annular end Citation7,14 and adjunct procedures including leaflet mobilization with chordal transfer, posterior leaflet extension, etc. are required Citation12. Sophisticated operative techniques require precise evaluation of the MR mechanism. The influence of different mechanisms of ischemic MR and the corresponding operative strategy on late results of ischemic MV repair, especially on LV functional changes, are not fully investigated.
The aim of the study was to define the anatomical–functional mechanisms of ischemic MR and to analyze correlations between the mechanisms of ischemic MR and LV function prior to and 1 year following MV repair.
Materials and methods
The study group was enrolled from 187 pts who underwent ischemic MV repair during 1998–2001 and consisted of 53 pts who met the inclusion criteria. Clinical inclusion criteria were: (1) previous MI, (2) functional ischemic MR ≥ grade II (functional ischemic MR was defined as MR that occurs with a structurally normal valve due to LV dysfunction and remodeling), (3) successful MV repair – residual MR < grade I intraoperatively, (4) echocardiographic follow-up 12 months after surgery (follow-up was unavailable in 63 pts due to social–economical reasons).
Clinical exclusion criteria were: (1) intrinsic valvular heart disease, (2) acute MI (including perioperative MI or MI during the follow-up period), (3) comorbid conditions (previous CABG, resection of ventricular aneurysm, valve replacement, etc.), (4) suboptimal echocardiographic windows.
Of these 53 pts, 50 were male, the mean age was 64.9±8.7 years, 92% were in NYHA class III–IV, and 100% had previous MI and 90% three-vessel CAD.
All pts underwent conventional multivessel CABG under cardiopulmonary bypass, using antegrade crystalloid cardioplegia. After complete revascularization the MV was approached via the right atrium and interatrial septum. MV repair was performed by the same surgeon. Functional and segmental MV analysis Citation15 in addition to a detailed assessment of papillary muscles was performed.
Echocardiographic investigation protocol included: (1) evaluation of MR mechanism, (2) quantification of MR, (3) evaluation of LV dimensions and function, and (4) pulmonary artery pressure (PAP) preoperatively, 10–14 d and 1 year after surgery. Clinical evaluation included assessment of NYHA functional class preoperatively and 1 year after surgery.
The MR mechanism was assessed preoperatively using transthoracic echocardiography, intraoperatively using the transesophageal approach and confirmed by the surgeon on the basis of the intraoperative morphological data. The echocardiographic definition of the mechanism of MR was based on the morphology of the LV and MV apparatus and peculiarities of the MR jet. The definition of MR mechanisms was performed according to Carpentier's functional classification (Type I–III), which is based on an assessment of the opening and closing motions of the MV leaflets: dysfunction Type I – annular dilation with normal leaflet motion, Type II – excessive leaflet motion due to chordal/papillary muscle elongation/rupture, Type III – restricted leaflet motion (during diastole – IIIa, during systole – IIIb) Citation14.
Mitral annulus diameter was measured at end-systole in parasternal long-axis and four-chamber views.
MR was graded by color Doppler mapping using the measurement of regurgitant jet area in the left atrium, size (r) of the proximal jet area and effective regurgitant orifice (ERO). Calculations of ERO were performed using the proximal isovelocity surface area (PISA) method at aliasing velocity of 33 cm/s. MR was considered mild (grade 1) when the regurgitant jet area in the left atrium was less than 4 cm2, PISA radius < 6 mm, ERO < 10 mm2; moderate (grade 2) when jet area was 4–8 cm2, PISA = 6–9 mm, ERO = 10–29 mm2; severe (grade 3) when jet area was > 8 cm2, PISA ≥ 9 mm, ERO ≥ 30 mm2Citation16,17.
LV end-diastolic and end-systolic diameter indices (EDDI and ESDI, respectively) were measured according to recommendations of the American Society of Echocardiography. LV ejection fraction (EF) was measured by the biplane Simpson disk method Citation18. LV regional wall motion assessment was performed by assigning a segmental score (1 = normal, 2 = hypokinetic, 3 = akinetic, 4 = dyskinetic) to each of the 16 LV segments and calculating wall motion score index (WMSI) Citation18.
Mean and systolic PAP were evaluated using the conventional method Citation19.
Statistical analysis
All data were expressed as mean±standard deviation. Clinical data were compared with the Student's t-test and two-sided test. A value of p<0.05 was considered statistically significant.
Results
The study population was divided into three groups according to the defined MR mechanism (): group 1 = 22 pts with predominantly Type I MR, group 2 = 11 pts with Type II MR and group 3 = 20 pts with Type IIIb MR. MR surgical repair was performed according to MR mechanism.
Table I. Mechanisms of functional ischemic MR and corresponding MV repair techniques.
Preoperative data
Demographic and clinical data are presented in . The groups were substantially homogeneous, except MI localization: anterior MI prevailed in group 1, inferior MI in groups 2 and 3. MR severity (MR grade and effective regurgitant area (ERA)) did not differ between the groups. LVEDDI and PAP did not differ significantly between the groups, but LVESDI was higher and LVEF lower in group 1 ().
Table II. Demographic and clinical data.
Table III. Echocardiographic data.
Postoperative data
Early after surgery MR grade was significantly reduced and did not differ between the groups. LVEDDI, LVESDI, WMSI and systolic PAP significantly decreased and LVEF markedly increased in group 3, LVEF and WMSI improved in group 2. There were no significant changes in LV morphometry and function in group 1. One year following surgery MR grade remained unchanged in groups 2 and 3 and increased in group 1. There were significant differences in MR grade between group 1 and the remaining groups. LV morphometry and function indices LVESDI, WMSI and PAP decreased, LVEF increased in group 3, the same tendencies were noted in group 2. LV morphometry and function indices remained unchanged in group 1. Significant differences in LVEDDI, ESDI, EF, WMSI and PAP appeared between group 1 and the remaining groups.
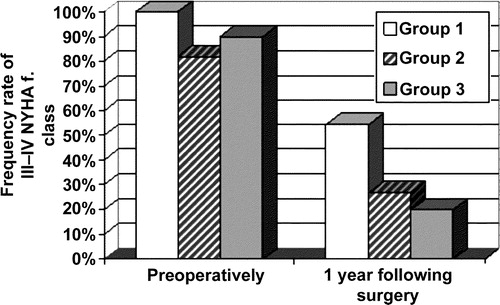
One year after surgery NYHA functional class substantially improved in group 3 – rate of NYHA functional class III–IV decreased from 90% to 20% and group 2 from 82% to 27% vs group 1 from 100% to 54.5% ().
Discussion
In the absence of papillary rupture, the precise mechanisms that cause MR secondary to myocardial ischemia are sometimes difficult to understand and impair subsequent reparative effects Citation20. Thus, careful assessment and understanding of the mechanisms causing ischemic MR is mandatory Citation21.
Analysis of the mechanisms of functional ischemic MR showed that 22/53 41, 5% of pts had Type I MR (annulus dilation), 11/53 20, 5% had Type II MR (commissural prolapse) and 20/53 38% had Type IIIb MR (restrictive motion of posterior leaflet). Our data are in agreement with the opinion that MV dysfunction, which is characterized by leaflet restriction with or without annulus dilation, is the most common mechanism of ischemic MR Citation12. Type II valve dysfunction resulting in leaflet prolapse is considered less commonly associated with ischemia MR Citation12. However, almost 1/4 of our study population presented with Type II MR – (A3, P3) commissural prolapse due to papillary muscle infarction and elongation.
Annulus undersizing is the most common approach for repair of ischemic MR, not only in the case of predominant annular dilation, but also in posterior leaflet restriction. It has been proved that annular reduction alters also subvalvular geometry, repositions papillary muscles closer to the mid-septal annulus, permits apical displacement of leaflet edges, increases leaflet coaptation length and substantially reduces MR Citation22–24. However, annulus undersizing in the case of commissural prolapse is not effective and only detailed analysis of the subvalvular apparatus permitted to employ the adjunct procedure – shortening of the ischemic papillary muscle and achieve optimal results (residual MR < grade I intraoperatively).
Precise echocardiographic evaluation has permitted two different mechanisms of Type IIIb MR to be defined: (1) posterior leaflet tethering due to inferobasal scar – increased distance between the annulus and papillary muscle, its displacement apically and posteriorally without ischemic damage and (2) posterior leaflet tethering due to inferior wall and papillary muscle infarction with anterior leaflet A3 bulging due to relative elongation of free edge chords and tethering of the strut chord. In the latter cases ring annuloplasty was insufficient and an additional procedure, shortening of the free edge chords, permitted the leaflet coaptation length to be increased. In the former cases, annulus undersizing was efficient in optimal reduction of MR.
The influence of different mechanisms of ischemic MR on late results of MV repair has not been fully investigated; thus, we undertook evaluation of correlations between the different mechanisms of ischemic MR and LV function prior to and late after surgery. Though MR severity did not differ between the groups, LV function preoperatively was slightly better preserved (LVESDI was smaller and LVEF higher) in pts with Type II and IIIb MR. Early after surgery optimal MV repair with complete revascularization resulted in marked LV function improvement in pts with Type IIIb MR (reduction in LVEDDI, LVESDI, WMSI and PAP, increase in LVEF) and Type II (increase in LVEF, reduction in WMSI) vs unchanged LV function in pts with Type I MR. At late follow-up the differences between the groups became even more evident. One year after surgery MR grade increased markedly in pts with Type I MR and significantly differed from the remaining groups. In group 1 (Type I MR) LV function, including LV dimensions, LVEF and PAP remained unchanged in comparison with preoperative data and even had a tendency to worsen. On the contrary, in pts with Type IIIb MR LV function further improved. The same tendencies were noted in pts with Type II MR, but not all changes were statistically significant due to a small number of pts in this group. Though, CABG grafts number per pt, MV repair efficiency intraoperatively was similar in all groups and preoperative LV function was only slightly better in pts with Type II and IIIb MR, following 1 year after surgery LV function (LVEDDI, LVESDI, LVEF, WMSI and PAP) was evidently better in these pts vs Type I pts. Moreover, MV remained optimally competent and positive NYHA functional class changes were also more marked in pts with Type II and IIIb MR late after surgery.
The data show that the mechanism of MR has impact on late MV repair results. However, the mechanism of ischemic MR is strongly related to the site of previous MI Citation25, LV function and remodeling Citation9,10,26,27. We have to note that in pts with Type I MR previous anterior MI prevailed, which causes more extensive ischemia/scar, minor involvement of the back positioned MV apparatus, but marked distortion of LV geometry Citation28 with displacement of both papillary muscles and further changes in mitral annulus. In pts with anterior MI, LV dilation has a major adjunctive role in the genesis of MR Citation25. Thus, Type I MR is usually the result of severely depressed LV function, markedly altered LV size and geometry (LV sphericalization) Citation29. LV systolic function is a strong predictor of poor prognosis Citation4,30 and it is reasonable to suppose that the outcome after surgical treatment of pts with low LVEF, distorted LV geometry and dilated annulus is mainly related to LV dysfunction severity.
Progression of MR in pts with predominant annulus dilation late after mitral annuloplasty may be the result of further LV remodeling and progression of LV dysfunction. It has been stated that LV remodeling precedes LV dilation and functional MR Citation31 and MR is a consequence, but not a cause of, postinfarction remodeling. Thus, operative techniques targeting LV geometry restoration and preventing further ischemic distortion of MV apparatus may be the more rational surgical solution.
On the other hand, in pts with Type II and IIIb MR inferior MI prevailed, which causes less extensive ischemia, but with the direct involvement of important components of the MV apparatus, including basal inferior LV wall and posterior papillary muscle. In these cases complete revascularization with appropriate MV repair was beneficial for positive LV remodeling.
Conclusions
Ischemic mitral regurgitation of Type I (due to predominant annular dilation) is associated with more marked LV dysfunction preoperatively, further deterioration of LV function and progression of mitral regurgitation late after MV repair.
Ischemic mitral regurgitation of Type IIIb (predominant restriction of posterior leaflet) and Type II (commissural prolapse) correlates with better preserved LV function preoperatively, marked incremental improvement in LV function and optimally competent MV late after surgery.
The present data, concerning correlations between the mechanisms of ischemic MR and LV function following MV repair, may have prognostic implications while considering the options of surgical treatment of ischemic MR.
References
- Grigioni F, Sarano ME, Zehr KJ, Bailey K, Tajik AJ, et al. Ischemic mitral regurgitation. Long term outcome and prognostic implications with quantitative Doppler assessment. Circulation 2001; 103: 1759–64
- Lamas GA, Mitchell GF, Flaker GC, Smith S, Gersh B, Basta L, Moye L, Braunwald E, Pfeffer MA, et al. Clinical significance of mitral regurgitation after acute myocardial infarction: Survival and ventricular enlargement investigations. Circulation 1997; 96: 827–33
- Adler DS, Goldman L, O'Neil A, Cook E, Mudge G, Shemin R, Disesa V, Cohn L, Collins J, et al. Long term survival of more than 2000 patients after coronary artery bypass grafting. Am J Cardiol 1986; 58: 195–202
- Kirklin JK, Naftel DC, Blackstone EH, Kirklin JW, Brown RC, et al. Risk factors for mortality after primary combined valvular and coronary artery surgery. Circulation 1989; 79: 1185–90
- Tahta SA, Oury JH, Maxwell JM, Hiro SP, Duran CM, et al. Outcome after mitral valve repair. J Heart Valve Dis 2002; 11: 11–20
- Akar AR, Dukas G, Szafranek A, Alexion C, Boehm M, Chin D, Sosnowski A, Spyt TJ, et al. Mitral valve repair and revascularization for ischemic mitral regurgitation: Predictors of operative mortality and survival. J Heart Valve Dis 2002; 11: 793–801
- David TE. Techniques and results of mitral valve repair for ischemic mitral regurgitation. J Card Surg 1994; 9: 274–7
- Yiu SF, Sarano ME, Triboilloy C, Seward J, Tajik AJ, et al. Determinants of the degree of functional mitral regurgitation in patients with systolic left ventricular dysfunction. A Quantitative Clinical Study. Circulation 2000; 102: 1400–6
- Otsuji Y, Handschumacher MD, Schwammenthal E, Jiang L, Song JK, Guerrero J, Vlahakes G, Levine RA, et al. Insights from three-dimensional echocardiography into the mechanism of functional mitral regurgitation: Direct in vivo demonstration of altered leaflet tethering geometry. Circulation 1997; 96: 1999–2008
- He S, Fontaine AA, Schwammenthal E, Yoganathan A, Levine RA, et al. An integrated mechanism for functional mitral regurgitation: Leaflet restriction vs. Coapting force – in vitro studies. Circulation 1997; 96: 1826–34
- Liel-Cohen N, Guerrero JL, Otsuji Y, Handschumacher MD, Rudski L, Hunziker P, Tanabe H, Scherrer-Crosbie M, Sullivan S, Levine RA, et al. Design of a new surgical approach for ventricular remodelling to relieve ischemic mitral regurgitation: Insights from three-dimensional echocardiography. Circulation 2000; 101: 2756–63
- Adams DH, Filsoufi F, Aklog L. Surgical treatment of the ischemic mitral valve. J Heart Valve Dis 2002; 11 Suppl 1: S21–5
- Schwammenthal E, Popescu AC, Popescu BA, Freimark D, Hod H, Eldar M, Feinberg M, et al. Mechanism of mitral regurgitation in inferior wall acute myocardial infarction. Am J Cardiol 2002; 90: 306–9
- Carpentier A. Cardiac valve surgery – the “French Correction”. J Thorac Cardiovasc Surg 1983; 86: 323–37
- Carpentier A, Chauvaud S, Fabiani JN, Deloche A, Relland J, Lessana A, D'Allaines C, Blondeau P, Piwnica A, Dubost C, et al. Reconstructive surgery of mitral valve incompetence: Ten-year appraisal. J Thorac Cardiovasc Surg 1980; 79: 338–48
- Dujardin KS, Enriquez-Sarano M, Bailey K, Nishimura R, Seward J, Tajik AJ. Grading of mitral regurgitation by Doppler echocardiography. Circulation 1997; 96: 3709–15
- Rossi A, Dujardin KS, Bailey KR, Seward J, Enriquez-Sarano M, et al. Rapid estimation of regurgitant volume by the proximal isovelocity surface area method in mitral regurgitation: Can continuous wave Doppler echocardiography be omitted?. J Am Soc Echocardiogr 1998; 11: 138–48
- Schiller NB, Shah PM, Crawford M, De Maria A, Deveraux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, et al. Recommendation of quantification of the left ventricle by two dimensional echocardiography. J Am Soc Echocardiogr 1989; 2: 358–67
- Kitabatake A, Inoue M, Asao M, Masuyama T, Tanouchi J, Morita T, Mishima M, Uematsu M, Shimazu T, Hori M, Abe H, et al. Noninvasive evaluation of pulmonary hypertension by a pulsed Doppler technique. Circulation 1983; 68: 302–9
- Gorman JH, Gorman RC, Jackson BM, Hiramatsu Y, Gikakis N, Kelley ST, Sutton MG, Plappert T, Edmunds LH Jr, et al. Distortions of the mitral valve in acute ischemic mitral regurgitation. Ann Thorac Surg 1997; 64: 1026–31
- Fasol R, Lakew F, Pfannmuller B, Slepian M, Joubert-Hubner E, et al. Papillary muscle repair surgery in ischemic mitral valve patients. Ann Thorac Surg 2000; 70: 771–7
- Timek TA, Lai DT, Tibayan F, Liang D, Rodriguez F, Daughters G, Dagum P, Ingels NB, Miller C, et al. Annular versus subvalvular approaches to acute ischemic mitral regurgitation. Circulation 2002; 106 Suppl: I-27–I-32
- Lai DT, Timek TA, Green GR, Glasson JR, Daughters GT, Liang D, Ingels NB Jr, Miller DC, et al. The effects of ring annuloplasty on mitral leaflet geometry during acute left ventricular ischaemia. J Thorac Cardiovasc Surg 2000; 120: 966–75
- Bolling SF, Pagani FD, Deeb GM, Bach DS, et al. Intermediate-term outcome of mitral reconstruction in cardiomyopathy. J Thorac Cardiovasc Surg 1998; 115: 381–6
- Lancellotti P, Lebrun F, Pierard LA. Determinants of exercise-induced changes in mitral regurgitation in patients with coronary artery disease and left ventricular dysfunction. J Am Coll Cardiol 2003; 42: 1921–8
- Kono T, Sabbah HN, Stein PD, Brymer JF, Khaja F, et al. Left ventricular shape as a determinant of functional mitral regurgitation in patients with severe heart failure secondary to either coronary artery disease or idiopathic dilated cardiomyopathy. Am J Cardiol 1991; 68: 355–9
- Kono T, Sabbah HN, Rosman H, Alam M, Jafri S, Stein PD, Goldstein S, et al. Left ventricular shape is the primary determinant of functional mitral regurgitation in heart failure. J Am Coll Cardiol 1992; 20: 1594–8
- Calafiore AM, Di Mauro M, Gallina S, Giammarco G, Iaco A, Teodori G, Tavarozzi I, et al. Mitral valve surgery for chronic ischemic mitral regurgitation. Ann Thorac Surg 2004; 77: 1989–97
- Stewart WJ, Sun JP, Mayer E, et al. Mitral regurgitation with normal leaflets results from apical displacement of coaptation, not annular dilation [abstract]. Circulation 1994; 90: I-311
- Seipelt RG, Schoendube FA, Vazquez-Jimenez JF, Doerge H, Voss M, Messmer BJ, et al. Combined mitral valve and coronary artery surgery: Ischemic versus non-ischemic mitral valve disease. Eur J Cardiothorac Surg 2001; 20: 270–5
- Howard RJ, Moe GM, Armstrong PW. Sequential echocardiographic-Doppler assessment of left ventricular remodelling and mitral regurgitation during evolving experimental heart failure. Cardiovasc Res 1991; 25: 468–74