Objectives
Intracoronary transplantation of different cell populations has been used in acute myocardial infarction (AMI) with promising results. The primary objective of the Autologous Stem cell Transplantation in Acute Myocardial Infarction (ASTAMI) study is to test whether intracoronary transplantation of autologous mononuclear bone marrow cells (mBMC) improves left ventricular ejection fraction (LVEF) after anterior wall AMI.
Design
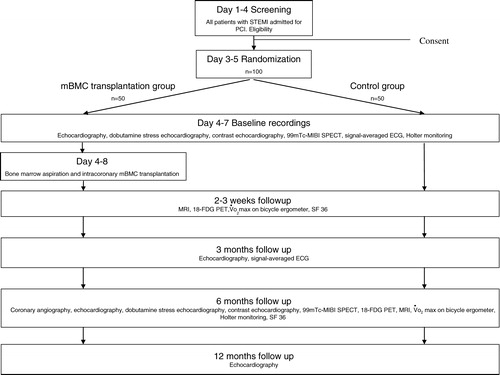
The ASTAMI study is a randomized, controlled, prospective study. One hundred patients with acute anterior wall ST-elevation myocardial infarction (STEMI) treated with acute percutaneous coronary intervention (PCI) are randomized in a 1:1 way to either intracoronary transplantation of autologous mBMC 5–8 d after PCI or to control. Left ventricular function, exercise capacity, biochemical status, functional class, quality of life and complications are validated at baseline and during a 12-month follow-up.
Results
By August 2004, out of 1004 patients with STEMI, 49 patients have been included in the study. Twenty-four patients have been randomized to intracoronary mBMC transplantation. Twenty patients had chest pain and 16 patients had ischemic ECG changes during the mBMC transplantation procedure. One patient had ventricular fibrillation 24 h after transplantation.
Conclusions
Intracoronary transplantation of autologous mBMC in the acute phase after AMI is feasible and seems safe in the short term.
Introduction
The main problems after acute myocardial infarction (AMI) are the consequences of loss of cardiomyocytes. Even though revascularization and best medical treatment improve prognosis, therapies aiming to regenerate lost myocardium are needed. Until recently, the heart has been regarded a post-mitotic organ without regenerating potential. However, myocardial hypertrophy is caused by cell hypertrophy and cell hyperplasia Citation1. Cardiomyocytes in cell cyclus and mitosis have been observed, and are significantly increased after large AMI Citation2. It has been estimated that all cardiomyocytes lost in AMI can be restored in a few months, but this process is restricted to the viable myocardium and its border zone Citation3. Cardiac stem cells have been identified Citation4, and cardiomyocytes continuously die in the process of necrosis and apoptosis and are replaced by new ones Citation3. However, AMI heals with scar formation Citation5. Especially large and transmural myocardial infarctions (MI) lead to remodelling, characterized by changes in geometry and size of the left ventricle. Remodelling is associated with reduced survival Citation6.
Cell transplantation
Myoblasts obtained from muscle biopsies have been transplanted to patients with chronic ischemic heart failure with subsequent improvement in ventricular function Citation7. However, these cells remain electrically isolated since gap junctions are not expressed, and this may predispose for the ventricular arrhythmias observed after myoblast transplantation. Bone marrow contains haematopoietic stem cells (HSC), mesenchymal stem cells (MSC) and endothelial precursor cells (EPC). After bone marrow transplantation, donor-derived cells have been identified in non-haematopoietic tissues like brain Citation8, liver Citation9 and striated muscle Citation10. In an experimental model of AMI in mice, bone marrow cells (BMC) were injected in the peri-infarct region. Left ventricular systolic dP/dt improved and cardiomyocytes, smooth muscle cells and endothelial cells were identified with transplanted cell markers Citation11. Furthermore, in male heart transplant recipients of female donor hearts, a significant number of Y-chromosome positive myocytes, arterioles and capillaries have been identified in the myocardial tissue at autopsy Citation12. In females who received male bone marrow, 0.23% Y-chromosome positive cardiomyocytes were found in heart specimens at autopsy Citation13. It is known that HSC can be found in peripheral blood under normal circumstances Citation14, and it has been postulated that progenitor cells “home” to myocardial tissue and differentiate to cardiomyocytes, smooth muscle cells and endothelial cells Citation15. The ability of adult stem cells to mature to cells different from their normal lineage development is referred to as plasticity. Recently, mononuclear cells expressing early cardiac, muscle and endothelial markers have been identified in peripheral blood Citation16. The number of these cells are significantly increased in the setting of ST-segment elevation myocardial infarction (STEMI), and there may be a population of tissue committed stem cells hiding out in different niches being mobilized by cytokine release induced by tissue damage. In conclusion, it seems like myocardial damage to a certain degree is repaired by intrinsic stem cells and circulating progenitor cells originating from bone marrow, but this process is insufficient in the setting of a large MI.
To augment the physiological process of tissue repair after AMI, studies of adult stem cell transplantation to the heart, both in animals and man, have been performed. The results have demonstrated positive effects on cardiac function Citation11, Citation17–22. This may be due to transdifferentiation of the transplanted cells to cardiomyocytes, although fusion of the transplanted cells with in situ viable cardiomyocytes cannot be ruled out Citation23. However, other mechanisms for the improvement in cardiac function may exist, e.g. secretion of local factors inducing angiogenesis, increased collagen formation and reduced apoptosis. This may in turn lead to reduced infarct expansion and remodelling and preservation of hibernating myocardium Citation24.
Human BMC transplantation studies in AMI
Autologous BMC have been transplanted to patients with chronic ischemic heart disease with promising results Citation25–27. The effect of transplantation of these cells in the setting of AMI have recently been investigated. Strauer et al. studied 10 patients transplanted with mononuclear bone marrow cells (mBMC) infused into the infarct-related coronary artery 5–9 d after acute percutaneous coronary intervention (PCI) for AMI and reported improved perfusion and regional wall motion in the infarct region at 3 months follow-up. In the TOPCARE-AMI study, either mBMC (n=23) or circulating progenitor cells (CPC) (n=23) were transplanted to the infarct-related artery 4.3 d after AMI utilizing the same methodology as in Strauer et al.'s study Citation18,19. Global left ventricular ejection fraction (LVEF) and regional function significantly improved, and infarct size measured by contrast-MRI was significantly reduced. The BOOST study was the first randomized trial to study intracoronary transplantation of BMC in the acute phase of MI Citation22. Principally the same protocol as in Strauer et al.'s study was used. There were 30 patients in the BMC therapy group and 30 control patients. At 6-month follow-up, LVEF by MRI was significantly increased to 56.7% vs 50.0% at baseline in the BMC group. In the control group LVEF was unchanged. MSC have been used in one study in humans Citation21. MSC cultured for 10 d were infused intracoronarily in the infarct-related artery nearly 18 d after PCI for AMI. The technique described by Strauer et al. was used for cell transplantation. Patients were randomized to MSC (n=34) or sham (saline, n=35) intracoronary infusions. At 3-month follow-up, LVEF on ventriculography had improved significantly to 67% vs 49% at baseline in the cell group whereas in the control group there was only a modest improvement (53% vs 48%). HSC can be mobilized to peripheral blood by cytokines, e.g. granylocyte-colony stimulating factor (G-CSF) and stem cell factor (SCF). This approach was used in the MAGIC trial Citation20, which indicated improvement in perfusion and systolic function at 6-month follow-up, but the study was stopped prematurely due to significant in-stent restenosis in a high proportion of treated patients.
The ASTAMI study
The promising results of intracoronary transplantation of autologous BMC in patients with AMI encouraged further controlled studies on human cell transplantation therapy. The Autologous Stem cell Transplantation in Acute Myocardial Infarction (ASTAMI) study is a double-centre, randomized, prospective and controlled study. The study is designed to test the effect of intracoronary transplantation of autologous mBMC in the left anterior descending artery (LAD) 5–8 d after successful acute PCI with stent for anterior wall AMI. Rikshospitalet University Hospital and Ullevål University Hospital do at present handle all patients with AMI eligible for acute PCI in the south and east health regions of Norway comprising approximately 2.5 million people, and this results in about 1000 acute PCI in STEMI annually. Patients fulfilling the inclusion and none of the exclusion criteria are consecutively invited to participate in the study. All patients admitted with STEMI are registered regardless of inclusion or exclusion from the study. The study design is given schematically in . Patients are included and randomized in a 1:1 way to either intracoronary transplantation of autologous BMC or control. We perform a permuted block randomization stratified on the two centres. All patients receive standard best medical therapy with ASA, clopidogrel, beta-blocker, statin and angiotensin-converting enzyme (ACE) inhibitor or angiotensin II receptor (ATII) blocker unless contraindicated. A Gp IIB/IIIA antagonist is used in conjunction with PCI when indicated as judged by the angiographer. All patients are offered general advice on diet, smoking and lifestyle change. All patients will follow post AMI rehabilitation in accordance with their local hospital conventions.
Objectives of the study
The primary objective of the study is to examine whether intracoronary mBMC transplantation improves LVEF after AMI assessed by ECG-gated single photon emission computed tomography (SPECT). Secondary objectives are to test whether mBMC treatment improves exercise capacity assessed by bicycle ergometry as well as quality of life assessed by the SF 36 formula. Additional objectives are 1) to test whether mBMC treatment reduces the extension of infarcted myocardium as judged by segmental analysis of left ventricular myocardium in terms of ischemia and viability by use of (a) echocardiography and dobutamine stress echocardiography with echo contrast and tissue velocity imaging techniques, (b) MRI with cine-loops, gadolinum contrast and late enhancement with gadolinum contrast and (c) scintigraphic techniques with SPECT and 18-FDG PET, 2) to analyse whether any improvement correlates to the number of cells injected (total mBMC/CD34+ cells) and finally 3) to test whether intracoronary mBMC transplantation affects vascular biochemical responses as judged by analysis of a wide range of circulating inflammatory and signalling variables.
Inclusion and exclusion criteria
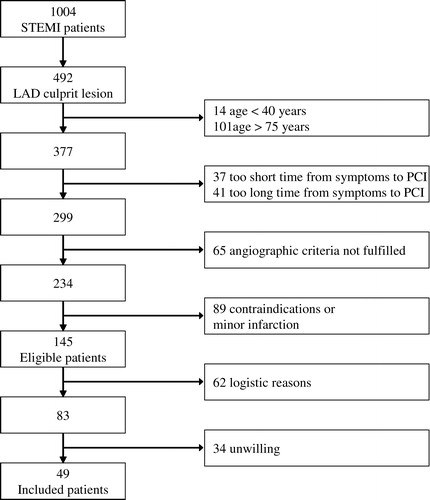
All patients with STEMI admitted for acute PCI (primary, facilitated or rescue) are screened for inclusion 1–4 d after PCI. Inclusion criteria are 1) age 40–75 years, 2) anterior wall AMI with 120–720 min from onset of symptoms to PCI, 3) ST-elevation on ECG according to WHO criteria, 4) angiographically significant stenosis on LAD proximal to the second diagonal branch, 5) successful PCI with stenting of culprit lesion, 6) ≥3 hypokinetic, akinetic or dyskinetic segments assessed by echocardiography in a standard 16 segment model and 7) creatine kinase MB (CK-MB) above three times upper reference value. Exclusion criteria are 1) previous MI with established significant Q-waves on ECG, 2) cardiogenic shock, 3) permanent pacemaker or other contraindication to MRI, 4) stroke with significant sequela, 5) short life expectancy due to extra cardiac reason, 6) uncontrolled endocrinological disturbance, 7) HIV and/or HBV/HCV positive serology and 8) mental disorder or other condition which interferes with patient possibility to comply with the protocol. A patient is included in the study if eligible and after giving written informed consent.
Bone marrow aspiration, preparation and mBMC transplantation
Patients randomized to intracoronary, autologous mBMC transplantation are aspirated for 50 ml of bone marrow 4–7 d after PCI by pelvic cristal puncture in local anaesthesia. Clotting is prevented with 10 000 IU heparin (Monoparin®, CP Pharmaceuticals, Wrexham, UK).
The bone marrow aspirate is diluted in 75 ml NaCl, and is gradient centrifuged on Lymphoprep (Axis-Shield, Oslo, Norway). The mBMC are washed three times with NaCl containing 5% autologous plasma prior to resuspension in 11 ml NaCl with 20% plasma for overnight storage. The next day, the mBMC suspension is filtered through a 70 µm sieve prior to quality control analysis and intracoronary transplantation. All cell preparation takes place in a laboratory approved according to good manufacturing practice (GMP) by the Norwegian Medicines Agency for ex vivo cell manipulation. Samples are tested for bacterial contamination, cell yield, viability, aggregate count and content of CD34+ cells and CD34+ cell subpopulations. Cell viability has to be above 90%, bacterial specimens should be negative and the proportion of cell aggregates ≥3 cells should be less than 10% of all cells to permit intracoronary transplantation.
The day after bone marrow aspiration, i.e. on d 5–8, the mBMC suspension is transplanted to the infarct-related coronary artery (LAD). The ostium of the left coronary artery is intubated with a 6 French guiding catheter. After administration of heparin 100 IU/kg body weight, a 0.5 mm oversized over-the-wire balloon catheter is advanced to the proximal part of the stent and inflated with very low pressures (<2 bar). One-third (ca 3 ml) of the mBMC suspension is then injected through the inner lumen of the catheter to the LAD and its side branches distal to the balloon. The balloon is then kept inflated for 90 s followed by 5 min deflation. The procedure is repeated twice. CK-MB mass, troponins and ECG are taken the day before and the day after the procedure. ECG with standard leads is monitored during the procedure and any chest pain and/or arrhythmias are registered. After the procedure, patients are monitored on telemetry for at least 24 h.
Power and statistics
The major endpoint is change in left ventricular function assessed by LVEF as estimated by scintigrams at baseline and 6-month follow-up. The outcome variable isBased on data on 93 patients with AMI investigated with scintigraphic techniques at Ullevål University Hospital, the estimated standard deviation of the ΔLVEF is 8.3%. Similar variability is estimated from biplane LVEF ultrasound. A clinically significant difference between treatment and control groups is considered at a ΔLVEF of 5% or more. For a type I error of 5% and a power of 80% with a pooled estimate of variance of SD = 8.3%, we will need 2×45 patients for the two arms of the study Citation28.
Analysis of the trial will be done according to the intention to treat strategy, i.e. all randomized patients will be kept in the study, and the analysis will be performed on the groups as randomized. Due to the relatively small number of patients in each arm, it is mandatory to consider major risk factors for outcome and thus perform an adjustment of efficacy by using adequate multivariate models as general estimated equation or ANOVA with repeated measure or multivariate linear regression models with outcome ΔLVEF difference between baseline and follow-up Citation29. All patient data and analysis results are managed in a database/analysis program (EpiData/EpiInfo, Centers for Disease Control and Prevention, Atlanta, GA, USA).
Approval and monitoring
The protocol has been approved by the Regional Committee for Medical Research Ethics and the Norwegian Medicines Agency according to GCP (good clinical practice) and GMP standards. To assess the safety of the procedure and the intracoronary mBMC treatment, the Steering Committee has appointed an independent Data and Safety Monitoring Board (DSMB). All investigations are continuously evaluated and adverse events and/or unexpected patient responses in terms of cardiovascular status, biochemical status, general well-being, need for rehospitalization, etc., are recorded at every follow-up visit. The accumulated results are consecutively made available to the DSMB. This committee has the right to advise the Steering Committee to first halt inclusion and subsequently terminate the study if a significant allocation of negative patient responses in the treatment group is observed. All serious adverse events and/or unexpected events will be reported to this committee and to the Norwegian Medicines Agency according to Norwegian Legislation.
Results
Patient enrolment started September 2003. By August 2004 we had screened 1004 STEMI patients for inclusion. Of these, 49 were included () and 24 were randomized to intracoronary mBMC transplantation. shows patients’ baseline characteristics. One aspirate was discarded because of contamination with Staphylococcus capitis. The patient accepted to repeat the aspiration procedure, which was successfully performed the next day. During the mBMC transplantation procedure, 20 patients noticed chest pain and 16 patients had reversible ischemic ECG changes after inflation of the balloon. There were no arrhythmias during the procedure, and no reinfarctions related to the mBMC transplantation. No increase in the levels of CK and CK-MB could be detected the morning after cell transplantation (). At discharge, all patients were taking ASA, clopidogrel, beta-blocker, statin and an ACE inhibitor or ATII blocker.
Table I. Baseline characteristics of the study population.
Table II. Biochemical and clinical parameters related to the mBMC transplantation.
Complications at 30 d after AMI
In the treatment group, one patient had reinfarction caused by stent thrombosis the day before planned mBMC transplantation, after bone marrow aspiration, SPECT and stress echocardiography were performed. The patient was treated immediately with new PCI. Owing to safety concerns, the patient did not receive treatment according to randomization. One patient had ventricular fibrillation 24 h after the procedure. He was successfully resuscitated and had an ICD implanted before discharge. One patient has been admitted twice with worsening heart failure. One patient has been admitted twice to hospitals not taking part in the study, diagnosed with non-coronary chest pain and vasovagal syncope, respectively.
In the control group, one patient had ventricular tachycardia 3 d after AMI and was cardioverted. One patient had elective PCI on the circumflex coronary artery. One patient has been admitted twice diagnosed with non-coronary chest pain and one patient was admitted with suspected stent thrombosis. Coronary angiography was performed and showed no evidence of stent thrombosis or restenosis and this patient was also diagnosed with non-coronary chest pain.
Discussion
The primary objective of the ASTAMI study is to investigate whether intracoronary transplantation of autologous mBMC improves left ventricular function after AMI. The study by Strauer et al. and the TOPCARE-AMI study were both non-randomized, and even though they indicate that treatment is safe and may improve myocardial function, they have convincingly demonstrated the feasibility of the treatment only Citation17–19. The BOOST study and the study by Chen et al. were randomized, however, both studies comprised patients without regards to infarct localization Citation21,22.
The ASTAMI study is randomized, but open-labelled because the Steering Committee found bone marrow aspiration and sham intracoronary infusions in the control group unethical. Sample size is calculated to achieve adequate power to assess the primary objective of the study.
In the ASTAMI study, inclusion and exclusion criteria were selected to obtain a relatively homogenous patient population with definite anterior wall infarction well suited for accurate evaluation of left ventricular global and regional function. In a standard 16-segment model for assessment of left ventricular function, the LAD territory schematically perfuses nine segments, the circumflex artery four segments and the right coronary artery three segments Citation30. LAD perfuses the apex, the anterior free wall, as well as the anterior and medial aspects of the septum which taken together represent the major portion of the left ventricular myocardium. Thus, AMI due to LAD occlusion tends to produce larger infarcts with more profound impact on left ventricular function, and subsequently a higher proportion of heart failure Citation31. Furthermore, wall motion analysis by echocardiography is more accurate when performed on the anterior than on the posterior ventricular segments Citation32. Thus, by choosing acute LAD-related infarctions as inclusion criterion in the present study, we intended to select a group of patients in whom treatment, if effective, would mean a clinical benefit, and in whom the accuracy of the imaging modalities is best. The inclusion criteria regarding duration of ischemia and time after start of symptoms, location of the stenosis, CK-MB mass and wall motion analysis on echocardiography were chosen to ensure that significant myocardial necrosis at baseline is present, thus establishing a potential to demonstrate any regeneration of myocardium. Exclusion criteria were selected to avoid confounding factors. With the methodology used, if there is no effect of intracoronary mBMC transplantation in this highly selected patient population, mBMC treatment can probably not be supposed to have positive effects in AMI patients with other infarct localizations.
In the studies that have been performed, different cell populations have been used. The different progenitor cells of the bone marrow constitute a small fraction of the mononuclear cell population and Strauer et al. used mBMC for transplantation Citation17. In the TOPCARE-AMI study either mBMC or CPC were used, and they found no evidence for differences in effect for the two cell populations Citation18,19. In the BOOST study, all nucleated BMC, not only the mononuclear fraction were used. Thus, a large amount of inflammatory cells were delivered to the infarcted myocardium, and this may have led to an unwanted inflammatory response. In the study by Chen et al., MSC were used Citation21,22. So far, there are no data from clinical or animal studies which indicate that a particulate cell population should be preferred for transplantation to the heart in the setting of AMI. In a rat infarction model, either myoblasts, CD133+ BMC or culture medium were injected into the scar. Injections of both cell populations led to improvement in LVEF 1 month after transplantation compared to controls, whereas there was no significant difference between the cell populations Citation33. Use of MSC requires cultivation for several days, making it impossible to use this cell population in the sub-acute phase of AMI. In the ASTAMI study, mBMC were chosen for intracoronary cell transplantation as this cell population contains all types of progenitor cells in the bone marrow that may have a potential for regeneration of myocardium. By removing polymorphonuclear cells from the bone marrow aspirate we avoid the possible unwanted effect of infusion of a large number of inflammatory cells to the heart.
There have been concerns regarding safety of cell transplantation in AMI. All studies with intracoronary cell transplantation to date have used a similar procedure to that described by Strauer et al. Citation17, a procedure which was also chosen in the ASTAMI study. Intracoronary transplantation of expanded MSC caused microinfarctions in dogs Citation34, but this may have been due to larger cell diameter than in freshly prepared BMC. So far, we and others Citation17–19, Citation21, Citation22 have not observed any reinfarctions related to the intracoronary cell transplantation procedure. To date, one patient in ASTAMI had ventricular fibrillation 24 h after intracoronary mBMC transplantation. In this population at high risk for ventricular arrhythmias Citation35, it is impossible to determine if this event had any relation to the treatment. In the studies that have been published, no tendency for malignant arrhythmias have been observed Citation17–19, Citation21, Citation22. The high incidence of in-stent restenosis in the MAGIC trial was probably due to high levels of inflammatory cells at the time of PCI as G-CSF was administered in advance of the procedure Citation21. Restenosis was also assessed in the BOOST study where no G-CSF was used and there was no evidence for increased rates of in-stent restenosis in the BMC group Citation22. So far in the ASTAMI study, at 30 d after AMI, there have not been any reinfarctions or stent thrombosis after transplantation of mBMC and generally, complication numbers are small in both groups.
Conclusion
Intracoronary, autologous mBMC transplantation is a promising therapy to improve left ventricular function after AMI. All human studies on cell transplantation after AMI to date have indicated positive effect on myocardial function, but patient numbers are small and only a few studies have been randomized. The ASTAMI study is designed to test in a robust way whether this therapy is as effective as it seems in the smaller studies that have been published. The treatment is feasible and seems safe in the short-term perspective.
References
- Linzbach AJ. Heart failure from the point of view of quantitative anatomy. Am J Cardiol 1960; 5: 370–82
- Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med 2001; 344: 1750–7
- Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Myocyte death, growth, and regeneration in cardiac hypertrophy and failure. Circ Res 2003; 92: 139–50
- Urbanek K, Quaini F, Tasca G, Torella D, Castaldo C, Nadal-Ginard B, et al. Intense myocyte formation from cardiac stem cells in human cardiac hypertrophy. Proc Natl Acad Sci USA 2003; 100: 10440–5
- Mallory GK, White PD, Salcedo-Salgar J. The speed of healing of myocardial infarction: A study of the pathologic anatomy in 72 cases. Am Heart J 1939; 18: 647–71
- Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 1990; 81: 1161–72
- Menasche P, Hagege AA, Vilquin JT, Desnos M, Abergel E, Pouzet B, et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol 2003; 41: 1078–83
- Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: Expression of neuronal phenotypes in adult mice. Science 2000; 290: 1775–9
- Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, et al. Liver from bone marrow in humans. Hepatology 2000; 32: 11–6
- Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science 1998; 279: 1528–30
- Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature 2001; 410: 701–5
- Quaini F, Urbanek K, Beltrami AP, Finato N, Beltrami CA, Nadal-Ginard B, et al. Chimerism of the transplanted heart. N Engl J Med 2002; 346: 5–15
- Deb A, Wang S, Skelding KA, Miller D, Simper D, Caplice NM. Bone marrow-derived cardiomyocytes are present in adult human heart: A study of gender-mismatched bone marrow transplantation patients. Circulation 2003; 107: 1247–9
- Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science 2001; 294: 1933–6
- von Harsdorf R, Poole-Wilson PA, Dietz R. Regenerative capacity of the myocardium: Implications for treatment of heart failure. Lancet 2004; 363: 1306–13
- Wojakowski W, Tendera M, Michalowska A, Majka M, Kucia M, Maslankiewicz K, et al. Mobilization of CD34/CXCR4+, CD34/CD117+, c-met+ stem cells, and mononuclear cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in patients with acute myocardial infarction. Circulation 2004; 110: 3213–20
- Strauer BE, Brehm M, Zeus T, Kostering M, Hernandez A, Sorg RV, et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation 2002; 106: 1913–8
- Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI). Circulation 2002; 106: 3009–17
- Britten MB, Abolmaali ND, Assmus B, Lehmann R, Honold J, Schmitt J, et al. Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (TOPCARE-AMI): Mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation 2003; 108: 2212–8
- Kang HJ, Kim HS, Zhang SY, Park KW, Cho HJ, Koo BK, et al. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: The MAGIC cell randomised clinical trial. Lancet 2004; 363: 751–6
- Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol 2004; 94: 92–5
- Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: The BOOST randomised controlled clinical trial. Lancet 2004; 364: 141–8
- Nygren JM, Jovinge S, Breitbach M, Sawen P, Roll W, Hescheler J, et al. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med 2004; 10: 494–501
- Forrester JS, Price MJ, Makkar RR. Stem cell repair of infarcted myocardium: An overview for clinicians. Circulation 2003; 108: 1139–45
- Tse HF, Kwong YL, Chan JK, Lo G, Ho CL, Lau CP. Angiogenesis in ischaemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation. Lancet 2003; 361: 47–9
- Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Mesquita CT, et al. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation 2003; 107: 2294–302
- Stamm C, Westphal B, Kleine HD, Petzsch M, Kittner C, Klinge H, et al. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet 2003; 361: 45–6
- Pocock, S. Clinical trials; A practical approach. New York: John Wiley; 1983.
- Kleinbaum, D. Applied regression analysis and other multivariable methods. Boston: PEWS-KENT; 1988.
- Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 1989; 2: 358–67
- Stone PH, Raabe DS, Jaffe AS, Gustafson N, Muller JE, Turi ZG, et al. Prognostic significance of location and type of myocardial infarction: Independent adverse outcome associated with anterior location. J Am Coll Cardiol 1988; 11: 453–63
- Takeuchi M, Araki M, Nakashima Y, Kuroiwa A. Comparison of dobutamine stress echocardiography and stress thallium-201 single-photon emission computed tomography for detecting coronary artery disease. J Am Soc Echocardiogr 1993; 6: 593–602
- Agbulut O, Vandervelde S, Al Attar N, Larghero J, Ghostine S, Leobon B, et al. Comparison of human skeletal myoblasts and bone marrow-derived CD133+ progenitors for the repair of infarcted myocardium. J Am Coll Cardiol 2004; 44: 458–63
- Vulliet PR, Greeley M, Halloran SM, MacDonald KA, Kittleson MD. Intra-coronary arterial injection of mesenchymal stromal cells and microinfarction in dogs. Lancet 2004; 363: 783–4
- Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346: 877–883
Appendix A
Steering Committee
Kolbjørn Forfang1 (chair), Svend Aakhus1, Harald Arnesen2, Torstein Egeland3, Knut Endresen1, Arnfinn Ilebekk4, Arild Mangschau2.
Study investigators
Svend Aakhus1, Michael Abdelnoor5, Harald Arnesen2, Pål Aukrust6, Reidar Bjørnerheim2, Magne Brekke7, Lorentz Brinch8, Jan E. Brinchmann3, Torstein Egeland3, Knut Endresen1, Jan Gunnar Fjeld9, Kolbjørn Forfang1, Haakon Kiil Grøgård4, Arnfinn Ilebekk4, Tor Ole Kjellevand1, Nils Einar Kløw7, Ketil Lunde1, Arild Mangschau2, Carl Müller10, Ingebjørg Seljeflot11, Hans Jørgen Smith12, Svein Solheim2, Eli Taraldsrud3. 1Department of Cardiology, Rikshospitalet University Hospital, Oslo, Norway; 2Department of Cardiology, Ullevål University Hospital, Oslo, Norway; 3Institute of Immunology, Rikshospitalet University Hospital, Oslo, Norway; 4Institute for Experimental Medical Research, University of Oslo, Norway; 5Unit of Epidemiology and Biostatistics, Centre for Clinical Research, Ullevål University Hospital, Oslo, Norway; 6Research Institute of Internal Medicine, Rikshospitalet University Hospital, Oslo, Norway; 7Department of Cardiovascular Radiology, Ullevål University Hospital, Oslo, Norway; 8Section of Haematology, Medical Department, Rikshospitalet Univeristy Hospital, Oslo, Norway; 9Department of Radiology, Section of Nuclear Medicine, Rikshospitalet University Hospital, Oslo, Norway; 10Department of Nuclear Medicine, Ullevål University Hospital, Oslo, Norway; 11Centre for Clinical Research, Ullevål University Hospital, Oslo, Norway; 12Department of Radiology, Rikshospitalet University Hospital, Oslo, Norway.
Data and Safety Monitoring Board
Knut Rasmussen13 (chair), Lars Wallentin14, Rune Wiseth15. 13Department of Cardiology, University Hospital of North Norway, Tromsø, Norway; 14Uppsala Clinical Research Centre, University Hospital, Uppsala, Sweden; 15Department of Cardiology, University Hospital of Trondheim, Norwegian University of Science and Technology, Trondheim, Norway.