Abstract
Objective Although sex hormones have potential cardioprotective effects, their effects on total antioxidant capacity (TAC) are not very well known. The aim of the study was to evaluate TAC in men who have decreased and normal testosterone levels and in women in menopausal and premenopausal period. Design Ninety-seven subjects with similar age intervals, men aged <45 years and female aged <50 years, were divided into four groups: 1) 10 men with normal testosterone levels, as control, 2) 36 men with decreased testosterone, 3) 19 women in menopause, surgically induced, and 4) 32 women in premenopausal period. Testosterone and estrogen levels were measured by chemiluminescence assay and TAC were measured by using a more recently developed automated measurement method. Results The TAC was significantly lower in Group 2 and Group 3 than those of Group 1 and Group 4 (ANOVA, p<0.001). A strong correlation between TAC, and testosterone and estrogen were found (r=0.807, p<0.001; r=0.685, p<0.001, testosterone and estrogen respectively). Conclusions The observed relationship between sex hormones and TAC may have a role in mechanism of their cardioprotective effect.
A number of studies have reported that there is an inverse relationship between the endogenous sex hormone level and major risk factors of atherosclerosis Citation1,2, as well as the presence and extent of coronary artery disease (CAD) Citation3.
Premenopausal women have been protected from coronary artery disease as compared to men of the same age Citation4. It is also known that in premenopausal women, the incidence of myocardial infarction and other complications related to atherosclerotic disease is lower than in men Citation5. The incidence of cardiovascular diseases after menopause is similar to that observed in males. These findings suggest a protective role for endogenous estrogen. In a recent study, it was shown that men with CAD have significantly lower concentrations of bioavailable testosterone than men with normal coronary angiograms and the prevalence of hypogonadism in a population of men with CAD is about twice that observed in the general population Citation3. In an animal model, castration increased aortic atheroma formation and testosterone replacement ameliorated this effect Citation6.
Testosterone and estrogen have effects on lipid profiles, endothelial cell function, vascular reactivity, homeostatic factors and binding free radicals Citation7,8. In addition to vascular and myocardial effects, sex hormones may act as an antioxidant Citation9. Based on the present findings, estrogen and testosterone show direct antioxidant effects by increasing the activities of antioxidant enzymes such as glutathione peroxidase and they also cause an increase in antioxidant vitamin levels and hence indirectly also contribute to antioxidant capacity Citation10.
Free radicals and oxidants are produced in metabolic and physiological processes. Oxidative effects of free radicals are controlled by endogenous antioxidants and also by exogenous antioxidants. Under some conditions, increases in oxidants and decreases in antioxidants cannot be prevented, and oxidative/antioxidative balance shifts towards the oxidative stress Citation11. Clinical and experimental studies have shown that oxidative stress and lipid peroxidation are involved in pathogenesis of atherosclerosis Citation12. A crucial step in the pathogenesis of atherosclerosis is the oxidative modification of low-density lipoprotein, mediated by reactive oxygen species (ROS) Citation13. A strong inverse association has been found between plasma antioxidants levels and prevalence of atherosclerosis, demonstrating that subjects with low antioxidant capacity levels may be at increased risk for coronary atherosclerosis Citation14. Recently the antioxidative effect of sex hormones and its cardioprotection have received considerable interest.
Plasma concentrations of antioxidants can be measured separately in the laboratory, but these measurements are time-consuming, labour-intensive and costly. Since antioxidative effects of antioxidant components of plasma are additive, the measurement of total antioxidant capacity (TAC) reflects the antioxidative status of plasma Citation15. We evaluated the total antioxidative status of plasma using a more recently developed automated measurement method Citation15.
The aim of this study was to examine the relationship between sex hormone and total antioxidant capacity in the male and female subjects with same age interval group.
Material and methods
Subjects
We included 97 subjects, men aged <45 years old and women <50 years old, in this study. Thirty-six consecutive voluntary men with newly diagnosed lower testosterone in urology clinic were referred to our clinic for the evaluation of TAC levels, and they were enrolled as the study group (Group I). Ten consecutive men, who were evaluated in urology clinic for urologic check up and were found as normal, were enrolled in the study as the control group for men (Group II).
The data of female study group were collected from subjects between March 2003 and May 2004. Women younger than 50 years of age with previous bilateral oophorectomy and hysterectomy earlier than at least 1 month before the enrollment was defined as surgically induced menopause. Thirty-two women with premenopausal period were enrolled to the study as the control group for women in Group III and 19 surgically induced menopausal women were enrolled to the study in Group IV.
Exclusion criteria were: congestive heart failure, diabetes mellitus, coronary artery disease, uncontrolled arterial hypertension, body mass index >30 kg/m2, smoking, hyperlipidemia, tumors, autoimmune and inflammatory diseases, renal and hepatic disorders, endocrine pathology and concomitant treatment affecting androgen metabolism (statins, calcium antagonists), as well as drug and/or alcohol abuse. We also excluded patients with supplementation of antioxidant, estrogen or testosterone therapy.
Age, serum TAC, estradiol and total testosterone concentrations were recorded. Body mass index was computed as weight divided by height squared (kg/m2). Written informed consent was obtained from all subjects prior to the study.
Blood sampling
Venous blood was withdrawn after an overnight fasting for at least 12 h. Blood was collected into glass tubes, allowed to clot at room temperature, centrifuged at 3000 g for 10 min, then serum was separated, frozen and kept at −81°C until analysis.
Measurement of total antioxidant capacity
TAC of serum was determined using a novel automated measurement method, developed by Erel Citation15. In this assay, a standardized solution Fe+ + -o-dianisidine complex reacts with a standardized solution of hydrogen peroxide by Fenton-type reaction, producing hydroxyl radical. These potent reactive oxygen species oxidize the reduced colorless o-dianisidine molecules to yellow-brown colored dianisidyl radicals at low pH. The oxidation reactions progress among dianisidyl radicals and further oxidation reactions develop. The color formation is increased with further oxidation reactions. Antioxidants in the sample suppress the oxidation reactions and color formation. The assay has excellent precision values, which are lower than 3%. The results are expressed as mmol Trolox equivalent/L.
Other assays
The levels of total testosterone and estradiol were measured by automated hormone analyzer using a chemiluminescence method (DPC, Immulite 2000).
Statistical analysis
Results were expressed as mean±SD or median (range) values for all continuous variables. Associations between sex hormones and other parameters such as TAC, age, were evaluated by bivariate correlation analysis. For multiple regression, factors showing a value p<0.05 in bivariate correlation analysis were selected (age, estradiol, and body mass index). A multivariate linear regression analysis was performed to predict TAC. The data were compared by one-way ANOVA followed by the Bonferroni multiple comparison test. A p<0.05 was considered statistically significant. Analysis was done with SPSS statistical software (Version 11.0).
Results
Sex hormones, TAC, body mass index and age data are shown in . No significant differences were detected in age and body mass index among the groups (p > 0.05). Total testosterone levels in female groups and estradiol levels in male groups were not measured. As expected, estrogen levels in the menopausal female group were lover than premenopausal group (p < 0.001, ). TAC was evaluated in each group.
Table I. Total antioxidant activity and sex hormones in males and females groups of subject
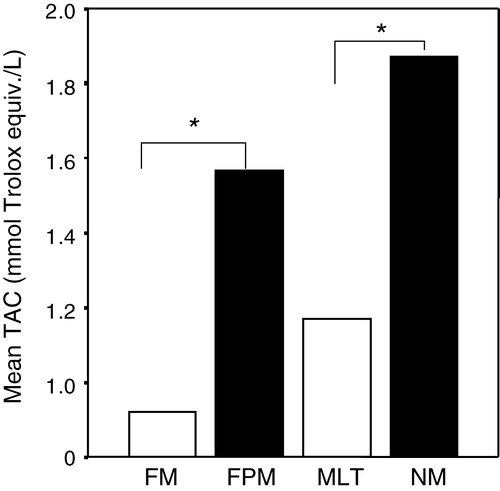
The level of TAC was higher in the male study group than in the female control group (p < 0.05). Serum TAC levels in menopausal group was lower compare to the female premenopausal group (p < 0.001). Similarly, in the male group with lower testosterone, TAC values were lower than the male control group (p < 0.001). No significant differences were found in TAC values in the lower testosterone male and female menopausal groups ().
Figure 1. Total antioxidant capacity (TAC) of male and female subjects. FM = female menopausal, FPM = female pre menopausal, MLT = male with lower testosterone, MN = male with normal testosterone. Values are means±SD for each group. *p<0.001
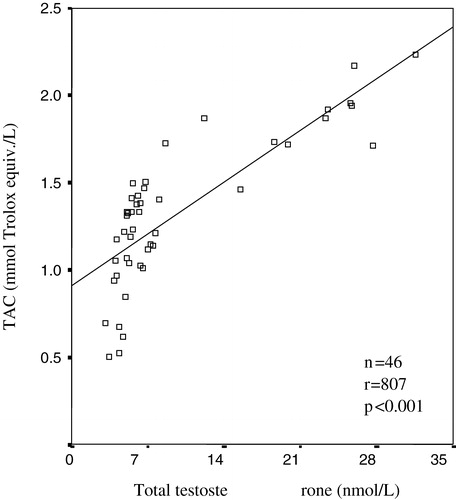
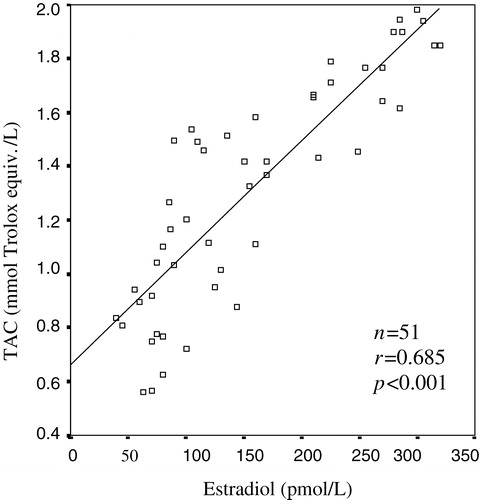
A strong positive correlation between TAC and total testosterone were detected in male groups (r = 0.807, p < 0.001) (). A significant correlation was found between serum TAC values and estradiol concentration by bivariate correlation test (r = 0.685, p < 0.001) ().
Bivariate and multivariate regression analyses for predicting for TAC in female and male subjects shown in . In multiple linear regression analyses, estradiol and body mass index were independent predictors factor of TAC in female groups (ß = 0.818, p < 0.001 vs ß = −0.156, p = 0.044). In the male group, total testosterone, age and body mass index were independent predictors factor of TAC (ß = 0.560, p < 0.001 vs ß = 0.262, p = 0.030 vs ß = −0.260, p = 0.025).
Table II. Bivariate and multivariate regression analyses for predicting for TAC in female and male subjects
Discussion
TAC was significantly lower in those with lower estradiol or testosterone concentrations and a significant positive relationship between TAC and plasma estradiol and total plasma testosterone of the same age males and females was found. TAC for men tended to be higher than in women.
While there are many studies on the relationship between sex hormones and antioxidants, the effects of sex hormones on the TAC are still rather inconsistent and incomplete. The aims of the present study were to evaluate the TAC of similar male and female control subjects and to determine whether there is a correlation between sex hormones and TAC. We evaluated the total antioxidative status of plasma using a more recently developed measurement method by Erel Citation15. In this study, we have demonstrated that a positive significant relationship between TAC, and estradiol, and total testosterone plasma levels of the same age males and females.
The antioxidant potential of various steroid hormones has been evaluated and it was shown that estrogens were natural antioxidants, while other steroids did not present significant antioxidant activity Citation16. It has been reported that antioxidant effects of sex hormones decrease the oxidants production in different cells Citation17. All these effects may contribute to the beneficial consequences of sex hormone replacement on the cardiovascular system Citation18. This effect may be of importance in protection against free radical mediated diseases. Contradictory results are also available concerning such activity of sex hormones Citation18. According to other publications, estrogen and testosterone did not influence superoxide generation Citation18. However, other studies suggest that estrogens have an inhibiting effect on superoxide production Citation19.
Furthermore, it has been reported that premenopausal women have lower cardiovascular disease rates compared to postmenopausal women, indicating that endogenous estrogens could have a protective role against atherosclerosis Citation20. The possible underlying mechanisms are not completely known. On the other hand, no reports have been published concerning the effects of the total antioxidant capacity test in this regard. In this study, we also investigated that whether there is a relationship between the estrogens and TAC. We have found a significant correlation between TAC and sex hormones. Additionally we have shown that the TAC was significantly higher in premenopausal females compared to menopausal females.
Our results provided further evidence that sex hormones have antioxidant activity. This finding suggests that the antioxidant potential of estrogen and testosterone may be an important contributor to the cardiovascular protection of the hormone. Taking the observations that sex steroids have an antioxidant capacity, their reduced levels in female with menopause and in males with lower testosterone may contribute to the accelerating development of atherosclerosis in this section of the population. Further researches about the antioxidative properties of sex hormones are needed especially controlling the effect of other confounding antioxidant.
References
- Zhao SP, Li XP. The association of low plasma testosterone level with coronary artery disease in Chinese men. Int J Cardiol 1998; 63: 161–64
- Simon D, Charles M, Nahoul K, et al. Association between plasma total testosterone and cardiovascular risk factors in healthy adult men: the Telecom Study. J Clin Endocrinol Metab 1997; 82: 682–85
- English KM, Mandour O, Steeds RP, Diver MJ, Jones TH, Channer KS. Men with coronary artery disease have lower levels of testosterone than those with normal coronary angiograms. Eur Heart J 2000; 21: 890–95
- Nabulsi AA, Folsom AR, White A, Patsch W, Heiss G, Wu KK, Szklo M. Association of hormone-replacement therapy with various cardiovascular risk factors in postmenopausal women. The Atherosclerosis Risk in Communities Study Investigators. N Engl J Med 1993; 328: 1069–75
- Matthews KA, Meilahn E, Kuller LH, Kelsey SF, Caggiula AW, Wing RR. Menopause and risk factors for coronary heart disease. N Engl J Med 1989; 321: 641–46
- Alexandersen P, Haarbo J, Byrjalsen I, Lawaetz H, Christiansen C. Natural androgens inhibit male atherosclerosis. A study of castrated, cholesterol fed rabbits. Circ Res 1999; 84: 813–19
- Maffei S, De Caterina R. Hormone replacement therapy and cardiovascular risk. G Ital Cardiol 1996; 26: 899–940
- English KM, Steeds R, Jones TH, Channer KS. Testosterone and ischemic heart disease: is there a link?. QJM 1997; 90: 787–91
- Massafra C, De Felice C, Gioia D, Buonocore G. Variations in erythrocyte antioxidant glutathione peroxidase activity during the menstrual cycle. Clin Endocrinol (oxf) 1998; 49: 63–67
- Massafra C, Gioia D, De Felice C, Picciolini E, De Leo V, Bonifazi M, Bernabei A. Effects of estrogens and androgens on erythrocyte antioxidant superoxide dismutase, catalase and glutathione peroxidase activities during the menstrual cycle. J Endocrinol 2000; 167: 447–52
- Young IS, Woodside JV. Antioxidants in health and disease. J Clin Pathol 2001; 54: 176–86
- Young IS, McEneny J. Lipoprotein oxidation and atherosclerosis. Biochem Soc Transact 2001; 29: 358–62
- Esterbauer H, Wag G, Puhl H. Lipid peroxidation and its role in atherosclerosis. Br Med Bull 1993; 49: 566–76
- Tribouilloy CM, Peltier M, Iannetta-Peltier MC, Zhu Z, Andrejak M, Lesbre JP. Relation between low-density lipoprotein cholesterol and thoracic aortic atherosclerosis. Am J Cardiol 1999; 84: 603–05
- Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem 2004; 37: 112–19
- Ayres S, Tang M, Subbiah MT. Estradiol-17 beta as an antioxidant: some distinct features when compared with common fat-soluble antioxidants. J Lab Clin Med 1996; 128: 367–75
- Laloraya M, Jain S, Thomas M, Kopergaonkar S, Pradeep Kumar G. Estrogen surge: a regulatory switch for superoxide radical generation at implantation. Biochem Mol Biol Int 1996; 39: 933–40
- Bekesi G, Kakucs R, Varbiro S, Racz K, Sprintz D, Feher J, et al. In vitro effects of different steroid hormones on superoxide anion production of human neutrophil granulocytes. Steroids 2000; 65: 889–94
- Arnal JF, Clamens S, Pechet C, et al. Ethinylestradiol does not enhance the expression of nitric oxide synthase in bovine endothelial cells but increases the release of bioactive nitric oxide by inhibiting superoxide anion production. Proc Natl Acad Sci USA 1996; 93: 4108–13
- Gruchow HW, Anderson AJ, Barboriak JJ, Sobocinski KA. Postmenopausal use of estrogen and occlusion of coronary arteries. Am Heart J 1988; 115: 954–63