Abstract
Objectives. Evaluate the prevalence, incidence and impact on prognosis of existing and new onset atrial fibrillation (AF) in patients with stable, symptomatic coronary artery disease. Design. Data from the 7 665 participants included in the ACTION (A Coronary disease Trial Investigating Outcome with Nifedipine GITS) trial was used. Results. Over a mean follow-up of 4.9 years, the incidence of recurrent AF in patients with AF at baseline (4.1%) was 13.5/100 patient-years and 1.64/100 patient-years for patients without baseline AF. Patients with AF at baseline had increased mortality and new overt heart failure. New onset AF was associated with increased morbidity and mortality and in particular soon after onset. [adjusted 30-day relative risk for mortality 23, 95% CI 14–36; for debilitating stroke 37, 95% CI 18–77; and for heart failure 54, 95% CI 32–93].The incidence of AF was not affected by treatment with nifedipine GITS. Conclusion. The presence of AF in patients with stable symptomatic CAD is an independent risk factor and in particular in the first 30 days for subsequent mortality and morbidity.
Atrial fibrillation (AF) affects approximately 1% of the general population and its prevalence increases with age Citation1, Citation2. In population-based studies, AF has been shown to be an independent predictor for all cause mortality and cardiovascular events Citation2–5. Compared to patients in sinus rhythm, the rate of stroke in non-rheumatic AF is two- to seven-fold higher Citation2. AF is frequently observed in patients with coronary artery disease (CAD) or myocardial infarction (MI), and is associated in the acute setting with a higher mortality and increased incidence of stroke compared to patients without AF Citation2, Citation6–9.
Both the short and long-term prognostic impact of AF in patients with stable, symptomatic CAD is poorly described, despite CAD being one of the most common cardiac conditions associated with AF Citation10, Citation11. Patients with CAD often have concomitant conditions predisposing to AF, such as hypertension, left ventricular hypertrophy, obesity, diabetes and diastolic dysfunction Citation12, Citation13. Treatment of such conditions may therefore also favorably affect the incidence of AF. The recently published ACTION (A Coronary disease Trial Investigating Outcome with Nifedipine GITS) trial compared the long-acting dihydropyridine calcium channel blocker nifedipine GITS (gastro-intestinal therapeutic system) with placebo in patients with symptomatic CAD but without overt heart failure Citation14. The ACTION study allowed us to perform an observational evaluation of (i) whether nifedipine, relative to placebo, had any effect on the incidence of AF, (ii) the impact of AF present at baseline on prognosis during a mean follow-up of almost five years, and (iii) the impact of new AF on the subsequent prognosis in patients without AF at baseline. As far as the latter is concerned, we distinguish between the impact on prognosis during the first 30 days after onset of AF, and the impact throughout the study.
Materials and methods
Study Population and follow-up
We analyzed all patients who participated in the ACTION trial, of which the design, methods and main results have been published previously Citation14, Citation15. Briefly, after signing informed consent, ambulatory patients aged 35 years or older with known stable symptomatic angina pectoris requiring treatment but without overt heart failure were randomly assigned to treatment with either nifedipine GITS (n = 3825) or matching placebo (n = 3840) on top of current treatment. Patients also had to have either a history of MI or proven CAD (either by coronary angiography (CAG) or a positive exercise or thallium test in the absence of CAG). The left ventricular (LV) ejection fraction (EF), estimated from two-dimensional echocardiography, performed locally, prior to starting study medication had to be at least 40%. Echocardiograms were analyzed centrally using the validated Simpson‘s biplane method Citation16.
After study medication was started, patients were seen at the out-patient clinic two weeks, six weeks and six months, and subsequently every six months. Any adverse experience or clinical event was reported as (serious) adverse event, using standard definitions and reporting procedures.
Study definitions
A 12-lead electrocardiogram (ECG) was recorded before starting study medication, and thereafter at every planned 6-monthly out-patient clinic visit. Investigators read ECGs and coded the abnormalities found. AF was considered present at baseline if the investigator had documented this diagnosis (or atrial flutter) in the case report form. This included AF coded as an abnormality in the baseline ECG. AF during follow-up was diagnosed if AF was coded by the investigator on a follow-up ECG, or if AF was reported on a (serious) adverse event report form.
The following pre-defined endpoints were considered as outcomes: all-cause death, MI, debilitating stroke, refractory angina, new overt heart failure, peripheral revascularization, coronary angiography (CAG), percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) Citation14.
Data Analysis
All analyses were based on intention to treat. The effects of nifedipine GITS were compared to placebo using Cox proportional-hazards analysis.
To assess which baseline characteristics were independent predictors of the occurrence of new AF during follow-up for patients with and without AF at baseline, Cox regression with a forward stepwise variable selection procedure and p < 0.05 as criterion for entry in the model was used.
We used modified Kaplan-Meier curves Citation17 to display the impact of new onset AF on the outcome of patients without AF at baseline. For the time prior to developing new AF, the patient was considered to belong the cohort without AF while those patients who developed new AF were considered part of the cohort with AF. To compare curves, we used Cox proportional-hazards analysis with new AF as the only time-dependent covariate. In addition, we used three different Cox multivariate regression models to evaluate the conditionally independent impact of AF on the pre-specified ACTION endpoints as described elsewhere Citation14. In the first model, the prognostic impact of presence of AF at baseline was assessed. In the second model, we assessed the long-term impact on outcome of new AF diagnosed during follow-up among patients without AF at baseline, using the occurrence of new AF as a time-dependent covariate. In a similar third model we truncated follow-up of patients with new AF to 30 days after its onset, and used the model-based hazard ratio as estimate of the risk ratio of event within 30 days comparing patients with and without new AF. All analyses for the impact of new AF on individual endpoints were adjusted for baseline covariates related to prognosis and for assignment to investigational drug.
The 30-day risk of event after onset of new AF was taken as the number of patients with event within this time span, divided by the number of patients who developed new AF. For comparison, we estimated the 30-day risk of event among patients without new AF by exponential approximation from the formula 30-day risk = 1–exp(-30 x daily event rate), taking the event rate as the number of patients with event divided by the total time at risk of event censored for new AF.
Results
Four point one percent (313 patients) of the 7 665 ACTION patients Citation14 were diagnosed with AF at baseline. Of these, 24% had AF on the baseline ECG while in the remainder the diagnosis was based on the clinical history. Of the 7 352 patients without AF at baseline, 7.8% (574 patients) developed AF over a mean follow-up of 4.95 years. In , key clinical features of patients with and without AF at baseline are compared. Patients with AF were older, were more often male, had a more complex clinical history and were treated with more drugs than patients without AF.
Table I. Key baseline characteristics by presence of atrial fibrillation*
The rate of recurrent AF during follow-up in patients with AF at baseline was 13.5 per 100 patient-years as opposed to a rate of new AF of 1.64 per 100 patient-years in patients without AF at baseline. Relative to placebo, nifedipine GITS had no effect on the occurrence of new AF for patients with [hazard ratio (HR) 1.11, 95% confidence internal (CI) 0.80–1.55] and without (HR 0.91, 95% CI 0.78–1.08) AF at baseline. The Cox regression analysis did not show any association between recurrent AF and investigational or concomitant treatment for patients with AF at baseline.
The last clinical event to occur within the four weeks before the diagnosis of new AF in the 574 patients without AF at baseline was MI in 6.8% (39/574), CABG in 26% (148/574), and new overt heart failure in 2.6% (15/574). In the remaining 65% (372/574) none of these events occurred during the four weeks before new AF.
Conditionally independent baseline predictors of new AF among 7 352 patients without AF at baseline were increasing age (1.77-fold risk increase per 10 years, p < 0.001), higher systolic blood pressure (1.07-fold risk increase per 10 mm Hg, p = 0.006), higher body mass index (1.04-fold risk increase per kg/m2, p = 0.002) and higher LV end-systolic volume (1.12-fold risk increase per 10 ml, p < 0.001) while patients with a history of coronary revascularization had a lower hazard (0.78-fold risk reduction, p = 0.006).
Adjusted for other factors, patients with AF at baseline had a significantly (p < 0.05) higher rate of death (1.42-fold) and new overt heart failure (1.67-fold) compared to patients without AF. The rate of debilitating stroke was 1.54-fold higher but this was not significant. The rate of myocardial infarction was similar (hazard ratio 1.17). Among patients with AF at baseline the rate of refractory angina was significantly lower (0.38-fold) while the need for coronary angiography was significantly higher (1.27-fold) than among patients without AF ().
Table II. Occurrence of events among patients with and without AF at baseline.
Modified Kaplan-Meier curves for total mortality and new overt heart failure among patients without AF at baseline are shown in A and B. The unadjusted hazard ratios comparing patients who developed new AF with those who did not were 4.1 for death and 7.1 for new overt heart failure. As shown in , the corresponding adjusted hazard ratios were 3.04 and 5.41 respectively. As shown in the same table, new AF had no (adjusted) effect on the rate of myocardial infarction but significantly increased the rate of debilitating stroke (3.53-fold), refractory angina (1.98-fold) and the need for coronary angiography, PCI and CABG.
Figure 1. Modified Kaplan-Meier Curves comparing all-cause mortality (Figure 1A) and new overt heart failure (Figure 1B) in patients with and without new AF during follow-up. For 7 352 patients without AF at baseline, numbers along the horizontal axis denote for the indicated time points the number of patients ‘at risk’ of death and new overt heart failure (Figure 1A and 1B respectively) by occurrence of new-onset atrial fibrillation (AF) prior to event. Also shown are the hazard ratio (HR) comparing patients with and without new-onset AF and its 95% confidence interval (CI), adjusted only for new-onset AF as a time-dependent covariate (c.f. statistical methods).
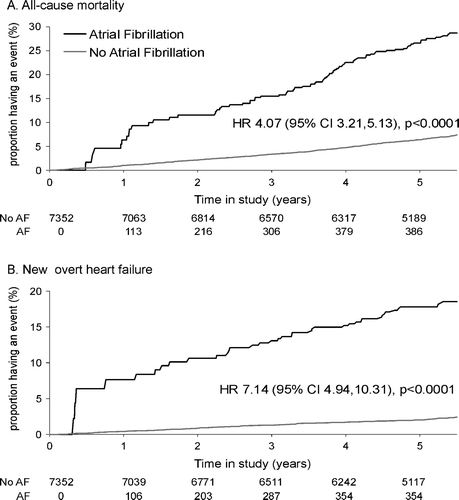
As shown in , the adjusted 30-day event risk of death after onset of new AF was increased 23-fold compared to the expected 30-day risk of death in a similar patient without new AF. The 30-date event risk of myocardial infarction was not significantly increased after onset of new AF but the risks of new heart failure, debilitating stroke and the other events considered were.
Table III. 30-day event risks before and after onset of new AF in 7 352 patients without AF at baseline.
Discussion
To our knowledge, this is the first report on the prevalence, incidence, impact and prognosis of AF during long-term follow-up of a large cohort of patients with stable symptomatic CAD. This post-hoc analysis concerns all 7 665 participants in the ACTION study. The selection criteria defined a broad spectrum of patients who require treatment for angina without overt heart failure, the only restriction being that patients who were treated with a calcium channel blocker that could not be stopped were excluded. Hence, we believe that this patient cohort is fairly representative for patients with symptomatic CAD seen in daily practice. The main findings are that patients with AF develop (recurrent) AF more often than patients without AF, and have a poor prognosis. The development of new-onset AF is not preceded by recent MI, CABG or heart failure in the majority of cases, but is nonetheless associated with a poor prognosis, in particular within the first 30 days.
The prevalence of AF in the present study is in agreement with the findings of previous studies Citation18. Compared to patients without AF, patients with AF at baseline in the present study more often had a history of MI and had bigger hearts. In patients with acute MI and clinical signs of heart failure participating in the OPTIMAAL study, the prevalence of AF at baseline was 12% Citation9. In TRACE, which included patients with acute MI and a LVEF < 35%, approximately 10% were not in sinus rhythm at baseline Citation19.
Neither nifedipine GITS nor concomitant treatments at baseline were associated with recurrent AF. Patients with AF at baseline had less refractory angina despite a higher referral rate for coronary angiography and subsequent CABG. Possibly, patients with ischemic cardiomyopathy and AF have dyspnea on exertion which is recognized clinically as angina and leads to referral for coronary angiography and subsequent CABG, followed by a lower subsequent incidence of refractory angina.
In patients without AF at baseline, the incidence of new AF was higher than in the general population Citation20 and was positively associated with age, systolic blood pressure, body mass index and LV end-systolic volume. This suggests that ischemic cardiomyopathy, reduced systolic function and metabolic syndrome may be involved in the development of new AF, Citation13, Citation21 and in the subsequent development of heart failure.
A distinction must be made between the presence of AF at baseline and the occurrence of new-onset AF in a patient not previously known with this condition. After adjusting for other factors related to prognosis, patients with a baseline AF history did not have an increased risk of MI or debilitating stroke, but had a moderately elevated risk of death and new overt heart failure. On the other hand, the prognostic implications of new-onset AF in patients without AF at baseline were more serious and especially when only the first 30 days after onset of AF were considered. Increased 30-day risks of mortality and stroke after onset of AF were also observed in the OPTIMAAL study Citation9 but to a lesser extent than in the present study. We postulate that new-onset AF in an otherwise stable patient with symptomatic angina may be related to unrecognized cardiovascular disorders associated with death, heart failure and stroke. This may explain the higher 30-day relative risks observed in this study compared to the OPTIMAAL study. Of note is that in only 35% of patients in our study, new-onset AF was preceded in the prior four weeks by either MI or CABG or heart failure, events which are known to be associated with the occurrence of AF.
As shown in recent experimental and clinical studies, myocardial and inflammatory vascular changes may also play a role Citation13, Citation21. Patients with AF at baseline in the present study represent prevalent cases who have survived a history of AF often of long duration without clinical events that would have precluded selection for participation in the ACTION trial. Because of this, the prognostic impact of prevalent AF should not be as serious as the impact of incident new-onset AF, whether or not preceded by antecedent associated clinical events.
Since both systolic blood pressure and left ventricular hypertrophy have been shown to be favorably influenced by calcium antagonists, comparisons between random allocation to nifedipine GITS or placebo as in the present study are of interest. Although new-onset AF was associated with an increased systolic blood pressure and increased LV end-systolic volume, nifedipine GITS had no effect on new-onset AF. In other studies, trandolapril and enalapril were shown to reduce the incidence of AF in post-MI patients with LV dysfunction Citation19 and heart failure Citation22 respectively. Captopril and losartan were equally effective in this regard in OPTIMAAL Citation9. ACTION was not designed to assess the efficacy of other treatments than nifedipine GITS. Nonetheless, we have performed Cox regression analyses for the impact of new AF adjusting for the use of angiotensin converting enzyme inhibitors or angiotensin receptor blockers, digoxin, anti-arrhythmics and anti-thrombotic drugs prescribed at baseline. Adjustment for these treatments did not substantially alter our results and we were unable to show that any of these reduced the risk of new-onset AF, or was associated with an improved outcome.
An important limitation of the present study is that we have no data on the evolution of cardiac function. End-systolic volume at baseline was an independent predictor of new-onset AF in patients without AF at baseline, and was also used as adjusting variable in multivariate Cox regression analysis for the prognostic impact of new-onset AF. Had we been able to use end-systolic volume as a time-dependent adjusting variable, the adjustment for the occurrence of any event that preceded the occurrence of new-onset AF would have been more reliable.
Another limitation might be that, as in the OPTIMAAL study Citation9, we were unable to distinguish between paroxysmal and permanent AF, both at baseline and during follow-up. Of patients classified as having AF at baseline, 24% also had AF on the baseline ECG. An unknown fraction of these 24% must have had permanent AF, while the remaining 76% are patients with paroxysmal AF. We were unable to show that the prognostic implications of AF at baseline differed between patients with and without AF on the baseline ECG.
The clinical implications of the distinction between prevalent AF as an additional finding and the occurrence of new AF in a patient without a history of AF are of considerable importance. AF as an additional finding requires the attention of the practicing physician; but new-onset AF in patients without a history of AF requires careful medical care. As soon as new-onset AF occurs, appropriate treatment to prevent further complications is indicated. ACTION patients were treated according to current clinical practice, which seemed inadequate in this subset of patients.
Conclusions
Both the presence of AF as an additional finding in patients with stable symptomatic CAD and new-onset AF are associated with an impaired prognosis, in particular within 30 days after onset of new AF. Treatment to prevent complications is therefore indicated, and patients without AF must be followed closely for new-onset AF. Long-acting nifedipine GITS has no effect on the development of AF.
We thank participating patients and the ACTION investigators, committee members and other study personnel as mentioned elsewhere for their contribution Citation14. ACTION was supported by Bayer Healthcare AG, Wuppertal, Germany.
The ACTION study was carried out by an independent Steering Committee and Research Group. The authors had full access to all data in the ACTION study and take responsibility for the integrity of the data and the accuracy of the data analysis. Bayer Healthcare AG had no role in the design and conduct of the study; or in the preparation, review and approval of this manuscript.
JEO, KAAF, JL and PAP-W have served as consultants to or received travel expenses, or funding for research from other pharmaceutical companies. BAK, SdB and JL are full-time employees of SOCAR Research SA, which managed the study.
References
- Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age, distribution, and gender of patients with atrial fibrillation: Analysis and implications. Arch Intern Med. 1995; 155: 469–73
- Fuster V, Ryden LE, Asinger RW, Cannom DS, Crijns HJ, Frye RL, et al. ACC/AHA/ESC guidelines for management of patients with atrial fibrillation. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences. Eur Heart J. 2001; 22: 1852–1923
- Gajewski J, Singer RB. Mortality in an insured population with atrial fibrillation. JAMA. 1981; 245: 1540–4
- Kannel WB, Abbot RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation; the Framingham Study. N Engl J Med. 1982; 306: 1018–22
- Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: The Framingham Heart Study. Circulation. 1998; 98: 946–52
- Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation; incidence, risk factors, and prognosis in the Manitoba follow-up study. Am J Med. 1995; 98: 476–84
- Crenshaw BS, Ward SR, Granger CB, Stebbins AL, Topol EJ, Califf RM. Atrial fibrillation in the setting of acute myocardial infarction: The GUSTO-I experience. Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries. J Am Coll Cardiol. 1997; 30: 406–13
- Rathore SS, Berger AK, Weinfurt KP, Schulman KA, Oetgen WJ, Gersh BJ, et al. Acute myocardial infarction complicated by atrial fibrillation in the elderly: Prevalence and outcomes. Circulation. 2000; 101: 969–74
- Lehto M, Snapinn S, Dickstein K, Swedberg K, Nieminen MS. Prognostic risk of atrial fibrillation in acute myocardial infarction complicated by left ventricular dysfunction: The OPTIMAAL experience. Eur Heart J. 2005; 26: 350–6
- Snow V, Weiss KB, LeFevre M, McNamara R, Bass E, Green LA, et al. Management of newly detected atrial fibrillation: A clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians. Ann Intern Med. 2003; 139: 1009–17
- O'Donnell M, Agnelli G, Weitz JI. Emerging therapies for stroke prevention in atrial fibrillation. Eur Heart J 2005; 7(Suppl C): C19–C27
- Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: A systematic review. Stroke. 2003; 34: 2741–8
- Gersh BJ, Tsang TSM, Barnes ME, Seward JB. The changing epidemiology of non-valvular atrial fibrillation: The role of novel risk factors. Eur Heart J 2005; 7(Suppl C): C5–C11
- Poole-Wilson PA, Lubsen J, Kirwan BA, van Dalen F, Wagener G, Danchin N, et al. Effect of long-acting nifedipine on mortality and cardiovascular morbidity in patients with stable angina requiring treatment (ACTION trial): Randomised controlled trial. Lancet. 2004; 364: 849–57
- Lubsen J, Poole-Wilson PA, Pocock SJ, van Dalen FJ, Baumann J, Kirwan BA, et al. Design and current status of ACTION: A Coronary disease Trial Investigating Outcome with Nifedipine GITS. Eur Heart J 1998; 19(Suppl I): 20–32
- Otterstad JE, Froeland G, St. John Sutton M, Holme I. Accuracy and reproducibility of biplane two-dimensional echocardiographic measurements of left ventricular dimensions and function. Eur Heart J. 1997; 18: 507–13
- Simon R, Makuch RW. A non-parametric graphical representation of the relationship between survival and the occurrence of an event: Application to responder versus non-responder bias. Stat Med. 1984; 3: 35–44
- The national heart, lung and blood institute working group on Atrial Fibrillation. Atrial fibrillation: Current understandings and research imperatives. J Am Coll Cardiol. 1993; 22: 1830–4
- Pedersen OD, Bagger H, Kober L, Torp-Pedersen C. Trandolapril reduces the incidence of atrial fibrillation after acute myocardial infarction in patients with left ventricular dysfunction. Circulation. 1999; 100: 376–80
- Savelieva I, Camm AJ. Clinical trends in atrial fibrillation at the turn of the millennium. J Intern Med. 2001; 250: 369–72
- Tamakoshi K, Yatsuya H, Kondo T, Hori Y, Ishikawa M, Zhang H, et al. The metabolic syndrome is associated with elevated circulating C-reactive protein in healthy reference range, a systemic low-grade inflammatory state. Int J Obes Relat Metab Disord. 2003; 27: 443–9
- Vermes E, Tardif JC, Bourassa MG, Racine N, Levesque S, White M, et al. Enalapril decreases the incidence of atrial fibrillation in patients with left ventricular dysfunction. Insights from the Studies of Left Ventricular Dysfunction (SOLVD) Trials. Circulation. 2003; 107: 2926–31