Abstract
Objectives. To evaluate the association between glycometabolic status in the acute phase and 2½ years later in patients with acute coronary syndrome (ACS). Methods. Non-diabetic patients (n = 762) presenting with ACS were prospectively followed up for 2½ years. Patients were stratified by admission plasma glucose (<6.1 mmol/l, 6.1 – 6.9 mmol/l and ≥7.0 mmol/l) and HbA1c (≤4.5%, 4.6 – 5.4% and ≥5.5%). The predictive value of glucose levels ≥ 7.0 mmol/l and HbA1c ≥ 5.5% for glycometabolic disturbance (i.e. diabetes or impaired fasting glycaemia (IFG)) was analysed. Results. Of 762 patients, 13% had a diagnosis of diabetes and 16% had IFG at follow-up. The prevalence of glycometabolic disturbance at follow-up increased with increasing plasma glucose at admission, from 19% in patients with < 6.1 mmol/l to 42% in patients with ≥ 7.0 mmol/l. Sixty-one percent of patients with HbA1c ≥ 5.5% had glycometabolic disturbance after 2½ years compared to only 25% of those with HbA1c < 5.5%. Conclusion. Non-diabetic patients with ACS and hyperglycaemia are at high risk for developing glycometabolic disturbance. HbA1c may be an even stronger predictor of glycometabolic disturbance than plasma glucose.
Among patients with acute myocardial infarction (AMI) 20 – 25% have diagnosed diabetes mellitus (DM) Citation1, Citation2 and approximately 25% have previously undiagnosed DM Citation3. Diabetes is a predictor of worse outcome after acute coronary syndrome (ACS) Citation4–6. Furthermore, acute phase hyperglycaemia is associated with adverse outcome in both diabetic and non-diabetic patients with ACS Citation7–10. Acute phase hyperglycaemia is common in patients with AMI and has been regarded as a stress response induced by the myocardial infarction Citation11, Citation12. However, prediction of long term glycometabolic status from acute phase hyperglycaemia is not well described.
Use of insulin to treat hyperglycaemia in patients with AMI has been reported to improve outcome and acute phase hyperglycaemia in these patients has been suggested to be a reflection of relative insulin deficiency Citation13. This relative insulin deficiency may be due to insulin resistance. Insulin resistance is common (52 – 86%) in patients with AMI Citation14 and is associated with increased risk of developing DM Citation12, Citation15.
Since DM is a major risk factor in cardiovascular disease, it is important to identify those patients at high risk of developing DM to allow early intervention. The aim of this study was to evaluate the association between acute phase hyperglycaemia and disturbed glycometabolic status 2½ years after an acute coronary syndrome. We also assessed the predictive value of HbA1c at admission for glycometabolic disturbance at follow-up.
Materials and methods
Study Population
Details on the study population have previously been described Citation16. Briefly, between September 15, 1995 and September 15, 1999 all patients with ACS admitted to the coronary care unit of Sahlgrenska University Hospital in Göteborg, Sweden, were evaluated for participation in a study on prognosis and its prediction in ACS. To enable long-term follow-up and repeated visits at our outpatient clinic only patients under the age of 80 and living within the hospital's catchment area were eligible. Patients transferred from other hospitals for tertiary care were not included. Patients with an AMI or chest pain or other symptoms suggestive of myocardial ischaemia were eligible for inclusion. The suspicion of myocardial ischaemia had to be supported by ECG changes on admission [ST-segment elevation ≥0.1 mV (0.2 mV in V1-V4) or ST-segment depression ≥0.1 mV or T wave inversion in at least two adjacent leads], biochemical markers above the upper reference level [creatine-kinase (CK)-MB 5 µg/l and/or troponin T 0.05 µg/l], or previously recognized coronary artery disease, such as AMI, prior percutaneous coronary intervention or coronary artery bypass grafting (CABG), stable or unstable angina pectoris with significant angiographic changes, or an exercise test suggestive of ischaemia. The exclusion criteria were severe non-cardiac disease with expected survival less than one year, and unwillingness to participate. A patient could only be included once. The study was approved by the ethics committee of Göteborg University.
In all there were 1 854 patients included in the study. Of these patients 110 were finally discharged with a diagnosis other than unstable angina or myocardial infarction and were excluded from this analysis since they did not fulfil the criteria for an acute coronary syndrome.
The enrolled 1 744 patients covered the whole spectrum of ACS. Based on ECG and biochemical markers of myocardial ischaemia and necrosis patients were diagnosed with ST-segment elevation AMI (ECG with ST-segment elevation or left bundle branch block and CK-MB > 10 µg/l and/or troponin T ≥0.15 µg/l), non-ST-segment elevation AMI (CK-MB > 10 µg/l and/or troponin T ≥0.15 µg/l) and unstable angina (CK-MB ≤ 10 µg/l and troponin T < 0.15 µg/l).
Data were prospectively collected from the hospital medical records, including information on previous clinical history, cardiovascular risk factors and medication. While hospitalized, the patients also had a detailed interview by an experienced study nurse. If information obtained at these interviews differed from those in the medical records a thorough work-up was done to resolve the discrepancies. Diabetes, hypertension and hypercholesterolemia were defined by clinical history recorded at the time of admission or registered in previous hospital records. Height and weight was measured and body mass index (BMI) was calculated as (body weight, kg)/(height, m)2.
Patients with previously known DM were excluded from the study.
Plasma glucose (random) was analysed at admission and HbA1c was analysed within 24 hours after admission. At follow-up after 2½ years, fasting plasma glucose was measured. Before the date of May 4, 1998, glucose measurements where done in whole blood (blood glucose). After that date measurements where done in plasma. A constant factor of 1.11 was used to convert measured glucose concentration in whole blood to the equivalent concentration in plasma Citation17. At 2½ year follow-up we classified patients as having diabetes if they during the follow-up period had been given the diagnosis diabetes mellitus (self reported or according to medical records or were receiving glucose lowering therapy) or if they had fasting plasma glucose values in the diabetic range (fasting plasma glucose ≥ 7.0 mmol/l). Patients were defined as having disturbed metabolic status if they had impaired fasting glycaemia (IFG) (i.e. plasma glucose 6.1 – 6.9 mmol/l, according to the WHO classification Citation18) or diabetes at follow-up.
For descriptive purposes patients were divided into three groups defined by admission plasma glucose values of < 6.1, 6.1 – 6.9 and ≥ 7.0 mmol/l. We also divided patients into three groups defined by HbA1c values of ≤ 4.5, 4.6 – 5.4 and ≥ 5.5%.
Statistics
Fisher′s exact test for proportions and Mann-Whitney U test for continuous variables were used for univariate comparisons between groups. Adjusted odds ratios were calculated using logistic regression, where adjustments were made for all variables with p < 0.20 in the univariate analysis of difference between groups. All p-values are two-tailed and considered significant if below 0.05.
Results
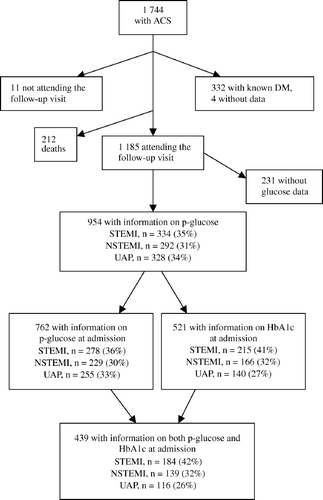
Of 1 744 patients with ACS, 332 (19%) had a previous diagnosis of diabetes mellitus. Information on diabetic status was not available for four patients. During follow-up 212 patients died and 11 patients failed to participate in the follow-up. The mean admission plasma glucose level was somewhat higher in patients who died during follow-up than in the patients evaluated at 2½ year follow-up. At follow-up, plasma glucose was available for 954 of the remaining 1 185 (81%). Among those 954 patients, measurements of admission plasma glucose and HbA1c were available for 762 (80%) and 521 (55%) respectively ().
Figure 1. Flow chart of studied patients (ACS = acute coronary syndrome; DM = diabetes mellitus; STEMI = ST-segment elevation myocardial infarction; NSTEMI = non-ST-segment elevation myocardial infarction; UAP = unstable angina pectoris).
Baseline characteristics and in-hospital complications
Among the 762 patients with information on plasma glucose at admission the median age was 65 years and 73% of the patients were men. Analysis of the baseline characteristics by admission plasma glucose level showed that those patients in group 3 (plasma glucose ≥ 7.0 mmol/l) were significantly less likely to have history of angina pectoris, CABG and PCI. Otherwise no significant differences in baseline characteristics were found. As for complications during hospitalisation, both congestive heart failure and atrial fibrillation were significantly more common among those with the highest glucose levels ().
Table I. Clinical characteristics and discharge diagnosis by admission plasma glucose concentrations
Admission plasma glucose in relation to type of ACS
There was a highly significant difference regarding index diagnosis between those with the highest admission plasma glucose levels and the others, with a proportion of ST-segment elevation AMI ranging from 20% in group 1 to 59% in group 3. Similarly the maximum CK-MB level and leucocyte count were higher in group 3. As a consequence there was an inverse relation between glucose levels and an index diagnosis of unstable angina, whereas non-ST-segment elevation AMI were more evenly spread across the admission plasma glucose groups ().
Admission plasma glucose and glycometabolic disturbance at follow-up
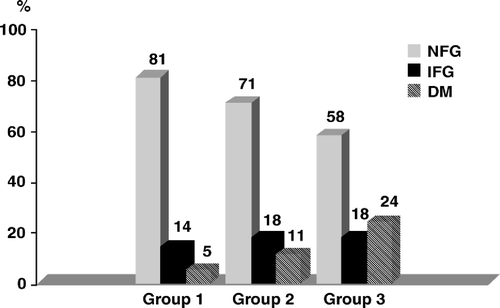
Of a total of 762 ACS patients without known DM at admission, 13% had DM at 2½ years follow-up and 16% had IFG. The prevalence of DM at 2½ years follow-up increased across the admission glucose groups, from 5% in group 1 to 24% in group 3. The prevalence of IFG at follow-up in group 1, 2 and 3 was 14%, 18% and 18% respectively ().
Figure 2. Glycometabolic status (NFG = normal fasting glucose; IFG = impaired fasting glucose; DM = diabetes mellitus) at 2½ year follow-up, after classification according to admission plasma glucose levels (group 1 = patients with plasma glucose < 6.1 mmol/l; group 2 = patients with plasma glucose 6.1 – 6.9 mmol/l; group 3 = patients with plasma glucose ≥ 7.0 mmol/l).
HbA1c and glycometabolic disturbance at follow-up
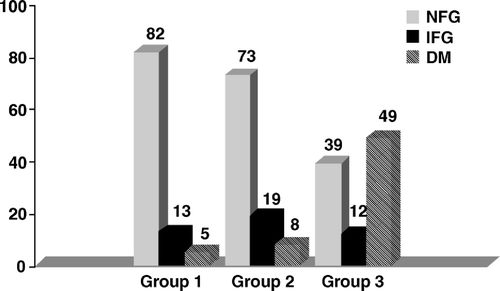
At admission HbA1c was measured in 521 of 954 patients. Among those with HbA1c ≥ 5.5%, 49% had developed DM after 2½ years, compared to 8% of those with HbA1c 4.6 – 5.4 and 5% of those with HbA1c ≤ 4.5% (). The odds ratio, adjusted for confounders (see Methods), for patients with admission HbA1c ≥ 5.5% relative to those with < 5.5% was 13.1 (95% CI: 7.1 – 24.2, p < 0.0001). The corresponding prevalence of IFG at follow-up was 12% in those with HbA1c ≥ 5.5%, 19% in those with HbA1c 4.6 – 5.4 and 13% in those with HbA1c ≤ 4.5%. The adjusted odds ratio of disturbed glycometabolic status for patients with HbA1c ≥ 5.5% relative to those with < 5.5% was 4.5 (95% CI: 2.7 – 7.4, p < 0.0001).
Figure 3. Glycometabolic status (NFG = normal fasting glucose; IFG = impaired fasting glucose; DM = diabetes mellitus) at 2½ year follow-up, after classification according to admission HbA1c (group 1 = patients with HbA1c ≤ 4.5%; group 2 = patients with HbA1c 4.6–5.4%; group 3 = patients with HbA1c ≥ 5.5%).
Prevalence of disturbed glycometabolic status at follow-up in correlation to HbA1c and plasma glucose at admission
Measurements both of admission plasma glucose and HbA1c as well as fasting plasma glucose at follow-up were available for 439 patients. There was a weak but significant correlation between plasma glucose and HbA1c at admission (r = 0.26, p < 0.0001). Sixty-six (15%) of the 439 patients had DM at follow-up and 16% had IFG. Among 46 patients who both had the highest admission plasma glucose (≥7.0 mmol/l) and HbA1c (≥5.5%), the prevalence of DM and IFG at follow-up was 67% and 13% respectively.
Discussion
Our results show that hyperglycaemia at admission is a strong predictor of glycometabolic disturbance at 2½ years in patients with ACS. Previous studies have indicated that acute phase hyperglycaemia in ACS is a response to adrenergic stress due to myocardial injury Citation10–12, Citation19. In our study hyperglycemia at admission correlated with the severity of myocardial injury when estimated with the maximum CK-MB level and the proportion of patients with ST-segment elevation AMI. Furthermore admission hyperglycaemia was associated with higher leukocyte count, which can also be a response to adrenergic stress. However the higher prevalence of disturbed glycometabolism at follow-up in the hyperglycaemic group supports the hypothesis that casual hyperglycaemia in patients with ACS should not only be regarded as a response to adrenergic stress related to the myocardial ischaemia but may be a sign of impaired glucose tolerance or undiagnosed DM.
In our study only 13% of the patients developed DM after 2½ years follow-up. In a recent report on patients admitted for acute coronary artery disease 22% of those without known DM had newly detected DM when an oral glucose tolerance test (OGTT) was performed. In the same study only 42% of the patients had normal glucose metabolism Citation20. Norhammar et al. found that in nondiabetic patients with AMI, 25% had diabetic 2-hour plasma glucose levels on OGTT and only 35% had normal glucose tolerance three months after discharge Citation3. However when the fasting blood glucose criteria were applied only 13% of the patients were diagnosed with DM, well in line with our findings. Epidemiological data from the DECODE study Citation21 suggest that if fasting glucose is used alone, 31% of diabetic subjects with a non-diabetic fasting glucose but a diabetic 2-hour plasma glucose ≥ 11.1, will not be diagnosed. Our study is a retrospective analysis of glycometabolic status and therefore some patients were classified as having DM based on one measurement of fasting plasma glucose ≥ 7.0 mmol/l. However, in real life the diagnosis of diabetes cannot be based on only one fasting plasma glucose value Citation18, Citation22. Consequently we do not know how many patients in our study classified as having DM at follow-up had true DM according to diagnostic criteria. However as OGTT was not performed the prevalence is most probably underestimated.
Our results show that HbA1c level ≥ 5.5% is a strong precdictive factor for disturbed glycometabolic status. After 2½ years almost a half of the patients with HbA1c ≥ 5.5% had developed DM and nearly two thirds had a disturbed glycometabolic status. Norhammar et al. Citation3 found that HbA1c and BMI were the only independent predictors of newly detected DM three months after an AMI.
We found a somewhat weak but still statistically significant correlation between HbA1c and admission plasma glucose. Tenerz et al. found no correlation between HbA1c and admission blood glucose in non-diabetic patients with AMI Citation19. In contrast Malmberg et al. showed that in patients with AMI, HbA1c was the most powerful predictor of blood glucose at admission Citation23.
In our study a majority of those patients who had both casual plasma glucose ≥ 7.0 mmol/l and HbA1C ≥ 5.5%, had at follow-up developed DM and four of five had a disturbed glycometabolic status. It makes sense that the patients at highest risk for developing DM are those who have both the highest long-term blood glucose levels (HbA1c) and a tendency to develope hyperglycaemia during stressful situations (e.g. ACS). Even though OGTT may be indicated in all patients with AMI Citation3, we believe that careful assessment of glycaemic status is particularly needed in those patients with ACS who are at highest risk for developing DM. We suggest that the combination of plasma glucose and HbA1c measurements can be a valuable tool for risk evaluation of later glycometabolic disturbance in patients with ACS.
Limitations
Even though patients with previously known diabetes at admission were excluded, the study group most likely included some patients with true but undiagnosed diabetes. The exclusion of patients who died during the follow-up period and patients where admission plasma glucose and/or HbA1c were not available may have affected the results of this study. Since the study started in 1995 the diagnostic criteria for non-ST-segment elevation AMI has changed. This means that some patients who in the study were classified as having unstable angina would today have the diagnosis non-ST-segment elevation AMI. This last point does however not influence the major findings of the study.
Conclusion
Non-diabetic patients with ACS and elevated admission plasma glucose levels (≥7.0 mmol/l) should be regarded as a high risk group for developing glycometabolic disturbance. This hyperglyceamia can not been regarded only as a stress response induced by the myocardial ischeamia. Patients with both acute phase hyperglycaemia and elevated levels of HbA1c at admission are at considerable risk for developing glycometabolic disturbance. We therefore recommend careful assessment of glycaemic status in this patient group.
This study was supported by the Swedish Heart and Lung Foundation, the Vardal Foundation, the Swedish Medical Research Council, the Göteborg University, the Västra Götaland Region, the Göteborg Medical Society and Sahlgrenska Foundation. There are no conflicts of interest or financial disclosure.
References
- Lifestyle and risk factor management and use of drug therapies in coronary patients from15 countries. Principal results from EUROASPIRE II Euro Heart Survey Programme.Eur Heart J. 2001;22:554–72.
- Tenerz A, Lonnberg I, Berne C, Nilsson G, Leppert J. Myocardial infarction and prevalence of diabetes mellitus. Is increased casual blood glucose at admission a reliable criterion for the diagnosis of diabetes?. Eur Heart J. 2001; 22: 1102–10
- Norhammar A, Tenerz A, Nilsson G, Hamsten A, Efendic S, Ryden L, et al. Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: A prospective study. Lancet. 2002; 359: 2140–4
- Malmberg K, Yusuf S, Gerstein HC, Brown J, Zhao F, Hunt D, et al. Impact of diabetes on long-term prognosis in patients with unstable angina and non-Q-wave myocardial infarction: Results of the OASIS (Organization to Assess Strategies for Ischemic Syndromes) Registry. Circulation. 2000; 102: 1014–9
- Norhammar A, Malmberg K, Diderholm E, Lagerqvist B, Lindahl B, Ryden L, et al. Diabetes mellitus: The major risk factor in unstable coronary artery disease even after consideration of the extent of coronary artery disease and benefits ofrevascularization. J Am Coll Cardiol. 2004; 43: 585–91
- McGuire DK, Newby LK, Bhapkar MV, Moliterno DJ, Hochman JS, Klein WW, et al. Association of diabetes mellitus and glycemic control strategies with clinical outcomes after acute coronary syndromes. Am Heart J. 2004; 147: 246–52
- Suleiman M, Hammerman H, Boulos M, Kapeliovich MR, Suleiman A, Agmon Y, et al. Fasting glucose is an importantindependent risk factor for 30-day mortality in patients with acute myocardial infarction: A prospective study. Circulation. 2005; 111: 754–60
- Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: A systematic overview. Lancet. 2000; 355: 773–8
- Wahab NN, Cowden EA, Pearce NJ, Gardner MJ, Merry H, Cox JL. ICONSInvestigators. Is blood glucose an independent predictor of mortality in acute myocardialinfarction in the thrombolytic era?. J Am Coll Cardiol. 2002; 40: 1748–54
- Foo K, Cooper J, Deaner A, Knight C, Suliman A, Ranjadayalan K, et al. A single serum glucose measurement predicts adverse outcomes across the whole rangeof acute coronary syndromes. Heart. 2003; 89: 512–6
- Oswald GA, Smith CC, Betteridge DJ, Yudkin JS. Determinants and importance of stress hyperglycaemia in non-diabetic patients with myocardial infarction. Br Med J (Clin Res Ed) 1986; 293: 917–22
- Zavaroni I, Bonini L, Gasparini P, Barilli AL, Zuccarelli A, Dall'Aglio E, et al. Hyperinsulinemia in a normal population as a predictor of non-insulin-dependent diabetes mellitus, hypertension, and coronary heart disease: The Barilla factory revisited. Metabolism. 1999; 48: 989–94
- Malmberg K, Ryden L, Efendic S, Herlitz J, Nicol P, Waldenstrom A, et al. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardialinfarction (DIGAMI study): Effects on mortality at 1 year. J Am Coll Cardiol. 1995; 26: 57–65
- Tenerz A, Norhammar A, Silveira A, Hamsten A, Nilsson G, Ryden L, et al. Diabetes, insulin resistance, and the metabolic syndrome in patients with acute myocardial infarction without previously known diabetes. Diabetes Care. 2003; 26: 2770–6
- Pradhan AD, Manson JE, Meigs JB, Rifai N, Buring JE, Liu S, et al. Insulin,proinsulin, proinsulin:insulin ratio, and the risk of developing type 2 diabetes mellitusin women. Am J Med. 2003; 114: 438–44
- Perers E, Caidahl K, Herlitz J, Sjolin M, Karlson BW, Karlsson T, et al. Spectrum of acute coronary syndromes: History and clinical presentation in relation to sex and age. Cardiology. 2004; 102: 67–76
- Burnett RW, D'Orazio P, Fogh-Andersen N, Kuwa K, Kulpmann WR, Larsson L, et al. IFCC recommendation on reporting results for blood glucose. Clin Chim Acta. 2001; 307: 205–9
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998; 15: 539–53
- Tenerz A, Nilsson G, Forberg R, Ohrvik J, Malmberg K, Berne C, et al. Basal glucometabolic status has an impact on long-term prognosis following an acutemyocardial infarction in non-diabetic patients. J Intern Med. 2003; 254: 494–503
- Bartnik M, Ryden L, Ferrari R, Malmberg K, Pyorala K, Simoons M, et al. Euro Heart Survey Investigators. The prevalence of abnormal glucose regulation in patients with coronary artery disease across Europe. The Euro Heart Survey on diabetes and the heart. Eur Heart J. 2004; 25: 1880–90
- The DECODE-study group, European Diabetes Epidemiology Group. Is fasting glucose sufficient to define diabetes? Epidemiological data from 20 European studies. Diabetes Epidemiology: Collaborative analysis of Diagnostic Criteria in Europe. Diabetologia. 1999; 42: 647–54
- Report of the expert committee on the diagnosis and classification of diabetes mellitus.Diabetes Care. 1997;20:1183–97.
- Malmberg K, Norhammar A, Wedel H, Ryden L. Glycometabolic state at admission:Important risk marker of mortality in conventionally treated patients with diabetesmellitus and acute myocardial infarction. Long-term results from the Diabetes andInsulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation. 1999; 99: 2626–32