Abstract
Blood lactate levels during cardiopulmonary bypass are often used to verify adequacy of perfusion. The present investigation aimed to propose a threshold for hyperlactatemia. Blood lactate levels in 5 121 cardiac surgical patients were retrospectively analysed by a review of database records. Hyperlactatemia was defined as a value equal to the 90th percentile of the identified lactate distribution at weaning from cardiopulmonary bypass. Patient demographics, background and outcome statistics were performed stratified on presence of hyperlactatemia. The threshold for hyperlactatemia was found to equal 2 mmol/l. Significant predictors of hyperlactatemia based on logistic regression modelling were age, complex surgery, duration of cardiopulmonary bypass, blood transfusion, acid base level, emergency operations, diabetes, vasoactive intervention, venous-blood-return to the heart-lung machine and renal function. Patients with hyperlactatemia required longer intensive care and postoperative ventilatory support. Complications were more frequent, especially: renal dysfunction, infections, respiratory and circulatory disorders. Hospital mortality was 13.3% compared to an overall level at 2.2%. The threshold for hyperlactatemia during cardiopulmonary bypass attained 2 mmol/l and predicted increased morbidity and mortality.
Lactic acidosis was first described as a clinical entity in the 1920's Citation1. Today, measurement of blood lactate is an established method to monitor and verify adequacy of organ and tissue oxygenation in a number of clinical situations, including the use of cardiopulmonary bypass (CPB) Citation2–5. Despite decades of refinements, CPB may have potential and unwanted effects on organ function and limit patient outcome Citation6. CPB initiates an immense systemic inflammatory reaction by activating a series of immunological defence systems of which all in theory may interfere with normal organ function Citation7, Citation8. To maintain normal tissue oxygenation organs rely on a continuous delivery of oxygen controlled by pump flow and the availability of oxygen dissolved in plasma and tied to haemoglobin Citation9. Under conditions when oxygen availability is limited energy supply is granted through anaerobic metabolism promoting the conversion of pyruvate to lactate. Hyperlactatemia may also occur without any apparent clinical signs of poor tissue oxygenation. Cohen and Wood Citation10 proposed to classify these two conditions as type A and type B hyperlactatemia, respectively.
The development of hyperlactatemia during CPB originates from a chain of physiological reactions of which most are scantily defined Citation4, Citation6. More generalised findings include a positive correlation between hyperlactatemia and the duration of CPB Citation11 presumably related to a time dependent deteriorating effect on normal circulatory and cellular functioning. Patients that develop hyperlactatemia are at greater risk verified by morbidity and mortality scores Citation2, Citation11. Of interest is that the significance of blood lactate as a predictor as such is mainly based on serial registrations made during the intensive care situation. To which extent hyperlactatemia during the period of CPB is predictive for morbidity and mortality remains to be clarified in more detail.
The reference interval for blood lactate is 0.5–2.2 mmol/l Citation12. Alert levels are normally set somewhat higher and vary depending on the particular clinical situation: a level exceeding 5 mmol/l exemplifies a situation of significant hyperlactatemia, whereas a level lower than 2 mmol/l in a critically ill patient generally is regarded as normal Citation13. However, what should define a normal reference interval for lactate during CPB is to date not defined. Is the normal reference interval for lactate also applicable during CPB or is there reason to believe that lactate values are deviant from the normal?
The present study aims to identify a threshold for hyperlactatemia during CPB and to determine risk factors and outcome variables associated with hyperlactatemia.
Material and methods
A retrospective database analysis of 5 121 cardiac surgical patients operated upon from year 2000 until 2005 were reviewed to identify factors related to lactate production during cardiopulmonary bypass.
Database structure and data management strategy
Entry to the database was performed by the category of staff primarily responsible for the patient. Patient records were generated on several occasions from admission until discharge from the hospital. The database covers a wide range of patient specific information from the entire perioperative period to delineate surgical, anaesthesiological and perfusion related aspects, followed by a detailed follow-up from the intensive care and the ward. Input of data to the database was based on predefined alternatives, typically on a nominal, ordinal or quotient level. No online or real-time transfer of information from patient monitors to the database was available. The database has been described earlier Citation14, Citation15.
Information specific for the heart-lung machine was post case registered into the database mostly based on discrete observations. No online or real time data collection was performed.
Management of anaesthesia and cardiopulmonary bypass
The patients were anaesthetised in a similar fashion using morphine for premedication, propofol for induction and fentanyl-pancuronium combined with isoflurane in oxygen/air mixture for maintenance. Normoventilation and adequate oxygenation was the objective.
The extracorporeal circuit comprised PVC tubing, a membrane oxygenator and a hard shell venous reservoir. Heparin coating and closed circuits were used in a small number of patients. Cardiotomy suction was used throughout. The circuit was primed with ringer acetate and mannitol to a total volume of approximately 1 500–2 000 ml. Pump flow was regulated to maintain the mixed venous oxygen saturation above 70%. A non-pulsatile pump mode was used in the great majority of cases. Mean arterial pressure was controlled to at least 50 mmHg using systemic vasoconstriction as needed, typically phenylephrine. Anticoagulation was achieved by administering a systemic dose of heparin (350 IU/kg) to elevate the activated clotting time in excess of 480 s. Mean body temperature was maintained at approximately 34°C in the majority of cases, except for complex surgical procedures under which the core temperature was further lowered.
Analysis of blood lactate levels during cardiopulmonary bypass
Arterial blood lactate levels during CPB were analysed on three occasions: 1) at initiation of CPB, 2) at start of re-warming the patient and 3) prior to weaning from bypass. Analysis of blood lactate was performed using the ABL 725 blood gas analyser (Radiometer A/S, Denmark). Results from the analysis were carefully noted on the perfusion chart and post case via the computer registered into the department's database.
Lactate analysed by the ABL 725 instrument is converting lactate by an interaction with oxygen to pyruvate and hydrogen peroxide. The release of hydrogen peroxide oxidised at the platinum anode generates a flux of electrons proportional to the lactate concentration Citation16.
The performance of the lactate measurement using the ABL 725 is reported analogous to an established laboratory reference Citation17.
Definition of hyperlactatemia
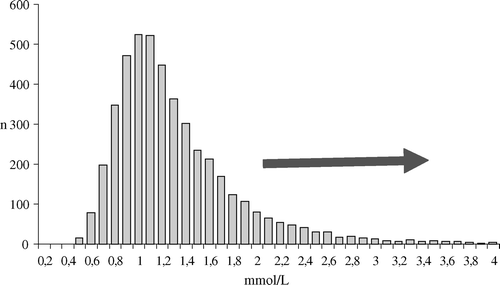
Weaning from CPB was set as the reference time point for analysis of lactate release during CPB (). Hyperlactatemia was defined as a lactate value equal to the 90th percentile of the derived sample distribution.
Statistical analysis
Variables associated with lactate release during CPB were initially analysed using univariate statistics: Student's t-test for continuous variables and the χ2 test or Fisher's exact test for categorical variables, whenever appropriate. Statistically significant variables from the univariate analysis were there upon included in a multivariate logistic regression model using a forward stepwise selection based on the likelihood ratio. Interaction between variables was considered in the model. Gender was used as controlling factor. A p-value less than 0.05 was set as statistically significant. Results were presented as means±the standard deviation, if not elsewise stated. Statistical analysis was performed using the SPSS statistical package version 13.0.
Results
Patient population and definition of hyperlactatemia
Characteristics of the patient population are described in . The lactate level after initiation of CPB was 1.04±0.64 mmol/l and rose to 1.26±0.88 at start of rewarming of the patient to eventually reach a peak mean value of 1.39±0.86 mmol/l at weaning from bypass. The sampling distribution of lactate at weaning from bypass is presented in . A threshold set at the 90th percentile to identify patients with hyperlactatemia was found to equal a lactate level greater than 2 mmol/l.
Table I. Patient related information.
Predictors of hyperlactatemia during CPB based on univariate statistics
Continuous variables associated with hyperlactatemia during CPB are presented in . The patients with hyperlactatemia were younger. Other risk factors were the duration of CPB, aortic cross clamp time, use of blood products, body temperature maintained during CPB and the patient's preoperative condition verified by the Higgins score. More than 60% of the patients requiring deep hypothermic circulatory arrest developed hyperlactatemia, corresponding to an odds ratio of 16. Still other risk factors for hyperlactatemia were emergency surgery, use of inotropic- and vasoactive drugs and complex surgery, exemplified by second time operations and non-CABG procedures. A direct influence of CPB on lactate production was seen among patients with a compromised venous return or backflow to the heart-lung machine: seven-teen percent of these patients developed hyperlactatemia compared to 7.9% where the venous return was completely normal. More than 50% of the patients that were difficult to wean from bypass had a lactate level over 2 mmol/l.
Table II. Univariate risk stratification of hyperlactatemia during cardiopulmonary bypass: continuous variables.
Predictors of hyperlactatemia during CPB based on logistic regression modelling
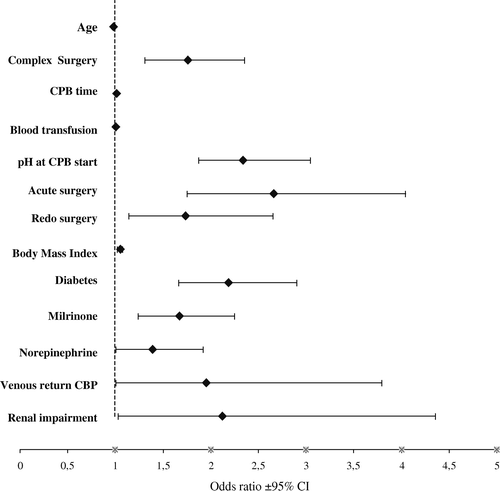
Based on logistic regression modelling the following predictors were identified to explain lactate release during cardiopulmonary bypass: age, complex surgery, duration of CPB, blood transfusion, pH during CPB, emergency operation, diabetes, use of milrinone and norepinephrine, venous return per CPB and renal function (). The model accounted for approximately one third (Nagelkerke r2=0.362) of the variation in lactate levels seen at weaning from CPB.
Figure 2. The figure presents the result from the logistic regression modelling based on a set of statistically significant pre- and intraoperative variables all associated with the development of hyperlactatemia at weaning from cardiopulmonary bypass. The model accounts for approximately 36% of the detected lactate variation (Nagelkerke r-square). Risk factors excluded from the model were: gender, volume load during CPB, haemoglobin concentration at CPB start, left ventricular function, Higgins score, preoperative anaemia, use of epinephrine and hypothermic circulatory arrest. Each variable's odds ratio (♦) is presented with its associated 95% confidence interval (CI). Dashed line indicates an odds ratio equal to 1. Definitions: Complex Surgery denotes all surgical procedures-isolated coronary artery bypass grafting excluded; Blood Transfusion performed per CPB; Milrinone and Norepinephrine treatment initiated to facilitate weaning from CPB; Venous return CPB categorised post bypass by the perfusionists as: normal, impaired or bad; Renal impairment based on the patient's preoperative creatinine level: (<141)–(141-167)–(>167) µmol/l.
The predictive value of hyperlactatemia during CPB on outcome
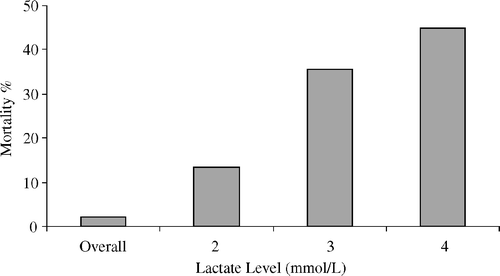
In patients fulfilling the criterion of hyperlactatemia, hospital mortality was 13.3%, compared to 2.2% for the entire cohort. A lactate level at 3 or 4 mmol/l, the hospital mortality pointed at 35.6% and 44.9%, respectively (). The 30-day mortality reached 14.6%, with a corresponding sensitivity of 48.6%. Patients who died during the hospital stay, 54% met the criterion of hyperlactatemia.
Figure 3. The positive predictive value of lactate for hospital mortality sampled at the time for weaning from cardiopulmonary bypass. The overall bar represents the mortality rate for all patients in the cohort regardless of lactate level. Sensitivity and specificity at 2 mmol/L: (54/98)%, 3 mmol/L: (44/99)% and 4 mmol/L: (30/99)%. Cut-off for lactate to obtain maximum sensitivity and specificity points at 2.4 mmol/L based on ROC-analysis.
Patients with hyperlactatemia at weaning from CPB stayed significantly longer in intensive care and required prolonged postoperative artificial ventilation. Also, the stay in hospital was extended: on average with more than one day (). The risk of postoperative renal failure increased fivefold (odds ratio 5.9). Other categories of postoperative complications with significantly increased risk scores were infections, respiratory and circulatory disorders ().
Table III. Outcome measures in patients with hyperlactatemia during cardiopulmonary bypass.
Table IV. The predictive value of hyperlactatemia at termination of cardiopulmonary bypass with reference to postoperative complications.
Discussion
The great majority of the patients exposed to cardiopulmonary bypass in this study demonstrated normal lactate levels. The proposed threshold for hyperlactatemia set at the 90th percentile of the identified distribution of lactate levels during cardiopulmonary bypass attained 2 mmol/L and is consistent with the upper normal range for blood lactate Citation12. Hence, based on this fairly large cohort, use of CPB seems in the normal case not to cause hyperlactatemia.
The patient's general preoperative condition represents a significant risk factor for hyperlactatemia during CPB as signified by Higgins score and the identified overrepresentation of conditions like diabetes, anaemia, impaired left ventricular- and renal function. Still other risk factors were emergency- or complex surgery. Similar findings have previously been proposed by Demers Citation2 and Maillet Citation11. Of interest, the single most significant risk factor for hyperlactatemia in our population was the use of deep hypothermic circulatory arrest, equivalent to an odds ratio of 16 and a sensitivity measure over 60%. Use of circulatory arrest is however indicated only in a very limited number of cases, why this specific risk factor did not qualify as a predictor in the final multivariate logistic regression model.
To our surprise low age appeared as a risk factor for hyperlactatemia. The contrary would have been expected Citation2. In theory, our finding could be attributed to regional hypoperfusion. Age and metabolism are related, why a younger patient normally would require a higher pump flow per body surface area. In our practice, flow control favours a dynamic adaptation governed by the oxygen extraction rate monitored online, rather than a fixed pump index. In the normal case the overall oxygen extraction rate is not allowed to exceed 30%. Despite our efforts to deliver a sufficient whole body perfusion, flow demands for individual organs may still have not been fulfilled. Regional hypoperfusion during CPB is a commonly reported phenomenon, especially during the phase of rewarming Citation18 predominately affecting the splanchnic regions Citation3 and gut Citation19.
Venous return or back-flow from the patient to the heart-lung machine was found to play a significant role on the detected lactate concentration. The magnitude of venous return is directly coupled to the delivery of arterialised blood and can in situations restrict maintenance of a normal blood flow. A concomitant built-up of central venous pressure in these situations may compromise organ perfusion even further. However, the resolution of information in our database did not permit a closer statistical analysis of possible real-time connections of compromised venous return and incidents of hypotension, pump flow restrictions or critical levels of venous saturation. Such an analysis would require online transfer of patient monitor data-yet not implemented in our historical database. On the other hand, hyperlactatemia based on venous blood flow restrictions during CPB is to the best of our knowledge a new observation and deserves a future re-examination to delineate our findings in more detail.
CPB may disturb hepatic perfusion and thereby indirectly provoke hyperlactatemia by altering lactate metabolism and clearance Citation4. Iatrogenic causes as the composition of priming solutions is still another co-factor to be considered. Use of ringer lactate may elevate the plasma lactate concentration Citation20 as well as use of stored packed red cells Citation21. Metabolic acidosis and hyperlactatemia are interrelated, but may exist independently Citation5. Metabolic acidosis seen during CPB is predominately caused by change in strong ion difference rather than hypoperfusion as for instance determined by the lactate concentration Citation22. The mayor component for developing acidosis during CPB is believed to be a concentration increase of chloride ions Citation5. We did not address ions shifts in this particular database analysis, but could indeed verify a significant association between pH and lactate levels. Patients may have different prognosis depending on the mechanism of acidosis, where a lactic acidosis is believed to be associated with a relatively poorer outcome than compared to chloride and strong ion differences Citation23.
There is beyond doubt a link between the duration of CPB and the amount of lactate released and detected in blood Citation2, Citation4, Citation11. The significance of the time component emerged at duration of CPB exceeding 2.5 hours in our material that also coincided with the group of patients who were difficult to wean from bypass. Hyperlactatemia at this time point may very well be explained both by deterioration related to CPB Citation7, but also as a direct pharmacological effect from various drugs Citation24. Phenylephrine was the drug of choice to treat vasodilatation during CPB, while milrinone and norepinephrine were more commonly used as circulatory support to facilitate weaning from CPB. All these drugs were overrepresented among patients with hyperlactatemia. To what extent a direct pharmacological effect can be attributed to the development of hyperlactatemia is difficult to state from a retrospective database analysis. However, based on the simple fact that this group of patients fulfilled the criterion for pharmacological support already from start due to a failing circulation would suggest an increased risk for hyperlactatemia.
Another important finding is that even a minor deviant blood lactate concentration, i.e. above 2 mmol/l observed at termination from CPB should alert the clinician about a more complicated patient course. We observed significantly higher postoperative complication rates than expected engaging a wide range of organ systems, especially with reference to renal function and infections. Stay in hospital and intensive care was also longer than normal, including the mean time for postoperative ventilatory support. The predicted hospital mortality rate was 13.3%, which was significantly higher than the actual overall rate at 2.3%-a more than fivefold increase. Our findings are in contrast with several previous reports Citation2, Citation11 in which typically much higher lactate concentrations were used for prediction of mortality and morbidity rates. Interestingly, Toraman and colleagues Citation25 set also the lactate threshold at 2 mmol/l and demonstrated in agreement with our results a predisposition for adverse outcome.
A limitation in this study is that lactate variations during the postoperative period not were addressed. However, it seems apparent from a digest of our findings that hyperlactatemia found at termination of cardiopulmonary bypass has a multi-factorial origin beginning with the patient's preoperative condition to be followed by type and course of surgical treatment. All these aspects will in turn have a significant impact on the cardiopulmonary bypass procedure and the associated risk for hyperlactatemia. Anaesthesiological interventions may also contribute. A lactate level >2 mmol/l at termination of CPB represent a deviant patient group or 10% of the investigated cohort. These patients sustained a significant risk for postoperative complications.
Based on findings from this study quality aspects of CPB would probably benefit from a method enabling monitoring blood lactate levels online.
References
- Clausen SW. Anhydremic acidosis due to lactic acid. Am J Dis Child. 1925; 29: 761
- Demers P, Elkouri S, Martineau R, Couturier A, Cartier R. Outcome with high blood lactate levels during cardiopulmonary bypass in adult cardiac operation. Ann Thorac Surg. 2000; 70: 2082–6
- Landow L. Splanchnic lactate production in cardiac surgery patients. Crit Care Med. 1993; 21(Suppl 2)S84–91
- Mustafa I, Roth H, Hanafiah A, Hakim T, Anwar M, Siregar E, et al. Effect of cardiopulmonary bypass on lactate metabolism. Intensive Care Med. 2003; 29: 1279–85
- Hayhoe M, Bellomo R, Liu G, McNicol L, Buxton B. The aetiology and pathogenesis of cardiopulmonary bypass-associated metabolic acidosis using polygeline pump prime. Intensive Care Med. 1999; 25: 680–5
- Boldt J, Piper S, Murray P, Lehmann A. Case 2-1999. Severe lactic acidosis after cardiac surgery: Sign of perfusion deficits?. J Cardiothorac Vasc Anesth. 1999; 13: 220–4
- Haisjackl M, Birnbaum J, Redlin M, Schmutzler M, Waldenberger F, Lochs H, et al. Splanchnic oxygen transport and lactate metabolism during normothermic cardiopulmonary bypass in humans. Anesth Analg. 1998; 86: 22–7
- Asimakopoulos G. Systemic inflammation and cardiac surgery: An update. Perfusion. 2001; 16: 353–60
- Clowes GH, Neville WE, Sabga G, Shibota Y. The relationship of oxygen consumption, perfusion rate, and temperature to the acidosis associated with cardiopulmonary circulatory bypass. Surgery. 1958; 44: 220–39
- Cohen RD, Woods HF. Lactic acidosis revisited. Diabetes. 1983; 32: 181–91
- Maillet JM, Le Besnerais P, Cantoni M, Nataf P, Ruffenach A, Lessana A, et al. Frequency, risk factors, and outcome of hyperlactatemia after cardiac surgery. Chest. 2003; 123: 1361–6
- Berkow R. Merck Manual of Diagnosis and Therapy16th edition. Merck & Co Inc, Rathway NJ 1992; 2581
- Mordes JP, Tranquada RE, Rossini AA. Lactic Acidosis. Intensive Care Medicine, JM Rippe, RS Irwin, JS Alpert, MP. Fink. Little, Brown and Company, Boston/Toronto/London 1991; 994–1000
- Åberg T, Svenmarker S, Hohner P, Hentschel J. Routine registration of deviations from the norm in cardiac surgery: A potent clinical research tool and quality assurance measure. Eur J Cardiothorac Surg. 1997;11:10–2,13–6.
- Åberg T, Hentschel J. Improved total quality by monitoring of a cardiothoracic unit. Medical, administrative and economic data followed for 9 years. Interactive Cardiovasc Thoracic Surg. 2004; 3: 33–40
- Sack DB. Carbohydrates. In: Tietz fundamentals of clinical chemistry. 4th ed. Burtis CA, Ashwood ER. Philadelphia: Saunders W. B.; 1999. p 351–74.
- Suen WW, Ridley B, Blakney G, Higgins TN. Comparison of lactate, bilirubin and hemoglobin F concentrations obtained by the ABL 700 series blood gas analyzers with laboratory methods. Clin Biochem. 2003; 36: 103–7
- Lindholm L, Bengtsson A, Hansdottir V, Lundqvist M, Rosengren L, Jeppsson A. Regional oxygenation and systemic inflammatory response during cardiopulmonary bypass: Influence of temperature and blood flow variations. J Cardiothorac Vasc Anesth. 2003; 17: 182–7
- Perner A, Jørgensen VL, Poulsen TD, Steinbrüchel D, Larsen B, Andersen LW. Increased concentrations of L-lactate in the rectal lumen in patients undergoing cardiopulmonary bypass. Br J Anaesth. 2005; 95: 764–8
- Himpe D, Neels H, De Hert S, Van Cauwelaert P. Adding lactate to the prime solution during hypothermic cardiopulmonary bypass: A quantitative acid-base analysis. Br J Anaesth. 2003; 90: 440–5
- Schroeder TH, Hansen M. Effects of fresh versus old stored blood in the priming solution on whole blood lactate levels during paediatric cardiac surgery. Perfusion. 2005; 20: 17–9
- Alston RP, Cormack L, Collinson C. Metabolic acidosis developing during cardiopulmonary bypass is related to a decrease in strong ion difference. Perfusion. 2004; 19: 145–52
- Gunnerson KJ, Saul M He S, Kellum JA. Lactate versus non-lactate metabolic acidosis: A retrospective outcome evaluation of critically ill patients. Crit Care 2006;10.
- Levy B, Bollaert PE, Charpentier C, Nace L, Audibert G, Bauer P, et al. Comparison of norepinephrine and dobutamine to epinephrine for hemodynamics, lactate metabolism, and gastric tonometric variables in septic shock: A prospective, randomized study. Intensive Care Med. 1997; 23: 282–7
- Toraman F, Evrenkaya S, Yuce M, Aksoy N, Karabulut H, Bozkulak Y, et al. Lactic acidosis after cardiac surgery is associated with adverse outcome. Heart Surg Forum. 2004; 7: E155–9