Abstract
Objectives. Preoperative carbohydrate administration attenuates insulin resistance. We studied effects of preoperative oral carbohydrate loading in elderly patients undergoing coronary artery bypass grafting. Design. Eighteen patients were assigned either to get a carbohydrate drink or to be controls. Perioperatively, glucose was administered. A gastric emptying test was performed. Glucose and insulin concentrations were measured. Levels of glucose, insulin and stress hormones were studied pre-, per- and postoperatively. Results and discussion. Preoperative carbohydrate loading did not affect stress hormones. Gastric residual after the carbohydrate drink was 11±3% (mean±SEM). Glucose concentration was lower before anaesthesia induction in the carbohydrate group, possibly due to increased insulin release. Insulin levels differed at baseline, induction and day six. All patients returned to baseline on day six. Conclusions. The study group was insulin resistant on postoperative day one and two. The effects were explainable by the traumatic stress response. No adverse effect was noted from the carbohydrate drink. If glucose is administered intravenously during surgery, there is no obvious advantage of preoperative carbohydrate loading on insulin resistance or stress hormone response.
It is increasingly common for elderly patients to undergo cardiovascular surgery. This group has a significant risk of postoperative complications and it is desirable to optimise the possible risk factors. Hyperglycaemia and insulin resistance are known factors associated with adverse prognosis Citation1–4. In the postoperative period after CABG, the patients are characterised by an acquired insulin resistance Citation5. Insulin resistance increases with age Citation6 and after fasting, trauma Citation7 and surgery Citation8, Citation9. Preoperative fasting constitutes additional stress superimposed on the surgical trauma and thereby affecting stress hormone response and insulin resistance. Decreased protein reserves contribute to make elderly patients more susceptible to complications and functional impairment Citation10. After surgery, old patients′ muscle strength falls to low levels and their postoperative recovery of strength is impaired Citation11.
Preoperative glucose or carbohydrate administration has been reported to attenuate insulin resistance and protein catabolism Citation8, Citation12 and to improve postoperative well-being as well as to reduce length of hospital stay Citation13. On the other hand, preoperative carbohydrate loading had no effect on insulin resistance after major surgery in an in vitro animal model Citation14.
The effects of carbohydrate loading have not been studied in elderly undergoing cardiovascular surgery. We investigated whether a preoperative oral carbohydrate load in elderly coronary artery bypass grafting (CABG) patients affected muscle strength, insulin resistance and stress hormone response.
Patients and methods
The investigation was approved by the Research Ethics committee and the Isotope committee of the Uppsala University Hospital. Eighteen patients, aged over 65, scheduled to undergo elective CABG, gave their informed consent to participate and were included in this investigation. Exclusion criteria were known diabetes mellitus, other metabolic disease or severely impaired respiratory, circulatory or renal function.
Study design
All patients were admitted to the hospital one or two days before surgery. On the day before surgery, dinner was served at 5 p.m. and tea or coffee at 8 p.m., thereafter the patients were not allowed any food or drink.
Patients were randomly assigned either to the carbohydrate or to the control group. Glycated haemoglobin concentration (HbA1c) was analysed on admission. Blood samples for concentrations of glucose, insulin, C-peptide, glucagon, cortisol, IGF-1, growth hormone (GH), epinephrine, norepinephrine, chromogranin A and pancreatic polypeptide (PP) were collected. Sampling times were in the morning on the day before surgery (baseline measurements), in the morning before surgery, before induction of anaesthesia and in the morning of the 1st, 2nd and 6th postoperative day (POD 1, 2 and 6 respectively). Thus, all patients were fasted overnight (12 h) before sampling.
Patients in the study group (n = 9) were given 400 ml of a 12.5% carbohydrate drink (Nutricia Preop®, Nutricia, Zoetermeer, The Netherlands) in the evening before surgery and in the morning on the day of surgery. Subsequently, gastric emptying measurement was performed as described below and simultaneous samples for plasma glucose and serum insulin were drawn at 0, 15, 30, 45, 60, 90 and 120 min. The control group (n = 9) was managed according to standard preoperative procedure and also fasted overnight prior to the surgery.
Anaesthesia, surgery and postoperative management
Anaesthesia and CABG were performed according to standard procedures. Anaesthesia was induced 3 – 5 h after the baseline measurements and carbohydrate intake. Surgery was started one hour after anaesthesia induction. Extra-corporeal circulation (ECC) was performed in moderate hypothermia, i.e. 32 to 34°C. In both groups a glucose infusion (100 mg/ml) was started as soon as the central venous catheter was placed and continued with a rate corresponding to 0.1 g glucose kg−1 h−1. Patients with a body mass greater than 75 kg were given 7.5 g glucose h−1. On POD 1 the intravenous glucose infusion was discontinued at 4 a.m. (4 h before the collection of blood samples). After blood sampling the patients were served breakfast and then managed according to routines throughout the study period.
Analytical methods
All assays were performed at the Clinical Chemistry Laboratory at Uppsala University Hospital. Plasma glucose was measured on an automated clinical chemistry instrument (Hitachi 912, Roche Diagnostics, Bromma, Sweden). HbA1c was measured with an automated HPLC system (Variant II, Bio-Rad Laboratories AB, Sweden). Serum concentrations of cortisol, GH, insulin, and C-peptide were measured on an automated fluorescence detection system (Autodelfia, Wallac Oy, Turku, Finland). Glucagon, Pancreatic polypeptide and IGF-1 were measured with a commercial RIA kit (Linco, St. Charles, MI, USA, EuroDiagnostica, Malmö, Sweden and Nichols Institute Diagnostics, San Juan Capistrano, CA, USA). Plasma epinephrine and norepinephrine were measured on a semi-automated HPLC-system with electrochemical detection (Tillqvist Analys AB, Sweden). Chromogranin A was measured with competitive radioimmunoassay Citation15.
HOMA
HOMA (homeostasis model assessment) was used to evaluate insulin resistance (HOMA-IR) and β-cell activity (HOMA-B) Citation16. HOMA, based on insulin and glucose determination in the fasting state, is easily obtained and simplifies longitudinal assessment of insulin sensitivity throughout the perioperative period. The measurements of HOMA were performed after an overnight fast at baseline, at induction and on POD 2 and 6.
Assuming that a subject with a normal weight, aged less than 35 years has a 100% β-cell function and an insulin resistance of 1, the values for the patients can be assessed from the insulin and glucose concentrations by the Citationequations 16:and (near approximation) Citation17:
Plasma glucose concentrations are given as mmol/l and serum insulin concentration as mU/l. HOMA-IR >1.0 indicates insulin resistance and HOMA-B <100% decreased β-cell function. We have also computed the ratio between baseline levels of glucose, insulin and HOMA and the values on POD 1, 2 and 6.
Gastric emptying measurements
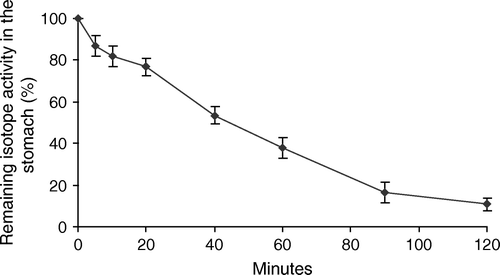
In the morning on the day of surgery, the patients in the study group ingested 400 ml of the carbohydrate drink mixed with 20 MBq 99mTc-labeled human albumin colloid (Solco Nanocoll, Solco Basle Ltd, Birsfelden, Switzerland). The patients were sitting up while drinking and recordings by gamma camera were made in the supine position at 0, 5, 10, 20, 40, 60, 90, and 120 min. Static anterior and posterior registrations were obtained with a double-headed gamma-camera (Maxxus, General Electric, Milwaukee, WI, USA) equipped with a low-energy general-purpose collimator. The acquisition was performed in a 128*128 matrix with an acquisition time of 2 min. In between recordings the patients were allowed to position themselves as they wanted. Evaluation of the gastric content was made on a Hermes workstation (Nuclear Diagnostics AB, Stockholm, Sweden). Regions of interest (ROI) were selected over the activity in the ventricle and the gastric content was calculated as geometric mean activity from anterior and posterior images . After correction for physical decay, the activity at the different recordings was expressed as a percentage of the initial value at time 0.
Respiratory function
We evaluated respiratory muscular function as peak expiratory flow (PEF), Pimax (maximum inspiratory flow) and Pemax (maximum expiratory flow) measurements before surgery and on POD 6. The ratio between the values on POD 6 and preoperative measurements was calculated. PEF was measured with a miniWright Peak flow meter (AIRMED, Clement Clarke Int. Ltd, Essex, England). Pimax and Pemax were assessed with PiMAX, (MPM Micro Medical Ltd, Medanz, Starnberg, Germany).
Statistical analysis
Results are presented as mean±SEM. ANOVA and global F-test were used to compare two and multiple groups of values. P < 0.05 was considered significant. Non-parametric statistics was used when appropriate.
Results
For background parameters see . There was no difference between the two groups with respect to age and body mass index (BMI), ECC data (total ECC-time and time with a crossclamp) as well as number of anastomoses and surgery time. HbA1c was 4.7±0.13 mmol/l in both groups.
Table I. Characteristics of patients and cardiopulmonary bypass data.
Glucose and insulin
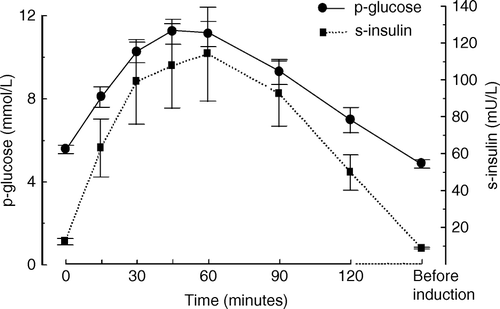
For plasma glucose and related parameters see . Plasma glucose concentration did not differ between the groups at baseline. Before induction of anaesthesia, plasma glucose concentration was lower in the carbohydrate group. On POD 1 and 2, fasting plasma glucose concentrations were elevated in a similar fashion in both groups. On POD 6, plasma glucose concentrations had returned to baseline in the controls and were slightly above baseline in the carbohydrate group.
Table II. Insulin, glucose, C-peptide and HOMA-IR and HOMA-B.
Insulin concentration at baseline was higher in the carbohydrate group (p < 0.02).
Before induction of anaesthesia, insulin concentration in both groups was lower than baseline, increasing on POD 1 and 2, subsequently returning to baseline on POD 6. The concentrations of C-peptide showed a similar pattern (). The ratios of insulin and glucose concentrations at POD 1, 2 and 6 and baseline did not differ between groups; for glucose 149±3 vs. 143±3%, 131±7% vs. 114±17% and 106±1% vs. 117±1% on POD 1, 2 and 6 respectively and for insulin 252±50 vs. 171±22%, 178±25 vs. 127±13% and 101±0.9 vs. 103±9% on POD 1, 2 and 6 respectively.
Table III. Stress related hormone response.
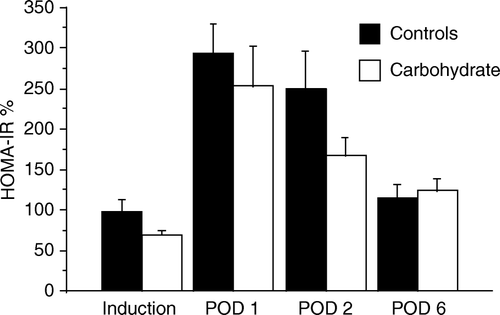
Insulin resistance, as measured by HOMA-IR, increased in both groups on POD 1 and 2 and returned to baseline on POD 6. There was no significant difference in the POD 1, 2 and 6 vs baseline ratio of HOMA-IR between the groups (). β-cell activity in the carbohydrate group, as measured by HOMA-B, showed a peak at induction. In the control group HOMA-B was lower, with no changes throughout the study period.
Stress hormones
For stress hormone concentrations see . No difference was seen between the groups at baseline. Epinephrine and norepinephrine concentrations were higher on POD 1 in both groups. The carbohydrate group had higher concentrations of norepinephrine on POD 1 than the controls. The concentrations of cortisol and glucagon were higher postoperatively than at baseline in both groups, with no obvious difference between groups. GH, chromogranin A and pancreatic polypeptide concentrations did not differ significantly between the two groups or over time. IGF-1 showed a decreasing trend postoperatively compared to baseline in both groups, however, no difference was observed between the groups.
Respiratory parameters
No differences were seen between the carbohydrate group and the controls in the relative dynamic respiratory parameters (POD 6 vs. baseline. The PEF ratio was 0.94±0.04 vs. 0.83±0.03, Pimax ratio was 0.71±0.09 vs. 0.84±0.08 and Pemax 0.91±0.05 vs. 0.96±0.10 for the control group and the carbohydrate group respectively.
Short term response to carbohydrate, gastric emptying
The patients responded to the carbohydrate drink with a temporary increase in plasma glucose and serum insulin concentrations (). Plasma glucose concentration reached a peak above 10 mmol/l between 30 and 60 min after the carbohydrate load. The serum insulin concentration curve showed a similar profile.
Figure 2. Plasma glucose concentrations and serum insulin concentrations during the gastric emptying study. Values are given as mean±SEM. The time scale is 0 – 120 minutes after ingestion of carbohydrate drink and before induction of anaesthesia (before ind).
After 120 min the remaining activity in the stomach was 11±3% ().
Discussion
We did not observe any beneficial effect of preoperative oral carbohydrate administration on the measures of insulin resistance. A possible positive effect could in our study group have been counteracted by several factors. A strong hormonal trauma response may obscure a beneficial effect. All patients had initially high levels of insulin, glucagon and cortisol, with a further increase on POD 1. On POD 6, the values had still not reached the baseline. The high levels of stress hormones in our study may be due to the fact that our patients are elderly Citation9, Citation18.
Our use of HOMA is motivated by the fact that the HOMA estimate of insulin resistance correlates with values obtained by euglycaemic clamp, fasting insulin concentration and hyperglycaemic clamp Citation16. Further, the estimate of β-cell function obtained by the HOMA correlates with that derived by the hyperglycaemic clamp and oral glucose tolerance test (R lies between 0.7 and 0.9) Citation17. The method has been applied in measurements of insulin resistance in subjects with or without maturity-onset diabetes Citation16, in prediction of re-stenosis after coronary stent placement in non-diabetic patients Citation19 and to assess insulin resistance in relation to the incidence of cardiovascular disease Citation20.
In the present investigation the relatively high levels of glucose and insulin suggest a high insulin resistance in the studied group. The patients were clearly insulin resistant on both POD 1 and 2, if the definitions by Hanley et al. Citation20 or Sekiguchi et al. Citation19 are used (HOMA-IR ≥ 2 and ≥ 3, respectively). Before induction of anaesthesia, the glucose concentration was lower and C-peptide higher in the carbohydrate group as compared to the controls. This was possibly due to an increased insulin release in response to the oral carbohydrate load.
The effect of glucose infusion
The perioperative administration of glucose intravenously could possibly have attenuated a positive effect of oral carbohydrate loading that has been described in other patients Citation13. We have several reasons for perioperative administration of glucose in the present patient group. Glucose is the preferred substrate for the ischemic heart Citation21, Citation22. Hypothermic cardioplegic arrest increases uptake and oxidation of glucose in the immediate reperfusion phase Citation21, Citation23. Without simultaneous glucose administration, perioperative infusion of exogenous insulin to keep blood glucose within the fasting range increases the risk of postoperative hypoglycaemia Citation24.
Markers of sympathetic and parasympathetic activity
The postoperative increase in norepinephrine tended to be more pronounced in the carbohydrate group. The Chromogranin A concentration showed a tendency towards increase throughout the investigation. Chromogranin A is stored and released together with catecholamines and can be interpreted as an indicator of sympathetic activity.
PP is secreted by the islets of Langerhan. The secretion is at least in part under cholinergic control and the level can thus be taken as an index of parasympathetic activity. The level of PP remained constant during the study.
The observed residual gastric activity of 11% is higher than what has been reported in a younger age group Citation25, however, it is a low value as compared to the healthy controls in a study by Delgado-Aros Citation26, The magnitude is modest and, taken together, these investigations suggest that it is safe to ingest a clear carbohydrate fluid 2 h before pre-medication. The slightly higher gastric retention than in Nygrens report can be due to an effect of elevated plasma glucose on gastric emptying that was observed when glucose was clamped at 8 mmol/l both in healthy subjects and diabetic patients Citation27.
Dynamic respiratory parameters with the patient as his own control have been used to quantify changes in muscle function Citation28. In the dynamic lung function tests both the controls and the carbohydrate group showed decreased values on POD 6. This is probably due both to the effect of surgery per se and to impaired muscle strength which has been shown after abdominal surgery Citation11. The finding is consistent with reports of impaired lung function up to three months after cardiac surgery Citation28.
Limitations
HOMA represents the basal conditions, as opposed to the clamping techniques that reflect the insulin sensitivity under hyperphysiological insulin concentrations.
Fasting hepatic glucose production may be taken to be more closely related to HOMA. A number of studies have however also shown a high correlation between HOMA and the euglycaemic clamps Citation29. Insulin concentrations and HOMA enabled us to assess insulin resistance in our patients during 7 days observation.
A significant spread in insulin sensitivity in non-diabetic patients undergoing surgery has been reported Citation30. A similar, although smaller variation could be seen among our patients () and there is a possibility that the absolute levels in individual patients did not properly reflect a difference between the groups.
For practical reasons we chose to sample catecholamines under resting conditions. However, catecholamine secretion varies throughout the day and is also influenced by a multitude of factors associated with environmental stress.
Conclusions
All patients exhibited insulin resistance on the first postoperative days. We did not observe any clearly adverse or beneficial effects of oral carbohydrate drink on insulin resistance or stress hormone response. This could be due to the fact that all these patients were given an adequate glucose infusion.
Nutricia® provided the Preop®-drink. The assistance of the staff of the Department of Nuclear Imaging, the ICU and the cardiothoracic surgical wards is gratefully acknowledged. RN Ingela Liberg assisted in the care and sampling of the patients. The Swedish Heart-Lung Foundation and the Research and Development Foundation of the Uppsala University Hospital have provided financial support.
References
- van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001; 345: 1359–67
- Norhammar A, Tenerz A, Nilsson G, Hamsten A, Efendic S, Ryden L, et al. Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: A prospective study. Lancet. 2002; 359: 2140–4
- Bartnik M, Ryden L, Ferrari R, Malmberg K, Pyorala K, Simoons M, et al. The prevalence of abnormal glucose regulation in patients with coronary artery disease across Europe. The Euro Heart Survey on diabetes and the heart. Eur Heart J. 2004; 25: 1880–90
- Zethelius B, Hales CN, Lithell HO, Berne C. Insulin resistance, impaired early insulin response, and insulin propeptides as predictors of the development of type 2 diabetes: A population-based, 7-year follow-up study in 70-year-old men. Diabetes Care. 2004; 27: 1433–8
- Farrer M, Fulcher G, Albers CJ, Neil HA, Adams PC, Alberti KG. Patients undergoing coronary artery bypass graft surgery are at high risk of impaired glucose tolerance and diabetes mellitus during the first postoperative year. Metabolism. 1995; 44: 1016–27
- Chen M, Halter JB, Porte D, Jr. The role of dietary carbohydrate in the decreased glucose tolerance of the elderly. J Am Geriatr Soc. 1987; 35: 417–24
- Carter EA. Insulin resistance in burns and trauma. Nutr Rev. 1998; 56: S170–S176
- Ljungqvist O, Thorell A, Gutniak M, Haggmark T, Efendic S. Glucose infusion instead of preoperative fasting reduces postoperative insulin resistance. J Am Coll Surg. 1994; 178: 329–36
- Brandi LS, Frediani M, Oleggini M, Mosca F, Cerri M, Boni C, et al. Insulin resistance after surgery: Normalization by insulin treatment. Clin Sci (Colch) 1990; 79: 443–50
- Hebuterne X, Bermon S, Schneider SM. Ageing and muscle: The effects of malnutrition, re-nutrition, and physical exercise. Curr Opin Clin Nutr Metab Care. 2001; 4: 295–300
- Watters, JM, Clancey, SM, Moulton, SB, Briere, KM, Zhu, JM. Impaired recovery of strength in older patients after major abdominal surgery. Ann Surg. 1993;218:380–90, Discussion 90–3.
- Soop M, Nygren J, Myrenfors P, Thorell A, Ljungqvist O. Preoperative oral carbohydrate treatment attenuates immediate postoperative insulin resistance. Am J Physiol Endocrinol Metab. 2001; 280: E576–E583
- Nygren J, Carlsson-Skwirut C, Brismar K, Thorell A, Ljungqvist O, Bang P. Insulin infusion increases levels of free IGF-I and IGFBP-3 proteolytic activity in patients after surgery. Am J Physiol Endocrinol Metab. 2001; 281: E736–41
- Strommer L, Isaksson B, Wickbom M, Arnelo U, Ostenson C, Herrington M, et al. Effect of carbohydrate feeding on insulin action in skeletal muscle after surgical trauma in the rat. Nutrition. 2001; 17: 332–6
- Stridsberg M, Hellman U, Wilander E, Lundqvist G, Hellsing K, Oberg K. Fragments of chromogranin A are present in the urine of patients with carcinoid tumours: Development of a specific radioimmunoassay for chromogranin A and its fragments. J Endocrinol. 1993; 139: 329–37
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28: 412–9
- Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004; 27: 1487–95
- Henriksen MG, Hessov I, Dela F, Hansen HV, Haraldsted V, Rodt SA. Effects of preoperative oral carbohydrates and peptides on postoperative endocrine response, mobilization, nutrition and muscle function in abdominal surgery. Acta Anaesthesiol Scand. 2003; 47: 191–9
- Sekiguchi M, Kurabayashi M, Adachi H, Hoshizaki H, Oshima S, Taniguchi K. Usefulness of insulin resistance measured by homeostasis model assessment in predicting restenosis after coronary stent placement in nondiabetic patients. Am J Cardiol. 2004; 93: 920–2
- Hanley AJ, Williams K, Stern MP, Haffner SM. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: The San Antonio Heart Study. Diabetes Care. 2002; 25: 1177–84
- Pietersen HG, Langenberg CJ, Geskes G, Kester A, de Lange S, Van der Vusse GJ, et al. Myocardial substrate uptake and oxidation during and after routine cardiac surgery. J Thorac Cardiovasc Surg. 1999; 118: 71–80
- Depre C, Vanoverschelde JL, Taegtmeyer H. Glucose for the heart. Circulation. 1999; 99: 578–88
- Lehot JJ, Piriz H, Villard J, Cohen R, Guidollet J. Glucose homeostasis. Comparison between hypothermic and normothermic cardiopulmonary bypass. Chest. 1992; 102: 106–11
- Chaney MA, Nikolov MP, Blakeman BP, Bakhos M. Attempting to maintain normoglycemia during cardiopulmonary bypass with insulin may initiate postoperative hypoglycemia. Anesth Analg. 1999; 89: 1091–5
- Nygren J, Thorell A, Jacobsson H, Larsson S, Schnell PO, Hylen L, et al. Preoperative gastric emptying. Effects of anxiety and oral carbohydrate administration. Ann Surg. 1995; 222: 728–34
- Delgado-Aros S, Camilleri M, Cremonini F, Ferber I, Stephens D, Burton DD. Contributions of gastric volumes and gastric emptying to meal size and postmeal symptoms in functional dyspepsia. Gastroenterology 2004; 127: 1685–94
- Schvarcz E, Palmer M, Aman J, Horowitz M, Stridsberg M, Berne C. Physiological hyperglycemia slows gastric emptying in normal subjects and patients with insulin-dependent diabetes mellitus. Gastroenterology. 1997; 113: 60–6
- Shenkman Z, Shir Y, Weiss YG, Bleiberg B, Gross D. The effects of cardiac surgery on early and late pulmonary functions. Acta Anaesthesiol Scand. 1997; 41: 1193–9
- Mather KJ, Hunt AE, Steinberg HO, Paradisi G, Hook G, Katz A, et al. Repeatability characteristics of simple indices of insulin resistance: Implications for research applications. J Clin Endocrinol Metab. 2001; 86: 5457–64
- Thorell A, Nygren J, Ljungqvist O. Insulin resistance: A marker of surgical stress [In Process Citation]. Curr Opin Clin Nutr Metab Care. 1999; 2: 69–78