Abstract
Aim. To examine whether changes in glycaemic control are related to the left ventricular function, in a group of type 1 diabetic patients. Methods. We included 19 consecutive type 1 diabetic patients selected for insulin pump therapy due to poor metabolic control. The outcome measures were HbA1c and systolic left ventricular strain rate as measured by Tissue Doppler echocardiography. Mean follow-up period was 45 days (range 35–50). All patients included had an ejection fraction > 55%. None had cardiac symptoms. Results. The mean age of the participants was 40 years (range 22–65). Following initiation of insulin-pump therapy, the mean HbA1c improved significantly from 8.6±1.4% to 7.6±1.1% (p < 0.01). The mean strain rate of 16 myocardial segments also improved significantly, from −1.58±0.30 s−1 to −1.80±0.4 s−1 (p < 0.01). In addition, there was a significant correlation between the changes in left ventricular function and the changes in HbA1c (delta-Strain rate vs. delta-HbA1c: r = 0.49, p < 0.01). Conclusion. Changes in glycaemic control resulting from insulin pump therapy seem significantly related to the systolic function of the left ventricle.
Reduced left ventricular contractile function in the long axis plane may be the earliest sign of cardiac dysfunction in the diabetic heart. Recent echocardiographic studies of type 2 diabetic patients emphasize the influence of poor glycaemic control on left ventricular systolic function, thus asserting that there is a connection between hyperglycemia and reduced left ventricular long axis function Citation1, Citation2. In a recent study of blood pressure reduction in type 2 diabetic patients, changes in the left ventricular function were actually more closely related to changes glycaemic levels than to changes in blood pressure Citation3.
At present, not much is known about this relation in type 1 diabetes. However, a recently published study, in which magnetic resonance imaging was employed, indicates that glycemia and left ventricular function are interrelated in type 1 diabetes as well Citation4. It is as yet unclear how closely changes in glycaemic control are related to left ventricular function in type 1 diabetic patients. Thus the aim of this study was to examine changes in the left ventricular function on the short term, in a group of type 1 diabetic patients during optimized glycaemic control.
Methods
We examined 20 consecutive type 1 diabetic patients selected for insulin pump therapy due to poor metabolic control. The patients were otherwise unmedicated. The main outcome yardstick was left ventricular strain rate (SR) in the long axis plane measured by Tissue Doppler echocardiography Citation5, Citation6. All participants had an ejection fraction > 55% and a fractional shortening > 25%. None of them had any cardiac symptoms, nor did they demonstrate diastolic dysfunction as defined as a mitral E deceleration time (DT) ≥ 140 ms and < 240 ms, an E/A ratio between 1.0 and 2.0, and a Tissue Doppler (TDI) derived E’/A’ ratio above 1.0.
Insulin therapy was based on insulin-aspart (Novo Nordisk A/S, Bagsværd, Denmark) and administered subcutaneously by a pump-system (Minimed 512, Medtronic, Northridge, CA, USA). Mean follow-up was 45 days (range 35–50). The study was conducted in accordance with the Helsinki II declaration and was approved by the local Ethics Committee.
All echocardiographic examinations in the study were performed by the same observer and all post-hoc off-line analyses were done blinded and in random order. Echocardiograms were performed with patients in euglycemia, on a GE Vivid Five (GE Healthcare, Horten, Norway) using a 2.5 MHz transducer. Images were obtained from the apical views during end-expiratory apnoea. The region of interest was enlarged to 9 times 9 pixels and Doppler scanning frame rates were kept between 140 ad 160 frames per second.
Strain rate was assessed as the mean value of three consecutive heart cycles measured in each of the 16 LV segments, equivalent to the wall motion score. No cine compound function was used. The strain rate was calculated over an offset (strain length) of 9 mm. The intra- and inter-observer variability of the SR measurements was previously determined to be 9±7% and 18±14%, respectively Citation7. These measurements were supplemented with systolic and diastolic velocity recordings of the mitral ring movement. The lateral mitral valve annulus provided the TDI sample area Citation8 and the presented values are also the mean value of three consecutive heart cycles. The left ventricular mass was calculated from the formula: LVM-0.8 [1.04 (Intraventricular septal thickness + LV diastolic diameter + Posterior Wall thickness)3 – (LV diastolic diameter)3] + 0.6 g Citation9.
Statistical analyses were done by paired t-test and Pearson correlations, and results are expressed as mean±standard deviation (SD).
Results
The mean age of the participants was 40 years (range 22–65), with a mean diabetes duration of 24 years (range 8–43). One patient was excluded due to the finding of a large atrial septal defect, leaving 19 patients available for follow-up. Seventeen of them were women.
The initiation of insulin pump therapy meant that the patients’ mean daily insulin dose was significantly reduced by a mean of 7 IE per day.
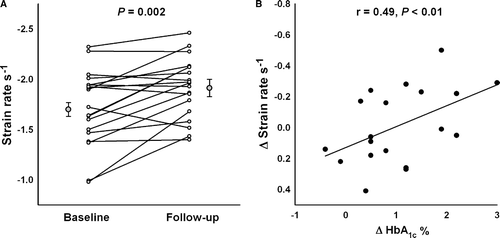
But it also led to a significant improvement in the patients’ HbA1c, and their mean left ventricular SR also improved significantly. However, the peak systolic velocity was not significantly improved. Left ventricular mass remained unchanged during the follow-up period, as did the measures of the diastolic function. Arterial blood pressures and heart rates were also unchanged during follow-up. The results are presented in .
Table I. Changes in glycaemic control and left ventricular function during follow-up.
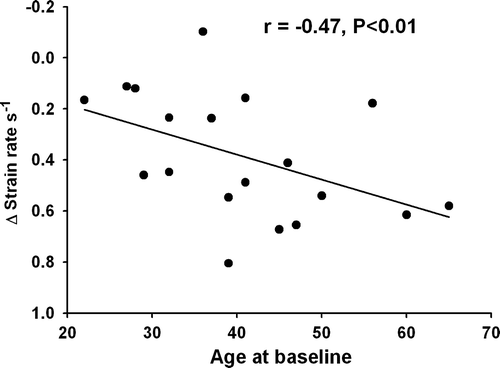
There was a significant correlation between the changes in left ventricular SR and the changes in HbA1c (ΔSR vs. ΔHbA1c: r = 0.49, p < 0.01, B), with improvement being more pronounced in the youngest part of the population. Patients younger than the mean age of 40 years improved significantly more during follow-up compared to the patients aged over 40 years (−1.40±0.32 s−1 to −1.74±0.25 s−1 vs. −1.76±0.40 s−1 to 1.82 s−1, p < 0.01) and there was a significant correlation between age at baseline vs. ΔSR; r = − 0.47, p < 0.05) ().
Discussion
The main finding in this study was a significant correlation between alterations in glycaemic control and changes in left ventricular function in a subset of type 1 diabetic patients. The findings did not seem influenced by heart rate or blood pressure which both remained unchanged during follow-up.
Previously, improved left ventricular function has been linked to a reduction in left ventricular mass in type 1 diabetic patients, partially induced by reduced hyperglycaemia Citation10, but in this study there were no changes in left ventricular mass. This implies that either the improved distribution of insulin or the reduction in glucose levels had a direct influence on the outcome.
Acute hyperinsulinemia administered via clamp techniques and combined with both hypo- and hyperglycaemia has been shown to improve left ventricular function in type 1 diabetic patients Citation11. However, this could partially be ascribed to the insulin mediated release of nor-epinephrine and epinephrine. Yet in the present study the patients’ insulin dosages were actually reduced, and this connection may not be of significance beyond experimental settings.
In fact, hyperglycemia may actually damage the left ventricle by bringing about non-enzymatic glycation of the myocardial wall. This, in turn, could be the result of structural changes in the myocardial wall due to collagen cross linking Citation12. Alternatively could reduction of the sarco(endo)plasmatic reticulum Ca2 + -ATPase influence the contractile forces of the cardiomyocyte. Both processes would be moderated by lowering blood glucose levels Citation13.
The most dramatic changes in left ventricular strain rate are to be found among the younger segment of the participants, which suggests that young diabetic patients with hyperglycemia may be more prone to reduced long axis function, but may also benefit substantially from optimized glycaemic control.
Surprisingly, the mean strain rate values were fairly high compared to similar patients with type 2 diabetesCitation14. This finding could partially be explained by lack of temporal smoothing in the image acquisition, but may also reflect that type 1 diabetic patients may have much less affection of the myocardium compared to type 2 diabetic patients. This issue needs further elucidation.
The peak systolic velocities did not change significantly during follow-up which probably reflects that strain rate is amore sensitive measure for the evaluation of the systolic function Citation15 and may also be more reproducible (Citation7, Citation16).
Limitations
Patients entering insulin pump therapy are scarce, which explains the limited amount of participants.
Newer echocardiographic equipment will provide better spatial resolution which may contribute with additional information.
Conclusion
Changes in glycaemic control seem significantly related to changes in left ventricular long axis function, with the connection being most pronounced in young patients with type 1 diabetes mellitus.
References
- Govind S, Brodin LA, Nowak J, Quintana M, Raumina S, Ramesh SS, et al. Isolated type 2 diabetes mellitus causes myocardial dysfunction that becomes worse in the presence of cardiovascular diseases: Results of the myocardial doppler in diabetes (MYDID) study 1. Cardiology. 2005; 103: 189–95
- Fang ZY, Schull-Meade R, Downey M, Prins J, Marwick TH. Determinants of subclinical diabetic heart disease. Diabetologia. 2005; 48: 394–402
- Andersen NH, Poulsen SH, Poulsen PL, Knudsen ST, Helleberg K, Hansen KW, et al. Effects of blood pressure lowering and metabolic control on systolic left ventricular function in Type II diabetes mellitus. Clin Sci (Lond) 2006; 111: 53–9
- Chung J, Abraszewski P, Yu X, Liu W, Krainik AJ, Ashford M, et al. Paradoxical increase in ventricular torsion and systolic torsion rate in type I diabetic patients under tight glycemic control. J Am Coll Cardiol. 2006; 47: 384–90
- Stoylen A, Heimdal A, Bjornstad K, Torp HG, Skjaerpe T. Strain rate imaging by ultrasound in the diagnosis of regional dysfunction of the left ventricle. Echocardiography. 1999; 16: 321–9
- Stoylen A, Skjaerpe T. Systolic long axis function of the left ventricle. Global and regional information. Scand Cardiovasc J. 2003; 37: 253–8
- Andersen NH, Poulsen SH. Evaluation of the longitudinal contraction of the left ventricle in normal subjects by Doppler tissue tracking and strain rate. J Am Soc Echocardiogr. 2003; 16: 716–23
- Fornander Y, Nilsson B, Egerlid R, Wandt B. Left ventricular longitudinal relaxation velocity: A sensitive index of diastolic function. Scand Cardiovasc J. 2004; 38: 33–8
- Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989; 2: 358–67
- Aepfelbacher FC, Yeon SB, Weinrauch LA, D'Elia J, Burger AJ. Improved glycemic control induces regression of left ventricular mass in patients with type 1 diabetes mellitus. Int J Cardiol. 2004; 94: 47–51
- Thuesen L, Christiansen JS, Schmitz O, Christensen NJ, Orskov H, Henningsen P. Increased myocardial contractility during intravenous insulin infusion in type 1 (insulin-dependent) diabetic patients: An echocardiographic study. Scand J Clin Lab Invest. 1988; 48: 275–84
- Norton GR, Candy G, Woodiwiss AJ. Aminoguanidine prevents the decreased myocardial compliance produced by streptozotocin-induced diabetes mellitus in rats. Circulation. 1996; 93: 1905–12
- Bidasee KR, Zhang Y, Shao CH, Wang M, Patel KP, Dincer UD, et al. Diabetes increases formation of advanced glycation end products on Sarco(endo)plasmic reticulum Ca2 + -ATPase. Diabetes. 2004; 53: 463–73
- Andersen NH, Poulsen SH, Eiskjaer H, Poulsen PL, Mogensen CE. Decreased left ventricular longitudinal contraction in normotensive and normoalbuminuric patients with Type II diabetes mellitus: A Doppler tissue tracking and strain rate echocardiography study. Clin Sci (Lond) 2003; 105: 59–66
- Marwick TH. Measurement of strain and strain rate by echocardiography: Ready for prime time?. J Am Coll Cardiol. 2006; 47: 1313–27
- Vinereanu D, Khokhar A, Fraser AG. Reproducibility of pulsed wave tissue Doppler echocardiography. J Am Soc Echocardiogr. 1999; 12: 492–9