Abstract
Purpose. The aim of this study was to determine whether the combination of 2 angiogenic growth factor, vascular endothelial growth factor 165(VEGF165) and angiopoietin-1 (Ang1), could increase angiogenesis and cardiomyocyte(CM) proliferation in an infarcted myocardium. Methods. Myocardial ischemia was induced in rats by ligation of the left anterior descending (LAD) coronary artery. Replication-deficient adenoviruses encoding VEGF165 (Ad-VEGF165), Ang1 (Ad-Ang1) or enhanced green fluorescence protein (Ad-EGFP) was injected into the ischemic myocardium immediately. Bromodexyuridine (BrdU) was administered intraperitoneally 1 week after ligation. One week later, the hearts were harvested and sectioned for hematoxylin-eosin (HE) and immunohistochemistry to evaluate densities of capillary, arteriole and double labelled BrdU(+) CM. M-mode echocardiography was used to evaluate the cardiac function. Results. Ang1 significantly increased collateral vessel formation. Both VEGF165 and Ang1 significantly increased densities of capillary and arteriole, as well as the number of double labelled BrdU(+) CM, and improved cardiac function. Conclusion. Our results suggest that the combination of VEGF165 and Ang1 can increase both myocardial angiogenesis and CM proliferation following myocardial ischemia in rats, leading to improved cardiac function.
Gene therapy to promote angiogenesis involves delivering the cDNA for a selected angiogen, such as VEGF, to the target tissue. This strategy potentially allows prolonged, localized angiogen expression sufficient to induce angiogenesis and provides “biological revascularization” of ischemic tissue, without the potential toxicity associated with excessive or systemic angiogen exposure.VEGF receptors are localized almost exclusively to vascular endothelial cells (ECs), and this specificity has made VEGF an attractive therapeutic target for stimulating new blood vessel formation. However, areas of increased vascularization induced by VEGF overexpression also show signs of increased inflammation, including vascular leakage, edema, and increased adhesion of leukocytes. The small GTP-binding protein Rac is a key component in mediation of VEGF-induced vascular permeability Citation1. In contrast, overexpression of Ang1 results in a prominent enlargement of vessels without signs of inflammation Citation2. Moreover VEGF and Ang1 show additive effects on angiogenesis, and produce leakage-resistant vessels with little inflammation Citation3. We have previously shown that VEGF165, Ang1 and VEGF165 + Ang1 decrease the myocardial infarct size on the seventh day following infarction Citation4. VEGF165 gene delivery promotes arteriologenesis and CM viability in infarcted myocardium Citation5.The aim of this study was to determine whether combination of Ad-VEGF165 and Ad-Ang1 could increase angiogenesis and CM proliferation in the infarcted myocardium.
Materials and methods
Animal model
Male Sprague-Dawley rats (Jiangsu Provincial Experiment Animal Center) weighing 250–300 g were intraperitoneally anesthetized with chloral hydrate (36 mg/100 g). An anterior thoracotomy was performed to open the pericardium. The LAD coronary artery was ligated 5 mm away from the origin of the aorta with a 6-0 silk suture (WL Gore & Associates, Inc.). Positive end-expiratory pressure was applied to fully inflate the lungs before the muscle layer and skin were closed separately. The animals were allowed to recover. BrdU (50 mg/kg, ICN Co.) was administered intraperitoneally 1 week after ligation, as the marker for proliferation and regeneration of CMs. Heart specimens of experimental animals were immediately fixed with 10% formaldehyde (50 ml). The experimental protocol was approved by the University Committee on Animal Care of Nanjin Medical University.
Adenoviral vectors and amplification
Ad-VEGF165 and Ad-Ang1 were generated by homologous recombination Citation4. Gene expression was driven by a cytomegalovirus promoter/enhancer. Ad-EGFP was used as a control for gene transfection. Ad-VEGF, Ad-Ang1 and Ad-EGFP were transfected into human embryonic kidney (HEK) 293A at 80% confluent. After 2 days in culture, cells were harvested by Trypsine/EDTA and lysed by freeze-thawing three times. After spinning down at 1 000 rpm for 5 min, the supernatant containing the viral particle was loaded onto a cesium chloride gradient. Viral particle-containing bands were extracted and dialyzed against phosphate-buffered saline (PBS) overnight. Viral titers were determined with Tissure Culture Infectious Dose 50(TCID50) method.
Intramyocardial gene transfection
All animals were randomly divided into seven groups (n = 12–18): Normal (no operation), Sham surgery (pericardium open only, without ligation), ligation (L), L + VEGF165(L + V), L + Ang1(L + A), L + EGFP(L + E), L + VEGF165 + Ang1(L + V+A). Immediately after the ligation of the LAD, adenoviruses were injected intramuscularly at the left anterior free wall adjacent to the ligated area through a 27-gauge needle. After the left ventricle was accessed, the needle was advanced along the left ventricular free wall and viruses were injected over a period of 5–10 s. Ad-VEGF165, Ad-Ang1, or Ad-EGFP was injected at108-PFU/rat in 0.1 ml PBS.
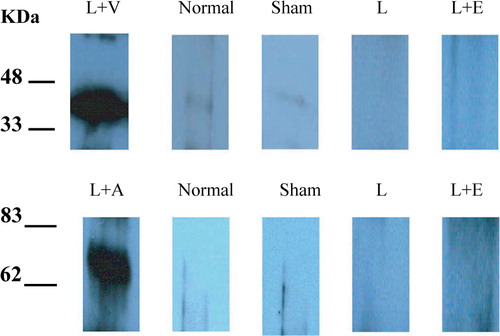
Expression of VEGF165 and Ang1 in rat hearts
Rat hearts were harvested 2 weeks after transfection. Heart tissues were dispersed in tris-buffered saline (TBS) and blood was washed out before lysing. Myocardial lysates were clarified by centrifugation at 12 000 g for 3 min before analyzing with 12.5% SDS-PAGE. Proteins were transferred to nitrocellulose membranes before blocking with 5% non-fat dried milk in TBS. Membranes were inmmunoblotted with VEGF165 and Ang1 antibodies (Neomarkers and R&D Systems, Inc.), and visualized by a chemiluminescence system (Amersham Biosciences) using horseradish peroxidase-conjugated anti-goat or anti-rabbit IgG (Sigma-Aldrich Corporation).
Left ventricle function
M-mode echocardiography (Philips Company, SONOS-5500) was used to evaluate the cardiac function of L + V+A and L groups at the day before surgery and the first week after surgery. Two-dimensionally guided M-mode recordings were obtained from the short axis view at the level of the chordae tendineae to inspect left ventricle systolic function using 5-12MHZ S-12 probe. Functional measures included diastolic left ventricular internal dimension (LVIDd), systolic left ventricular internal dimension (?LVIDs), diastolic left ventricular posterior wall dimension (?LVPWd), systolic left ventricular posterior wall dimension (?LVPWs), diastolic interventricular septal dimension (?IVSd), systolic interventricular septal dimension (?IVSs). Data was transferred on-line to a computer for subsequent analysis; the ejection fraction (EF), fractional shortening (FS) and thickening of IVSs and LVPWs (ΔT%) were also calculated.
Measurement of capillary and arteriolar density
Capillaries were categorized by their size (>40 µm; 20–40 µm; < 20 µm), a thin layer of endothelial cells and a lack of smooth muscle cells at a total magnification of 400x. Similarly, arterioles were identified and recognized by their size (>40 µm) and a thin layer of smooth muscle cells at a total magnification of 100×. Four non-overlapping random fields were selected from the infarcted area; images were captured and digitally stored.Counting was performed in a blind manner by two separate investigators.
HE and immunohistochemical staining
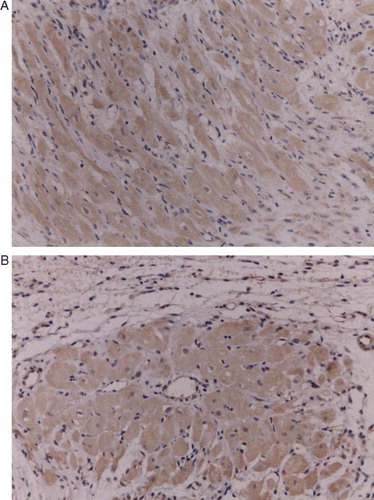
Two weeks after LAD ligation, the hearts were harvested, paraffin-embedded and sectioned (4 µm thick) for HE staining and immunohistochemical examination. Anti-myosin heavy chain (MHC) (Chemicon Co.) and anti-BrdU (Neomarkers Company) were used to stain BrdU(+) CMs. The total number of double-stained Brdu(+) CM is an indication of proliferation. C-Kit-receptor of stem cell factor (SCF), also called CD117, is commonly expressed on the surface of hematopoietic stem cells in marrow, particularly pluripotent HSC. Anti-c-Kit (ZYMED Co.) was used to examine the expression of c-Kit.
Statistical analysis
All results are expressed as mean±SD. Cell counts were analyzed by one-way analysis of variance (ANOVA); differences in left ventricle function were analyzed by a group t-test. A value of p < 0.05 was considered statistically significant.
Results
VEGF and Ang1 protein expression
Expression of VEGF and Ang1 protein were determined by Western blot analysis of protein extracts obtained from myocardium harvested at 2 week after ligation ().
Left ventricle function
EF value in the L group was reduced 13.72±1.85% 1 week after surgery compared with normal control, while that in L + V+A group was only decreased 5.95±2.14% compared with normal control. FS values in the L group was reduced 14.8±1.65% 1 week after surgery compared with normal control, while that in L + V+A group was only decreased 6.19±1.64% compared with normal control. All data suggested that combination of VEGF165 and Ang1 significantly improved cardiac function 1 week after LAD ligation by 56%. The values of ΔT% of IVSs in both groups were significantly lower than normal 1 week after surgery (, A,B & C)
Figure 2. A,B & C. M-mode echocardiography (Philips Company, SONOS-5500) was used to evaluate the cardiac function of L + V+A and L groups at the day before surgery (normal, A) and the first week (L + V+A, B; L, C). Two-dimensionally guided M-mode recordings were obtained from the short axis view at the level of the chordae tendineae to inspect left ventricle systolic function using a 5-12MHZ S-12 probe.
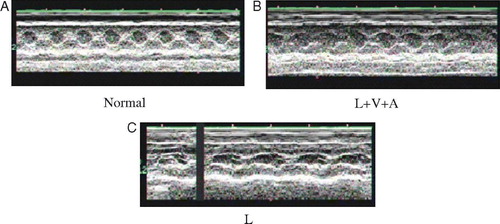
Table I. Measurements of left ventricular function and structure
Capillary diameter and quantitative measurement of capillary density
Both VEGF165 and Ang1 and their combination significantly increased the number of capillaries in the infarcted area after 2 weeks. The combination of VEGF165 and Ang1 was statistically greater than all groups except for VEGF165 alone. More important, the number of capillaries (20–40 µm) was significantly elevated following the combination of VEGF165 and Ang1 or Ang1 separately (, A,B,C & D)
Figure 3. A,B,C,D,E & F. Capillaries of L + V+A and L groups were identified and recognized by their size (<20 µm, A,B; 20–40 µm, C,D), a thin layer of endothelial cells and a lack of smooth muscle cells at a total magnification of 400×. Similarly, arterioles were identified and recognized by their size (>40 µm) and a thin layer of smooth muscle cells at a total magnification of 100×(E,F).
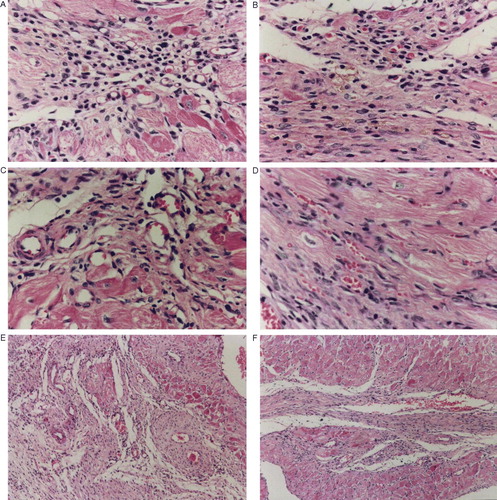
Table II. Capillary and arteriolar density
The combination of VEGF165 and Ang1 induced the production of significantly more arterioles in the infarct area than all groups after 2 weeks (, E & F).
BrdU (+) CMs
CMs positive for BrdU, a marker of cellular proliferation, were present in all experimental groups. These actively proliferating cardiac cells were mostly detected in the zone of the infarct, possibly suggesting an ongoing process of active regeneration of the myocardium after infarction. Viable tissue quantitation indicated that the combination of VEGF165 and Ang1 caused a significant improvement in myocardial viability at the second week (, A & B).
Figure 4. A,B. Immunohistological double staining for BrdU(+) cell nuclei and β-MHC(400×): ellipse type, intense red cytoplast (AEC), nucleus with cavity forms, karyotheca and cell membrane should be complete, nucleolus of BrdU(+) nuclear submits dark purple color (NBT), dyed deeply, and are located l in the center of nuclear.
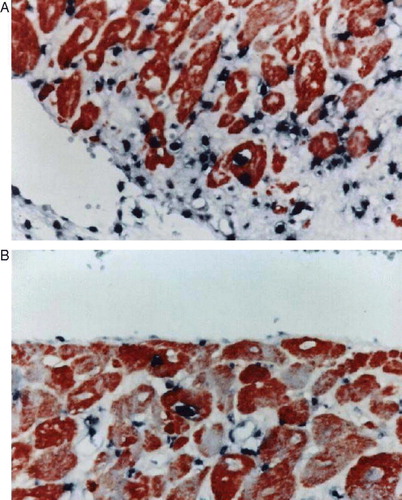
Table III. Cells regenerated at second week
Discussion
Induction of new blood vessel formation by gene transfer remains a very attractive clinical possibility for the treatment of ischemia in tissues not susceptible to conventional revascularization procedures. Co-expression of VEGF165 with Ang1, reported by Arsic and coworkers, did not display synergistic angiogenic effects but remarkably reduced leakage of vessels produced by VEGF165 alone Citation6. Nevertheless, Shyu and coworkers reported synergistic effects of Ang1 and VEGF on neoangiogenesis in a hypercholesterolemic rabbit model with acute hind limb ischemia Citation7. Our results suggest that the combination of VEGF and Ang1 gene therapy can increase both myocardial angiogenesis and CM proliferation following myocardial ischemia in rats, leading to improved cardiac function.
The mechanism underlying this synergistic response is unclear. However, Ang1 reduces VEGF-stimulated leukocyte adhesion to ECs by reducing ICAM-1, VCAM-1, and E-selectin expression Citation8. The combination of VEGF with Ang1 reduces levels of interleukin-6, a marker of inflammation, further than VEGF alone Citation7. More importantly, Ang1 also inhibits EC apoptosis via the Akt/surviving pathway Citation9.
BrdU(+) CMs and expression of c-Kit in the infarcted area indicate that vasculogenesis possibly plays the important role in this synergistic response. Vasculogenesis is the process of blood vessel formation that occurs during embryonic development and involves the differentiation of angioblasts into blood islands that fuse to form the primitive vascular network Citation10. These angioblast-like cell precursors, which are derived from the bone marrow, can circulate in the adult vasculature and contribute to physiological and pathological neovascularization in the adult Citation11. These circulating stem cell precursors are able to invade tissues and differentiate into several cell types after ischemic damage of the heart or of the skeletal muscle, and might constitute a reservoir for tissue and vessel regeneration of important therapeutic value Citation12. Very little is currently known about the actual molecules triggering recruitment, activation and differentiation of these cells.
As evidenced of CMs regeneration, BrdU(+) CMs have another interesting meaning. Traditionally, CMs are regarded as terminally differentiated cells, which are associated with their permanent withdrawal from the cell cycle with loss of their capacity for renewal Citation13. Where did BrdU(+) CMs come from? Expression of c-Kit in infarcted area has shown that mobilization of the marrow stem cells by VEGF165 and Ang1 possibly plays important role in these mechanisms. Both hematopoietic stem cells (HSC) and mesenchymal stem cells (MSC) exist in adult marrow, as do the angioblast or endothelial progenitor cells (EPC) that caused vasculogenesis during the embryonic period Citation14. EPC and HSC have VEGF receptors and Ang1 as marker (KDR, Tie2) Citation15, Citation16. Under certain circumstances, such as in ischemic cardiomyocytes or trauma, adult marrow stem cells could be mobilized to peripheral blood Citation17. Hattori and coworkers have demonstrated that the elevation of VEGF165 and/or Ang-1 plasma levels by Ad vectors results in robust mobilization of CEPs and hematopoietic progenitors and HSCs to the peripheral circulation. Although VEGF165 alone could also induce splenomegaly and increase BM cellularity, the sustained elevation of both VEGF165 and Ang1 was essential to induce significant remodeling of BM vascular architecture, with concomitant to the extramedullary organs, resulting in profound splenomegaly. Elevation of Ang1 alone did not affect the size of the spleen or cellularity of the BM Citation18. Marrow stem cells could differentiate into ECs, smooth muscle cells (SMCs) and CMs-like cells in the infarcted myocardium Citation19.
Our results also showed that the combination of VEGF and Ang1 gene therapy increased CMs proliferation in the myocardium following myocardial ischemia. The ability to repair or regenerate ischemic or damaged myocardium presents a major challenge in the treatment of cardiovascular disease. This has seen an explosion of interest in the past decade – firstly, to harness the potential of cell transplantation technology to repair damaged myocardium, and secondly, to find mechanisms of coaxing endogenous CMs to re-enter the cell cycle. Recently, there have been breakthroughs in both areas of research. These include the identification and characterization of bone marrow-derived adult somatic stem cells as potential exogenous cellular sources of both CMs and new blood vessels, and the discovery that in certain circumstances CM proliferation can occur Citation13, Citation20, Citation21. The source of replicating CM proliferation is uncertain, but they may arise from primitive stem cells, whose origin (cardiac or non-cardiac) is unclear.
The clinical use of bone marrow-derived mesenchymal stem cells to regenerate lost CMs appears to be most advantageous. However, cell transplantation strategies to replace lost myocardium are limited by the inability to deliver large numbers of cells that resist peritransplantation graft cell death and differentiate into CMs. Treatment of myocardial ischemia with bone marrow-derived mesenchymal stem cells overexpressing hepatocyte growth factor or Akt would be a novel strategy that can both restore local blood flow and regenerate lost CMs Citation22, Citation23.
Our studies indicated that the angiogenic growth factors, VEGF165 and Ang-1, could improve the cardiac function in rats with myocardial infarction. However, newly formed vessels were mainly capillaries, which have unclear ability to improve blood perfusion. The values of ΔT% of IVSs in L + V+A and L groups were not statistically different during the first week, both values being significantly lower than normal. This shows that blood perfusion in the ischemic area had not improved. Zhou and coworkers reported that VEGF165 and Ang1 could decrease myocardial infarct size, which was related to the activation of myocardial PI-3K and Bcl-2 pathways Citation4, both of which play roles in antiapoptosis and cell survivals.
In summary, enhancing the cardiac function following myocardial infarction in rats through using VEGF and Ang1 may have therapeutic potential for human heart disease. Our study suggests that latent mechanisms include: promoting angiogenesis and vasculogenesis, mobilizing stem cells homing to ischemic area and differentiating into CMs and direct protection of CMs in the infarct area.
The protective effects of these two angiogenic growth factors on myocardium may have clinical potential, especially combination therapy. Recently, the results of human trials only with VEGF gene therapy in patients with ischemic heart disease have been disappointing Citation24. It has made people realize the importance of combination gene therapy. However, there are still two weak points in our research that we want to address in future efforts: firstly, we did not explore the exact mechanism and cellular signal pathways in this synergistic effects; secondly, as echocardiographic measurements indicate, the rats only had moderate infarct size 1 week after surgery, and we have to carry out further study of the cardiac function at other time points in the future.
References
- Eriksson A, Cao R, Roy J, Tritsaris K, Wahlestedt C, Dissing S, et al. Small GTP-binding protein Rac is an essential mediator of vascular endothelial growth factor-induced endothelial fenestrations and vascular permeability. Circulation. 2003; 107: 1532–8
- Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999; 286: 2511–4
- Thurston G. Complementary actions of VEGF and angiopoietin-1 on blood vessel growth and leakage. J Anat. 2002; 200: 575–80
- Zhou, L, Ma, W, Yang, Z, Zhang, F, Lu, L, Ding, Z, et al. VEGF165 and angiopoietin-1 decreased myocardium infarct size through phosphatidylinositol-3 kinase and Bcl-2 pathways. Gene Ther. 2005;12:196–202. Erratum in: Gene Ther. 2005; 12:552
- Ferrarini, M, Arsic, N, Recchia, FA, Zentilin, L, Zacchigna, S, Xu, X, et al. Adeno-associated virus-mediated transduction of VEGF165 improves cardiac tissue viability and functional recovery after permanent coronary occlusion in conscious dogs. Circ Res 2006;98:954–61, Epub 2006 Mar.
- Arsic N, Zentilin L, Zacchigna S, Santoro D, Stanta G, Salvi A, et al. Induction of functional neovascularization by combined VEGF and angiopoietin-1 gene transfer using AAV vectors. Mol Ther. 2003; 7: 450–9
- Shyu KG, Chang H, Isner J M. Synergistic effect of angiopoietin-1 and vascular endothelial growth factor on neoangiogenesis in hypercholesterolemic rabbit model with acute hindlimb ischemia. Life Sci. 2003; 73: 563–79
- Kim I, Moon SO, Park SK, Chae SW, Koh GY. Angiopoietin-1 reduces VEGF-stimulated leukocyte adhesion to endothelial cells by reducing ICAM-1, VCAM-1, and E-selectin expression. Circ Res. 2001; 89: 477–9
- Papapetropoulos A, Fulton D, Mahboubi K, Kalb RG, O'Connor DS, Li F, et al. Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/surviving pathway. J Biol Chem. 2000; 275: 9102–5
- Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitor. Nature. 2000; 408: 92–6
- Rabbany SY, Heissig B, Hattori K, Rafii S. Molecular pathways regulating mobilization of marrow-derived stem cells for tissue revasculariation. Trends Mol Med. 2003; 9: 109–17
- Orlic D. Adult bone marrow stem cells regenerate myocardium in ischemic heart disease. Ann NY Acad Sci. 2003; 996: 152–7
- Hierlihy AM, Seale P, Lobe CG, Rudnicki MA, Megeney L A. The post-natal heart contains a myocardial stem cell population. FEBS Lett. 2002; 530: 239–43
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997; 275: 964–7
- Ziegler BL, Valtieri M, Porada GA, De Maria R, Muller R, Masella B, et al. KDR receptor: A key marker defining hematopoietic stem cells. Science. 1999; 285: 1553–8
- Yuasa H, Takakura N, Shimomura T, Suenobu S, Yamada T, Nagayama H, et al. Analysis of human TIE2 function on hematopoietic stem cells in umbilical cord blood. Biochem Biophys Res Commun. 2002; 298: 731–7
- Moore, MA, Hattori, K, Heissig, B, Shieh, JH, Dias, S, Crystal, RG, et al. Mobilization of endothelial and hematopoietic stem and progenitor cells by adenovector-mediated elevation of serum levels of SDF-1, VEGF, and Angiopoietin-1. Ann NY Acad Sci. 2001;938:36–45; Discussion 45–7.
- Hattori K, Dias S, Heissig B, Hackett NR, Lyden D, Tateno M, et al. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J Exp Med. 2001; 193: 1005–14
- Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infracted myocardium. Pediatr Transplant. 2003; 7(Suppl 3)86–8
- Deb A, Wang S, Skelding KA, Miller D, Simper D, Caplice N M. Bone marrow-derived cardiomyocytes are present in adult human heart: A study of gender-mismatched bone marrow transplantation patients. Circulation. 2003; 107: 1247–9
- Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001; 344: 1750–7
- Duan HF, Wu CT, Wu DL, Lu Y, Liu HJ, Ha XQ, et al. Treatment of myocardial ischemia with bone marrow-derived mesenchymal stem cells overexpressing hepatocyte growth factor. Mol Ther. 2003; 8: 467–74
- Mangi, AA, Noiseux, N, Kong, D, He, H, Rezvani, M, Ingwall, JS, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infracted hearts, Nat Med. 2003;9:1195–201. Epub 2003 Aug 10.
- Stewart, DJ, Hilton, JD, Arnold, JM, Gregoire, J, Rivard, A, Archer, SL, et al. Angiogenic gene therapy in patients with nonrevascularizable ischemic heart disease: A phase 2 randomized, controlled trial of AdVEGF(121) (AdVEGF121) versus maximum medical treatment, Gene Ther 2006;13:1503–11. Epub 2006 Jun 22.