Abstract
Objective. To study whether and how a lipophilic and a hydrophilic β-adrenoceptor antagonist affects ventricular fibrillation (VF) after coronary artery occlusion in a rabbit model with high sympathetic and low cardiac vagal activation. Design. Rabbits were treated for 3 weeks (series 1) or 2 hours (series 2) with metoprolol, atenolol or control vehicle. Finally the animals in series 1 were exposed to coronary artery occlusion. Heart rate response to cholinergic blockade was studied in series 2. Results. The incidence of postocclusion VF in metoprolol animals was lower (p<0.05) than that in atenolol or control animals. The two β-blockers caused similar reductions of heart rate, arterial pressure and myocardial ischemia. However, metoprolol animals had more respiratory sinus arrhythmia higher baroreflex sensitivity and more pronounced tachycardic response to cholinergic blockade than atenolol animals. Conclusion. Metoprolol reduced the incidence of VF by a better maintained discharge than atenolol in efferent cardiac vagal nerves, possibly due to inhibition of central nervous β1 adrenoceptors modulating vagal nervous outflow.
Several large clinical trials have shown that long-term administration of certain β-adrenoceptor blocking drugs improves survival after myocardial infarction Citation1. The results suggest that an important contributing factor for the mortality reduction is prevention of ventricular fibrillation (VF), the most frequent cause of sudden cardiac death Citation2. The mechanisms involved in prevention of VF by β-blockers are unclear. It is generally believed that a major factor is cardiac β-blockade counteracting the electrical instability and ischemia in the heart Citation3. An alternative view has been presented by Skinner et al. Citation4, Citation5 based on results obtained in studies on stress-related VF in conscious pigs. These authors suggest a major role for β-adrenoceptor blockade within the central nervous system leading to reduced electrical instability of the ischemic myocardium by altered autonomic neural activity from the brain to the heart.
Three factors have been shown to augment the electrical instability of the heart and predispose to VF: 1) myocardial ischemia, 2) high sympathetic and 3) low vagal activation of the heart Citation6. Interestingly, a similar autonomic nerve discharge pattern characterizes the “defense reaction”, which is expressed in many psychosocial stress situations believed to predispose to VF Citation7.
The present study aimed at studying whether and how β-blockade affects VF after coronary artery occlusion in a rabbit model with high cardiac sympathetic tone and low cardiac vagal tone obtained by anesthesia with α-chloralose Citation8. Two β1 selective antagonists, metoprolol and atenolol, were chosen because they have about the same potency and degree of β1 selectivity Citation9, but differ as regards lipophilicity and blood-brain barrier passage Citation10. Two series of experiments were carried out. The first series comprised pretreatment with the β-blockers for 3 weeks. In the terminal experiment a coronary artery was occluded, degree of myocardial ischemia, changes of arterial pressure, heart rate, baroreceptor sensitivity and respiratory sinus arrhythmia (RSA) were estimated and related to occurrence of VF. In the second series the β-blockers were given for 2½ hours under maintained chloralose anesthesia and the changes of efferent cardiac vagal activity were estimated from the heart rate response to cholinergic blockade.
Material and methods
Series 1
Male New Zealand white rabbits (2.8–3.7 kg) were treated for 3 weeks with atenolol (0.3 mg/(kg×h)), metoprolol tartrate (0.4 mg/(kg×h)) or saline vehicle (controls) by subcutaneously implanted osmotic minipumps (Alzet 2ML4, Palo Alto, California, USA). In the terminal experiment anesthesia was induced by i.v. injection of methohexital sodium (20 mg/kg, Brietal, Lilly, Indianapolis, Indiana, USA) followed by α-chloralose (Aldrich, Steinheim, West Germany) as i.v. maintenance anesthesia (initial dose 100 mg/kg over 10 min followed by 40 mg/(kg×h). The body temperature was kept at 38±0.4°C by an automatic thermoregulator connected to a waterheated operating table. The trachea was cannulated and the animals were mechanically ventilated with 70% room air and 30% oxygen (tidal volume 20±2 ml, 30 inflations/minute). Blood gases and blood pH were kept in the normal range by means of tidal air adjustments and bicarbonate administration. The intratracheal pressure was recorded with a Statham P23 ID transducer (Gould Inc, Glen Burnie, Maryland, USA). After thoracotomy a positive end expiratory pressure of 3 mm Hg was applied to prevent occurrence of pulmonary athelectases. Catheters were placed in the abdominal aorta and the caval vein via the right femoral vessels to allow blood pressure measurement (Statham, P23 ID, Gould Inc, Glen Burnie, Maryland, USA) and blood sampling, respectively. An intravenous infusion of Ringer solution (5 ml/(kg×h)) was given throughout the experiment.
Subcutaneous needle electrodes were applied for ECG recordings of chest leads corresponding to CM1, CM3, CM5 and CM7 (chest manubrium leads). A leftsided thoracotomy was performed at the 4th intercostal space. The pericardium was incised and a fine silk thread attached to a curved reverse cutting micropoint needle was placed around the circumflex coronary artery below the point where it emerged from under the left atrial appendix Citation11. Both ends of the cord were passed through a 10 cm long plastic tubing, which enabled later artery occlusion from the outside. The thoracic incision was then closed. Heparin was given (100 U/kg i.v.) and a 60 min stabilization period followed.
Determination of area at risk
Immediately after the death of the animal (VF or i.v. injection of saturated KCl) the heart and the first part of the ascending aorta were removed. The aorta was tied to a perfusion system of the Langendorff type. The coronary artery system was perfused with 50 ml of 0.1% solution of fluorescein in physiological saline at a pressure of 100 mm Hg. This was followed by perfusion of 20 ml of 10% formaldehyde. The atria and the right ventricle were removed and the left ventricle was cut perpendicular to the base-apex axis into six slices. During inspection in ultraviolet light the slices were cut along the sharp margins between fluorescein-perfused myocardium and regions which had been eliminated from perfusion by the previous coronary artery occlusion. The weight of the nonperfused segments was expressed as per cent of the weight of the whole left ventricle, and the obtained value indicated the area at risk.
Determinations of β-blocker levels in plasma and CSF
For the determination of the plasma level of metoprolol or atenolol, 2 ml blood was taken into a heparinized tube. The tube was immediately centrifuged and the plasma stored at −20°C until analysis. Since it was unfeasible to take simultaneous samples of cerebrospinal fluid (CSF) and blood in the instrumented animals, such samplings were made in an additional group of 20 anesthetized rabbits pretreated with either β-blocker for 3 weeks as in series 1. CSF samples (0.5 ml) were obtained by cisterna magna puncture. The analysis of metoprolol was made according to Ervik et al. Citation12 and that of atenolol by a technique involving liquid chromatography and fluorescence detection.
Determinations of catecholamines
Arterial samples (1 ml) for analysis of catecholamine levels were taken into glass tubes containing EGTA and glutathione. The samples were centrifuged immediately and the plasma kept frozen at −70°C until analysis. The concentrations of norepinephrine and epinephrine were determined by liquid chromatography Citation13.
Inclusion criteria
In the third week of treatment the plasma level of metoprolol or atenolol was determined. Animals with plasma levels within a predetermined range (metoprolol 100–400 nM/l and atenolol 800–3200 nM/l) were included in the terminal experiment.
The terminal experiment was carried out on 45 rabbits, nine of which were excluded from the final analysis (see below). All experiments on rabbits treated with metoprolol or atenolol were blinded and pairwise randomized. Before the study code was broken, the myocardial area at risk was determined and formed the basis for the decision to include the experiment into the final analysis. Six of the control animals were studied in a blind and randomized manner paralell to the β-blocker treated rabbits. The remaining controls were non-blinded. Experiments carried out before the start of this study indicated that untreated animals with a myocardial area at risk <20% do not fibrillate, while a high incidence of VF occur in animals with a larger area at risk. It was therefore predetermined to exclude animals with area at risk<20% from final analysis. Eight animals with an area at risk of 7–16% were excluded (3 controls, 3 metoprolol and 2 atenolol). None of these fibrillated. One additional atenolol animal was excluded because the area at risk evaluation indicated septal involvement. This rabbit, which died in VF, also showed disturbances of atrioventricular conduction.
The final analysis comprised 12 rabbits in each of the three treatment groups.
Experimental protocol
During the initial 30 min period before occlusion variables were determined by computerized evaluation of recordings from a 30 s period every 5 min. The values recorded in the first two and the last (7th) periods were averaged for computerized determination of the pre-occlusion levels of arterial pressure, heart rate, heart rate variability and ECG configuration. In the third and fourth periods a rapid i.v. injection of phenylephrine was given for evaluation of baroceptor sensitivity. Immediately after the fifth period arterial blood samples were taken for analysis of plasma concentrations of Na+ and K+, norepinephrine and epinephrine. Blood gases and pH were analyzed and kept within normal range.
After abrupt occlusion of the circumflex coronary artery the hemodynamic variables were fed to the computer by 30 s recordings collected every minute for 15 min, followed by recordings every 5 min until 30 min after the coronary occlusion or until VF occurred.
Data analysis
ECG and pressure variables were recorded on a Grass polygraph (Model 7PCM12, Quincy, Massachusetts, USA). As indicated above, 30 s sequences of the signals were analyzed by a computerized system Citation14 for a detailed evaluation of ECG, arterial pressure, heart rate and of respiratory sinus arrhythmia (RSA, i.e. artificial ventilation arrhythmia). The sampling frequency was 500 Hz.
RSA was measured by determining the R-R interval variability per respiratory cycle (s.d. R-R). The pre-occlusion value for s.d. R-R was based on the average of 45 respiratory cycles in each animal. The corresponding 5 min post-occlusion value was based on the mean of 15 respiratory cycles.
The baroreflex sensitivity was determined from the beat-beat changes of systolic arterial pressure and R-R interval following rapid i.v. injection of phenylephrine (2 or 4 µg/kg). The peak increase of systolic pressure occurred 8–12 s after the phenylephrine injection, the peak increase of R-R intervals was observed with a delay of 2–3 beats. The correlation between systolic pressures and R-R intervals (2 or 3 beats) recorded in the 20–25 beats from the onset to the peak of the pressor effect of phenylephrine was determined. In all experiments the correlation coefficient showed a p-value <0.05, and the slope of the regression line was taken to represent baroreceptor reflex sensitivity in terms of ms/mm Hg.
The evaluation of ECG:s was based on a computerized average of all normal sinus beats occurring in a 30 s sampling period. In each ECG lead the ST20 (ST level 20 ms after end of the QRS complex) was determined. All arrhythmias were visually evaluated.
Series 2
In 24 anesthetized rabbits either metoprolol, atenolol or control vehicle was given by i.v. infusion. Anesthesia was induced with methohexital sodium (20 mg/kg i.v.) and maintained with alpha-chloralose (70 mg/kg during 10 min followed by 20 mg/(kg×h)). I.v. injections of vecuron bromide (0.1 mg/kg) were given 30 min prior to each of the three recordings (before, 1 and 2 hours, respectively, after start of β-blocker infusion).
The preparation was the same as in series 1, except for thoracotomy and coronary artery dissection. Positive expiratory pressure was not applied. Like in series 1, recordings were made of ECG, arterial pressure, heart rate, RSA and intratracheal pressure. Every 5 min recordings over 30 s were sampled by the computer system for effect valuations.
The protocol included initial control recordings for 20 min followed by administration of either metoprolol tartrate (0.3 mg/(kg×h) constant i.v. infusion), atenolol (the same dosage as for metoprolol) or control vehicle. The drugs were studied in randomized experiments. After 2 h infusion, i.v. injections of methscopolamine bromide were given (initial dose 0.1 mg/kg, followed by 0.2, 0.4 and 0.8 mg/kg every 5 min). The effects of methscopolamine were recorded every minute.
Arterial blood samples for analysis of metoprolol or atenolol were taken 2½ h after start of infusion. The β-blocker level in CSF after 2½ h was also determined.
Statistical analysis
The comparisons with respect to the occurrence of VF were performed by Fisher's exact test. All other comparisons in series 1 and 2 and test of correlation coefficients were carried out by Student's t-test. Two-sided tests were used. All data are reported as the means±sem. A statistically significant difference was based on a p-value < 0.05.
Results
Series 1
In the third week of treatment plasma levels of metoprolol and atenolol were 262±23 nM/l and 1440±235 nM/l, respectively. No β-blocker could be detected in control animals.
Before coronary artery occlusion
a) Hemodynamics. and show the pre-occlusion values of heart rate and mean arterial pressure. The heart rate in controls was 267±8 beats/min. Metoprolol and atenolol reduced heart rate to the same extent (about 40 beats/min). The mean arterial pressure was 77±4 mm Hg in controls, and about 15 mm Hg less in the β-blocker treated animals. Plasma concentrations of norepinephrine and epinephrine () were similar in the three groups. Plasma potassium levels were close to 3.0 mM/l with no significant difference between the three groups ().
Figure 1. Rabbits, chloralose anesthesia. Series 1. Pre-occlusion levels of heart rate, mean arterial pressure and s.d. of R-R intervals per respiratory cycle.
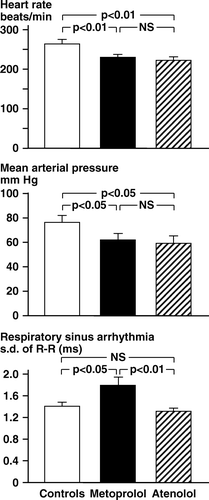
Table I. Rabbits anesthetized with chloralose – Series 1.
Table II. Rabbits anesthetized with chloralose – Series 1.
b) Respiratory sinus arrhythmia (RSA). The animals expressed RSA; the R-R intervals reached a nadir during lung inflation (higher heart rate) and a peak during deflation (lower heart rate).The pre-occlusion s.d. R-R in metoprolol rabbits was higher (p < 0.05) than the values recorded in the atenolol and the control groups ( and ). The concomitantly recorded peak intratracheal inflation pressures were similar in the three groups ().
c) Baroreflex sensitivity. The phenylephrine injection increased arterial pressure 22±2 mm Hg after the lower, and 33±2 mm Hg after the higher dose (no difference between the three groups). The pressor response to phenylephrine was associated with heart rate reduction, allowing determination of the baroreceptor reflex sensitivity. After 4 µg/kg phenylephrine there was a higher sensitivity in the metoprolol group than in the atenolol animals (p < 0.05, ). A corresponding difference was observed after 2 µg/kg phenylephrine.
d) After coronary occlusion. The occlusion of the circumflex coronary artery resulted in similar areas at risk, estimated to 37±3, 35±3 and 33±3% of the left ventricular mass in the control, metoprolol and atenolol groups, respectively. All included animals showed transmural ischemia confined to the free wall of the left ventricle.
e) Hemodynamics and respiratory sinus arrhythmia. As shown in the heart rate and mean arterial pressure levels observed 5 min after occlusion differed only slightly from pre-occlusion values. The arterial pulse pressure was, however, reduced by 10–15% in all groups (p < 0.05). After the occlusion the RSA showed a statistically significant decrease in the control and atenolol groups (), while no corresponding change occurred in the metoprolol group. Thus, the differential RSA observed before occlusion in metoprolol animals and the other two groups was augmented 5 min after occlusion. Except for RSA, there were no significant differences between the metoprolol and the atenolol groups as regards postocclusion hemodynamic variables.
f) Electrocardiographic changes. Coronary artery occlusion was consistently followed by ST elevation in all precordial ECG leads, most pronounced in lead CM3. The ST elevation was apparent within 1 min and grew in magnitude in the following minutes. shows the mean increase of ST20 observed in the four leads 5 min after occlusion at which point no VF had occurred. The mean ST20 elevation in the control group was 0.51±0.07 mV, whereas in both β-blocker groups the increase of ST20 was approximately 50% less (p < 0.05). Values for the metoprolol and atenolol groups were not significantly different.
g) Arrhythmias. Essentially all animals had regular sinus rhythm during the 30 min period after coronary occlusion until a lethal VF occurred. After the coronary artery occlusion ventricular extrasystolic beats (VES) occurred in 16 of the 36 rabbits. The incidence of VES was not significantly different in the three treatment groups, nor was it discriminating for later occurrence of VF. The occurrence of VES showed a distinct time distribution in two periods, first in the initial 2 min after occlusion, and then from the 6th to the 15th min. In the latter time period 23 of the 36 rabbits died in VF. No instance of spontaneous reversal of VF was observed. In 18 of the 25 fibrillating animals VF was initiated by an early VES during regular sinus rhythm. In the remaining seven fibrillating animals, the final VF was preceeded by short runs of ventricular tachycardia or by several multifocal VES.
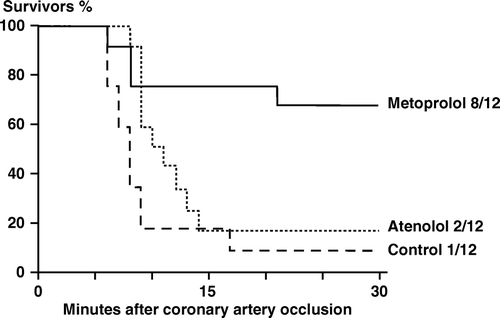
h) Survival rate. shows the percentage survivors in the three groups 30 min after coronary occlusion. Almost all control and atenolol animals died in VF between the 6th and the 15th minute. In metoprolol animals the incidence of VF was less, and eight of 12 animals (67%) survived the 30 min period. The corresponding survival rates were 17% (2 of 12) in the atenolol group and 8% (1 of 12) in the control group. The difference in survival between the metoprolol animals and the other two groups was statistically significant (p < 0.05).
Figure 2. Rabbits, chloralose anesthesia. Series 1. Rabbits (percent) surviving in the three treatment groups during the 30-minute period after coronary artery occlusion. The difference in survival between the metoprolol animals and the other two groups was statistically significant (p < 0.05).
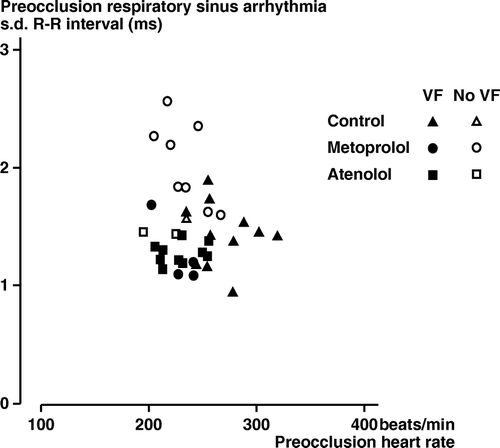
i) Occurrence of VF as related to other variables. shows individual pre-occlusion values for heart rate and s.d.R-R. An inverse correlation was found between the pre-occlusion s.d. R-R and subsequent VF (s.d. R-R in 11 non-VF 1.9±0.12 ms, in 25 VF 1.3±0.04 ms, p < 0.001). Furthermore, pre-occlusion s.d. R-R in the metoprolol rabbits without VF (2.0±0.13 ms) was higher (p < 0.01) than in the metoprolol animals with VF (1.3±0.14 ms). No significant correlations were found between pre-occlusion heart rate, baroreceptor reflex sensitivity or arterial pressure variables and subsequent VF.
Figure 3. Rabbits, chloralose anesthesia. Series 1. Pre-occlusion values for heart rate and s.d. R-R per respiration in controls (Δ), metoprolol (○) and atenolol rabbits (□). Filled symbols: Animals, which fibrillated after the subsequent coronary artery occlusion. Open symbols: Animals without VF after the coronary artery occlusion.
Five minutes postocclusion there was a significant correlation between subsequent non-VF (n = 11) and VF (n = 25) as regards postocclusion values for s.d. R-R (1.8±0.19 ms vs.1.2±0.04 ms, p < 0.001). No such correlation was found for area at risk (34±3% in non-VF vs.35±2% in VF). Again postocclusion s.d. R-R in the non-fibrillating metoprolol animals (2.0±0.22 ms) was significantly higher (p < 0.01) than in the fibrillating metoprolol rabbits (1.3±0.08 ms).
Series 2
The plasma levels of metoprolol and atenolol were 340±50 nM/l and 1900±380 nM/l, after 2½ h infusion, respectively.
Heart rate reductions elicited by metoprolol and atenolol were similar (), and apparent already after 5 min. Mean arterial pressure was not significantly changed by the β-blockers. During the 2 h period of atenolol or control vehicle infusions s.d. R-R was not significantly changed (). In the metoprolol animals, however, there occurred a delayed increase of s.d. R-R, which was statistically significant after 2 h of metoprolol infusion. The peak intratracheal pressure during lung inflation was similar in the three groups (). The injection of methscopolamine 2 h after the start of β-blocker infusion elicited an increased heart rate and a concomitant decrease of s.d. R-R in all animals (peak effect usually occurring after 0.2 or 0.4 mg/kg). The peak heart rate response to the muscarinic receptor antagonist was higher in the metoprolol-treated rabbits than in those given atenolol (33±6 vs. 16±4 beats/minute, p < 0.05). The simultaneous reduction of s.d. R-R was more pronounced in the metoprolol than the atenolol group (0.99±0.10 vs. 0.50±0.19 ms, p < 0.05).
Table III. Rabbits anesthetized with chloralose – Series 2.
β-blocker concentrations in cerebrospinal fluid, relation to plasma levels
In some rabbits given short term (2½ h) or long term (3 weeks) administration of metoprolol or atenolol simultaneous samples were taken of arterial blood and CSF for determination of β-blocker concentrations. The metoprolol level in CSF was close to that in plasma both in the short term (250±29 vs. 310±62 nM/l, n = 5) and in the long term study (270±30 vs. 260±21 nM/l, n = 10). The CSF concentration of atenolol was, however, only 5–10% of the plasma drug level (short term study; 75±22 vs. 1760±300 nM/l, n = 6; long term study: 160±4 vs. 1750±280 nM/l, n = 10).
Discussion
In the rabbit model – with high sympathetic and low cardiac vagal activation Citation15 –metoprolol reduced the incidence of VF after coronary artery occlusion. The preventive effect of metoprolol was apparently associated with favourable effects on three major risk factors for VF: compared to control metoprolol reduced myocardial ischemia, decreased sympathetic activation of the heart and maintained cardiac vagal activity. Atenolol also reduced myocardial ischemia and cardiac sympathetic activation but was ineffective as regards prevention of VF. The efferent cardiac vagal activity was, however, significantly lower in rabbits given atenolol.
Metoprolol and atenolol are β1 adrenoceptor antagonists with a β1/β2 adrenoceptor affinity ratio of about 20:1 Citation9. Results obtained in vitro Citation9 indicate that metoprolol and atenolol should elicit the same inhibition of β1 adrenoceptor mediated stimuli at a plasma level ratio of about 1:5. In the present experiments the plasma level ratio of metoprolol and atenolol was indeed 1:5–1:6. It was therefore expected that the two blockers elicited similar effects on heart rate and arterial pressure. The plasma levels of the two β-blockers attained in the present rabbit study are within the range observed in patients on long-term treatment with metoprolol or atenolol Citation16, Citation17.
Metoprolol and atenolol differ as regards distribution to the brain. Thus, the CSF levels of metoprolol corresponded to the plasma drug concentration, whereas those of atenolol were only 5–10% of the plasma drug level. These results are in general agreement with previous reports, indicating a lower blood-brain passage for the hydrophilic atenolol than for the more lipophilic metoprolol Citation10.
The coronary artery occlusion resulted in elimination of coronary perfusion in a transmural region of the left ventricular free wall, corresponding to about 35% of left ventricular mass in all three groups.
The ST segment elevation recorded by precordial skin electrodes in the early phase after coronary occlusion has been shown to reflect the degree of myocardial ischemia Citation18. The ST segment elevation in the metoprolol and atenolol experiments was similar, being only half as great as in controls. These findings indicate that the two β-blockers exerted the same anti-ischemic effect.
Thus, metoprolol and atenolol exerted similar effects on the heart rate, arterial pressure and myocardial ischemia development. The two β1 blockers elicited, however, markedly differential effects on respiratory sinus arrhythmia and prevention of VF.
Respiratory sinus arrhythmia is mainly caused by variations of the activity in efferent cardiac vagal nerves Citation19, and the RSA recorded in artificially ventilated animals also reflects the discharge rate in efferent cardiac vagal nerves Citation20. In the present study we compared RSA, as s.d. R-R per respiratory cycle, in rabbits treated with metoprolol or atenolol at constant ventilation rate, equal average levels of intratracheal pressure during inflation, heart rate and arterial pressure. In such conditions RSA can be expected to reflect the “tonic” activation of the efferent cardiac vagal nerves Citation23. The level of s.d. R-R was significantly higher in the rabbits given metoprolol than in those given atenolol both before and after the coronary occlusion. These results indicate that metoprolol animals had a higher level of efferent cardiac vagal activity than atenolol treated animals in spite of similar signs of myocardial ischemia. The significantly more marked increase of heart rate and decrease of s.d. R-R after cholinergic blockade in the metoprolol rabbits compared to the atenolol animals also support the conclusion that the efferent cardiac vagal activation was higher in the metoprolol group.
In general the baroreflex sensitivity was very low in the present experiments; it is well established that the “defense reaction” includes a bulbar inhibition of reflex bradycardia responses Citation7. The higher values for s.d. R-R and baroreflex sensitivity observed in the metoprolol animals may indicate that reflexes – with afferents in blood vessels and heart involving efferent cardiac vagal nerves – operated more efficiently than in atenolol rabbits both before and after coronary occlusion.
All in all, our results support the conclusion that the vagal activation of the heart was higher in metoprolol rabbits than in atenolol and control animals.
VF
In the present study 11 of 12 control rabbits developed VF after coronary occlusion. The site at which the circumflex coronary artery was occluded Citation11 causes ischemia of the left ventricular wall, moderate hemodynamic changes, maintained sinus rhythm with few VES, but a high incidence of VF within 15 min after the occlusion. The same effect pattern was observed in the present experiments.
During the last three decades both clinical and experimental studies indicate that ventricular fibrillation can be enhanced by sympathetic and antagonized by vagal activity Citation21. Psychosocial stress factors have been shown to serve as important triggers for lethal arrhytmias, both experimentally Citation22 and in real life catastrophies Citation23 and excessive sympathetic activation has been identified as a major precipitating factor Citation21. Vagal nerve activation seems to increase the electrical stability of the heart via pre- and postjunctional inhibition of the adrenergic activation and a prolongation of the refractory period in the ventricular muscle mediated by muscarinic receptors Citation27.
The importance of central nervous β-adrenergic mechanisms for triggering VF is suggested by results obtained in conscious pigs by Skinner et al. Citation4, Citation5. These authors showed that after acute coronary artery occlusion the incidence of VF was increased if the pig was exposed to a psychologically stressful environment. This increased cardiac vulnerability could be prevented by cryogenic blockade of the frontocortico-brainstem pathway involved in stress reactions or by l-propranolol (0.05 mg/kg) injected into the cerebral ventricles. It is of interest that the results of adrenoceptor binding studies have shown a relatively high density of β1 adrenoceptors in the frontal cortex Citation24.
All in all the present results indicate that the metoprolol-induced prevention of VF involved β-adrenoceptor blockade in the heart but also in CNS. The central nervous action of metoprolol was apparently of crucial importance for the VF prevention, and associated with a better maintained vagal activation.
The results of clinical prevention trials with β-blockers support the tenet that β-blockade in the central nervous system contributes to the reduction of VF. Thus, three lipophilic β-blockers, metoprolol, propranolol and timolol decrease the incidence of sudden death and total mortality in patients after myocardial infarction Citation2. This is in contrast to the hydrophilic β-blocker sotalol, which reduced the incidence of reinfarction but did not affect the rate of sudden death Citation25.
Two lipophilic β-blockers, bisoprolol and metoprolol, reduced total mortality and sudden death in patients with heart failure Citation26, Citation27. In patients with essential hypertension metoprolol reduced the incidence of total mortality and sudden death Citation28, while atenolol did not affect all-cause mortality, cardiovascular mortality or myocardial infarction incidence Citation29. Thus it should be of great interest, both concerning the pathophysiology and the therapy of ischemic heart disease, to know the results of a direct clinical comparison of a lipophilic and a hydrophilic β-blocker as regards prevention of sudden death.
Acknowledgements
We thank Britt-Marie Jacobson, Lars Johansson and Per-Olof Lagerström for excellent measurements of plasma concentration of catecholamines, atenolol and metoprolol. We are also very grateful to Anita Carlsson for dedicated secretarial assistance.
References
- Freemantle N, Cleland J, Young P, Mason J, Harrison J. β-blockade after myocardial infarction: Systematic review and meta regression analysis. Br Med J. 1999; 318: 1730–7
- Julian DG. Towards prevention of coronary death from ventricular fibrillation. Circulation. 1976; 54: 360–4
- Bigger JT, Coromilas J. How do β-blockers protect after myocardial infarction?. Ann Intern Med. 1984; 101: 256–8
- Skinner JE. Regulation of cardiac vulnerability by the cerebral defense system. J Am Coll Cardiol. 1985; 5: 88B–94B
- Parker GW, Michael LH, Hartley CJ, Skinner JE, Entman ML. Central β-adrenergic mechanisms may modulate ischemic ventricular fibrillation in pigs. Circ Res. 1990; 66: 259–70
- Lown B. Sudden cardiac death: Biobehavioral perspective. Circulation 1987; 76(Suppl)1: 186–96
- Folkow B. Physiology of behaviour and blood pressure regulation in animals. In: Julius S, Bassett DR Handbook of hypertension. Behavioural factors in hypertension Amsterdam: Elsevier;Vol 9. 1987.
- Åblad B, Bjurö T, Björkman J-A, Edström T, Olsson G. Role of central nervous β-adrenoceptors in the prevention of ventricular fibrillation through augmentation of cardiac vagal tone. J Am Coll Cardiol. 1991;17( Suppl):165. ( Abstract)
- Åblad B, Abrahamsson T, Hultberg E, Majcherczyk S, Nerme V, Sjölander M. Pharmakologische Grundlagen. In: β-Rezeptoren-blockade. Die klinische Bedeutung der β1-Selektivität. München: Ed FW Lohmann; 1984.
- van Zwieten PA, Timmermans PBMWM. Comparison between the acute hemodynamic effects and brain penetration of atenolol and metoprolol. J Cardiovasc Pharmacol. 1979; 1: 85–96
- Coker SJ. The anesthetised rabbit as a model for ischemia and reperfusion induced arrhythmias: Effects of quinidine and bretylium. J Pharmacol Methods. 1989; 21: 263–79
- Ervik M, Kylberg-Hanssen K, Johansson L. Determination of metoprolol in plasma and urine using high-resolution gas chromatography and electron-capture detection. J Chromatogr. 1986; 381: 168–74
- Eriksson BM, Persson BA. Determination of catecholamines in rat heart tissue and plasma samples by liquid chromatography with electrochemical detection. J Chromatogr. 1982; 288: 143–54
- Axenborg JE. BIOLAB – A computerized on-line system for physiological measurements in experimental animals. Computer Methods Prog Biomed. 1989; 28: 75–85
- Pettersson K, Bejne B, Björk B, Strawn WB, Bondjers G. Experimental sympathetic activation causes endothelial injury in the rabbit thoracic aorta via β1-adrenoceptor activation. Circ Res. 1990; 67: 1027–34
- Bengtsson C, Johnsson G, Regårdh CG. Plasma levels and effects of metoprolol on blood pressure and heart rate in hypertensive patients after an acute dose and between two doses during long-term treatment. Clin Pharmacol Ther. 1975; 17: 400–8
- Leonetti G, Terzoli L, Bianchini C, Sala C, Zanchetti A. Time-course of the anti-hypertensive action of atenolol: Comparison of response to first dose and to maintained oral administration. Eur J Clin Pharmacol. 1980; 18: 365–74
- Capone AJ, Most AS, Sydlik PA. Precordial ST segment mapping. A sensitive technique for the evaluation of myocardial injury?. Chest. 1975; 67: 577–82
- Eckberg DL. Human sinus arrhythmia as an index of vagal cardiac outflow. J Appl Physiol. 1983; 54: 961–6
- Potter EK. Inspiratory inhibition of vagal responses to baroreceptor and chemoreceptor stimuli in the dog. J Physiol. 1981; 316: 177–90
- Schwartz PJ. The autonomic nervous system and life threatening arrhythmias. The nervous control of the heart, JT Shephard, SF Vatner. Harwood Academic Publishers GmbH, Amsterdam 1996; 329–355
- Verrier RL, Lown B. Behavioural stress and cardiac arrhythmias. Ann Rev Physiol. 1984; 46: 155–76
- Rozanski A, Blumenthal J A, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999; 99: 2192–217
- Flügge G, Ahrens O, Fuchs E. β-adrenoceptors in the Tree Shrew Brain I. Distribution and characterization of [125I] Iodocyanopindolol Binding Sites. Cell Mol Neurobiol. 1997; 17: 401–15
- Julian DG, Prescott RJ, Jackson FS, Szekely P. Controlled trial of sotalol for one year after myocardial infarction. Lancet. 1982; 319: 1142–7
- CIBIS-II Investigators and Committees. The cardiac insufficiency bisoprolol study II (CIBIS II): A randomized trial. Lancet. 1999;353:9–13.
- MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL randomised intervention trial in congestive heart failure. Lancet. 1999;353:2001–7.
- Olsson G, Tuomilehto J, Berglund G, Elmfeldt D, Warnold I, Barber H, et al. Primary prevention of sudden cardiovascular death in hypertensive patients. Mortality results from the MAPHY study. Am J Hypertens. 1991; 4: 151–8
- Carlberg B, Samuelsson O, Lindholm LH. Atenolol in hypertension: Is it a wise choice?. Lancet. 2004; 364: 1684–9