Abstract
Objective. Oxygen-dependent changes in vascular diameters may be detrimental when the endothelium is dysfunctional. Design. Endothelial responsiveness was evaluated by brachial ultrasound and flow-mediated/nitroglycerin-mediated dilation (FMD/NMD). FMD/NMD was investigated in males with increased risk of cardiovascular disease (mean age 44±2 years, n =10) and matched controls without risk factors (44±2 years, n =10). FMD/NMD was assessed during normoxia (21% O2, 79% N2), while inhaling hypoxic gas (12.5% O2, FMDHyp/NMD), and 100% O2 supplementation (FMDO2/NMD). In a second study we addressed the effect of lipid lowering. Twenty persons with cardiovascular risk (mean age 50±2 years) were treated with atorvastatin (80 mg/day) and FMD/NMD was measured during normoxia, hypoxia and oxygen supplementation before, after 1 day and 3 months. Results. Oxygen supplementation evoked vasoconstriction, while FMDHyp/NMD was reduced compared to FMD/NMD. Atorvastatin significantly lowered total cholesterol, LDL cholesterol, and ADMA after 1 day of treatment, while triglycerides, ApoB and hsCRP were lowered after 3 months. Atorvastatin did not change FMD/NMD irrespective of oxygen tension. Conclusion. Irrespective of risk factors or atorvastatin, hypoxia reduced endothelial vasodilation while oxygen supplementation evoked vasoconstriction.
Oxygen is a powerful modulator of arterial diameter. Hypoxia causes vasodilation while hyperoxia leads to vasoconstriction in conductance arteries and in the microvasculature Citation1. Myocardial function may directly (by changes in myocardial blood flow) or indirectly (through alterations in preload and afterload) be affected by changes in oxygen tension. We have previously demonstrated that hypoxia dilates swine coronary arteries in vitro and alters smooth muscle metabolism in the coronary artery wall Citation2. In healthy volunteers we have recently reported that low oxygen tension increases tissue-Doppler peak strain in the left ventricle, while oxygen supplementation decreases peak strain indicating a poorer left ventricular performance Citation3.
Endothelial dysfunction is an early event in atherosclerosis characterised by a decreased synthesis of vasodilators during hypoxia Citation4. Both hypoxia and hyperoxia induce oxidative stress and formation of reactive oxygen species and oxidative stress decreases endothelium-dependent vascular responses Citation5. HMG-CoA reductase inhibitors (statins) reduce the risk of vascular disease in clinical trials and may reverse detrimental consequences of oxidative stresses on the endothelium and improve flow-mediated dilation (FMD) Citation5, Citation6. If the endothelium is involved in the vascular responses to changes in oxygen tension, persons with cardiovascular risk factors could have a reduced ability to counteract the circulatory consequences of systemic (e.g. sleep-disordered breathing, air line travel, high altitude stay) and regional (coronary artery stenosis) hypoxia and demonstrate increased vasoconstriction to hyperoxia. The clinical importance of this apparent contradiction between tissue hypoxia in cardiovascular emergencies and the goals of oxygen therapy remains to be settled.
The aim of the present study was two-fold: 1) To investigate how oxygen-dependent brachial artery diameter changes are related to endothelial function and cardiovascular risk; 2) to study the effect of aggressive lipid lowering therapy on endothelium-dependent and -independent vascular diameter changes to hypoxia and oxygen supplementation. We used brachial artery FMD to address the involvement of conductance artery endothelial function. We investigated persons with a high cardiovascular risk score and a high likelihood of endothelial dysfunction and matched controls with no significant cardiovascular risk and supposedly normal endothelial function.
Methods
Participants protocol 1
We studied 10 men (mean age 44±2 years) at risk for endothelial dysfunction. The subjects either had familial hypercholesterolemia or significant cardiovascular risk defined as a minimum of 5% risk of fatal cardiovascular disease in the next ten years in accordance with European 2003 Guidelines Citation7. The diagnosis of familiar hypercholesterolemia was based on clinical criteria (high LDL-cholesterol), xanthomas, family history of hypercholesterolemia and premature ischemic heart disease (IHD) or by genetic testing. Ten healthy men (mean age 44±2 years) without significant cardiovascular risk factors served as controls. Exclusion criteria in both groups were: known IHD, arrhythmia, heart valve disease, diabetes, liver disease, malignant or other severe concomitant disease and ongoing statin or phosphodiesterase type 5 inhibitor therapies. All participants were studied in the morning in fasting state. None were smokers.
Participants protocol 2
Twenty subjects (5 females, 15 males, mean age 50±2 years) with a high cardiovascular risk but no overt IHD, fulfilling criteria as the risk subjects above, were included. Seven of the risk subjects in protocol 1 also participated in protocol 2. Sequential examinations of premenopausal women were performed at the same time in menstrual cycle.
Ethics
All participants gave informed consent before the study and the protocols were approved by the local ethics committee.
Ventilation system
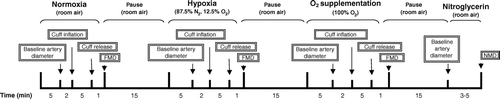
During the examination participants were in the supine position while breathing through a latex mask. A ready-mixed gas was delivered through a Siemens Elema 900C (Siemens Medical Solutions, Göteborg, Sweden) respirator that also measured tidal volume and respiration frequency. The peripheral arterial saturation and end tidal CO2 (PECO2) were measured by a Novametrix model 7100 capnograph (Carlsbad, California, USA). Normoxia was achieved by inhaling room air (21% O2, 79% N2). Hypoxia was achieved by inhalation of a mixture of 87.5% N2 and 12.5% O2, a mixture that corresponds to an altitude of 4000 meters above sea level. Oxygen was supplemented by inhalation of 100% O2.
When respiratory steady-state was achieved, an additional 5 minutes was awaited before brachial artery scanning during continuous inhalation of the gas. Respiratory steady state was defined as a constant arterial oxygen saturation (SaO2) and a PECO2 within an interval of 10%. Every minute during the examination respiratory rate, PECO2 and peripheral oxygen saturation was recorded. Each examination was performed using the sequence normoxia, hypoxia and oxygen supplementation. Fifteen minutes of room air breathing was allowed between normoxia and hypoxia and between hypoxia and oxygen supplementation.
Brachial ultrasound
The brachial ultrasound examination was performed with a Siemens Sonoline G50 vascular ultrasound apparatus (Siemens Medical Solutions, Erlangen, Germany) with a 10 MHz multifrequency linear probe at the lower part of the non-dominant upper arm during simultaneous ECG-monitoring. After 5 min at each respiratory steady-state baseline diameter was measured. FMD was induced by inflation for 5 min to 250 mmHg and then deflation of a sphygmanometer cuff around the forearm. After the last scan during oxygen supplementation the participant was allowed to breathe room air for 15 min and a final scanning sequence was performed before and 3–5 min after administration of 0.3 mg of nitroglycerin sublingually. Ultrasound images were recorded digitally and transferred to computer for off-line analysis.
Technicians blinded to the participants’ clinical status measured brachial artery diameters using commercially available software (Brachial Analyzer, Medical Imaging Applications, Iowa, USA). The arterial diameter was measured according to the R-wave in the ECG and measured as the distance between the two media layers. FMD was expressed as the actual change in diameter in millimeters (hyperemia diameter at 60 seconds–baseline diameter) and as percent change from baseline (100x[hyperemia diameter at 60 seconds–baseline diameter]/baseline diameter). FMD peaked at 60 s with no differences between risk subjects and controls (data not shown). The so-called FMD/NMD ratio Citation8, where NMD is nitroglycerin-mediated dilation, was also calculated. When we calculated FMD during different oxygen tensions and divided it by the NMD it represented ratios of absolute changes in flow-mediated diameter and nitroglycerin-mediated diameter. Because of slight but significant changes in baseline diameters during hypoxia and oxygen supplementation, FMD/NMD was not in complete accordance with the ratio defined by Mancini et al. Citation8. A requirement for the Mancini ratio is that baseline diameters for FMD and NMD are practically the same. Therefore, we found it prudent to designate the ratio FMDHyp/NMD during hypoxia and FMDO2/NMD during oxygen supplementation in order to emphasize that these ratios represent relative changes.
Blood sampling and analyses
All blood samples were collected from the left cubital vein after at least 10 hours of overnight fasting. Plasma lipids and lipoproteins (total cholesterol, HDL cholesterol and triglycerides) were measured by routine laboratory methods with LDL-cholesterol calculated by the Friedwald formula (LDL = total cholesterol – HDL – (triglycerides/2.2)). Apolipoprotein B (ApoB), lipoprotein (a) [Lp(a)] levels and hsCRP were determined as previously described Citation9. Concentrations of L-arginine, NG-NG-dimethyl-L-arginine (asymmetric dimethylarginine, ADMA), and NG-NG′-dimethyl-L-arginine (symmetric dimethylarginine, SDMA) were measured by high-perfomance liquid chromatography (HPLC, fluorescence detector) and precolumn derivation with o-pthaldialdehyde (OPA) according to previous descriptions Citation10. L-homoarginine was used as internal standard.
Design
The sequence of changes in inspired oxygen, FMD, and NMD for protocol 1 and each visit in protocol 2 is depicted in . In protocol 1 blood samples were drawn and brachial ultrasound examination was performed as described above. In protocol 2 all persons were examined before statin therapy with brachial ultrasound and blood samples. Subsequently subjects were treated with 80 mg of atorvastatin (generously supplied by Pfizer, Denmark) per day for three months. Brachial ultrasound and blood samples were repeated after 24 hours and after 3 months of statin therapy.
Statistical analysis
In a previous pilot study with 40 healthy volunteers we found a standard deviation on FMD of 3%. If we hypothesized a similar standard deviation in this study, a power of 0.80 and α = 0.05 our aim was to be able to detect a difference in FMD of 4.5%. A population of ten persons was needed in protocol 1 and 20 persons were needed in protocol 2 to detect this difference.
All data are presented as mean±SEM. Comparisons between groups (protocol 1) were made with an unpaired Students t-test. Respiratory variables during hypoxia and oxygen supplementation were compared using a paired Students t-test. Heart rate during hypoxia and oxygen supplementation was compared with normoxic values using a paired Students t-test. Baseline brachial artery diameters during normoxia, hypoxia, oxygen supplementation and after nitroglycerin administration were compared with a one-way repeated-measures analysis of variance (ANOVA). The Dunnets test was used post hoc to identify pairwise differences in comparison to normoxia. The effect of atorvastatin treatment on plasma lipids, lipoproteins and arginine metabolites were compared with a one-way repeated-measures ANOVA.
The role of group (significant risk versus controls) and normoxia, hypoxia and oxygen supplementation on FMD/NMD were evaluated by two-way repeated-measures ANOVA with Huynh-Feldt epsilon correction. Comparisons of the responses to changes in FMD/NMD at baseline, day 1 and after 3 months of atorvastatin during normoxia, hypoxia and oxygen supplementation were made with two-way repeated-measures ANOVA. Linear correlation analysis (Pearson) was used to test correlations between ultrasound measurements and coronary risk factors. Differences were considered statistically significant when p < 0.05 (two-sided).
Results
Baseline characteristics of the participants are listed in . The two groups in protocol 1 were similar with regard to age, height, body mass index, and systolic and diastolic blood pressure. The average weight of persons with significant cardiovascular risk was higher and so were values for total cholesterol, LDL cholesterol, and apolipoprotein B, while HDL cholesterol values were lower in risk subjects. Two subjects with significant cardiovascular risk received cardiovascular medication for hypertension; one subject was treated with amlodipine and one subject received enalapril.
Table I. Baseline data, protocol 1
There were no between-group differences in respiratory variables in protocol 1 and there were no significant differences between identical parameters at the three visits in protocol 2 (). During normoxia, hypoxia and oxygen supplementation there were no significant changes in respiratory rate and PECO2 but SaO2 was significantly lower during hypoxia compared to oxygen supplementation (both protocols). As in our previous study Citation3 heart rate increased with hypoxia (protocol 1 and 2) and decreased during oxygen supplementation (protocol 2).
Table II. Respiratory data and heart rate, protocol 1
Brachial artery reactivity
Cardiovascular risk subjects had a non-significant trend towards a higher brachial artery diameter at baseline compared to controls (4.98±0.17 mm vs. 4.56±0.11, p = 0.06). We therefore report brachial artery reactivity as the recently recommended FMD/NMD ratio, where NMD is nitroglycerin-mediated dilation, as this index is independent of baseline arterial diameter Citation8. In the brachial artery reactivity results are listed. Baseline diameters were significantly smaller during oxygen supplementation compared to normoxia and significantly bigger during nitroglycerin administration. Moreover, baseline nitroglycerin-mediated diameter was significantly bigger in cardiovascular risk subjects than in controls (protocol 1).
Table III. Brachial artery diameters, protocol 1
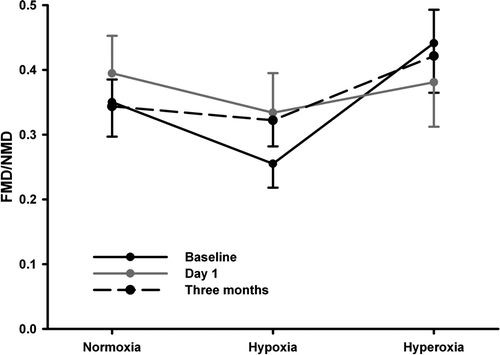
In protocol 1 oxygen tension (normoxia, hypoxia, or oxygen supplementation) significantly affected FMD/NMD (p = 0.03) and in post hoc testing FMDHyp/NMD was significantly lower than FMDO2/NMD (p = 0.01, ). In protocol 2 oxygen tension (normoxia, hypoxia, or oxygen supplementation) also significantly affected FMD/NMD (p = 0.04) while the post hoc test was only borderline significant (p = 0.06) for FMDHyp/NMD being lower than FMD/NMD (). Atorvastatin treatment was not associated with changes in FMD.
Figure 2. Brachial artery reactivity expressed as FMD/NMD in subjects with a high cardiovascular risk profile and in matched healthy controls without significant risk factors. Subjects were examined at normoxia, during hypoxia (inhalation of 87.5% N2 and 12.5% O2, FMDHyp/NMD) and during oxygen supplementation by inhaling 100% O2 (FMDO2/NMD). FMDHyp/NMD was significantly lower than FMD/NMD (*, p = 0.04).
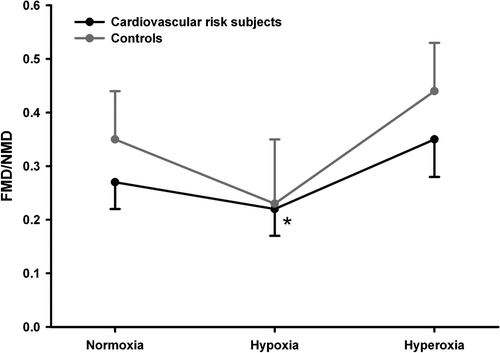
Figure 3. FMD/NMD in subjects with a high cardiovascular risk profile examined at normoxia, during hypoxia (FMDHyp/NMD) and during oxygen supplementation (FMDO2/NMD). Subjects were examined at three occasions: baseline, one day after initiation of therapy with atorvastatin 80 mg o.d. and after 3 months of atorvastatin therapy.
Effect of atorvastatin on plasma lipids and lipoproteins
The effect of atorvastatin on lipids and lipoproteins is shown in . Atorvastatin significantly lowered total cholesterol, LDL cholesterol, ADMA, SDMA and the ADMA/l-arginine ratio 1 day after the initial dose and after 3 months. Triglycerides, ApoB and hsCRP were lower after 3 months on atorvastatin. There were no correlations between FMD/NMD and biochemical markers.
Table IV. Effect of atorvastatin treatment on plasma lipids, lipoproteins and arginine metabolites, protocol 2
Discussion
The main findings of the present study were that hypoxia reduced endothelial responsiveness as expressed by FMDHyp/NMD while oxygen supplementation was a brachial vasoconstrictor at baseline. Atorvastatin 80 mg once daily improved biochemical markers of endothelial function but had no effect on FMD.
Cardiovascular disease and oxygen
Endothelium-dependent vasodilation is reduced in patients with IHD and such patients are believed to have a reduced ability to counteract the circulatory consequences of systemic hypoxia. We therefore found it relevant to investigate the effects of hypoxia and oxygen supplementation (hyperoxia) on brachial artery reactivity in subjects at risk for cardiovascular disease.
Patients with IHD are prone to experience variations in oxygen tension. A myocardial infarction or decompensated heart failure may be accompanied by local and generalized hypoxia and spontaneous nocturnal hypoxia with desaturation for hours is a frequent phenomenon in patients with severe coronary artery disease Citation11. Patients with coronary artery disease also respond differently to high altitudes than healthy persons. At 2 500 m above sea level there is a significant decrease in exercise-induced reserve in patients with coronary artery disease indicating that compensatory mechanisms might be exhausted even at moderate altitudes Citation12.
Both hypoxia and hyperoxia are oxidative stressors. Oxidative stress impairs endothelial function via an inactivation of nitric oxide (NO), a reduced expression of endothelial nitric oxide synthase (eNOS) and an increased amount of reactive oxygen species Citation13.
Hypoxia and vascular reactivity
In this study hypoxia did not dilate the brachial artery while FMDHyp/NMD was reduced in comparison to FMD/NMD. This contrasts our previous in vitro findings of hypoxic vasodilation in swine coronary arteries Citation2 and our findings from tissue-Doppler echocardiography in humans where hypoxia increased tissue-Doppler peak strain in the left ventricle Citation3. These apparent discrepancies could be explained by the fact that hypoxia activates sympathetic vasoconstriction and exerts more pronounced microvasculature effects in vivo. Moreover, we cannot exclude that hypoxia influences arterial diameter through central effects and chemoreceptors. However, our present findings are in line with previous studies of rat endothelial function after chronic intermittent hypoxia showing reduction in nitric oxide bioavailability and reduced vasodilation Citation14.
Oxygen supplementation and vascular reactivity
Oxygen supplementation is part of first-line treatment for patients with acute cardiovascular disease – a treatment that is regularly administered to many emergency patients whether they are hypoxic or not with a risk of inducing hyperoxia and less favourable outcome Citation15. Previously, we found that oxygen supplementation worsened systolic myocardial performance in healthy male volunteers while tissue Doppler measures of diastolic function were unaffected Citation3. This supports that changes in myocardial performance to oxygen administration are secondary to changes in vascular tone and this is in line with the brachial artery diameter reduction during oxygen administration before cuff application in the present study. Any likely contribution to increased vascular resistance during hyperoxia from the microvasculature could not be directly addressed by brachial artery ultrasound. Mak et al. Citation16 found that hyperoxia impaired acetylcholine-mediated increase in forearm blood flow and that this could be prevented by administration of vitamin C. We could not confirm that oxygen supplementation affected FMD/NMD but this could be related to our methodology. With the use of an occlusion cuff distal to the site of FMD measurement and 5 min of occlusion duration, FMD is primarily NO dependent Citation17 while venous occlusion strain gauge plethysmography, as used by Mak et al. Citation16, is confined to a more distal vascular bed where vasodilating prostaglandins are part of reactive hyperemia Citation18.
Statin therapy
As expected, atorvastatin lowered total cholesterol and LDL cholesterol. This effect was significant after the initial dose and a further reduction was seen after 3 months. There was no initial effect on triglycerides and ApoB but after 3 months of treatment both were significantly lowered. In line with previous findings Citation19 hsCRP was also lowered after 3 months of therapy. Our finding that ADMA was reduced after just one dose of atorvastatin was surprising. In other studies on hypercholesterolemic subjects atorvastatin Citation20, simvastatin Citation21, or pravastatin treatment Citation22 had no effect on ADMA levels. However, in one recent study Citation23 6 weeks of rosuvastatin treatment reduced ADMA in subjects with hypercholesterolemia to the same extent as found in our study. The statins are structurally different and further investigation should address whether the effect on ADMA plasma levels of statins is related to the structure of the statin and/or the maximal lipid lowering effect of the drug.
Previous studies have shown an improvement in FMD in patients with IHD after statin treatment Citation6. Statins may also have other so called pleiotropic effects and results from Parker et al. Citation24 indicate that different statins might have different effects on endothelial function. They examined the effect of 8 days of statin treatment in rat aortas and found that vascular relaxation was inhibited with simvastatin (sevenfold higher EC50 for acetylcholine induced relaxation) and atorvastatin (twofold increase) but not pravastatin. The authors conclude that under some conditions the direct action of lipophilic HMG-CoA reductase inhibitors may unbalance NO and O2- fluxes and promote oxidant stress, compromising potentially beneficial vascular effects of e-NOS upregulation Citation24. The results from this animal study are contrasted by a recent study comparing the effects of simvastatin and ezetimibe on FMD Citation25. Despite similar reductions of LDL cholesterol by simvastatin and ezetimibe, FMD was markedly improved after simvastatin but not after ezetimibe treatment Citation25. Comparable findings were seen in another recent study comparing 8 weeks of therapy with simvastatin (improvement) and pravastatin (no change) on FMD in patients with IHD Citation26.
Clinical implications
Although this study was conducted in cardiovascular risk patients and not in patients with overt IHD the findings highlight important consequences of tissue hypoxia and oxygen supplementation with possible consequences for such patients. At sea level, many patients with IHD have a partial pressure of oxygen in the arterial blood <95 mmHg and are on the steep portion of the oxyhemoglobin-dissociation curve Citation12. With a significant part of older-age people remaining active, deeds such as airline travel at normal cabin pressure or participation in altitude sports considerably increase the risk of hypoxia for IHD patients. Physical activity and hypoxia may lead to a decrease in coronary flow reserve Citation12 and our findings indicate that a hypoxia-induced decline in endothelial responsiveness may help to explain reduced coronary flow reserve in IHD patients that already suffer from endothelial dysfunction. The only randomized trial to study routine administration of oxygen in uncomplicated myocardial infarction found elevated markers of myocardial damage in the oxygen treatment group Citation15 and no evidence of benefit. Our findings of baseline vasoconstriction and our previous findings of deterioration of myocardial function with oxygen supplementation Citation3 are in accordance with this and further questions the uncritical use of oxygen therapy. So with Shakespeare (As You Like It, 1600): “can one desire too much of a good thing?” – the answer must be yes in case of oxygen.
Limitations
The brachial artery ultrasound FMD assessment is limited by inherent age-related and biological variation and lack of correlation with severity of specific disease states e.g. coronary artery disease. Because FMD is dependent on brachial artery diameter at baseline Citation8 we chose to report the FMD/NMD parameter also because it is diameter independent Citation8. Measurement of FMD has inter-and intraobserver variability. We tried to reduce this as all off line analyses were performed with state-of-the-art software by technicians blinded for subject identity. Repetitive reactive hyperemia, as in this study, has no effect on FMD Citation27. We chose to perform all experiments using the same sequence of normoxia-hypoxia-oxygen in order to familiarize the subjects with the study environment. We cannot exclude that some carry-over effect from hypoxia could have affected results of oxygen supplementation. We chose to measure blood pressure before but not during testing. In order to keep the study environment as quiet as possible and we found this justified from our previous demonstration that blood pressure is unaffected by the applied levels of hypoxia and hyperoxia Citation3. The lack of respiratory data during normoxia was due to the fact that only hypoxia and oxygen supplementation was carried out with the inhalation system and the accompanying monitoring devices. Another potential criticism is that we did not control CO2 levels. CO2 has a potent vasodilatory effect in the cerebral circulation but a negligible effect in heart and femoral vasculature Citation28. We cannot rule out that some of our findings were secondary to changes in ventilation although a statistically significant difference in PECO2 was only found at baseline in protocol 2 ().
The number of subjects in the present studies was relatively small and we did not introduce a placebo-arm in protocol 2 (statin treatment). We might have been over-optimistic to expect a difference in FMD of 4.5%. This was based on a few pilots and the scarce literature on FMD and oxygen Citation16. Roughly we found a 3% difference in FMD between hypoxia and normoxia with substantial inter-individual variation (0–8%). Furthermore, the study was not powered to detect differences in FMD/NMD.
Conclusion
We found that a low oxygen tension reduced endothelium-dependent vasodilatory capacity and that oxygen supplementation was a vasoconstrictor at baseline. While this is probably of no importance for healthy subjects at sea level it may be serious for people with IHD experiencing hypoxia or receiving oxygen therapy.
Acknowledgements
We wish to thank Keld Sørensen, MD and Bente Mortensen for invaluable help with brachial ultrasound methodology. The study was supported by the Danish Heart Foundation (no 03-1-2-12-22050 and 04-10-B93-A142-22182), the John and Birthe Meyer Foundation, Karen Elise Jensens Fond, the Novo Nordisk Foundation, and the Danish Medical Research Council.
References
- Waring WS, Thomson AJ, Adwani SH, Rosseel AJ, Potter JF, Webb DJ, et al. Cardiovascular effects of acute oxygen administration in healthy adults. J Cardiovasc Pharmacol. 2003; 42: 245–50
- Frøbert O, Bagger JP, Simonsen U, Lund S, Gravholt CH. Insulin increases glycolysis without further vasodilation in porcine coronary arteries exposed to hypoxia. Clin Sci (Lond) 2004; 107: 213–20
- Frøbert O, Moesgaard J, Toft E, Poulsen SH, Sogaard P. Influence of oxygen tension on myocardial performance. Evaluation by tissue Doppler imaging. Cardiovasc Ultrasound. 2004; 2: 22
- Barreto-Filho JA, Consolim-Colombo FM, Guerra-Riccio GM, Santos RD, Chacra AP, Lopes HF, et al. Hypercholesterolemia blunts forearm vasorelaxation and enhances the pressor response during acute systemic hypoxia. Arterioscler Thromb Vasc Biol. 2003; 23: 1660–6
- Vecchione C, Brandes RP. Withdrawal of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors elicits oxidative stress and induces endothelial dysfunction in mice. Circ Res. 2002; 91: 173–9
- Jarvisalo MJ, Toikka JO, Vasankari T, Mikkola J, Viikari JS, Hartiala JJ, et al. HMG CoA reductase inhibitors are related to improved systemic endothelial function in coronary artery disease. Atherosclerosis. 1999; 147: 237–42
- De Backer G, Ambrosioni E, Borch-Johnsen K, Brotons C, Cifkova R, Dallongeville J, et al. European guidelines on cardiovascular disease prevention in clinical practice. Third Joint Task Force of European and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J. 2003; 24: 1601–10
- Mancini GB. Reporting measures of endothelial dysfunction independent of baseline arterial diameter. Am J Cardiol. 2004; 94: 1222–3
- Madsen T, Christensen JH, Blom M, Schmidt EB. The effect of dietary n-3 fatty acids on serum concentrations of C-reactive protein: A dose-response study. Br J Nutr. 2003; 89: 517–22
- Boger RH, Bode-Boger SM, Szuba A, Tsao PS, Chan JR, Tangphao O, et al. Asymmetric dimethylarginine (ADMA): A novel risk factor for endothelial dysfunction: Its role in hypercholesterolemia. Circulation. 1998; 98: 1842–7
- Smith HL, Sapsford DJ, Delaney ME, Jones JG. The effect on the heart of hypoxaemia in patients with severe coronary artery disease. Anaesthesia. 1996; 51: 211–8
- Wyss CA, Koepfli P, Fretz G, Seebauer M, Schirlo C, Kaufmann PA. Influence of altitude exposure on coronary flow reserve. Circulation. 2003; 108: 1202–7
- Aikawa M, Sugiyama S, Hill CC, Voglic SJ, Rabkin E, Fukumoto Y, et al. Lipid lowering reduces oxidative stress and endothelial cell activation in rabbit atheroma. Circulation. 2002; 106: 1390–6
- Phillips SA, Olson EB, Morgan BJ, Lombard JH. Chronic intermittent hypoxia impairs endothelium-dependent dilation in rat cerebral and skeletal muscle resistance arteries. Am J Physiol Heart Circ Physiol. 2004; 286: H388–H393
- Rawles JM, Kenmure AC. Controlled trial of oxygen in uncomplicated myocardial infarction. Br Med J 1976; 1: 1121–3
- Mak S, Egri Z, Tanna G, Colman R, Newton GE. Vitamin C prevents hyperoxia-mediated vasoconstriction and impairment of endothelium-dependent vasodilation. Am J Physiol Heart Circ Physiol. 2002; 282: H2414–H2421
- Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: Implications for the assessment of endothelial function. J Physiol. 2005; 568: 357–69
- Engelke KA, Halliwill JR, Proctor DN, Dietz NM, Joyner MJ. Contribution of nitric oxide and prostaglandins to reactive hyperemia in human forearm. J Appl Physiol. 1996; 81: 1807–14
- Kent SM, Flaherty PJ, Coyle LC, Markwood TT, Taylor AJ. Effect of atorvastatin and pravastatin on serum C-reactive protein. Am Heart J 2003; 145: e8
- Shinohara K, Shoji T, Kimoto E, Yokoyama H, Fujiwara S, Hatsuda S, et al. Effect of atorvastatin on regional arterial stiffness in patients with type 2 diabetes mellitus. J Atheroscler Thromb. 2005; 12: 205–10
- Pereira EC, Bertolami MC, Faludi AA, Salem M, Bersch D, Abdalla DS. Effects of simvastatin and L-arginine on vasodilation, nitric oxide metabolites and endogenous NOS inhibitors in hypercholesterolemic subjects. Free Radic Res. 2003; 37: 529–36
- Eid HM, Eritsland J, Larsen J, Arnesen H, Seljeflot I. Increased levels of asymmetric dimethylarginine in populations at risk for atherosclerotic disease. Effects of pravastatin. Atherosclerosis. 2003; 166: 279–84
- Lu TM, Ding YA, Leu HB, Yin WH, Sheu WH, Chu KM. Effect of rosuvastatin on plasma levels of asymmetric dimethylarginine in patients with hypercholesterolemia. Am J Cardiol. 2004; 94: 157–61
- Parker RA, Huang Q, Tesfamariam B. Influence of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase inhibitors on endothelial nitric oxide synthase and the formation of oxidants in the vasculature. Atherosclerosis. 2003; 169: 19–29
- Landmesser U, Bahlmann F, Mueller M, Spiekermann S, Kirchhoff N, Schulz S, et al. Simvastatin versus ezetimibe: Pleiotropic and lipid-lowering effects on endothelial function in humans. Circulation. 2005; 111: 2356–63
- Ling MC, Ruddy TD, deKemp RA, Ukkonen H, Duchesne L, Higginson L, et al. Early effects of statin therapy on endothelial function and microvascular reactivity in patients with coronary artery disease. Am Heart J. 2005; 149: 1137
- Harris RA, Padilla J, Rink LD, Wallace JP. Variability of flow-mediated dilation measurements with repetitive reactive hyperemia. Vasc Med. 2006; 11: 1–6
- Ainslie PN, Ashmead JC, Ide K, Morgan BJ, Poulin MJ. Differential responses to CO2 and sympathetic stimulation in the cerebral and femoral circulations in humans. J Physiol. 2005; 566: 613–24