Abstract
Objectives. To investigate the protective effect of tumor necrosis factor receptor (TNFR) gene modified mesenchymal stem cells (MSCs) transplantation against inflammation and cardiac dysfunction following acute myocardial infarction (AMI). Design. MSCs were extracted from the tibias and femurs of rats and transfected with recombinant adeno-associated viral (rAAV) expressing EGFP (enhanced green fluorescent protein) or p75 (human 75 kilodalton) TNFR at multiplicity of infection of 105 particles/cell. Rats with AMI induced by occlusion of the left coronary artery were randomized to MSCs-TNFR transplantation group, MSCs-EGFP transplantation group and MI control group. Results. The effects of MSCs-TNFR transplantation on cardiac inflammation and left ventricular dysfunction were observed after 2 weeks of MI. We found that: 1) MSCs-TNFR transplantation attenuated protein production and gene expression of inflammatory cytokines TNF-α, IL-1β and IL-6; 2) MSCs-TNFR transplantation inhibited cardiomyocytes apoptosis and 3) MSCs-TNFR transplantation improved left ventricular function. Conclusions. The experimental data show that transplantation with rAAV-TNFR transfected MSCs improves left ventricular function following MI through anti-apoptotic and anti-inflammatory mechanisms.
Many researchers have investigated cell transplantation and considered it as an alternative treatment for heart disease Citation1–4. Mesenchymal stem cells (MSCs) are probably more suitable for cardiac cell therapy given their multipotency and immunomodulatory properties. While recent studies reported that MSCs prevent left ventricular remodeling of ischemic heart through paracrine signaling Citation5–7. MSCs combined with genetic modification may be a promising solution that could improve protective effect of MSCs on ischemic heart disease. Furthermore, MSCs may become an effective vehicle for gene therapy whose secreted gene products can exert paracrine or endocrine actions that may result in further therapeutic benefits. Tumor necrosis factor alpha (TNF-α) is an inflammatory mediator that is important in the pathophysiology of several cardiac diseases, including heart failure and ischemia-reperfusion injury Citation8, Citation9. Recent data indicate that inhibition TNF-α potentially render it biologically inactive and exhibit the beneficial effect in ischemic disease Citation10, Citation11. Meanwhile, recombinant adeno-associated virus(rAAV)-based gene therapy represents a promising approach for the treatment of heart diseases. Therefore, we transplanted MSCs transfected with rAAV-TNFR following myocardial infarction, and the objectives of our study were firstly to investigate whether MSCs-TNFR transplantation may decrease the production of gene and protein expression of some crucial inflammatory cytokines such as TNF-α, IL-1β and IL-6, and secondarily to study the protective effect of MSCs-TNFR transplantation against cardiac dysfunction.
Materials and methods
Animals
Healthy male Sprague-Dawley rats weighing 200–250 g were obtained from the experimental animal center of Wuhan University. This study was approved by the Ethic Committee of Wuhan University School of Medicine. All animals received humane care according to the Guide for the Care and Use of Laboratory Animals published by National Institute of Health (NIH publication NO. 85-23, revised 1996).
Isolation and culture of bone marrow stromal cells (MSCs)
After rats were anesthetized with 20% sodium urethane (1.0 g/kg ip injection), bone marrow cells were extracted from the tibias and femurs of rats and were suspended in Dulbecco modified Eagle medium (DMEM; Invitrogen, Paisley, UK) with 2% heat-inactivated fetal calf serum (FCS, GibcoBRL), penicillin G (100 U/ml), and streptomycin (100 µg/ml). Cells were then introduced into 25 cm2 flask (Corning, MA) and incubated with 95% air and 5% CO2 at 37°C. Medium was replaced every 4 days. Non-attached cells were discarded and adherent cells were retained. Each primary culture was replated to three new flasks when MSCs grow to approximately 70–80% confluency.
rAAV vector construction and MSCs transfection
MSCs were transduced with rAAV expressing EGFP or p75 (human 75 kilodalton) TNFR. Cytomegalovirus (CMV) rAAV-EGFP vector was kindly provided by Vector Gene Technology Company Limited and rAAV-TNFR:Fc vector was constructed as described previously Citation12. The effective viral titer was 4.0×1014 L−1. The medium was removed and MSCs were washed once with 1 ml of pre-warmed L-DMEM (DMEM supplemented with 2% FCS). Then cells were infected with rAAV at multiplicity of infection of 105 particles/cell in 200 µl serum-free Dulbecco modified Eagle medium at 37°C for 2 h, then cultured with normal medium for 24 h.
Myocardial infarction models preparation and MSCs implantation
Rats underwent myocardial ischemia by occlusion of the left coronary artery. Briefly, rats were anesthetized with 20% sodium urethane (1.0 g/kg ip injection). The chest was opened and the ribs were gently spread. The heart was quickly expressed out of the thoracic cavity. After opening the pericardium, a small plastic snare was threaded through the ligature and placed in contact with the heart. Ligation of the LAD was performed 1 to 2 mm distal to the line between the left border of the pulmonary conus and the right border of left atrial appendage. Then the heart was repositioned to the chest. MI was assessed by electrocardiograph. Experimental animals were randomized for MI control group (n = 12), MSCs-rAAV-EGFP group (n = 12) and MSCs-rAAV-TNFR group (n = 12). The transplantation was performed at 1 h after induction of MI. Multiple intramyocardial injections of 100 µl DMEM basal medium without cells (MI group) or containing 1×107 MSCs were carried out in the free wall of the left ventricle (MSCs-rAAV-EGFP group and MSCs-rAAV-TNFR group).
Echocardiographic study
After 2 weeks of coronary reperfusion, left ventricular function was assessed by echocardiography (8 MHz probe, GE, VIVID7, USA). Rats were sedated (100 mg/kg ketamine, 1.5 mg/kg xylazine) and transducer was applied to the left hemithorax. The heart was visualized by using 2-dimensional mode with the axial view of the left ventricle. Left ventricular end diastolic diameters (LVDd), left ventricular end-diastolic volumes (LVEDV), ejection fraction (EF) and fractional shortening (FS) were calculated using standard formulas, respectively.
Hemodynamic measurements
Hemodynamics was measured with lead 2000, B type, multichannel physiologic recorder (Jinjiang Tongyong Industry Limited Company, Sichuan, China) and blood samples were obtained. The cannula was inserted through the right carotid artery into the left ventricle to monitor the left ventricular end-systolic pressure (LVESP), left ventricular end-diastolic pressure (LVEDP) and left ventricular +dp/dt (maximum rate of pressure rise).
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL)
After 2 weeks of MSCs transplantation, parts of tissue samples in infarcted region were collected and embedded in paraffin, then cut into slices of 5 µm thick for TUNEL. TUNEL assay was performed according to the manufacturer's protocol (Roche Applied Science, Indianapolis, IN). From each section, ten light microscopic fields were used to identify TUNEL-positive cells. A total of 3 000 nuclei per section were examined by a blinded observer. Myocytes were considered to be TUNEL positive when the nuclei were identified staining dark brown.
Enzyme-Linked Immunosorbent Assay
The levels of serum TNF-α was determined using an ELISA kit (Quantikine, R & D Systems).
Werstern blot of TNF-α, IL-1β, IL-6, Bcl-2 and Bax protein
After 2 weeks of MSCs transplantation, tissue samples obtained from the infracted myocardial tissues were flash frozen in liquid nitrogen. Briefly, these samples were homogenized on ice in 0.1% Tween 20 homogenization buffer with a protease inhibitor. Samples containing 30 µg of total protein were subjected to electrophoresis using a 10% SDS–Tris–Glycine polyacrylamide gel (Invitrogen, San Diego, CA). Proteins were electrophoretically transferred to nitrocellulose membranes, then blocked with 5% defatted milk for 2 h at 37°C. The cytokines protein expressions were detected by using a 1:500 dilution of TNF-α, IL-1β, IL-6, Bcl-2 and Bax (mouse monoclonal, Santa cruz, California) antibody as the first antibody. A 1:5000 dilution of horseradishperoxidase-conjugated rabbit anti mouse IgG (Santa Cruz, California) were then used as the second antibody and developed with the enhanced chemiluminescence western blotting detection system (Pierce Company Product).
RNA isolation and semi-quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) analysis of TNF-α,IL-1β, IL-6 mRNA expression
Total RNA was reverse transcribed into cDNA after isolation using TRIzol reagent. The sense primer and the antisense primer in rat for TNF-α,IL-1β, IL-6 and GAPDH were as follows: TNF-α sense 5′-TGGCCCAGACCCTCACA-3′ and antisense 5′-TGCCCGGACTCCGTGAT-3′, IL-1β sense 5′-ATGGCAACTGTCCCTGAACTCAACT-3′ and antisense 5′-CAGGACAGGTATAGATTC AACCCCTT-3′, IL-6 sense 5′-CCAGTTGCC TTCTTGGGACTGATG-3′ and antisense 5′-ATTTTCTGACCACAGTGAGGA ATG-3′, GAPDH sense 5′-GAAACCTGCCAAGTATGA TG -3′ and antisense 5′- ACCAGGAAATGAGCTTCACA-3′. Each cycle consisted of denaturation for 40 seconds at 94°C, annealing for 40 s at 55°C, extension for 1 minute at 72°C, final extension for 5 min at 72°C, followed by 35 cycles of PCR amplification. Each PCR product was separated by electrophoresis on 1.5% agarose gel. The density of each PCR band was analyzed with a densitometer (ImageMaster VDS, Vilber Lourmat, Germa)
Statistical analysis
Data were presented as mean±SD. The multivariate ANOVA was used to determine the overall difference between the three independent groups. The statistical significance between groups was determined using a post hoc Bonferroni/Dunn test. A value of p<0.05 was considered to indicate statistical significance.
Results
Improvement of cardiac function
We analyzed parameters of echocardiography. EF, FS, LVESP as well as dp/dtmax were significantly improved by MSCs-EGFP and MSCs-TNFR group compared with MI group (p < 0.05). Meanwhile, LVDd, LVEDV and LVEDP were decreased (p < 0.05). MSCs-TNFR group further improved EF, FS, LVESP as well as dp/dtmax(p < 0.01), and dereased LVDd, LVEDV and LVEDP compared with MSCs-EGFP group (p < 0.05) in the volume 2, (Table 1).
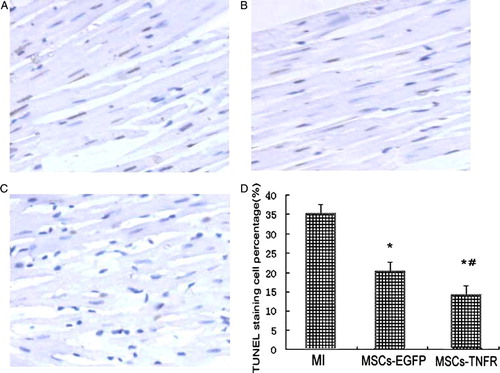
Decrease of apoptosis of cardiomyocytes
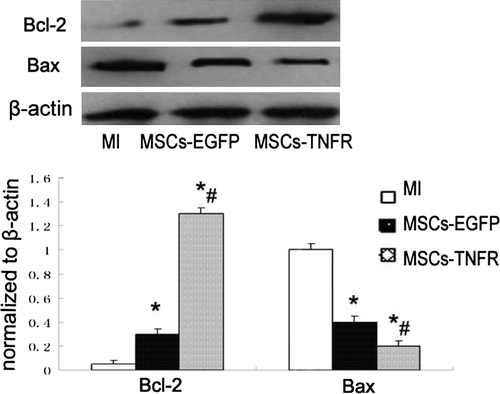
We investigated whether MSCs transfected with rAAV-TNFR:Fc has an anti-apoptotic role in myocardial cell injury with rat AMI. After rats was given with MSCs-rAAV-TNFR:Fc, the decrease of TUNEL-positive nuclei staining in the myocardium was clearly observed, by comparison with MI control group and MSCs-EGFP group (p<0.01). MSCs-EGFP group also reduced apoptosis compared with MI group (p < 0.05) (). By analysis of Western blot, the changes of upregulation of Bcl-2 and downregulation of Bax were examined in MSCs-TNFR group at 2 w ().
Figure 1. MSCs-TNFR prevents myocardial cell apoptosis in MI. Sectioned myocardium tissues from free wall treated with medium without cells (A) or MSCs-rAAV-EGFP (B) or MSCs-rAAV-TNFR:Fc (C) were analyzed by TUNEL staining. Magnification: ×400. (D) Quantitative analysis of TUNEL positively stained myocardial cells (%) was measured using the positive apoptotic nuclei staining in infarcted myocardial tissues. Results (n = 12) are presented as means±s.e.m. *p < 0.05 compared with MI control group; #p < 0.05 compared with MSCs-EGFP group.
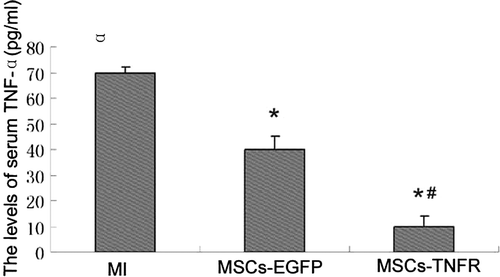
Attenuation of serum levels of TNF-α
Compared to MI control group, serum levels of TNF-α was significantly reduced after MSCs-rAAV-EGFP or MSCs-rAAV-TNFR:Fc transplantation (p<0.01). Furthermore, MSCs-TNFR group decreased serum levels of TNF-α compared with MSCs-EGFP group (p < 0.05) ().
Inhibition of protein production and gene expression of inflammation cytokines TNF-α, IL-1β and IL-6
We investigated the effects of MSCs transfected with rAAV-TNFR:Fc on TNF-α, IL-1β and IL-6 production in myocardial infarction. Cytoplasmic TNF-α, IL-1β and IL-6 mRNA isolated from the infracted area was analyzed by RT-PCR. The results showed that MSCs-EGFP and MSCs-TNFR group significantly reduced TNF-α, IL-1β and IL-6 mRNA expression (p < 0.05). MSCs-TNFR group further decreased these inflammatary cytokines expression compared with MSCs-EGFP group (p<0.01) (). Western blotting analysis also showed the similar fashion ().
Figure 4. Rt-PCR analyzed TNF-α,IL-1β and IL-6 gene expression from free wall of MI rats in each group. *p < 0.05 compared with MI control group; #p < 0.05 compared with MSCs-EGFP group.
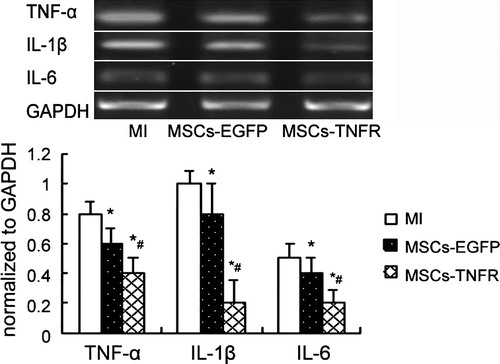
Figure 5. Western blot analyzed TNF-α,IL-1β and IL-6 protein production from free wall of MI rats in each group. *p < 0.05 compared with MI control group; #p < 0.05 compared with MSCs-EGFP group.
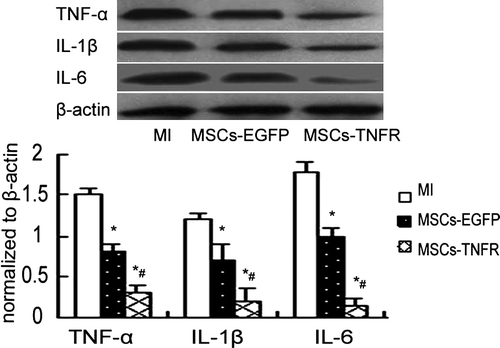
Table I. Cardiac function parameter and hemodynamics analysis.
Discussion
In this study, we focused on the therapeutic potential of MSCs transfected with rAAV-TNFR:Fc transplantation in the myocardial infarction. The main findings in our research included: 1) MSCs-TNFR transplantation attenuated protein production and gene expression of inflammatory cytokines TNF-α,IL-1β and IL-6; 2) MSCs-TNFR transplantation inhibited cardiomyocytes apoptosis and 3) MSCs-TNFR transplantation improved left ventricular function.
It has been demonstrated that cellular cardiomyoplasty (CCM) has been an alternative treatment for ischemic heart disease. Many researchers have investigated cell transplantation and considered it as an alternative treatment for heart disease. A variety of cell types have been proposed as useful candidates, such as fetal cardiomyocytes Citation1, Citation2, embryonic stem cells Citation3, skeletal muscle satellite cells, and so on Citation4. Among these, mesenchymal stem cells (MSCs) are probably more suitable for cardiac cell therapy given their multipotency and immunomodulatory properties.
Accumulating reports showed that the cardioprotective effects of MSCs may be mediated not only by their differentiation into cardiomyocyte-like cells and vascular cells, but also by their ability to supply large amounts of angiogenic, anti-apoptotic and mitogenic factors Citation5–7. Furthermore, recent several studies reported that no significant regeneration of cardiac myocytes was observed after MSC treated, while left ventricular function was improved Citation13, Citation14. These results indicated that MSCs prevent left ventricular remodeling of ischemic heart through paracrine signaling. Moreover, unknown numbers of newly regenerated cells may die by apoptosis during tissue remodeling and morphogenesis Citation15. MSCs combined with genetic modification may be a promising solution that could improve survival, proliferative capacity, or differentiation of the stem cells and so on. Furthermore, MSCs may become an effective vehicle for gene therapy whose secreted gene products can exert paracrine or endocrine actions that may result in further therapeutic benefits.
In our study, we observed that MSCs treatment substantially suppressed the production of inflammatory cytokines, such as TNF-α, IL-1β and IL-6, at both the transcriptional and post-transcriptional levels. Previous study demonstrated that inflammatory cytokines (TNF-α, IL-1β, IL-6 and so on) trigger the pathophysiological alterations characteristic of cardiac remodeling such as replacement of the infarcted area by scar, myocyte hypertrophy, switch to fetal gene programs, myocyte loss through apoptosis, alterations of the extracellular matrix and endothelial dysfunction Citation16. Therefore, anti-inflammation therapy may be a novel therapeutic intervention against cardiac remodeling.
TNF-α is an inflammatory mediator that is important in the pathophysiology of several cardiac diseases, including heart failure and ischemia–reperfusion injury Citation8, Citation9. After acute MI, TNF-α is released initially from ischemic cardiomyocytes and subsequently from activated leukocytes Citation17, Citation18. In addition, TNF-α induces apoptosis in cardiomyocytes Citation19. Some researchers reported that transgenic mice with cardiac-specific overexpression of TNF-α tend to present myocarditis, cardiomegaly, cardiac dysfunction, and congestive heart failure Citation20, Citation21. Recent data indicate that TNF inhibitor, soluble TNF-α receptor gene or Etanercept, a fusion protein of TNFR type II and the Fc portion of human IgG, specifically binds TNF-α, potentially rendering it biologically inactive Citation10, Citation11. More strategies aimed at recombinant adeno-associated virus (AAV) vector, which is one of the most promising delivery vehicles under development for gene therapy given its several advantages such as efficiently direct long-term transgene expression in multiple tissues, little or no inflammation or cellular immune response Citation22, Citation23. Therefore, we transplanted MSCs transfected with rAAV-TNFR and found gene expression and protein production of TNF-α and other inflammatory cytokines IL-1β and IL-6 were decreased significantly. IL-1β, IL-6 also plays key role in triggering the pathophysiological alterations characteristic of cardiac dysfunction Citation16. Therefore, anti-inflammation may be a novel therapeutic intervention against ischemic heart disease.
Taken together, these reports and our observation support the beneficial results of MSCs-rAAV-TNFR transplantation with their anti-inflammation and left ventricular function improvement potential following myocardial infarction.
Acknowledgements
We thank Jun Cai (Department of Cardiology, Chaoyang Hospital, Peking, China), Xi Wang, Ping Hu, Yanhong Tang and Hong-gang Chu (Department of Cardiology, Renmin Hospital, Wuhan University School of Medicine, Wuhan, China) for great assistance. This project was supported by fund from Department of Education of Hubei Province (T200606), Department of Science and Technology of Hubei Province (2005ABA0085).
References
- Li RK, Mickle DA, Weisel RD, Mohabeer MK, Zhang J, Rao V, et al. Natural history of fetal rat cardiomyocytes transplanted into adult rat myocardial scar tissue. Circulation. 1997;96(9 Suppl): II-179-86; Discussion 186–7.
- Scorsin M, Hagege A, Vilquin JT, Fiszman M, Marotte F, Samuel JL, et al. Comparison of the effects of fetal cardiomyocyte and skeletal myoblast transplantation on postinfarction left ventricular function. J Thorac Cardiovasc Surg. 2000; 119: 1169–75
- Min JY, Yang Y, Converso KL, Liu L, Huang Q, Morgan JP, Xiao YF. Transplantation of embryonic stem cells improves cardiac function in postinfarcted rats. J Appl Physiol. 2002; 92: 288–96
- Menasche P, Hagege AA, Scorsin M, Pouzet B, Desnos M, Duboc D, et al. Myoblast transplantation for heart failure. Lancet. 2001; 357: 279–80
- Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002; 105: 93–8
- Shake JG, Gruber PJ, Baumgartner WA, Senechal G, Meyers J, Redmond JM, et al. Mesenchymal stem cell implantation in a swine myocardial infarct model: Engraftment and functional effects. Ann Thorac Surg. 2002; 73: 1919–26
- Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003; 9: 1195–201
- Bryant D, Becker L, Richardson J, Shelton J, Franco F, Peshock R, et al. Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor-alpha. Circulation. 1998; 97: 1375–81
- Gurevitch J, Frolkis I, Yuhas Y, LifschitzMercer B, Berger E, Paz Y, et al. Anti-tumor necrosis factor-alpha improves myocardial recovery after ischemia and reperfusion. J Am Coll Cardiol. 1997; 30: 1554–61
- Kubota T, Bounoutas GS, Miyagishima M, Kadokami T, Sanders VJ, Bruton C, et al. Soluble tumor necrosis factor receptor abrogates myocardial inflammation but not hypertrophy in cytokine-induced cardiomyopathy. Circulation. 2000; 101: 2518–25
- Sugano M, Tsuchida K, Hata T, Makino N. In vivo transfer of soluble TNF-alpha receptor 1 gene improves cardiac function and reduces infarct size after myocardial infarction in rats. FASEB J. 2004; 18: 911–3
- Sandalon Z, Bruckheimer EM, Lustig KH, Rogers LC, Peluso RW, Burstein H. Secretionof a TNFR:Fc fusion protein following pulmonary administration of pseudotyped adeno-associated virus vectors. J Viro. 2004; 78: 12355–65
- Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. J Viro. 2006; 98: 1414–21
- Noiseux N, Gnecchi M, Lopez-Ilasaca M, Zhang L, Solomon SD, Deb A, et al. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther. 2006; 14: 840–50
- Geng YJ. Molecular mechanisms for cardiovascular stem cell apoptosis and growth in the hearts with atherosclerotic coronary disease and ischemic heart failure. Ann N Y Acad Sci. 2003; 1010: 687–97
- Ono K, Matsumori A, Shioi T, Furukawa Y, Sasayama S. Cytokine gene expression after myocardial infarction in rat hearts. Possible implication in left ventricular remodeling. Circulation. 1998; 98: 149–56
- Irwin MW, Mak S, Mann DL, Qu R, Penninger JM, Yan A, et al. Tissue expression and immunolocalization of tumor necrosis factor-alpha in postinfarction dysfunctional myocardium. Circulation. 1999; 99: 1492–8
- Akatsu T, Nakamura M, Satoh M, Hiramori K. Increased mRNA expression of tumour necrosis factor-α and its converting enzyme in circulating leucocytes of patients with acute myocardial infarction. Clin Sci (Lond) 2003; 105: 39–44
- Song W, Lu X, Feng Q. Tumor necrosis factor-alpha induces apoptosisvia inducible nitric oxide synthase in neonatal mouse cardiomyocytes. Cardiovasc Res. 2000; 45: 595–602
- Bryant D, Becker L, Richardson J, Shelton J, Franco F, Peshock R, et al. Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor-alpha. Circulation. 1998; 97: 1375–81
- Kubota T, McTiernan CF, Frye CS, Slawson SE, Lemster BH, Koretsky AP, et al. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-α. Circ Res. 1997; 81: 627–35
- Xiao X, Li J, Samulski RJ. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Viro. 1996; 70: 8098–108
- Zaiss AK, Liu Q, Bowen GP, Wong NC, Bartlett JS, Muruve DA. Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors. J Viro. 2002; 76: 4580–90