Abstract
Objectives. To investigate the mobilization of non-haematopoietic and haematopoietic cells from the bone marrow induced by intramyocardial VEGF gene therapy and G-CSF treatment alone or in combination in patients with chronic ischemic heart disease (IHD). Secondly, we tested the hypothesis that the quantity of circulating stem cells correlated with improvement in symptoms. Design. We treated I) 16 patients with intramyocardial placebo injections, II) 16 patients with intramyocardial VEGF-A165 gene injections, III) 13 patients with low dose G-CSF, and IV) 16 patients with intramyocardial VEGF-A165 gene followed by high dose G-CSF. Results. Circulating CD34+ cells and CD45 − CD34 − cells increased by G-CSF in a dose-dependent manner, but did not increase with VEGF gene therapy. The CD45 − /CD34− cells subgroups increased in both G-CSF treated groups. No association was found between the concentration of mobilized stem cells in the circulation and improvement in symptoms. Conclusions. G-CSF mobilized both haematopoietic and non-haematopoietic cells from the bone marrow in a dose-dependent manner in patients with chronic IHD. However, even high levels of circulating stem cells did not improve symptoms of IHD.
In recent clinical trials, vascular endothelial growth factor (VEGF) delivered as plasmid DNA percutaneously by a catheter-based intramyocardial approach, have been demonstrated to be safe and to be associated with a reduction in angina and an increase in exercise time or an improvement in regional wall motion in “no-option patients” with chronic myocardial ischemia Citation1, Citation2. These tendencies to a therapeutic benefit of VEGF gene transfer were initially considered to be due to angiogenesis, only. However, clinical data seem to suggest that VEGF gene transfer is accompanied by mobilization of bone marrow derived stem cell and regulates differentiation of the hemoangioblast into an endothelial cell lineage, thereby inducing vasculogenesis as well as angiogenesis in patients with coronary artery disease Citation3.
Bone marrow derived haematopoietic stem cells have the potential to differentiate into endothelial cells and create new blood vessels and could hereby be involved in myocardial vasculogenesis Citation4. Recently, several small clinical safety studies with mononuclear cell solutions (MNC) from the bone marrow have in some but not all studies suggested a beneficial effect on myocardial function and on symptoms in acute and chronic myocardial ischemia Citation5–7. However, it has been discussed whether this is “true” stem cell therapy, since only 2% of the injected cells are stem cells Citation6.
It is generally accepted that the multipotent mesenchymal stem cell (MSC) can be isolated from the bone marrow, expanded, and stimulated to differentiation into blood vessels, cardiac or other tissues Citation8. Based on results from animal studies, MSCs could potentially induce vasculogenesis in patients with myocardial ischemia and establish new contractile myocardium in patients with scar tissue and dilated cardiomyopathy Citation9, Citation10.
Mobilization of bone marrow stem cells by granulocyte colony stimulating factor (G-CSF) might facilitate regeneration of myocardial tissue and improve cardiac function after acute myocardial infarction (AMI) Citation11. We recently reported that mobilization of bone marrow stem cells by G-CSF alone or in combination with vascular endothelial growth factor gene therapy in patients with severe chronic ischemic heart disease improved clinical symptoms of myocardial ischemia. However, there was no improvement in perfusion Citation12, Citation13.
The primary aim of this study was to investigate the mobilization of non-haematopoietic (CD45 − /CD34 − ) and haematopoietic stem cells from the bone marrow, induced by local intramyocardial VEGF gene therapy and G-CSF treatment alone or in combination in patients with severe chronic occlusive coronary artery disease. Secondly, we aimed to test the hypothesis that the quantity of circulating stem cells correlated with improvement in symptoms.
Materials and methods
Patients and design
We studied four patient cohorts from three previously published trials: group I: 16 patients treated with intramyocardial placebo injections, group II: 16 patients treated with intramyocardial injections of 0.5 mg VEGF-A165 plasmid Citation2, group III: 13 patients treated with subcutaneous 5 µg/kg body weight G-CSF daily for 6 days (low dose G-CSF group) Citation12, and group IV: 16 patients treated with intramyocardial injection of 0.5 mg VEGF-A165 plasmid followed one week later by subcutaneous 10 µg/kg body weight G-CSF daily for 6 days (high dose G-CSF group) Citation13. All patients had severe chronic occlusive coronary artery disease. Inclusion criteria were identical in the three trials: Reversible ischemia on an adenosine stress single photon emission computerized tomography (SPECT), a coronary arteriography demonstrating at least one main coronary vessel from which new collaterals/vessels could be supplied, age above 18 years, Canadian Cardiovascular Society angina classification (CCS) ≥3. Excluded were patients with an ejection fraction <0.40, unstable angina pectoris, acute myocardial infarction within the last 3 months, diabetes mellitus with proliferative retinopathy, diagnosed or suspected cancer disease, chronic inflammatory disease and fertile women. Before inclusion, patients had a screening of blood tests, urinary tests, stools for occult blood x 3, and chest x-ray. Patients with diabetes mellitus had an opthalmoscopy, and a mammography was performed in all women. All patients were scheduled to undergo clinical examination, Seattle Angina Pectoris Questionnaire (SEQ), and exercise test, before the treatment and 3 months after. All patients (except one patient in the placebo group who died) went through the 3 months follow-up.
This study protocol complied with the Declaration of Helsinki and was approved by the regional Ethical Committee (02-078/00, 02-053/01) and Danish Medicines Agency (2612-1490, 2612-1782). All patients received oral and written information about the study, and signed an informed consent.
Treatment with G-CSF, VEGF-A165 and placebo plasmid
Detailed treatment protocols have been published previously Citation2, Citation12, Citation13. In short, electromechanical evaluation of the left ventricle was performed with the NOGA® system (Biosense Webster A/S, Cordis, Johnson & Johnson). The intramyocardial injections of VEGF-A165 or placebo plasmid were done with the 8-french-sized Myostar® mapping-injection catheter (Biosense Webster A/S, Cordis, Johnson & Johnson) inserted into the left ventricle via the groin Citation2. Ten 0.3 ml injections were given around and within the area with reversible ischemia with a total dose of 0.5 mg phVEGF-A165 or placebo plasmid. The plasmid contained a cytomegalovirus promotor/enhancer to drive VEGF-A165 expression. The placebo plasmid was identical to the plasmid VEGF-A165 except for the VEGF-A165 gene, which had been cut out. G-CSF (Neupogen, Amgen Europe BV, Breda, The Netherlands) was administered as a subcutaneous injection once daily for 6 days during hospitalization at a dose of either 5 µg/kg body weight (low dose G-CSF group) or 10 µg/kg body weight (high dose G-CSF group).
Enumeration of haematopoietic stem cells and non-haematopoietic mesenchymal stem cells (MSC) by flow cytometry
Using flow cytometry, circulating haematopoietic stem cells CD34+ cells and non-haematopoietic CD45 − /CD34− cells were measured before (“baseline”) and on day 7, 14, 21 and 28 after VEGF or placebo treatment in VEGF gene transfer (II) and placebo (I) group, and were measured before (“baseline”) and on day 3, 7, 14, and 28 after G-CSF treatment in G-CSF (III) and VEGF + G-CSF (IV) group.
The concentration of circulating non-haematopoietic cells in blood was quantified as CD45 and CD34 double-negative (CD45 − /CD34 − ) cells by multiparametric flow cytometry. A panel of monoclonal antibodies and one polyclonal antibody were used, including anti-CD45 (HI30 clone, IgG1; Becton Dickinson (BD) Mountain View, CA, USA) and anti-CD34 (8G12 clone, IgG1; BD) to exclude haematopoietic cells, as well as anti-CD105 (Endoglin, NI-3A1, clone, IgG1; Ancell), -CD31 (PECAM-1, platelet endothelial cell adhesion molecule, WM59 clone, IgG1; BD), VEGF R2 (KDR; R&D Systems), -CD144 (VE-cadherin; BMS158FI Polyclonal; BenderMed), anti-CXC chemokine receptor 4 (CXCR4; 12G5 clone, IgG2a; R&D Systems, Minneapolis, Minn). Analytic gates were used to enumerate total number and subset of circulating CD45 − /CD34− cells at a FACS Calibur (Becton Dickinson, San Jose, CA).
Plasma SDF-1 and VEGF-A
Plasma SDF-1 and VEGF-A concentrations were measured in duplicate by a colorimetric enzyme linked immunosorbent assay (ELISA) kit (R&D Systems). The lower limits of detection were 18 pg/ml for SDF-1 and 10 pg/ml for VEGF-A
Statistical analysis
Data are presented as mean ± standard error of the mean. Continuous variables were compared using the Kruskal-Wallis test and categorical variables were compared using Fisher's exact test. Correlation coefficients were tested using Spearman's Rank Correlation. The comparisons between baseline and follow-up data measured within patient's group by the Wilcoxon signed-ranks test for paired samples. All data were analysed using SPSS statistical analysis program (SPSS version 11.0, SPSS INC., Chicago, Il.). Values of p < 0.05 were considered significant.
Results
Patient characteristics
The demographic characteristics of the patients are presented in . All patients had severe stable angina pectoris and limited exercise capacity. They were treated with more than one anti-angina drug. Moreover, they all had documented stress induces myocardial ischemia on a SPECT. All patients had previously been treated with at least one coronary artery by-pass operation or percutaneous coronary intervention.
Table I. Demographics and clinical data of the patients treated with Placebo, VEGF, low dose G-CSF (lG-CSF) and VEGF-A165- combined with high dose G-CSF (hG-CSF).
Effect of VEGF and G-CSF on circulating haematopoietic stem cells
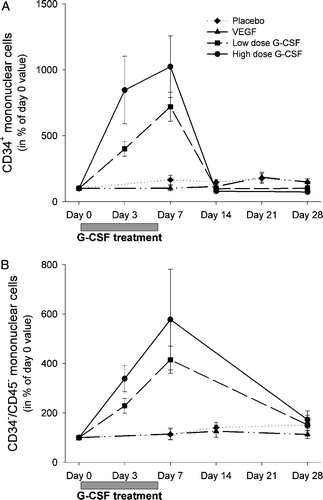
VEGF-A gene therapy did not increase the number of circulating CD34+ stem cells compared to placebo (A). A significant increase in circulating CD34+ stem cells was found during the G-CSF treatment (group III and IV), with normalization after one week in both groups (A). In addition, a G-CSF dose dependent mobilization was found with enhanced mobilization of CD34+ cell in peripheral blood on day 3 and 7 after 10 µgram/kg G-CSF treatment compared to 5 µgram/kg G-CSF treatment (A). At all other time points the CD34+ levels were similar between the four groups.
Figure 1. A) Circulating CD34+ cells (103/mL) from baseline to day 28. B) Increase in circulating CD45 − CD34− relative to the baseline value in each group. Data in panel A are previously published. Citation12, Citation13
Effect of VEGF and G-CSF on circulating non-haematopoietic CD45 − /CD34 − cells
There was no significant mobilization of circulating CD45 − /CD34− cells after VEGF gene therapy (group II) compared with placebo (group I; B). Circulating CD45 − /CD34− cells increased significantly in a dose-dependent manner after G-CSF treatment and normalized one week after G-CSF treatment (group III and IV; B).
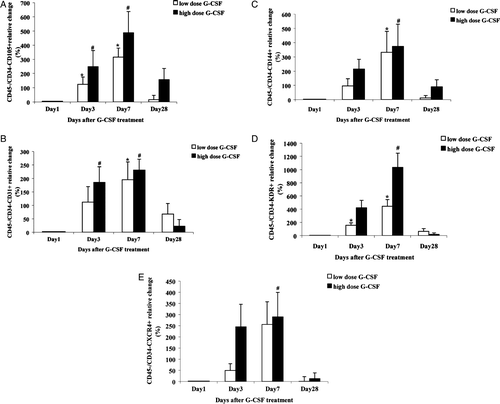
Analysis for a range of subpopulations among the G-CSF mobilized CD45 − /CD34− cells identified significant variations for CD105+, CD31+, CD144+ and KDR+ cell. There was a significant increase in these sub-types during both low and high dose G-CSF treatment (A-D). In addition, relative increase of circulating CXCR4 (the SDF-1 receptor) positive CD45−CD34− cells was significant one week after high dose G-CSF treatment (E) and the cells tended to be significantly increased in the low dose G-CSF group too (p = 0.07, E). The increase in all the measured CD45 − /CD34− cell sub-populations seemed dependent on the G-CSF dose (A-E).
Figure 2. Relative increase in circulating CD45 − CD34− subtype (A) CD105+ and CD45-CD34- cell types with endothelial markers (B) CD31+ (C) CD144+ (D) KDR+ and (E) CXCR4+ in the first month after G-CSF treatment. *p < 0.05 day 3 and day 7 versus day 1 in low dose group. #p < 0.05 day 3 and day 7 versus day 1 in high dose group.
Plasma VEGF-A and SDF-1
The baseline concentrations of plasma VEGF-A varied between the groups. Plasma VEGF-A increased significantly in the two VEGF-A treated groups and in the placebo group one week after the intramyocardial injections (). SDF-1 was lower at baseline in the control group compared to the three active treated groups (). VEGF gene therapy tended to increase plasma SDF-1 one week after the treatment (p = 0.07, ). However, plasma SDF-1 also increased significantly in the placebo group. SDF-1 decreased non- significantly in the high dose G-CSF group and significantly during low dose G-CSF treatment (group IV and III).
Table II. Plasma concentrations of VEGF and stromal-derived factor-1 (SDF-1) in patients treated with placebo, plasmid VEGF-A165, G-CSF or VEGF-A165- combined with G-CSF the first month after treatment.
Effects of mobilized stem cells on clinical outcome
The correlation between the clinical symptoms of the ischemic heart disease and the mobilized cells are shown in . There seemed to be no association between the mobilization of CD34+ cells or CD45 − /CD34− cells by G-CSF and the improvement in symptoms.
Table III. Correlations between peak value of mobilized cells and change in clinical characteristics from baseline to follow-up for patients treated with G-CSF or VEGF-A165- combined with G-CSF.
Discussion
The main results of this trial were that G-CSF treatment mobilized both haematopoietic and non- haematopoietic stem cells from the bone marrow into the circulation in a dose-dependent manner and that local intramyocardial VEGF-A165 therapy did not mobilize bone marrow stem cells. However, we identified no association between the concentration of mobilized stem cells in the circulation and improvement in symptoms of ischemic heart disease.
In small clinical studies intramyocardial gene transfer of VEGF demonstrated clinical evidence of improved myocardial perfusion Citation1, Citation14, Citation15. However, the first large double-blind placebo-controlled study with VEGF gene transfer could not demonstrate comparable clinical effects Citation2. The reason for this discrepancy is not known. However, Kalka et al. Citation3 suggested that VEGF gene therapy affects the ischemic tissue through mobilization of bone marrow derived stem cells to the peripheral blood and maybe also by directly influencing the engraftment of the circulating stem cells into ischemic tissue and thus promoting vasculogenesis Citation3. However, in opposition to Kalka et al. we could not detect a significant mobilization of bone marrow-derived cells by intramyocardial VEGF gene transplantation thus offering a potential explanation for the lacking improvement in myocardial perfusion in the Euroinject One Trial Citation2. The lack of cell mobilization could be caused by an inadequate chemokinetic effect due to the low dose of VEGF gene transplantation (0.5 mg of DNA compared to 4 mg in the Kalka trial Citation3) chosen for safety reasons. A higher VEGF plasmid dose or more effective gene transfection methods should be considered in future clinical angiogenic therapy studies.
Recently, the differentiation of haematopoietic stem cells into new cardiomyocytes has been challenged Citation16. This issue may call into question the mechanism of clinical stem cell-based trials Citation5–7. Therefore, more attention has been focused on the utilization of bone marrow derived CD45 − /CD34 − cells, which are capable of differentiation into many cell types Citation12 and which have been found to induce vasculogenesis and improve cardiac function in animals with acute myocardial infarction or chronic ischemic myocardium Citation9, Citation10. The participation of the mesenchymal subtype CD45 − /CD34 − /CD105+ cell in vasculogenesis and microvascular development in ischemic tissue is supported by results demonstrating that human bone marrow-derived CD45 − /CD34 − /CD105+ are capable of differentiating into endothelial cells in vitro Citation17. However, even though we found an increase in the CD45 − /CD34 − /CD105+ and the cell populations related to the endothelial cell lineage (CD31/CD144/KDR) after G-CSF treatment we could not find evidence of induced vasculogenesis in the ischemic myocardium of the patients studied. These results could indicate that cell mobilization alone is not sufficient for myocardial repair.
The increase in circulating CD34+ stem cell and CD45 − /CD34− cells was higher in the group treated with a high dose G-CSF compared to the low dose G-CSF treated group. This indicates that G-CSF mobilized not only haematopoietic stem cells but also non-haematopoietic CD45 − /CD34 − cells in a dose-dependent manner. However, a high proportion of the patients had low concentration of circulating CD34+ cell following especially the low dose G-CSF treatment. This phenomenon is well known from clinical haematology and can potentially bias efficacy trials of G-CSF. In the present study, 35% and 25% of patients, treated with low dose and high dose G-CSF respectively, failed to mount adequate CD34+ cell response. This result underscores the importance of the dose-dependent effect of G-CSF stimulation.
Our recently published clinical trials demonstrated that mobilization of bone-marrow stem cell with G-CSF treatment or combined with VEGF gene transplantation were safe but did not improve cardiac function Citation12, Citation13, Citation18. This despite the marked increase in blood concentration of both haematopoietic and non-haematopoietic cells in patient with both chronic myocardial ischemia ( and ) and acute myocardial infarction Citation19. Additionally, we could not detect a clinical effect of G-CSF when controlling for the inter-individual difference in cell mobilization (). The lack of effect of G-CSF mobilized bone marrow stem cells might be due to the lack of a significant myocardial SDF-1 homing signal immediately after AMI and also in chronic myocardial ischemia Citation20. This is supported by the finding of unchanged myocardial SDF-1 m-RNA levels in both chronic and acutely ischemic myocardium obtained from biopsies during by-pass surgery Citation21. Also, recent evidence suggests that G-CSF treatment impairs the migratory response to SDF-1 of endothelial progenitor cells Citation22.
We identified a surprising increase in both VEGF-A and SDF-1 following intramyocardial injection of placebo-plasmid. We find it likely that these increases are caused by the small myocardial damage inflicted by intramyocardial injections. This information once again underscores the importance of a proper placebo-treatment group for interpretation of efficacy data, since the procedure itself can potentially augment a neovascularization response.
The presented data are potentially limited by the fact that patients were not randomized into the four treatment arms. In addition, early changes in cell mobilization in patients treated with VEGF or placebo could have been missed since the first blood samples were obtained a week after treatment. However, the differences in cell mobilization between the groups are substantial, and we find it unlikely that these would be caused by minor differences in patient-populations or blood sampling time.
In conclusion, G-CSF mobilizes haematopoietic stem cells and non-haematopoietic CD45 − /CD34− cells from the bone marrow in a dose–dependent manner to levels of relevance for clinical stem cell trials in patients with severe coronary artery disease. In opposition, intramyocardial VEGF-A165 plasmid therapy (0.5 mg DNA) does not mobilize bone marrow stem cells. However, mobilized stem cells in the circulation did not seem to improve symptoms of ischemic heart disease. Thus, mobilization with G-CSF alone or combined with VEGF gene therapy does not seem to be an effective strategy for stem cell therapy in patients with severe chronic ischemic heart disease.
Acknowledgements
We are very thankful to Professor Christer Sylvén, Department of Cardiology and the Gene Therapy Centre, Huddinge University Hospital, Stockholm, Sweden for the delivery of the VEGF-A165 plasmid. This study was supported with unrestricted grants from the Research Foundation at Rigshospitalet, Lundbeck Foundation, Aase og Ejnar Danielsens Foundation, and The Danish Heart Foundation. There are no conflicts of interest.
References
- Losordo DW, Vale PR, Hendel RC, Milliken CE, Fortuin FD, Cummings N, et al. Phase 1/2 placebo-controlled, double-blind, dose-escalating trial of myocardial vascular endothelial growth factor 2 gene transfer by catheter delivery in patients with chronic myocardial ischemia. Circulation. 2002; 105: 2012–8
- Kastrup J, Jørgensen E, Ruck A, Tägil K, Glogar D, Ruzyllo W, et al. Direct intramyocardial plasmid vascular endothelial growth factor-A165 gene therapy in patients with stable severe angina pectoris. A randomized double-blind placebo-controlled study: The Euroinject One trial. J Am Coll Cardiol. 2005; 45: 982–8
- Kalka C, Masuda H, Takahashi T, Gordon R, Tepper O, Gravereaux E, et al. Vascular endothelial growth factor(165) gene transfer augments circulating endothelial progenitor cells in human subjects. Circ Res. 2000; 86: 1198–202
- Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001; 410: 701–5
- Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006; 355: 1199–209
- Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Mesquita CT, et al. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation. 2003; 107: 2294–302
- Schächinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006; 355: 1210–21
- Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004; 95: 9–20
- Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005; 111: 150–6
- Tang YL, Zhao Q, Zhang YC, Cheng L, Liu M, Shi J, et al. Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul Pept. 2004; 117: 3–10
- Ince H, Petzsch M, Kleine HD, Schmidt H, Rehders T, Korber T, et al. Preservation from left ventricular remodeling by front-integrated revascularization and stem cell liberation in evolving acute myocardial infarction by use of granulocyte-colony-stimulating factor (FIRSTLINE-AMI). Circulation. 2005; 112: 3097–106
- Wang Y, Tägil K, Ripa RS, Nilsson JC, Carstensen S, Jørgensen E, et al. Effect of mobilization of bone marrow stem cells by granulocyte colony stimulating factor on clinical symptoms, left ventricular perfusion and function in patients with severe chronic ischemic heart disease. Int J Cardiol. 2005; 100: 477–83
- Ripa RS, Wang Y, Jørgensen E, Johnsen HE, Hesse B, Kastrup J. Intramyocardial injection of vascular endothelial growth factor-A165 plasmid followed by granulocyte-colony stimulating factor to induce angiogenesis in patients with severe chronic ischaemic heart disease. Eur Heart J. 2006; 27: 1785–92
- Sylvén C, Sarkar N, Ruck A, Drvota V, Hassan SY, Lind B, et al. Myocardial Doppler tissue velocity improves following myocardial gene therapy with VEGF-A165 plasmid in patients with inoperable angina pectoris. Coron Artery Dis. 2001; 12: 239–43
- Rosengart TK, Lee LY, Patel SR, Sanborn TA, Parikh M, Bergman GW, et al. Angiogenesis gene therapy: Phase I assessment of direct intramyocardial administration of an adenovirus vector expressing VEGF121 cDNA to individuals with clinically significant severe coronary artery disease. Circulation. 1999; 100: 468–74
- Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004; 428: 664–8
- Oswald J, Boxberger S, Jorgensen B, Feldmann S, Ehninger G, Bornhauser M, et al. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004; 22: 377–84
- Ripa RS, Jørgensen E, Wang Y, Thune JJ, Nilsson JC, Søndergaard L, et al. Stem cell mobilization induced by subcutaneous granulocyte-colony stimulating factor to improve cardiac regeneration after acute ST-elevation myocardial infarction: Result of the double-blind, randomized, placebo-controlled stem cells in myocardial infarction (STEMMI) trial. Circulation. 2006; 113: 1983–92
- Ripa, RS, Haack-Sørensen, M, Wang, Y, Jørgensen, E, Mortensen, S, Bindslev, L, et al. Bone marrow-derived mesenchymal cell mobilization by granulocyte-colony stimulating factor after acute myocardial infarction: Results from the Stem Cells in Myocardial Infarction (STEMMI) Trial. Circulation. 2007; 116((11 suppl)): I24–30.
- Kastrup J, Ripa RS, Wang Y, Jorgensen E. Myocardial regeneration induced by granulocyte-colony-stimulating factor mobilization of stem cells in patients with acute or chronic ischaemic heart disease: A non-invasive alternative for clinical stem cell therapy?. Eur Heart J. 2006; 27: 2748–54
- Wang Y, Gabrielsen A, Lawler PR, Paulsson-Berne G, Steinbruchel DA, Hansson GK, et al. Myocardial gene expression of angiogenic factors in human chronic ischemic myocardium: Influence of acute ischemia/cardioplegia and reperfusion. Microcirculation. 2006; 13: 187–97
- Honold J, Lehmann R, Heeschen C, Walter DH, Assmus B, Sasaki K, et al. Effects of granulocyte colony simulating factor on functional activities of endothelial progenitor cells in patients with chronic ischemic heart disease. Arterioscler Thromb Vasc Biol. 2006; 26: 2238–43