Abstract
Objectives. To investigate whether human veins responded to surgical handling with acute remodelling by measuring matrix metalloproteinase 9 (MMP9) expression and activity. Design. Saphenous veins were collected from 24 patients (12 stable angina, 12 unstable angina) undergoing coronary artery bypass grafting. Expression of MMP9 and its regulators (plasminogen activators, plasminogen activator inhibitor-1, tissue inhibitor of metalloproteinase-1) was evaluated by semiquantitative RT-PCR in veins sampled at the start of and after surgical preparation, while protein was detected by western blotting. The proteolytic activity of MMP9 was analyzed by zymography. Results. Gene (p=0.01) and protein (p=0.001) expression of MMP9 increased after surgical manipulation of vein grafts in all patients, accompanied by increased pro-MMP9 (p=0.04), but not active MMP9 (p=0.6). Grafts from stable patients had increased gene (p=0.05) and protein (p=0.006) expression, as well as increased pro- (p=0.04) and active (p=0.04) MMP9. Grafts from unstable patients increased only in MMP9 protein expression (p=0.05). The MMP9 regulators were unchanged. Conclusions. Surgical handling of vein grafts increased expression and activity of MMP9. However, the surgery-induced increase was attenuated in veins from unstable patients.
Saphenous veins are the most commonly used conduit for coronary artery bypass grafting (CABG), and are used in other revascularization procedures. Vein graft failure is a clinical problem, where intimal thickening due to migration and proliferation of vascular smooth muscle cells causes low graft patency in a long term perspective Citation1. This may be due to having the vein in arterial position with high pressure and pulsatile flow. Possibly the surgical procedure of graft harvesting and handling per se may contribute to the remodelling process. Matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases possessing catalytic activity against extracellular matrix components, and contribute to tissue remodelling in several pathologies Citation2. MMPs may be involved in the procedure of vein graft remodelling through causing matrix degradation, which stimulates smooth muscle cell migration and proliferation Citation3. MMPs may also be associated with the phenotypic change of smooth muscle cells which occurs during migration and proliferation Citation4.
MMPs are synthetized as inactive proenzymes, and are proteolytically cleaved to become active proteases. Plasmin has been suggested to be the important activator of MMP9. The activation procedure can be inhibited by plasminogen activator inhibitor-1 (PAI-1) through the inactivation of the tissue and urokinase type plasminogen activators (tPA and uPA), whereas the activated MMP is inhibited by tissue inhibitor of metalloproteinases (TIMPs) Citation2.
Unstable coronary syndromes may be initiated by rupture of an atherosclerotic plaque. However, patients with unstable coronary syndromes also have a systemic inflammatory response with increase of the acute phase reactant C-reactive protein, soluble leukocyte adhesion molecules, and markers of leukocyte activation Citation5–7. We have previously found an increased expression of MMP9 in the plaques from patients with unstable angina, concomitant with a systemic increase of MMP9 antigen in plasma Citation8. However, whether increased plasma MMP9 was cause or consequence of instability was not determined.
The present study aimed to examine whether routine surgical handling of vein grafts would induce MMP9 expression, thus potentially initiate the process of vein graft remodelling. Furthermore, we investigated if there was any difference in the vascular response to surgical handling between patients with stable and unstable coronary syndromes.
Materials and methods
Patient population
Twelve consecutive patients with a diagnosis of unstable angina and 12 consecutive patients with chronic stable effort angina were included in this study. Patients with other accompanying diseases than those associated with risk factors for cardiovascular disease were excluded (i.e.malignancies, rheumatoid arthritis, chronic obstructive lung disease), as well as patients treated with non-antianginal medication. The only exceptions to the latter were patients with diabetes mellitus type II treated with oral glucose lowering medication. The clinical data are shown in . The main differences between the two patient groups were that patients with unstable angina had suffered more myocardial infarctions (). The project was approved by the Regional Ethics Committee of the Karolinska Hospital, and follows the recommendations from the Helsinki Declaration. All patients gave informed consent to participate. Unstable angina was defined as chest pain at rest resistant to oral nitroglycerin; upon hospitalization, transient electrocardiographical evidence of myocardial ischemia was found despite optimal medication including intravenous nitroglycerin and low molecular weight heparin (Fragmin®). They were scheduled for acute revascularization by CABG. The time interval between occurrences of symptoms until surgery ranged from 2 days to 3 weeks. Stable patients had excertional angina and a positive exercise stress-test, and were scheduled for elective CABG.
Table I. The clinical characteristic of patients with stable and unstable angina undergoing acute or elective coronary artery bypass grafting with sampling of saphenous veins used in the present study.
Anesthesia and surgery
All patients were anesthetized according to clinical routine of the department. After premedication with morphine, anesthesia was induced with fentanyl, midazolam and propofol, and muscular relaxation was achieved with pancuronium or atracurium. Volume-controlled ventilation with 40–50% O2 in air was performed. Anesthesia was maintained with intermittent fentanyl and isoflurane and continuous propofol was used as a supplement when needed.
The majority of the patients were operated through standard median sternotomy with cardiopulmonary bypass (CPB) (). Heparin 300 IU/kg was administered to obtain activated clotting time (ACT) over 480 seconds before start of CPB. As vein grafts collected before the start of CPB were the subject of the study, details of the conductance of CPB and surgical procedures on the heart are not given. Mean arterial pressure was kept over 50 mmHg and norepinephrine was given if required to maintain blood pressure. Antegrade, cold blood cardioplegia was used. Rewarming was initiated when the last distal anastomosis was started and the patients were weaned from CPB when the nasopharyngeal temperature was above 36°C.
Table II. Some surgical characteristics of patients with stable and unstable angina undergoing acute or elective coronary artery bypass grafting with sampling of saphenous veins used in the present study.
Patients operated off pump initially received heparin 150 IU/kg and ACT was kept above 300 s while the anastomoses were performed. All proximal anastomoses were performed by the use of a side-biting clamp both in on and off pump surgery. Protamine was administered at the end of the operation to fully reverse the heparin effect.
Vein collection
The first biopsy was obtained from the distal end of the saphenous vein shortly after skin incision, before any further dissection, handling, or storage. After harvesting of the whole graft material, it was prepared, checked for leakage, and stored in Ringer acetate without any additives at room temperature until implantation. Leakage was checked with high pressure flushing (100–150 mmHg) with a 10 ml syringe filled with Ringer's acetate. Leaks were repaired with 8-0 Prolene (Ethicon, Sommerville, NJ). The second biopsy was sampled after completion of the last proximal anastomosis (see for time frame). The biopsies were immediately transferred into RNAase-free tubes and frozen on dry ice in the operation theatre.
Semiquantitative RT-PCR
Frozen tissue was homogenized in a microdismembrator, and mRNA extracted using a Dynabeads mRNA direct kit (Dynal, Oslo, Norway) following the manufacturer's procedure. Single stranded cDNA synthesis was performed by superscript II (Life Technologies, Paisley, UK) in the presence of RNasin (Promega, Madison, Wisconsin, USA). The semiquantitative RT- PCR was carried out for each patient individually by relating different genes in the linear phase of the amplification reaction to the reference gene histone H3, with identical amounts of cDNA in each sample. Each reaction was run in a volume of 25 µl. Primers were added to a final concentration of 0.2 µmol/l to the reaction mixture consisting of cDNA (1 µl per reaction), dNTP (6.25 mmol/l), MgCl2 (1.5 mmol/l), 2.5 µl 10×PCR buffer, 0.02 U Taq Polymerase (all Life Technologies) and 5µCi 33P-dATP (Amersham Pharmacia Biotech, Buckinghamshire, UK). The primer for H3, 5′-CCA CTG AAC TTC TGA TTC GC (base position 282-301) and 5′- GCG TGC TAG CTG GAT GTC TT (base position 476-495) gave an amplification product of 215 base pairs. The MMP9 primer (5′-ACCTGGTTCAACTCACTCCG, 5′-GCCTCTGTAGGTTCGACGTG) gave a product of 181 base pairs, while the TIMP-1 primer (5′-3′, 5′-CTG TTG TTG CTG TGG CTG ATA, 5′-CCG TCC ACA AGC AAT GAG T) gave a product of 481 base pairs. The tPA (5′- CTG ATG ATG CCC ACC AAA GTC, 5′- CTG CAG CTG AAA TCG GAT TCG T) and PAI-1 (5′- ATG CAG ATG TCT CCA GCC CTC AC, 5′- CTC CAC CTC TCT CGG TCT AAG T) primers gave amplification products of 368 and 472 base pairs respectively (all sequences are 5′-3′). The PCR reaction was performed with a hot start at 94°C for 2 min, 60°C for 30 s, 72°C for 45 s, followed by cycles of 30 s at 94°C, 30 s at 60°C, and 45 s at 72°C. After initial studies on number of cycles and the linearity of the amplified products, we selected to run H3 samples for 27 cycles, while MMP9, TIMP-1 and PAI-1 were run for 33 cycles, and tPA for 36 cycles. Control PCR of master mix without cDNA with the primers was routinely used, and no contamination detected. A radiolabelled DNA ladder was synthetized using the Gibco 100 base pair DNA ladder and T4 DNA polymerase kit (Life Technologies). The PCR products of paired samples (first and second biopsy) from stable and unstable patients were separated by electrophoresis on a 5% polyacrylamide gel and quantified using a phosphoimager (BioImaging Analyzer System BAS 1000, Fuji). The ratio of the optical density of test gene band and H3 was calculated.
Western blotting
Cytoplasmatic proteins were extracted by homogenizing frozen tissue in lysis buffer (1% SDS, 1 mM Na vanadate, 1 mM protease inhibitor phenylmethylsulphonyl fluoride), and insoluble material was removed by centrifugation. Protein content was determined using the bicinchonic acid reagent (Pierce, Rockford, IL, USA) with bovine serum albumin as a standard. The lysates were mixed with 5×Laemmli buffer at a ratio of 5:1, boiled, and proteins were electrophoresed under reducing conditions (50 µg/lane for MMP9, 45 µg/lane for TIMP-1, 45 µg/lane for tPA, 45 µg/lane for uPA, 45 µg/lane for PAI-1) followed by transfer to presoaked nitrocellulose membranes (Hybond-C pure; Amersham Life Science). The membranes were stained with Ponceau solution to evaluate equal protein loading, and a digital image obtained. Thereafter membranes were blocked in PBS/Dulbecco′s (Gibco BRL, Life Technology) with 0.1% Tween and 5% nonfat dry milk followed by incubation with a mouse monoclonal anti-MMP9 antibody (Chemicon, Temecula, California, USA) diluted 1/1000, a mouse monoclonal anti −TIMP-1 antibody (Chemicon) diluted 1/1000, goat polyclonal anti-tPA antibody (Chemicon) diluted 1/1200, goat polyclonal anti-uPA antibody (Chemicon) diluted 1/1200, or a mouse monoclonal anti-PAI-1 antibody (Diagnostica Inc., Greenwich, CT, USA) diluted 1/1000. A rabbit anti-mouse or a mouse anti-goat (both StressGen Biotechnologies) IgG-horseradish peroxidase 1/1000 and an enhanced chemiluminescence substrate kit (Pierce, Rockford, IL, USA) were used for visualization. The bands were scanned into a digital image. Band densities were quantified and the test band corrected for amount of protein shown by Ponceau (TINA 2.0, Strauberhardt, Germany).
Zymography
Gelatinase activity in tissue extracts of paired veins in the stable and unstable patients was measured by zymography. To correct for variations in tissue segment size, all tissue extracts were diluted to the equivalent of 65 mg/ml with extraction medium. Tissue extracts were then subjected to electrophoresis on SDS/polyacrylamide gels containing 1mg/ml gelatin. After electrophoresis, SDS was removed from the gels by washing twice for 30 minutes each with an aqueous solution of 2.5% Triton X-100. The gels were then incubated overnight at 37°C in a reaction buffer containing 50 mmol/l Tris-HCl with pH 7.5, 5 mmol/l CaCl22H2O, and 0.05% Brij -35. Zones of gelatinolytic activity appeared as clear bands against the Coomassie blue–stained background. All gels were calibrated with high-molecular-weight standards. The cell line THP-1 secreting both MMP-2 and MMP-9 was used as an internal positive control, and allowed quantification of proteolytic activity across several gels to be statistically compared. MMP-9 proteins detected by zymography were quantified by densitometric scanning of wet gels by using a GS-690 imaging densitometer (Bio-Rad). The ratio between the optical density of test sample bands and positive cell lines were calculated in all the patients.
Data analysis
Data are shown as dot-plots for stable and unstable patient groups separately and jointly, indicating the individual change from the first to the second biopsy. The difference (for gene expression) or ratio (for protein expression and enzyme activity) between the second and first value is also illustrated by a box-plot for each group. The statistical significance of the measured change is tested for each group separately by a paired t-test on log-scale, or (for gene expression) by a Wilcoxon signed rank test. P-values are given, and the t-tests are supplemented by confidence intervals when the effect was found significant. The p-value level for this was taken to be 5%. A simple regression analysis for duration of symptoms and gene expression of MMP9 as well as activity (zymoygraphy) was performed, but no correlation was found.
Results
Clinical outcome
One patient in each group suffered a postoperative myocardial infarction evident as increased troponin-T and a new Q-wave on the ECG (listed as major postoperative complication in ). Three of the stable patients were drained for pleural fluid during their stay in the ward (minor postoperative in hospital complications in ). Otherwise, the revascularizations were successful and there were no other in-hospital postoperative events.
Gene expression of MMP9, TIMP-1, tPA and PAI-1
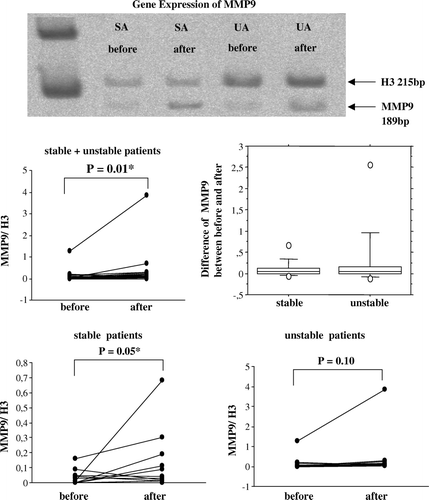
Extracted mRNA was reverse-transcribed to cDNA and amplified by PCR using primers for MMP9, TIMP-1, tPA and PAI-1 with histone H3 as a reference gene. A representative polyacrylamide gel with MMP9 products of paired veins (before and after surgical handling) from one patient with stable angina and one with unstable angina is shown in . The gels with the other genes are not shown. The band densities were measured and the ratio between test genes and H3 was calculated in all the patients. The gene expressions of MMP9 before and after surgical handling, and their differences, are shown in . It is seen that a substantial increase can take place, but more typically the change is small. When comparing the sample taken at the start of surgical handling with the sample taken at the end of the procedure, an increased gene expression was found (p = 0.01). Veins from stable patients had a significantly increased gene expression of MMP9 (p = 0.05), while veins from unstable patients did not (p = 0.10). Gene expression of TIMP-1, tPA, and PAI-1 were not induced by surgical handling (results not shown).
Figure 1. A representative polyacrylamide gel with RT-PCR products of MMP9 from human saphenous veins sampled before and after surgical preparation for CABG from patients with stable (SA) or unstable angina (UA). The band densities were measured and the ratio between test genes and reference gene H3 were calculated in total of 24 patients (12 stable, 12 unstable). MMP9 gene expression for stable and unstable patient groups jointly and separately is illustrated by dot-plots, showing the individual change from the first to the second biopsy. The difference in MMP9 between the second and first biopsy is illustrated by a box-plot for each group. Gene expression of MMP9 was increased after surgical handling in all patients. When the patients were subdivided into populations of stable and unstable angina, MMP9 gene expression was significantly increased in veins from stable, but not unstable patients. Note that the scale of relative gene expression on the dot-plots are different.
Western blotting
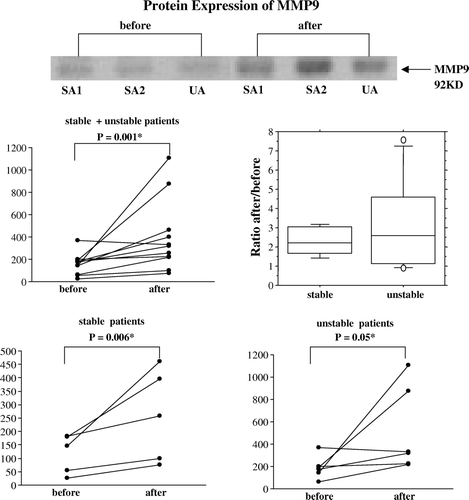
A representative immunoblot with an antibody against MMP9 (92 kD) of paired veins from two stable and one unstable patient is shown in . The band densities were measured and the ratio between test bands and Ponceau was calculated in all patients. The MMP9 protein expression at the start of and end of surgical handling, and their ratios, are shown in . MMP9 protein increased due to surgical handling when all patients were compared (p = 0.001). The patient groups are small, but all stable patients show an increased MMP9 expression (p = 0.006). Also veins from patients with unstable angina increased after surgical handling (p = 0.05), although these patients were more variable and shows huge increases as well as small decreases. In the stable group the log-scale-estimated population factor of increase (ratio After/Before) is 2.2, with a 95% confidence interval of (1.4, 3.4). The immunoblots with antibodies against TIMP-1, tPA, uPA, PAI-1 are not shown, as there were no changes induced in the expression of these proteins by surgical handling, and no difference between the two subgroups. The baseline expression of all proteins was similar between the two groups.
Figure 2. A representative immunoblot showing protein extracts from human saphenous veins sampled before and after surgical preparation for CABG from patients with stable (SA) or unstable angina (UA). MMP9 protein is illustrated by dot-plots, showing the individual change from the first to the second biopsy for stable (n = 5) and unstable patient groups (n = 6) jointly and separately. The ratio between the second and first value is shown by a box-plot for each group. Protein expression of MMP9 was increased by surgical handling of the veins of all patients. When the patients were subdivided into groups according to plaque stability, an increase of MMP9 was found after surgical vein graft handling in stable patients, while this difference becomes smaller in veins from unstable patients. In the stable group the log-scale-estimated population factor of increase (ratio After/Before) is 2.2, with a 95% confidence interval of (1.4, 3.4). Note that the scale of relative protein expression on the dot-plots are different.
Zymography
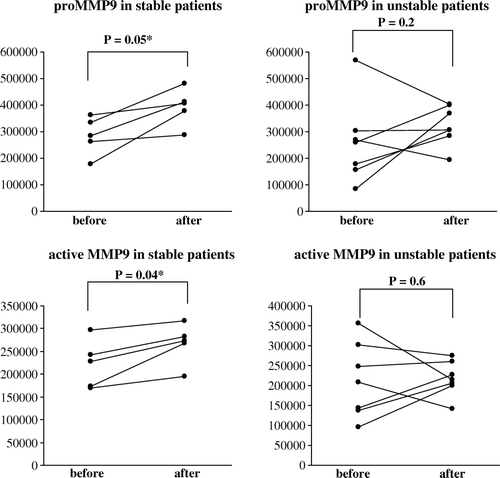
A representative zymogram of MMP9 activities in tissue extracts obtained before and after surgical handling from stable and unstable patients is shown in . The diagrams show that surgical preparation induced an increase of proMMP9 in the combined group (p = 0.04), while active MMP9 was not increased (). When subdividing the patient groups in stable and unstable patients, a greater activity of both proMMP9 and active MMP9 was observed in the stable group (p = 0.05 and p = 0.04, respectively), but for the unstable group the picture was too mixed for any statistical significance (). For the stable group the effects were small but consistent; For proMMP9 the log-scale-estimated population factor of increase (ratio After/Before) is 1.4, with a 95% confidence interval of (1.0, 1.9) (). For active MMP9 the corresponding estimate was 1.2, with confidence interval (1.0, 1.4) (). The covariation in the increase in proMMP9 and in active MMP9 was positive and statistically significant for the stable group (log-scale correlation coefficient r = 0.9), but negative and significant for the unstable group (r = − 0.8), indicating that for the latter, a change in one of the substances occurred at the expense of an opposite change in the other. Baseline MMP9 activity was similar in both groups. Neither pro-MMP2 nor active MMP2 was influenced by surgical handling.
Figure 3a. A representative zymography gel shows the pro-MMP9 and activated MMP9 in tissue extracts from human saphenous veins sampled at the start of and end of surgical preparation for CABG from two patients with stable (SA) and one patient with unstable angina (UA). proMMP9 as well as active MMP9 are illustrated by dot-plots, showing the individual change from the first to the second biopsy for patients with stable (n = 5) and unstable (n = 7) angina pectoris. The ratio between the second and first value is shown as a box-plot for each group. Pro-MMP9, but not active MMP9, was increased in all patients after surgical handling. For proMMP9 the log-scale-estimated population factor of increase (ratio After/Before) was 1.4, with a 95% confidence interval of (1.0, 1.9). For active MMP9 the corresponding estimate was 1.2, with confidence interval (1.0, 1.4). Note that the scale of pro- and active MMP9 on the dot-plots are different.
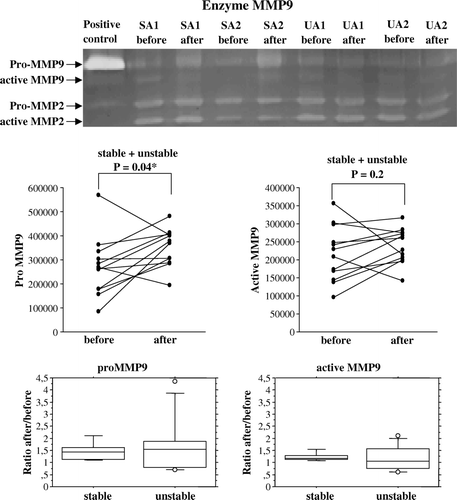
Figure 3b. The patients shown in were subdivided into populations with stable and unstable angina, and the values of pro- and active MMP9 are shown as dot-plots. An increase of pro- and active MMP9 was found after surgical vein graft handling in stable patients, while this was blunted in veins from unstable patients.
Discussion
Our major findings were that in saphenous veins prepared for coronary artery bypass grafting, an increased MMP9 expression and activity was induced. Regulators of MMP9 were not influenced. Unstable angina preceding surgery had little impact on any of the investigated factors involved in tissue remodelling, but dampened the increased MMP9 expression and activity in response to surgical handling. This may potentially indicate that the process of vein graft remodelling leading to loss of graft patency may start already in the process of graft handling.
To the authors’ knowledge, ours is the first paper to document what happens to MMP9 expression in human saphenous veins during routine procedures of surgical harvesting and handling. In the present study we found that surgical preparation of vein grafts increased MMP9 expression in the vessel wall. This is in accordance with related previous studies by others; Mechanical stretching of vein grafts increased MMP9 expression Citation9, while human saphenous veins in culture secrete MMPs after injury Citation10. Surgical preparation of grafts includes stimuli such as contact with air, dissection itself, mechanical distension, and exposure to storage medium and room temperature Citation11. We did not dissect which of the stimuli which was of importance. Surgical mechanical injury, including adventitial stripping, side branch ligation, vein distention, and storage in heparinized blood, was previously found to increase cell proliferation Citation12 and intimal thickness Citation13 compared to non-injured veins.
Neointima formation is a characteristic feature of restenosis after angioplasty and in vein grafts used in coronary artery bypass surgery Citation10. Like in restenosis and atherosclerosis, the main source of intimal thickness in vein grafts is thought to be the proliferation and migration of cells from the underlying media into the intima layer Citation1. Studies of vascular smooth muscle cells in vivo Citation14 and of restenosis in animal models Citation15 show that migration and proliferation depend on the matrix-degrading activity of MMP-enzymes, especially the gelatinases MMP2 and MMP9, which degrade basement membrane collagens and elastin Citation16. Increased MMP9 activity in the media of surgically prepared, but not freshly isolated veins correlates with the presence and location of subsequent smooth muscle cell proliferation Citation10. The present study showed an increased expression and proteolytic activity of MMP9 after surgical preparation. The increased ability for matrix degradation coincides with smooth muscle cell phenotypic modulation detected by in situ zymography in others’ studies Citation4, indicating that the activation of MMP9 may contribute to neointima thickening.
MMP9 is synthetized as a proenzyme and needs proteolytic cleavage at specific sites to become an active protease. Plasminogen activator expression may be important for destabilizing the fibrous cap of the atherosclerotic plaque Citation17, directly because plasmin is a proteolytic enzyme capable of degrading some components of extracellular matrix, and indirectly by initiating the degradation of other components through activating MMP Citation18. In the present study, venous expression of uPA, tPA, PAI-1, or TIMP-1 was not influenced by surgical preparation or by unstable coronary syndroms. Thus, other factors rather than plasminogen activators and inhibitors may predominantly regulate the MMP9 activity in the human veins.
We previously found increased expression of MMP9 in the plaques from patients with unstable angina, concomitant with a systemic increase of MMP9 antigen in plasma Citation8. In the present study the baseline expression of MMP9 and its regulators was similar in veins from patients with stable and unstable coronary syndromes, indicating that no general tissue remodelling process is apparent in patients with unstable angina. One exiting difference between patient groups, however, was that the increase of MMP9 expression caused by surgical handling was blunted in veins from patients with unstable angina. For the latter, it may be indicated that a change in one of the substances occurred at the expense of an opposite change in the other. Theoretically patients with unstable angina undergoing coronary artery bypass grafting may have improved longterm patency of vein grafts. This may be explained by a possible preconditioning-like effect of unstable angina, which induces general molecular changes of protective substances such as heat shock protein 72 (HSP72) and endothelial nitric oxide synthase (eNOS) Citation19. Other studies show that patients with unstable angina prior to acute myocardial infarction have improved outcome: reduced mortality, reduced severe congestive heart failure and shock, smaller infarcts as evaluated by CK-MB leakage, and less Q-wave activity than patients with acute infarction of sudden onset Citation20–22. Recent studies on preconditioning indicate that general organ protective effects are evoked, protecting not only the target organ of preconditioning but also remote organs Citation23. To the authors’ knowledge, no association of graft patency and unstable angina has been investigated.
The present study illustrates that surgical preparation of saphenous veins induced an increased expression and proteolytic activity of MMP9. The finding that this was modified in patients with preoperative unstable angina supports the possibility that a general organ protective effect of unstable angina similar to that found by ischemic preconditioning is present. Modifications of procedures for surgical handling may be important for the longterm patency of vein grafts.
Limitations
The present study describes expression of MMP9 at two frozen time points; at the start of surgical handling and when the saphenous veins were anastomosed to the heart. We did not do an angiographic follow up of our patients, and can only speculate on the long term outcome of this expression. The unstable group was heterogenous regarding duration of symptoms before surgery. Although we found no correlation between symptoms and expression/activity of MMP9, more fine-tuned methods may detect such a difference. Furthermore, the finding that unstable angina blunted the response to surgery is at this time a preliminary observation, and will be subject to future studies to fully understand the implications. One difference between stable and unstable patients was preoperative medication in the form of intravenous nitroglycerin and low molecular weight heparin, and even though there was no baseline difference in expression between groups we cannot exclude the possibility that the drugs themselves rather than the instability may have influenced MMP9 expression. In depth analysis of MMP expression and its regulation in the vascular wall must be performed in animal experimental studies to further understand the regulation and evaluate the clinical implications of its inhibiton.
Acknowledgements
The excellent help with statistics and manuscript handling by Rolf Sundberg is gratefully acknowledged. This project was supported by grants from the Swedish Medical Research Council (11235, 12660 and 12665), AFA insurance, the Swedish Heart-Lung Foundation, the Fredrik o Ingrid Thuring Foundation, the King Gustaf V and Queen Victoria Foundation, and the Karolinska Institutet. Further support was obtained by the Norwegian Research Council, Norwegian Health Association and the University of Oslo.
References
- Davies M, Hagen PO. Pathobiology of intimal hyperplasia. Br J Surg. 1994; 81: 1254–69
- Watanabe N, Ikeda U. Matrix metalloproteinases and atherosclerosis. Curr Atheroscler Rep. 2004; 6: 112–20
- Fernandez-Ortiz A, Fuster V. Pathophysiology of coronary artery disease. Clin Geriatr Med. 1996; 12: 1–21
- Johnson JL, van Eys GJ, Angelini GD, George SJ. Injury induces dedifferentiation of smooth muscle cells and increased matrix-degrading metalloproteinase activity in human saphenous vein. Arterioscler Thromb Vasc Biol. 2001; 21: 1146–51
- Mehta JL, Saldeen TG, Rand K. Interactive role of infection, inflammation and traditional risk factors in atherosclerosis and coronary artery disease. J Am Coll Cardiol. 1998; 31: 1217–25
- Crea F, Biasucci LM, Buffon A, Liuzzo G, Monaco C, Caligiuri G, et al. Role of inflammation in the pathogenesis of unstable coronary artery disease. Am J Coll Cardiol. 1997; 80: 10E–16E
- Tousoulis D, Davies G, Stefanadis C, Toutouzas P, Ambrose JA. Inflammatory and thrombotic mechanisms in coronary atherosclerosis. Heart. 2003; 89: 993–7
- Chen F, Eriksson P, Hansson GK, Herzfeld I, Klein M, Hansson L-O, et al. Expression of matrix metalloproteinase 9 and its regulators in the unstable coronary atherosclerotic plaque. Int J Mol Med. 2005; 15: 57–65
- Meng X, Mavromatis K, Galis ZS. Mechanical stretching of human saphenous vein grafts induces expression and activation of matrix-degrading enzymes associated with vascular tissue injury and repair. Exp Mol Pathol. 1999; 66: 227–37
- George SJ, Zaltsman AB, Newby AC. Surgical preparative injury and neointima formation increase MMP-9 expression and MMP-2 activation in human saphenous vein. Cardiovasc Res. 1997; 33: 447–59
- Newby AC, George SJ. Proposed roles for growth factors in mediating smooth muscle proliferation in vascular pathologies. Cardiovasc Res. 1993; 27: 1173–83
- Soyombo AA, Angelini GD, Bryan AJ, Newby AC. Surgical preparation induces injury and promotes smooth muscle cell proliferation in a culture of human saphenous vein. Cardiovasc Res. 1993; 27: 1961–7
- Soyombo AA, Angelini GD, Newby AC. Neointima formation is promoted by surgical preparation and inhibited by cyclic nucleotides in human saphenous vein organ cultures. J Thorac Cardiovasc Surg. 1995; 109: 2–12
- Pauly RR, Passaniti A, Bilato C, Monticone R, Cheng L, Papadopoulos N, et al. Migration of cultured vascular smooth muscle cells through a basement membrane barrier requires type IV collagenase activity and is inhibited by cellular differentiation. Circ Res. 1994; 75: 41–54
- Bendeck MP, Zempo N, Clowes AW, Galardy RE, Reidy MA. Smooth muscle cell migration and matrix metalloproteinase expression after arterial injury in the rat. Circ Res. 1994; 75: 539–45
- Senior RM, Griffin GL, Fliszar CJ, Shapiro SD, Goldberg GI, Welgus HG. Human 92- and 72-kilodalton type IV collagenases are elastases. J Biol Chem. 1991; 266: 7870–5
- Lee RT, Libby P. The unstable atheroma. Arterioscler Thromb Vasc Biol. 1997; 17: 1859–67
- Murphy G, Atkinson S, Ward R, Gavrilovic J, Reynolds JJ. The role of plasminogen activators in the regulation of connective tissue metalloproteinases. Ann NY Acad Sci. 1992; 667: 1–12
- Valen G, Hansson GK, Dumitrescu A, Vaage J. Unstable angina activates myocardial heat shock protein 72, endothelial nitric oxide synthase, and transcription factors NFkappaB and AP-1. Cardiovasc Res. 2000;47:49–56.
- Kloner RA, Shook T, Przyklenk K, Davis VG, Junio L, Matthews RV, et al. Previous angina alters in-hospital outcome in TIMI 4. A clinical correlate to preconditioning?. Circulation. 1995; 91: 37–45
- Hirai T, Fujita M, Yamanishi K, Ohno A, Miwa K, Sasayama S. Significance of preinfarction angina for preservation of left ventricular function in acute myocardial infarction. Am Heart J. 1992; 124: 19–24
- Ottani F, Galvani M, Ferrini D, Sorbello F, Limonetti P, Pantoli D, et al. Prodromal angina limits infarct size. A role for ischemic preconditioning. Circulation. 1995; 91: 291–7
- Przyklenk K, Darling CE, Dickson EW, Whittaker P. Cardioprotection ‘outside the box’– the evolving paradigm of remote preconditioning. Basic Res Cardiol. 2003; 98: 149–57