Abstract
Objective. To investigate the three-year prognosis in refractory angina pectoris. Patients. Two hundred and forty three patients were screened at six European university hospitals for a gene therapy trial. In 150 patients refractory angina pectoris could be confirmed. Vital status was assessed after a mean of 33 months. Results. At baseline, mean age was 63±9 years and mean LVEF was 52±12%. In 61% there was a history of myocardial infarction and 83% had previously been revascularised. Mortality was 5.5% at one year and 13.5% at three years. New revascularisation options were found in 9.5% of the 243 screened patients at baseline coronary angiography. Additionally, in 6% of the trial patients the protocol-specified angiogram after three months revealed new significant stenosis, which was treated by percutaneous intervention. Conclusions. Annual mortality in refractory angina pectoris seems higher than in stable angina pectoris in general, but substantially lower than after myocardial infarction. The “refractoriness” of these patients should be re-evaluated regularly, as new revascularisation options often occur.
A considerable proportion of stable angina pectoris patients are not suitable for revascularisation by coronary bypass surgery or percutaneous coronary intervention (PCI) and remain symptomatic despite optimal medical treatment. It has been estimated that 100 000 new patients per year fall into this category in the United States Citation1. These patients have been called “end-stage coronary artery disease” Citation2, “no-option” or “refractory angina pectoris” as defined by a European Society of Cardiology joint study group Citation3 suggesting a high mortality and low potential for improvement. This view mainly stems from smaller patient series and trials, with reported mortality up to 17% per year Citation4.
This patient group has received increased attention as several “unconventional” treatment modalities have been investigated. Examples of new treatments are Transmyocardial Laser Revascularisation Citation5, Citation6 Spinal Cord Stimulation Citation7, Enhanced External Counterpulsation Citation8 and treatment with angiogenic proteins or gene therapy Citation9, Citation10. Some of these treatments are not possible to test in a blinded fashion and others include a procedural risk or high cost. In order to better understand the merits of these new treatments, we sought to investigate the natural course in a cohort of patients with a diagnosis of refractory angina pectoris.
Specifically we were interested in mortality and the frequency of new revascularisation options.
Methods
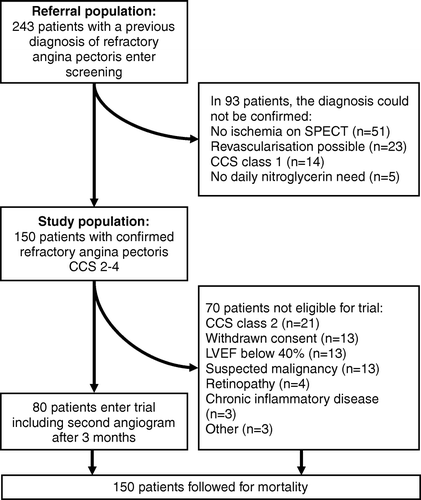
Two hundred and forty three patients with a clinical diagnosis of chronic refractory angina pectoris were screened for the gene therapy trial EUROINJECT ONE in 2001–2002 Citation11, Citation12. Before screening, all patients had a current clinical diagnosis of refractory angina pectoris (no further revascularisation options), based on coronary angiogram and stress tests in all patients. Screening was performed as second opinion and included single photon emission computed tomography (SPECT), echocardiography, coronary angiography and exercise test.
The definition of refractory angina pectoris in this study was: stable angina pectoris class 2–4 with angiographic verified non treatable coronary stenosis and a positive stress test. In 93 cases the refractory angina pectoris diagnosis could not be confirmed (). In the remaining 150 patients with a confirmed diagnosis of refractory angina pectoris, vital status was assessed by interrogation of hospital records and national population registers after a mean of 33 (range 20–47) months after screening. Of the 150 patients 70 were excluded from participation in the EUROINJECT ONE trial.
Thus 80 patients were included in the trial, with 1:1 randomization to percutaneous intramyocardial injections of either plasmid encoding the angiogenic protein VEGF-A165 or to placebo. After three months the left ventricular wall motion and myocardial perfusion improved in the active group compared to placebo Citation11, Citation12. In the 80 trial patients, clinical follow-up data was available for one year after treatment, including a repeat coronary angiogram after 3 months and all serious adverse events.
All patients had given their informed consent. The study was approved by the local ethics boards and conformed to the declaration of Helsinki.
Statistics
Results are presented as mean with standard deviation. A p-value <0.05 was considered significant.
Results
Baseline characteristics
In the 150 patients with confirmed refractory angina pectoris, mean age was 63 years and LVEF was below 40% in 10% of the patients. Coronary bypass surgery had previously been performed once in 45% and twice or more in 19%. More details are found in .
Table I. Baseline characteristics in 150 patients.
Mortality
Among the 150 patients, mortality was 5.5% at one year, 9.8% at two years and 13.5% at three years follow-up. In univariate analysis, higher age (p = 0.0004) was the only baseline factor significantly associated with mortality at long time follow-up.
Revascularisation
Although all patients had a diagnosis of refractory angina pectoris, baseline coronary angiography revealed new revascularisation options in 23 cases (9.5% of the 243 screened patients). Six patients underwent bypass surgery and 17 PCI.
Among the 80 patients included in the EUROINJECT ONE trial four had a myocardial infarction (one fatal) and eight developed unstable angina pectoris (one treated with PCI) during twelve months follow-up. The protocol-specified coronary angiogram after three months revealed a new revascularization target (a new stenosis or progression of a previously non-significant lesion) in five patients, which were treated by PCI.
Discussion
Patients with chronic stable angina who are unsuitable for revascularisation have been regarded as “refractory/end-stage/no option” with a poor prognosis. In order to come close to the “real world” population, we studied not only the patients included in a clinical trial on refractory angina pectoris, but also those excluded but with a confirmed diagnosis of refractory angina pectoris. This study is unique as it followed-up all screened patients with confirmed refractory angina pectoris, and not only the patients included in the trial, thus avoiding the common selection bias in clinical trials.
Mortality in our 150 refractory angina pectoris patients was 5.5% at one year and 13.5% at three years follow-up. This is in keeping with previous trials where mortality ranged between 3 and 10% per year with a sample size of n = 92–112 Citation5–7, Citation13 but lower than the 10% annual mortality reported by Mukherjee et al. Citation1. Mortality in a registry (n = 59) was as high as 17% after one year Citation4. Previous trials and also a population-based registry closely resemble our material in demographics including high-risk features such as depressed left ventricular function, non-use of betablockers and diabetes Citation5, Citation7, Citation9, Citation10. Mean age in the present study was 63 years, similar to most trials, but almost 10 years lower than a population based registry Citation14.
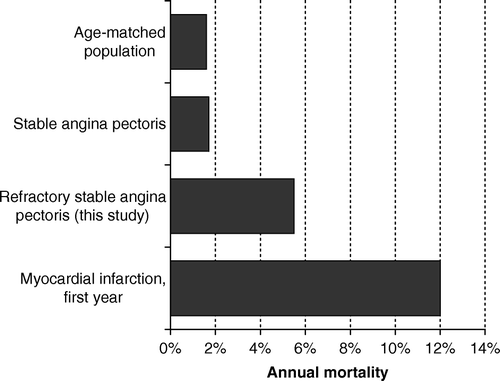
The observed annual mortality of around 5% in refractory angina pectoris needs to be put into context (). First, the total population annual mortality in men and women aged 63 (the mean age in our study) was 1.6% and 1.1% in Denmark Citation15, the country contributing with the largest number of patients to this study. Secondly, in non-refractory stable angina pectoris, annual mortality has been as low as 1.7%, exceeding a matched reference population only in the three first years after diagnosis, and only in men Citation16. Finally, myocardial infarction had a one-year mortality of 12% in Swedish patients aged 60–69 in 2002 Citation17.
Figure 2. Annual mortality in subsets of patients with ischemic heart disease. See text for references.
Based on the findings of baseline coronary angiography 9.5% of the 243 screened patients could be revascularised, despite having a previous diagnosis of refractory angina pectoris. One explanation is that the threshold for when revascularisation is appropriate differs from one surgeon and PCI operator to another. Alternatively, new lesions may have developed. The latter explanation is supported by findings at the 3 months follow-up angiogram in the trial patients. This resulted in a protocol-driven revascularisation rate of 6% due to progression of coronary stenosis. Similarly, revacularization rates of 6% after six months, 12% after one year and 20% after five years have been found in previous trials on refractory angina pectoris Citation6, Citation13, Citation18 Thus, not being a candidate for revascularisation at a certain time does not rule out future such possibilities. On the other hand, it is unclear how aggressive the revascularisation strategy should be in these patients. As 70 of the 150 patients in this study were not included in the EUROINJECT trial, we do not have detailed clinical follow-up data on them, which might have been helpful in this regard.
Conclusions
Patients with a diagnosis of refractory angina pectoris have a comparatively favourable prognosis. New revascularisation options frequently occur. The merits of new and alternative treatments in refractory angina pectoris must be evaluated with this in mind, since no alternative treatment yet has been shown to be clearly superior to placebo. The terms “no option” and “end stage” angina pectoris inadequately describe this condition and the ” refractoriness” should be questioned on a regular basis. High-risk procedures such as redo coronary artery surgery should therefore be used selectively in these patients. They may be regarded as survivors of their disease, who despite extensive and long-standing coronary artery disease generally have a preserved left ventricular function.
Acknowledgements
This study is a result of the EUROINJECT ONE study group. The following investigators made important contributions to this study: Jens Kastrup, Erik Jørgensen and Hanne Graulund (Cardiac Catheterization Laboratory, University Hospital Rigshospitalet, Copenhagen, Denmark); Wytold Ruzyllo and Zbigniew Chmielak (Institute of Cardiology, Warszawa, Poland); Dariusz Dudek and Grzegorz Heba (Institute of Cardiology, Jagellonian University, Krakow, Poland); Hans Erik Bøtker and Søren Steen Nielsen (Department of Cardiology, Skejby University Hospital, Aarhus, Denmark); Mariann Gyöngyösi and Dietmar Glogar (Department of Cardiology, University of Vienna, Austria); Viktor Drvota and Michael Uchto (Karolinska Institutet, Karolinska University Hospital Huddinge, Stockholm, Sweden).
This study was supported by grants from the Danish Heart Foundation, the Research Foundation at Rigshospitalet, the Danish Medical Research Council, the Swedish Research Council, the Swedish Heart and Lung Foundation, the King Gustav V and Queen Victoria 80-Year Foundation, the Karolinska Institute Foundations and the Belvén Foundation, Medicon Valley and WeAidYou.
None of the authors have any financial conflicts of interest.
References
- Mukherjee D, Bhatt DL, Roe MT, Patel V, Ellis SG. Direct myocardial revascularization and angiogenesis--how many patients might be eligible?. Am J Cardiol. 1999; 84: 598–600, A8
- Lauer B, Junghans U, Stahl F, Kluge R, Oesterle SN, Schuler G. Catheter-based percutaneous myocardial laser revascularization in patients with end-stage coronary artery disease. J Am Coll Cardiol. 1999; 34: 1663–70
- Mannheimer C, Camici P, Chester MR, Collins A, DeJongste M, Eliasson T, et al. The problem of chronic refractory angina; report from the ESC Joint Study Group on the Treatment of Refractory Angina. Eur Heart J. 2002; 23: 355–70
- Mukherjee D, Comella K, Bhatt DL, Roe MT, Patel V, Ellis SG. Clinical outcome of a cohort of patients eligible for therapeutic angiogenesis or transmyocardial revascularization. Am Heart J. 2001; 142: 72–4
- Burkhoff D, Schmidt S, Schulman SP, Myers J, Resar J, Becker LC, et al. Transmyocardial laser revascularisation compared with continued medical therapy for treatment of refractory angina pectoris: A prospective randomised trial. ATLANTIC Investigators. Angina Treatments-Lasers and Normal Therapies in Comparison. Lancet. 1999; 354: 885–90
- Oesterle SN, Sanborn TA, Ali N, Resar J, Ramee SR, Heuser R, et al. Percutaneous transmyocardial laser revascularisation for severe angina: The PACIFIC randomised trial. Potential Class Improvement From Intramyocardial Channels. Lancet. 2000; 356: 1705–10
- Ekre O, Eliasson T, Norrsell H, Wahrborg P, Mannheimer C. Long-term effects of spinal cord stimulation and coronary artery bypass grafting on quality of life and survival in the ESBY study. Eur Heart J. 2002; 23: 1938–45
- Arora RR, Chou TM, Jain D, Fleishman B, Crawford L, McKiernan T, et al. The multicenter study of enhanced external counterpulsation (MUST-EECP): Effect of EECP on exercise-induced myocardial ischemia and anginal episodes. J Am Coll Cardiol. 1999; 33: 1833–40
- Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, et al. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. 2003; 107: 1359–65
- Grines CL, Watkins MW, Helmer G, Penny W, Brinker J, Marmur JD, et al. Angiogenic Gene Therapy (AGENT) trial in patients with stable angina pectoris. Circulation. 2002; 105: 1291–7
- Kastrup J, Jørgensen E, Rück A, Tägil K, Glogar D, Ruzyllo W, et al. Direct intramyocardial plasmid vascular endothelial growth factor-A165 gene therapy in patients with stable severe angina pectoris A randomized double-blind placebo-controlled study: The Euroinject One trial. J Am Coll Cardiol. 2005; 45: 982–8
- Gyöngyösi M, Khorsand A, Zamini S, Sperker W, Strehblow C, Kastrup J, et al. NOGA-guided analysis of regional myocardial perfusion abnormalities treated with intramyocardial injections of plasmid encoding VEGF A-165 in patients with chronic myocardial ischemia. Subanalysis of the EUROINJECT-ONE multicenter double-blind randomized study. Circulation. 2005; 112(suppl I)I-157–I-65
- Allen KB, Dowling RD, Angell WW, Gangahar DM, Fudge TL, Richenbacher W, et al. Transmyocardial revascularization: 5-year follow-up of a prospective, randomized multicenter trial. Ann Thorac Surg. 2004; 77: 1228–34
- Ekre, O. Severe angina pectoris and spinal cord stimulation. Thesis. Institute of cardiovascular diseases. Göteborg: Göteborg University; 2003.
- Statistical Yearbook 2003. Copenhagen: Statistics Denmark; 2003.
- Hjemdahl P, Eriksson SV, Held C, Forslund L, Näsman P, Rehnqvist N. Favourable long term prognosis in stable angina pectoris: An extended follow up of the angina prognosis study in Stockholm (APSIS). Heart. 2006; 92: 177–82
- Stenestrand, U, Wallentin, L. RIKS-HIA Report 2005 (Register of Information and Knowledge about Swedish Heart Intensive care Admissions). UppsalaSweden: Uppsala Clinical Research Center; 2005. http://www.ucr.uu.se/rikshiaint/document/RiksHia_Report_2004_EN1.pdf ( accessed 20 Nov 2006).
- Simons M, Annex BH, Laham RJ, Kleiman N, Henry T, Dauerman H, et al. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: Double-blind, randomized, controlled clinical trial. Circulation. 2002; 105: 788–93