Abstract
B-type natriuretic peptide (BNP) is a diagnostic and prognostic marker in heart failure (CHF) patients. Aim. To assess the relation between BNP, diastolic function and exercise capacity in CHF patients. Methods. Fifty CHF patients underwent cardiopulmonary exercise testing. BNP levels were determined at baseline and at peak exercise. Patients were divided in two groups: with lower (<14 ml/kg/min) or higher (≥14 ml/kg/min) peak oxygen consumption (VO2). Results. Seventeen patients with lower peak VO2 showed larger incidence of restrictive pattern of the transmitral flow (7/17 vs 4/33, p =0.036). E/Ea ratio was inversely related with peak VO2 (r =−0.419, p =0.004) and directly related with BNP levels at baseline (r =0.449, p =0.001) and at peak exercise (r =0.475, p =0.001). LV ejection fraction was similar in the two groups. Independent predictors of exercise tolerance were E/Ea ratio (p =0.003), lg BNP at baseline (p =0.034) and increase in lg BNP during exercise (p =0.038). Conclusions. In CHF patients, E/Ea ratio is a predictor of exercise tolerance and is related with BNP level at rest and at peak exercise.
In the management of patients with chronic heart failure (CHF), risk stratification is crucial to define the optimal treatment strategy Citation1. New York Heart Association (NYHA) functional class and peak oxygen consumption have been traditionally considered powerful predictors of survival in CHF patients Citation2. More recently, B-type natriuretic peptide (BNP) has been demonstrated to bear diagnostic and prognostic information in patients with CHF, and it is able to predict the risk of death and cardiovascular events Citation3. Secreted from the cardiomyocytes in response to ventricular stress, BNP has a fundamental role in cardiovascular remodeling and volume homeostasis, being also related with systolic and diastolic left ventricular (LV) function Citation4. In addition, BNP level is inversely related with exercise capacity and, in combination with peak oxygen consumption is able to improve risk stratification of patients with stable CHF Citation5.
Abnormalities of diastolic function may play a major role in patients with signs and symptoms of CHF Citation6. Interestingly, progression of diastolic dysfunction is reflected by BNP level changes. In fact, a BNP rise is directly related to an increase in LV filling pressures, which can be detected by the presence of diastolic abnormalities on echocardiography Citation7.
Nevertheless, in patients with CHF the impact of diastolic dysfunction on neuro-endocrine alterations and exercise tolerance has not been fully investigated. Accordingly, we aimed to assess the relationship between exercise tolerance, diastolic function and BNP levels, evaluated both at rest and at peak exercise in patients with stable chronic heart failure.
Methods
Patients population
Fifty-seven consecutive patients with stable CHF were enrolled from the division of cardiology and heart failure outpatient clinic of our Institution. The diagnosis of CHF was confirmed by the presence of a LV ejection fraction <45% at the echocardiography. All patients had typical symptoms of CHF and were classified according to the NYHA functional class. All patients were in sinus rhythm, clinically stable for at least six weeks before the study, and on optimal and maximally tolerated pharmacological therapy, according to the current guidelines Citation8. Coronary angiography was performed in all patients. Seven patients were subsequently excluded: 2 patients for poor echogenicity, 2 patients for technical problems during cardiopulmonary exercise testing, and 3 patients because of exercise-induced ischemia. Fifty patients, aged 66±9 years (37 males), were finally included in the study. Study protocol was approved by the local ethical committee, and all patients provided written informed consent before entering the study.
Echocardiography
Each patient underwent M-mode and 2-dimensional echocardiography, followed by color flow imaging and pulsed and continuous wave Doppler ultrasound study. Echocardiograms were performed using a Hewlett-Packard ultrasonic scanner (Philips 5500, Handover, MA) equipped with 2.5 MHz probe. M-mode echocardiograms were obtained from the 2-dimensional images under direct anatomic visualization. Left atrial, LV end-diastolic and end-systolic diameters were measured according to the guidelines of the American Society of Echocardiography Citation9. LV ejection fraction was evaluated with biplane transthoracic echocardiography by the modified Simpson rule using second harmonic imaging Citation9.
Diastolic function was further assessed by transmitral flow patterns and Tissue Doppler. Pulsed mitral Doppler measurements were obtained with the transducer in the apical four-chamber view by positioning a 1–2 mm sample volume between the tips of the mitral valve leaflets in diastole, with the Doppler beam aligned perpendicularly to the plane of the mitral annulus Citation10. From mitral inflow patterns we derived early peak filling velocity (E), late peak filling velocity (A), early to late filling ratio, deceleration time of the mitral E-wave. Transmitral LV filling patterns were classified as: normal, abnormal relaxation, pseudonormal and restrictive Citation10.
The Tissue Doppler signals were recorded at a sweep speed of 100 mm/s. From the apical four-chamber view, a 5-mm sample volume was placed at the lateral and at the septal corner of the mitral annulus. The following measurements were made: peak systolic velocity (Sa), early (Ea) and late (Aa) diastolic velocities at the mitral annulus, and the ratio between early wave mitral flow and mitral anulus early diastolic velocity (E/Ea). The E/Ea ratio was expressed as the average between septal and lateral corner of the mitral annulus. The echocardiographic examinations were performed by a single experienced operator (Q.C.). Measurements were made in three to five cardiac cycles, and values averaged subsequently Citation11. Color Doppler flow imaging was used to identify and semiquantitatively estimate mitral regurgitation.
Measurement of BNP plasma levels
In all patients blood was collected in EDTA tubes from antecubital vein after 20 min of supine rest and at peak exercise Citation12. We used a rapid bedside test, based on an immunofluorometric assay (Triage BNP, Biosite Diagnostics, San Diego, California), for quantitative determination of BNP.
Exercise testing
All patients underwent cardiopulmonary exercise testing on a treadmill according to the modified Bruce protocol, with expired gas measurement. Oxygen uptake, carbon dioxide production, and minute ventilation were measured using a breath-by-breath gas analysis. A 12-lead electrocardiogram was continuously registered and blood pressure was recorded every minute by a cuff sphygmomanometer. Peak oxygen uptake was determined as the highest value in the terminal phase of exercise. Exclusion criteria were exercise-limiting diseases other than cardiac, like peripheral vascular disease or degenerative joint disease and relevant primary pulmonary disease. According to peak oxygen consumption, CHF patients were divided in two groups: 1) patients with lower exercise tolerance (peak oxygen consumption < 14 ml/kg/min); 2) patients with higher exercise tolerance (peak oxygen consumption ≥ 14 ml/kg/min) Citation5, Citation13.
Statistics
Data were expressed as mean±standard deviation for continuous variables and as numbers (percent) for categorical variables. The BNP levels were presented as median and total range (lowest and highest measured value).
Normal distribution of all continuous variables, including% differences from peak to baseline, was tested using the one sample Kolmogorov-Smirnov test. Logarithm (Lg) transformation was performed on BNP values because of nonlinear distribution. Regression analysis is used to assess correlations between peak oxygen uptake with continuous variables, using Pearson's coefficient. The association of lower and normal peak oxygen uptake with continuous variables was analyzed by unpaired Student's t-test, and with categorical variables by chi-square test with Yate's correction for continuity. Independent predictors of exercise tolerance were assessed by the means of multiple linear regression analysis.
A probability value of <0.05 was considered statistically significant. All statistical calculations were performed by SPSS for Windows, release 12.0 (Chicago, Illinois).
Results
Clinical and echocardiographic data
The mean LV ejection fraction of the studied population was 34±8% and mean LV end-diastolic diameter was 62±5 mm. Eight percent of the patients were in NYHA class I, 74% in class II and 18% in class III. Ischemic heart disease was the cause of LV systolic dysfunction in 28 patients (56%). The median and total range for BNP levels was 144 pg/ml (16–2220).
Cardiopulmonary exercise testing
All patients performed symptom-limited exercise: the reason for discontinuing exercise was either shortness of breath or fatigue. Heart rate (+41±14% over baseline, p < 0.001) and systolic blood pressure (+27±12% over baseline p < 0.001) increased significantly during exercise.
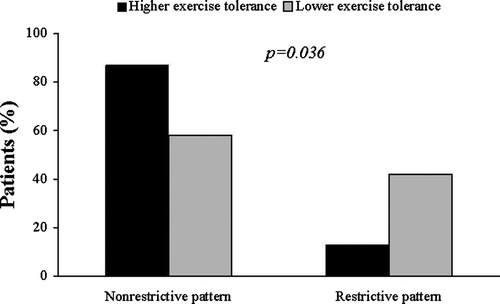
According to the peak oxygen consumption, 17 patients showed lower (peak VO2<14 ml/kg/min) and 33 patients showed higher peak oxygen consumption (peak VO2≥14 ml/kg/min). The clinical and echocardiographic characteristics of the two groups are shown in .
Table I. Clinical and echocardiographic characteristics of the HF patients with peak oxygen uptake ≥14 ml/kg/min (higher exercise tolerance) and with peak oxygen uptake <14 ml/kg/min (lower exercise tolerance).
Diastolic function and exercise tolerance
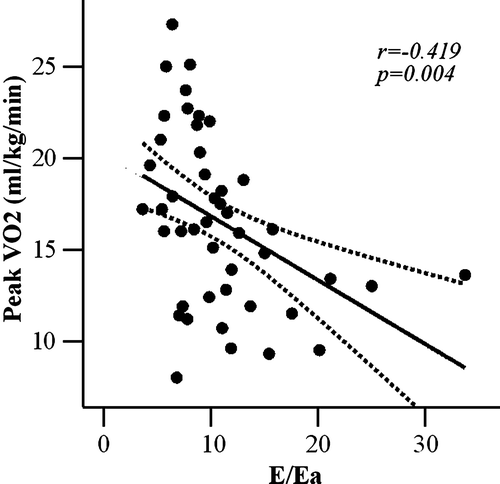
An abnormal transmitral LV filling relaxation pattern was observed in 36 patients (72%), while in 3 patients (6%) was observed a pseudonormal pattern, and in 11 patients (22%) a restrictive transmitral pattern. Patients with lower peak oxygen consumption had higher incidence of restrictive LV filling pattern compared with patients with higher peak oxygen consumption (7/17 vs 4/33, p = 0.036, ). An inverse relationship was found between peak oxygen consumption and E/Ea ratio (r = − 0.419, p = 0.004, ), confirming the relationship between exercise tolerance and diastolic dysfunction,
BNP and exercise tolerance
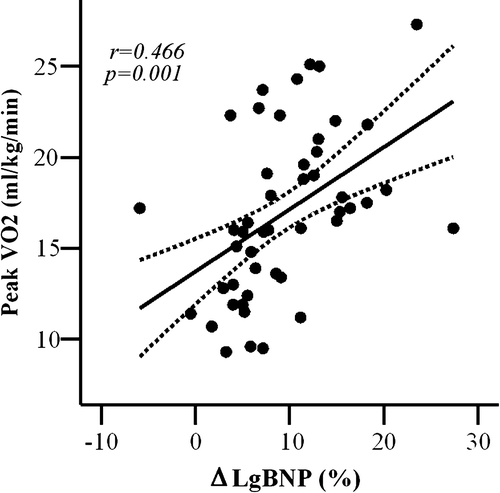
Because of nonlinear distribution of BNP values, logarithm transformation was performed. Peak oxygen consumption was inversely related to Lg value of BNP levels at baseline (r = − 0.448, p = 0.001, ) and at peak exercise (r = − 0.313, p = 0.03); in addition, peak oxygen consumption was directly related with% increase of Lg BNP during exercise (r = 0.466, p = 0.001, ).
Figure 3. Lg BNP at baseline plotted as a function of peak oxygen consumption (peak VO2). Curved lines show 95% confidence intervals for the fitted line.
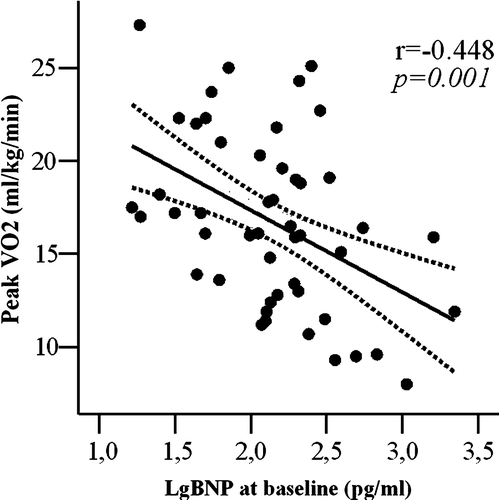
Figure 4. Increase in lg BNP levels during exercise (□ Lg BNP%) plotted as a function of peak oxygen consumption (peak VO2). Curved lines show 95% confidence intervals for the fitted line.
Based on the analyzed parameters, at multiple linear regression analysis independent predictors of exercise tolerance in patients with CHF were E/Ea (Beta: −0.393, p = 0.003), lg BNP at rest (Beta:−0.304, p = 0.034) and% increase in Lg BNP levels during exercise (Beta:0.288, p = 0.039); age, gender, LV end-diastolic diameter did not influence the exercise tolerance.
There were no differences in LV ejection fraction in the two patient groups with lower or higher peak oxygen consumption. LV ejection fraction did not predict exercise tolerance at multiple linear regression analysis.
Diastolic function and BNP
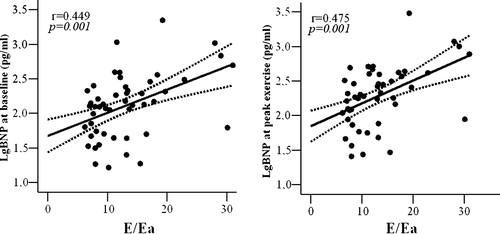
A significant relationship was found between diastolic dysfunction and BNP levels: restrictive transmitral LV filling pattern was, in fact, significantly related with BNP levels, either at rest (p = 0.001) or at peak exercise (p = 0.001). In addition, E/Ea ratio was directly related with BNP levels at baseline (r = 0.449, p = 0.001, ) and at peak exercise (r = 0.475, p = 0.001, ).
Figure 5. A. Early wave mitral flow (E) and early diastolic velocity mitral annulus (Ea) ratio plotted as a function of Lg BNP at baseline. Curved lines show 95% confidence limits for the fitted line. B. Early wave mitral flow (E) and early diastolic velocity mitral annulus (Ea) ratio plotted as a function of Lg BNP at peak exercise. Curved lines show 95% confidence intervals for the fitted line.
Discussion
In the present study we demonstrate that diastolic function and BNP levels can predict exercise tolerance in patients with stable chronic heart failure. Moreover, oxygen consumption can be predicted by changes in BNP levels during exercise, the latter being influenced also by diastolic dysfunction.
Diastolic function and exercise tolerance
Diastolic dysfunction influences signs and symptoms of CHF Citation6. It can be assessed by measuring transmitral flow patterns at the echocardiography, which are able to provide diagnostic and prognostic information in CHF patients Citation14, independently of peak oxygen consumption Citation15. Accordingly, in our study we demonstrated that patients with CHF and lower exercise tolerance have a more severe diastolic dysfunction, as confirmed by transmitral LV filling patterns (). However, transmitral flow patterns are influenced by multiple factors, including LV relaxation rate, atrial and ventricular compliance, and left atrial pressure Citation10. Therefore, mitral annular motion, assessed by tissue Doppler, has been introduced to correct for the influences of preload and myocardial relaxation on transmitral flow with excellent predictive value of diastolic filling in CHF patients Citation11. In addition, Hadano et al Citation16 demonstrated that E/Ea ratio is a strong predictor of reduced exercise tolerance. Accordingly, we found an inverse relationship between exercise tolerance and E/Ea ratio (), confirming the E/Ea ratio as the strongest predictor of exercise tolerance at multiple regression analysis.
Systolic function and exercise tolerance
LV ejection fraction was reduced in all CHF patients recruited, but comparable in the 2 different groups, irrespective of their exercise tolerance (Table I). This finding is supported by previous studies showing that ejection fraction failed to predict maximal exercise capacity Citation17, Citation18. Szlachcic et al. Citation18 observed that systolic function was poorly related with peak oxygen consumption in CHF patients, however, when analyzing only patients with severely impaired exercise tolerance, they showed a relationship between ejection fraction and peak oxygen consumption. Nevertheless, in a larger patient population, Cohn et al. demonstrated that LV ejection fraction alone was not related to peak oxygen consumption and it was unable to predict unfavorable outcome Citation2.
BNP and exercise tolerance
BNP release plays an important physiological role in facilitating cardiovascular performance during exercise Citation19. The increase in circulating BNP levels should be reflective of an increase in filling pressures and aims at promoting diuresis, natriuresis, arterial vasodilatation, and inhibition of sympathetic nervous activity Citation4. In our CHF patients, BNP levels, measured both at rest and at peak exercise, were inversely related with peak oxygen consumption (). Percent BNP increase during exercise was significantly higher in patients with better exercise tolerance, and directly related with peak oxygen consumption (). The increase of BNP with exercise was associated with better exercise capacity also in hypertensive patients with normal systolic function and BNP levels at baseline Citation20. In addition, McNair et al. showed that the percent change in BNP levels immediately after exercise was higher in patients with mild CHF and lower BNP levels at baseline, compared to patients with severe CHF and significantly higher BNP levels at baseline Citation21. Kruger et al. demonstrated a decrease in BNP levels at peak exercise in CHF patients that were older and in higher NYHA functional class Citation22. In agreement with these findings, in the present study CHF patients with lower exercise tolerance showed minimal or absent BNP increase. We can speculate that these latter patients, already presenting with higher BNP levels at rest, have exhausted their myocardial reserve of BNP during exercise. This can be due to the fact that BNP has minimal granule storage and its release appears to be directly related with ventricular volume expansion and pressure overload Citation23.
Diastolic function and BNP
In CHF patients with poorer exercise tolerance we observed a more severe diastolic dysfunction at rest (,) that results in higher BNP levels at baseline (). Matsumoto et al. demonstrated a greater increment in BNP during exercise in patients with dilated cardiomyopathy compared with patients with mitral stenosis, indicating that LV diastolic pressure is more important that left atrial pressure in determining BNP rise with exercise Citation24. Accordingly, at multiple regression analysis we demonstrated that E/Ea ratio and BNP increase during exercise are the strongest determinant of exercise tolerance in patients with CHF. In addition, diastolic dysfunction, assessed by transmitral flow patterns and Tissue Doppler, is directly related to BNP levels at baseline and at peak exercise (). Our results are in agreement with previous studies showing that BNP levels reflect the presence of severe diastolic dysfunction Citation7, Citation19, Citation24 and they are directly related with LV filling pressure Citation7.
Clinical implication
In this study, we demonstrated that diastolic dysfunction assessed by tissue Doppler and BNP levels at rest and the BNP changes during exercise are independent predictors of exercise tolerance. The possibility to obtain these crucial information very easily with standard echocardiography and blood sample collection, provides additional important diagnostic and prognostic tools in the risk stratification of CHF patients.
Study limitations
This study is an observational analysis of patients with stable chronic heart failure, prospectively, non-blinded design. Sample size is relatively small, and larger studies are warranted to further confirm our findings. A heterogeneous CHF patient population was recruited, even though this would better reflect the clinical usefulness of BNP and E/Ea in a “real-world” CHF patients.
Conclusion
In patients with stable chronic heart failure, diastolic dysfunction at rest is a predictor of exercise tolerance and is related to BNP levels both at rest and at peak exercise. A semiquantitative assessment of diastolic function may be part of the standard echocardiographic assessment in chronic heart failure patients.
Acknowledgements
The technical support of Mr. Felice de Rosa has been greatly appreciated.
References
- Redfield MM. Heart failure–an epidemic of uncertain proportions. N Engl J Med. 2002; 347: 1442–4
- Cohn JN, Johnson GR, Shabetai R, Loeb H, Tristani F, Rector T, et al. The V-HeFT VA Cooperative Studies Group. Ejection fraction, peak exercise oxygen consumption, cardiothoracic ratio, ventricular arrhythmias, and plasma norepinephrine as determinants of prognosis in heart failure. Circulation. 1993; 87: VI5–16
- Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004; 350: 655–63
- Adams KF, Jr, Mathur VS, Gheorghiade M. B-type natriuretic peptide: From bench to bedside. Am Heart J. 2003; 145: S34–S46
- de Groote P, Dagorn J, Soudan B, Lamblin N, McFadden E, Bauters C. B-type natriuretic peptide and peak exercise oxygen consumption provide independent information for risk stratification in patients with stable congestive heart failure. J Am Coll Cardiol. 2004; 43: 1584–9
- Bonow R, Udelson JE. Left ventricular diastolic dysfunction as a cause of congestive heart failure. Ann Intern Med. 1992; 117: 502–10
- Lubien E, DeMaria A, Krishnaswamy P, Clopton P, Koon J, Kazanegra R, et al. Utility of B-natriuretic peptide in detecting diastolic dysfunction. Circulation. 2002; 105: 595–601
- Swedberg C, Cleland J, Dargie H, Drexler H, Follath F, Komajda M, et al. The task force for diagnosis and treatment of chronic heart failure of the European Society of Cardiology. Guidelines for diagnosis and treatment of chronic heart failure: Update 2005. Eur Heart J. 2005; 26: 1115–40
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: A report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005; 18: 1440–63
- Nishimura RA, Tajik AJ. Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography is the clinician's rosetta stone. J Am Coll Cardiol. 1997; 30: 8–18
- Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. Doppler tissue imaging: A noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997; 30: 1527–33
- Fischer Y, Filzmaier K, Stiegler H, Graf J, Fuhs S, Franke A, et al. Evaluation of a new rapid bedside test for quantitative determination of B-type natriuretic peptide. Clin Chem. 2001; 47: 591–4
- Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Jr, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991; 83: 778–86
- Hansen A, Haass M, Zugck C, Krueger C, Unnebrink K, Zimmermann R, et al Prognostic value of Doppler echocardiographic mitral inflow patterns: Implications for risk stratification in patients with congestive heart failure. J Am Coll Cardiol. 2001;37:1049–55.
- Pinamonti B, Di Lenarda A, Sinagra G, Camerini F. Restrictive left ventricular filling pattern in dilated cardiomyopathy assessed by Doppler echocardiography: Clinical, echocardiographic and hemodynamic correlations and prognostic implications. J Am Coll Cardiol. 1993; 22: 808–15
- Hadano Y, Murata K, Yamamoto T, Kunichika H, Matsumoto T, Akagawa E, et al Usefulness of mitral annular velocity in predicting exercise tolerance in patients with impaired left ventricular systolic function. Am J Cardiol. 2006;97:1025–8.
- Pepi M, Agostoni PG, Marenzi GC, Doria E, Guazzi M, Lauri G, et al The influence of diastolic and systolic function on exercise performance in heart failure due to dilated cardiomyopathy or ischemic heart disease. Eur J Heart Failure. 1999;1:161–7.
- Szlachcic Z, Massie B, Kramer B, Topie N, Tubau J. Correlates and prognosis implication of exercise capacity in chronic congestive heart failure. Am J Cardiol. 1985; 55: 1037–42
- Clarkson PB, Wheeldon NM, MacFadyen RJ, et al. Effects of brain natriuretic peptide on exercise hemodynamics and neurohormones in isolated diastolic heart failure. Circulation. 1996; 93: 2037–42
- Mottram PM, Haluska BA, Marwick TH. Response of B-type natriuretic peptide to exercise in hypertensive patients with suspected diastolic heart failure: Correlation with cardiac function, hemodynamics, and workload. Am Heart J. 2004; 148: 365–70
- McNairy M, Gardetto N, Clopton P, Garcia A, Krishnaswamy P, Kazanegra R, et al Stability of B-type natriuretic peptide levels during exercise in patients with congestive heart failure: Implications for outpatient monitoring with B-type natriuretic peptide. Am Heart J. 2002;143:406–11.
- Kruger S, Graf J, Merx MW, Stickel T, Kunz D, Hanrath P, et al. Brain natriuretic peptide kinetics during dynamic exercise in patients with chronic heart failure. Int J Cardiol. 2004; 95: 49–54
- Nakagawa O, Ogawa Y, Itoh H, Suga S, Komatsu Y, Kishimoto I, et al. Rapid transcriptional activation and early m RNA turnover of BNP in cardiocyte hypertrophy: Evidence for BNP as an “emergency” cardiac hormone against ventricular overload. J Clin Invest. 1995; 96: 1280–7
- Matsumoto A, Hirata Y, Momomura S, Suzuki E, Yokoyama I, Sata M, et al. Effects of exercise on plasma level of brain natriuretic peptide in congestive heart failure with and without left ventricular dysfunction. Am Heart J. 1995; 129: 139–45