Abstract
Objectives. We recently demonstrated reduced exercise capacity in phlebotomy treated genetic haemochromatosis in spite of normal systolic function. The present objective was to investigate diastolic function at rest. Design. Diastolic function was echocardiographically assessed in 132 phlebotomy treated genetic haemochromatosis patients and 50 controls. Results. Patients had higher body mass index and heart rate, higher transmitral early (E) (11.2±2.6 versus 10.4±2.2 cm) and atrial (A) (5.7±1.6 versus 5.0±1.6) velocity time integrals, pulmonary venous systolic peak velocity (0.58±0.12 versus 0.54±0.13 m/s) and ratio of E to spectral tissue Doppler E′ velocity (6.3±1.6 versus 5.6±1.4, all p <0.05). Independently of age, heart rate, systolic blood pressure and body weight, having haemochromatosis remained statistically significantly associated with higher E (β =0.27) and A (β =0.18) velocity time integrals, pulmonary venous systolic peak velocity (β =0.21), and E/E′-ratio (β =0.25) in separate multivariate analyses (all p <0.05). In the youngest age tertile, patients had longer isovolumic relaxation time and lower E′ than controls. Conclusion. Our findings are compatible with mildly impaired diastolic function in treated haemochromatosis, with delayed relaxation in the younger tertile, and an elevated filling pressure and pseudonormalisation with increasing age.
For almost 100 years following the first descriptions of haemochromatosis, there was no effective treatment against the iron overload, and dilated cardiomyopathy was the most frequent cardiac manifestation. From around 1950 repeated phlebotomy has been offered, and since then cardiac complications have been rare and the population of treated patients large, especially in Northern Europe. We recently reported that haemochromatosis treated with regular phlebotomy influences longitudinal left ventricular function, and is associated with reduced peak oxygen uptake Citation1, Citation2. Myocardial deposit disease often impedes with diastolic function before systolic impairment can be detected Citation3, and details about diastolic function in large series of treated patients with haemochromatosis have not been reported. Thus, the aim of the present study was to assess diastolic left ventricular function in haemochromatosis patients receiving regular phlebotomy treatment.
Material and methods
Patients
All 170 haemochromatosis patients who were treated regularly with phlebotomy at the Blood bank at Haukeland University Hospital in October 2001 were identified through the patient administrative system and invited to participate. One hundred and fifty three responded, all positively. They all had genetic haemochromatosis, meeting the major criterion of s-ferritin > 300 µg/L (men) and > 250 µg/L (women) respectively, and additionally at least one minor criterion (C282Y homozygous or heterozygous, H63D homozygous; or transferrin saturation > 50% (men) and > 40% (women) respectively.) One C282Y homozygous patient had been blood donor more than ten times before s-ferritin was ever measured, and was included even though the highest s-ferritin measured was below the diagnostic limit. The exclusion criteria were coronary heart disease, valvular heart disease, diabetes mellitus, and daily use of any cardiovascular medication. Thus, 21 patients were excluded, and the remaining 132 make up the population of the present study, together with 50 healthy, sex and age matched, blood donors serving as controls. The study was approved by the Regional ethical committee, and written informed consent was obtained from all participants.
The participants filled out a standardised questionnaire otherwise used for patients with coronary disease in our department, providing self-reported, relevant information about coronary risk factors, ethanol consumption, physical exercise, other diseases and medical drug treatment.
Blood samples were taken from fasting patients and analysed for haematological indices, electrolytes, liver and heart enzymes. Also ferritin, transferrin, iron, and the genotype of the HFE gene (presence or absence of the two most important mutations C282Y and H63D) were determined. Two thirds of the patients were genotyped using gene electrophoresis and restriction enzyme technique, one third using a real time polymerase chain reaction DNA analysis. Blood samples were drawn on the day of echocardiography, which for both patients and controls followed atleast 5 days after the last phlebotomy.
Height, weight and blood pressure were measured, and 12-lead ECG recorded. Due to ethical considerations the controls did not have their genotype analysed, but otherwise underwent an identical protocol.
Echocardiography
All participants were examined in the left recumbent position with an Acuson Sequoia c256 echocardiograph (Acuson, Mountainview, California, USA) using a 2.5–3.5 MHz transducer and 2nd harmonic imaging. The recordings were stored on analogous videotapes and digitally in Dicom format on a KinetDx computer (Siemens, München, Germany). All but 4 of the echocardiograms were performed by one physician. Off-line measurements were made with Image Arena® (TomTec, Unterschleissheim, Germany).
Left ventricular dimensions were measured in parasternal long-axis two-dimensional view, according to the guidelines of the American Society of Echocardiography Citation4. Left ventricular mass was calculated according to Devereux’ formula Citation5 and indexed for body surface area. Left atrial diameter was measured from the trailing edge of the atrioaortic complex to the leading edge of the posterior wall. Based on apical four-chamber and apical long-axis views left ventricular volumes and ejection fraction were calculated from modified Simpson's biplane formula Citation4, Citation6. When long-axis view was unavailable, four-chamber view was used for single-plane left ventricular volume.
All spectral Doppler recordings were displayed with 100 mm/s sweep speed. Transmitral flow was recorded with pulsed wave Doppler between the mitral cusp tips in apical four-chamber view, using low gain and a gate of 3 mm Citation7. The early (E) and atrial (A) waves were traced for peak velocities and for velocity time integrals. Isovolumic relaxation time was recorded in apical five-chamber view with a gate of 5 mm and measured from the leading edge of the aortic valve closure spike to the leading edge of the mitral valve high intensity echo. On the pulsed wave Doppler recording from a right pulmonary vein the peak velocity and the velocity time integrals were measured on the systolic and on the diastolic components, using the time of lowest velocity between them as the separation line. Duration and peak velocity of the A wave were measured Citation8. Spectral tissue Doppler was recorded as previously described Citation2 and early diastolic tissue velocity (E’) measured. We used the average E’ from lateral and septal corner of apical four-chamber and posterior and septal of apical long-axis views, and calculated the E/E′-ratio.
Statistics
The data were analysed with SPSS 12.0.0 (SPSS Inc, Chicago, USA). Values are given as mean±S.D. Between-group comparisons were made by independent t-test for continuous variables and by χ2 test for nominal variables. Correlations were assessed using Pearson's correlation coefficient and multiple regression analyses using an enter method.
In order to avoid interpretational problems due to age-related changes in diastolic parameters and disease alterations, we decided in advance to analyse diastolic parameters separately in age tertiles. Tertile limits were 42.6 and 52.3 years, respectively.
Results
The demographic data for the 132 patients and 50 controls are given in , which also summarizes the biochemical data. s-Ferritin ranged from 6 to 377 µg/L among the patients, and from 7 to 240 among the controls. The s-ferritin measured at the time when haemochromatosis was diagnosed, ranged from 126 to 3 460 µg/L, with mean value 705 and median 475 µg/L. Only 19 of the patients had ever measured s-ferritin >1 000 µg/L, 8 had >2 000 µg/L.
Table I. Demographic and biochemical data.
ECG demonstrated sinus rhythm in all participants, and no cases of bundle branch block. QRS-duration was shorter among the patients (98±12 versus 104±15 ms, p = 0.02), which was probably related to left ventricular size, as QRS-duration correlated with left ventricular end-diastolic diameter (r = 0.177, p = 0.018).
The left ventricular end-diastolic diameter was smaller among the patients, but left ventricular mass index and systolic function did not differ between the groups (). Higher left ventricular mass index correlated with lower E, longer E deceleration time, larger atrium, lower E′, and higher heart rate.
Table II. Echocardiographic dimensions and systolic function.
E- and A-velocity time integrals, A-velocity, E/E′-ratio, and pulmonary venous systolic velocity were higher among the patients (). Age, heart rate, and systolic blood pressure are known to influence diastolic Doppler parameters, and body weight was significantly higher in the patient group. Thus, heart rate, age, weight, and systolic blood pressure were chosen as covariates for multiple regression models, with E and A velocity time integrals, pulmonary venous systolic peak velocity, and E/E′-ratio entered separately as dependent variable. In these four models, having haemochromatosis was independently associated with higher transmitral E and A velocity time integrals, pulmonary venous systolic peak velocity, and E/E′-ratio, and remained so also when left ventricular mass index was entered among the independent covariates ().
Table III. Diastolic functional parameters.
Table IV. β coefficients from multiple regression models for four different diastolic function parameters: Transmitral E and A velocity time integrals, pulmonary venous S velocity, and ratio of E to spectral tissue Doppler E′ velocity. P < 0.001 for all R2.
E/E′-ratio correlated with s-ferritin (r = 0.242, p = 0.003) and transferrin saturation (r = 0.178, p = 0.032), while the other parameters of diastolic left ventricular function did not. Peak s-ferritin, available only in the patient group, did not correlate with any diastolic parameters.
The study population was further analysed after splitting into age tertiles. In the youngest tertile, patients had longer isovolumic relaxation time and lower E′ than controls (). Multiple regression models in the youngest tertile showed longer isovolumic relaxation time to be associated with haemochromatosis independently of significant contributions from higher age and systolic blood pressure, and lower weight (multiple r2=0.21, p = 0.026), and lower E′ to be associated with haemochromatosis independently of significant contributions from higher age and systolic blood pressure (multiple r2=0.39, p < 0.001). shows how differently E and A velocity, E′, and E/E′-ratio correlated with age among controls and patients. Among patients E velocity did not diminish, and A velocity and E/E′-ratio increased more than among controls.
Figure 1. Haemochromatosis patients: dark circles, solid regression lines. Controls: open circles, dashed regression lines. A. Transmitral early diastolic peak velocity decreased less among haemochromatosis patients (E = 0.77–0.00032×age) than among controls (E = 1.00–0.0058×age). B. Transmitral atrial peak velocity increased more among haemochromatosis patients (A = 0.29 + 0.0070×age) than among controls (A = 0.43 + 0.0027×age). C. Spectral tissue Doppler early diastolic peak velocity decreased similarly in the two groups (patients: E′ = 18.0–0.12×age; controls: E′ = 21.0–0.16×age). D. E/E′-ratio increased more among haemochromatosis patients (E/E′ = 3.38 + 0.063×age) than among controls (E/E′ = 4.44 + 0.024×age).
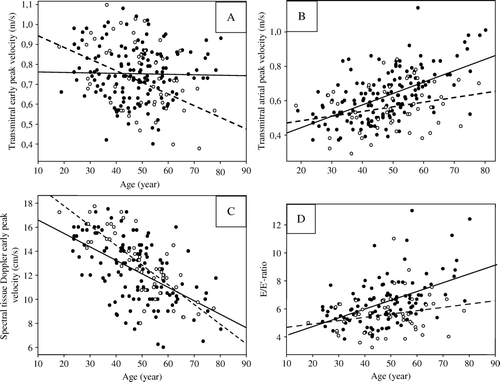
Table V. Diastolic parameters, split between the younger (A) and older (B) tertiles of participants.
Discussion
This is among the first studies to evaluate left ventricular diastolic function in a large series of phlebotomy treated haemochromatosis patients. Several findings point to mildly impaired diastolic function among the patients: 1) higher E/E′-ratio, 2) increased systolic pulmonary venous peak velocity, and 3) larger A wave, and 4) impaired relaxation in the youngest tertile.
With increasing age, E decreased in the control group as expected, while it remained unchanged among the patients. Thus in the oldest tertile, E was higher among patients than among controls, probably representing pseudonormalisation among the patients, indicative of increased filling pressure. On the other hand, spectral tissue Doppler E′ decreased with age, as expected Citation9, and this decrease was similar in the two groups. Thus, the E/E′-ratio, which has recently been suggested as a superior echocardiographic indicator of early diastolic left ventricular filling pressure, particularly in persons with normal ejection fraction Citation10, was higher among the patients, and increasingly so with age. Left ventricular relaxation is known to be retarded by increasing mass, but in multiple regression analysis in the entire study population, higher E/E′-ratio remained associated with haemochromatosis independently of contribution from increasing age and systolic blood pressure, also when left ventricular mass index was added to the model.
The pulmonary venous inflow had, for all age groups combined, a larger systolic component, whether measured as peak velocity or velocity time integral. In multiple regression model higher systolic pulmonary venous inflow was associated with haemochromatosis, independently of significant contribution from increasing age. Thus, the difference seems to reflect a slower diastolic relaxation, shifting the pulmonary venous inflow from early diastole to systole Citation11.
The A velocity and velocity time integral were larger among the patients. In a multiple regression model larger A velocity time integral remained associated with haemochromatosis, independently of significant contributions from increasing age, and independently of left ventricular mass index. These findings suggest that a larger volume of blood remained in the atrium during early diastole and flew transmitrally in late diastole during the atrial contraction, a previously described consequence of delayed relaxation in early diastole Citation12. Yet, we found no sign of increased end-diastolic pressures, as the transmitral A wave duration did not differ between the groups. Thus, the patients apparently had a diastolic pattern of slow relaxation, rather than of reduced compliance, suggesting – if the underlying cause should be iron accumulation in the myocardium – that disturbed metabolism is the mechanism, rather than the direct mechanical consequences of the deposits.
Impaired diastolic relaxation appears in three different patterns; delayed relaxation, pseudonormalisation, and restrictive pattern, in the order of increasing severity Citation11. Longer isovolumic relaxation time and lower E′, as were found among the patients in the youngest tertile, is a typical pattern of mildly impaired relaxation at this age, and in multiple regression models they remained associated with haemochromatosis, independently of significant contributions from increasing age and systolic blood pressure, both well known covariates of myocardial relaxation Citation9, Citation13–15.
Smaller, previous studies have yielded conflicting results regarding haemochromatosis influence on myocardial function Citation16, Citation17. The definition of the study population may have crucial importance for the results, providing a possible explanation to different findings in different studies. Palka et al. Citation16 studied 18 patients, of whom 22% were medically treated for clinical congestive heart failure, and reported increased end-diastolic left ventricular volume and decreased ejection fraction, decreased systolic and early diastolic tissue velocities by spectral tissue Doppler, and prolonged atrial reversal in the pulmonary veins, but almost no differences by conventional parameters for diastolic function. Shizukuda et al. Citation17 described 43 asymptomatic, C282Y homozygous patients, reporting larger A among the patients, otherwise mainly no group differences, and concluding that there were no statistically significant abnormalities of Doppler left ventricular relaxation in their haemochromatosis groups, compared to their controls. The present work, by far the largest echocardiographic study of haemochromatosis patients, confirms the tendency of non-specific, mildly impaired diastolic function, causing significant differences in some parameters at rest, as well as reduced exercise capacity Citation1.
Clinical implications
All participants in this study, patients as well as controls, were free from clinical signs and symptoms of heart failure. HFE mutations have low penetrance. The risk of developing clinical end organ disease from haemochromatosis is suggested to be around 1%, even for C282Y homozygous persons Citation18, Citation19, or 1–2 patients in our population. It has been estimated that organ manifestations secondary to iron overload develop only when the total removable body iron exceeds 20–40 g iron (equivalent to s-ferritin 2 700–5 300 µg/L) Citation20, while only a few of our patients had ever had s-ferritin >2 500 µg/L. Moreover, most of them were de-ironed already before the echocardiographic examination. In the cases of small, yet statistically significant differences between the groups, the mean values of either group still were within normal ranges, as were most of the individual values. Thus, echocardiographic findings corresponded well to the clinical and biochemical situation. We interpret this as signs of subtle differences between treated haemochromatosis patients and healthy blood donors, mainly an impaired early diastolic relaxation and a higher filling pressure, traceable at a group level, even when not at an individual level. Long-term follow-up studies of phlebotomy treated haemochromatosis patients using exercise echocardiography and evaluation of diastolic left ventricular function including spectral tissue Doppler are needed for evaluation of clinical implication of our findings.
Limitations
There was no strict randomisation procedure for selecting which blood donors should be invited as controls. Even though the control group was matched for sex and age, this lack of random selection may have influenced our results.
The findings of this study do not tell anything about whether therapeutic phlebotomy prevents haemochromatosis related myocardial damage, as it is a case-control study rather than a randomised, prospective study.
ERRATA
[10.1080/14017430802203811]
The iFirst version of this article published online ahead of print on 26 Jun 2008 contained an error in Figure 1. In panel B the text “Transmitral early peak velocity (m/s)”. should have read “Transmitral atrial peak velocity (m/s)” There was also an error in Table 2. Left ventricular mass index, the values for patients and controls had switched places. Under patients the value should be 72±18, under controls the value should be 77±19. The corrected version is shown in this issue.
Acknowledgements
We thank Jorunn Vadheim at the Blood Bank, Haukeland University Hospital, for organising the patient flow, Knut Liseth for the clinical examinations and follow-up, and Britt Gjellefall and Sarita K. Frette for technical assistance during the echocardiographic examinations. The biochemical analyses were performed at the Clinical Biochemical Laboratory and HFE-gene testing at the Centre for Medical Genetics and Molecular Medicine, Haukeland University Hospital.
References
- Davidsen ES, Liseth K, Omvik P, Hervig T, Gerdts E. Reduced exercise capacity in genetic haemochromatosis. Eur J Cardiovasc Prev Rehabil. 2007; 14: 470–5
- Davidsen ES, Hervig T, Omvik P, Gerdts E. Left ventricular long-axis function in treated haemochromatosis 2007 (submitted).
- Zabalgoitia M, Ismaeil MF, Anderson L, Maklady FA. Prevalence of diastolic dysfunction in normotensive, asymptomatic patients with well-controlled type 2 diabetes mellitus. Am J Cardiol. 2001; 87: 320–3
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006; 7: 79–108
- Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am J Cardiol. 1986; 57: 450–8
- Malm S, Sagberg E, Larsson H, Skjærpe T. Choosing apical long-axis instead of two-chamber view gives more accurate biplane echocardiographic measurements of left ventricular ejection fraction: A comparison with magnetic resonance imaging. J Am Soc Echocardiogr. 2005; 18: 1044–50
- Appleton CP, Jensen JL, Hatle LK, Oh JK. Doppler evaluation of left and right ventricular diastolic function: A technical guide for obtaining optimal flow velocity recordings. J Am Soc Echocardiogr. 1997; 10: 271–92
- Rossvoll O, Hatle LK. Pulmonary venous flow velocities recorded by transthoracic Doppler ultrasound: Relation to left ventricular diastolic pressures. J Am Coll Cardiol. 1993; 21: 1687–96
- Tighe DA, Vinch CS, Hill JC, Meyer TE, Goldberg RJ, Aurigemma GP. Influence of age on assessment of diastolic function by Doppler tissue imaging. Am J Cardiol. 2003; 91: 254–7
- Quiñones MA. Assessment of diastolic function. Prog Cardiovasc Dis. 2005; 47: 340–55
- Cohen GI, Pietrolungo JF, Thomas JD, Klein AL. A practical guide to assessment of ventricular diastolic function using Doppler echocardiography. J Am Coll Cardiol. 1996; 27: 1753–60
- Kitabatake A, Inoue M, Asao M, Tanouchi J, Masuyama T, Abe H, et al. Transmitral blood flow reflecting diastolic behavior of the left ventricle in health and disease–a study by pulsed Doppler technique. Jpn Circ J. 1982; 46: 92–102
- Munagala VK, Jacobsen SJ, Mahoney DW, Rodeheffer RJ, Bailey KR, Redfield MM. Association of newer diastolic function parameters with age in healthy subjects: A population-based study. J Am Soc Echocardiogr. 2003; 16: 1049–56
- Pela G, Bruschi G, Cavatorta A, Manca C, Cabassi A, Borghetti A. Doppler tissue echocardiography: Myocardial wall motion velocities in essential hypertension. Eur J Echocardiogr. 2001; 2: 108–17
- Wilkenshoff UM, Hatle L, Sovany A, Wranne B, Sutherland GR. Age-dependent changes in regional diastolic function evaluated by color Doppler myocardial imaging: A comparison with pulsed Doppler indexes of global function. J Am Soc Echocardiogr. 2001; 14: 959–69
- Palka P, Macdonald G, Lange A, Burstow DJ. The role of Doppler left ventricular filling indexes and Doppler tissue echocardiography in the assessment of cardiac involvement in hereditary hemochromatosis. J Am Soc Echocardiogr. 2002; 15: 884–90
- Shizukuda Y, Bolan CD, Tripodi DJ, Yau YY, Nguyen TT, Botello G, et al. Significance of left atrial contractile function in asymptomatic subjects with hereditary hemochromatosis. Am J Cardiol. 2006; 98: 954–9
- Beutler E, Felitti VJ, Koziol JA, Ho NJ, Gelbart T. Penetrance of 845G( A (C282Y) HFE hereditary haemochromatosis mutation in the USA. Lancet. 2002; 359: 211–8
- Åsberg A, Hveem K, Thorstensen K, Ellekjær E, Kannelønning K, Fjøsne U, et al. Screening for hemochromatosis: High prevalence and low morbidity in an unselected population of 65,238 persons. Scand J Gastroenterol. 2001; 36: 1108–15
- Limdi JK, Crampton JR. Hereditary haemochromatosis. QJM. 2004; 97: 315–24