Abstract
Background. YKL-40 is involved in remodelling and angiogenesis in non-cardiac inflammatory diseases. Aim was to quantitate plasma YKL-40 in patients with ST-elevation myocardial infarction (STEMI) or stable chronic coronary artery disease (CAD), and YKL-40 gene activation in human myocardium. Methods and results. We included 73 patients: I) 20 patients with STEMI; II) 28 patients with stable CAD; III) 15 CAD patients referred for coronary by-pass surgery. YKL-40 mRNA expression was measured in myocardium subtended by stenotic or occluded arteries and areas with no apparent disease; and IV) 10 age-matched healthy controls. Plasma YKL-40 was significantly increased in patients with STEMI (88 µg/l, median) and CAD (66 µg/l) compared to controls (16 µg/l, p<0.01 for both). Plasma YKL-40 correlated with CRP at baseline in STEMI (r=0.53, p=0.02) and CAD patients (r=0.41, p=0.031).YKL-40 gene expression was similar in ischemic and non-ischemic myocardium. Conclusions. Plasma YKL-40 was significantly increased in patients with STEMI and stable CAD. Further studies will define the role of YKL-40 as a clinically useful marker for myocardial ischemia, remodelling and maybe prognosis.
It has become increasing clear, that cardiovascular disease has a significant inflammatory component. The contribution of inflammation in the acute stage of myocardial infarction and during remodeling after acute myocardial infarction has attained increasing recognition. A number of clinical studies have investigated serum CRP in cardiovascular disease Citation1, Citation2 and high serum CRP has been found useful as a predictor of myocardial infarction, peripheral arterial disease Citation3, Citation4.
YKL-40 is a 40 kDa plasma glycoprotein and a member of “mammalian chitinase-like proteins” Citation6, which is secreted in vitro by differentiated and activated monocytes Citation7 and neutrophils Citation8, vascular smooth muscle cells (VSMC) Citation9, cancer cells Citation6 and arthritic chondrocytes Citation10. Macrophages in atherosclerotic plaques express YKL-40 mRNA, particularly macrophages that have infiltrated deeper in the lesion, and the highest YKL-40 expression is found in macrophages in the early lesion of atherosclerosis Citation11.
In patients, YKL-40 has been found elevated in plasma or serum in different diseases characterized by inflammation, increased extracellular remodelling and ongoing fibrosis such as infections Citation12, endotoxaemia Citation13, rheumatoid arthritis Citation10, inflammatory bowel disease Citation14, giant cell arteritis Citation15, and type II diabetes Citation16.
It has recently been discovered, that serum YKL-40 is elevated in patients with coronary artery disease, and there was an association between serum YKL-40 and the extent of coronary artery disease defined by the number of diseased vessels assessed by coronary angiography Citation17. It could therefore be speculated that YKL-40 is involved in the inflammatory and remodelling processes in both acute and chronic coronary artery disease. The finding, that YKL-40 expression also has been demonstrated in the developing rat fetal heart, further supports this hypothesis Citation9.
The aims of the present study was to test the hypotheses, Citation1 that plasma YKL-40 is increased in patients with acute myocardial infarction or chronic stable coronary artery disease; Citation2 that there is an association between plasma YKL-40 and markers of acute myocardial injury and serum CRP; and Citation3 that the myocardial activation of YKL-40 gene expression would be increased in the myocardium subtended by stenotic or occluded arteries, when compared to non-ischemic myocardium.
Patients and methods
Patient population
We included:
Twenty consecutive patients with ST-elevation myocardial infarction (STEMI) (17 men and 3 women; mean age 58 years) treated with percutaneous coronary intervention (PCI) within 12 hours after the onset of symptoms (STEMI group). The STEMI-diagnosis was based on typical chest pain at rest lasting > 30 minutes, and the presence of ST segment elevations > 0.4 mV in 2 or more contiguous leads on a standard 12-lead electrocardiogram and a serum CK-MB level greater than twice the normal value;
Twenty eight patients with severe chronic stable coronary artery disease (25 men and 3 women; mean age 62 years) (chronic ischemia group); reversible ischemia at an adenosine stress single photon emission computerized tomography (SPECT); age above 30 years; Canadian Cardiovascular Society angina classification (CCS) 2-3; left ventricular ejection fraction ≥40%; and coronary angiography with significant coronary artery disease ineligible for revascularization. Excluded were patients with unstable angina pectoris, acute myocardial infarction within the last three months, NYHA class IV, diabetes mellitus with proliferative retinopathy, diagnosed or suspected cancer disease and chronic inflammatory disease;
Fifteen patients (13 men and 2 women; mean age 68 years) with stable coronary artery disease, who underwent coronary bypass surgery (CABG group) were enrolled for analysis of YKL-40 mRNA expression in the myocardium. Transmural myocardial biopsies were taken during by-pass operation as described below; and
Ten patients (9 men and 1 women; mean age 55 years), referred on the basis of suspicion for angina pectoris, but who showed to have a normal coronary angiogram (control group).
Blood samples
In the STEMI group, a baseline sample of 10 ml venous blood samples was obtained within 24 hours after admission at day 1 as baseline, and followed by blood samples day 3, 7 and 28 after onset of STEMI. In the stable coronary artery disease group and in the control group 10 ml venous blood samples were obtained after 15 min in resting supine position. Blood samples were colleted in ice-chilled glass. EDTA plasma concentration of YKL-40 was determined by a commercial two-site, sandwich-type enzyme-linked immunosorbent assay (Quidel, CA, USA) using streptavidin coated microplate wells, a biotinylated-Fab monoclonal capture antibody, and an alkaline phosphatase-labeled polyclonal detection antibody. The sensitivity of the ELISA was 20 µg/l. The intra-assay and inter-assay CVs were <5.0% and <10.2%, respectively. CRP was measured with an immunoturbidimetric analysis.
Biopsy of myocardium
In the CABG group transmural myocardial biopsies were taken from areas of angiographic defined hypoperfusion and chronic ischemic myocardium caused by significant coronary artery stenosis > 70%, and from an area of normally perfused ventricular tissue (no coronary artery stenosis). Biopsies were taken by a 20 mm 14-gauge Tru-cut biopsy needle three times during cardiopulmonary bypass (CPB) grafting. Thus, each patient served as his or hers own control. No biopsies were taken from grossly infarcted fibrotic myocardium. Biopsies were taken: I) at baseline before initiation of cardioplegia; II) immediately after termination of CPB grafting; and III) 30 minutes after blood reperfusion of the heart. Biopsy sites were closed with polypropylene sutures. All specimens were immediately frozen in liquid nitrogen at −140°C after sectioning and stored at −80°C until analysis.
High-density oligonucleotide micro-array technology
Total RNA isolated from 6 myocardial biopsies collected from each of 6 patients was included in the microarray analysis protocol. A total of 33 microarray analyses were performed due to 3 samples drop out. In order to obtain sufficient material for oligonucleotide micro-array analysis, RNA amplification was performed on 0.1 µg of total RNA using the poly-A directed T7-promoter/polymerase based “linear” amplification protocol described by Scheidl et al. Citation18 and the standard Affymetrix protocol by using a T7 polymerase based kit (ENZO Bioarray™, High Yield™ RNA Labelling Kit, Farmingdale, NY, US distributed by Affymetrix) to generate biotin-labelled cRNA probe. Then, micro-DNA arrays were performed on Affymetrix HG_U133 A Genechip® (Affymetrix GeneChip® Expression Analysis. Technical Manual. Affymetrix Inc., USA) and analysed as previous described previously Citation19. From the analysis of these 33 Affymetrix U133A global gene arrays, based on biopsies from 6 patients, we probed for changes of YKL-40 and a group of genes related to inflammation. Data were analyzed for changes in gene expression comparing chronic ischemic tissue with normally perfused tissue as well as changes during the course of acute ischemia and reperfusion.
Gene expression by Real-time Quantitative PCR
In order to measure gene expression by quantitative PCR, cDNA was synthesized from 0.5 µg of RNA from each biopsy sample using a standard protocol and then diluted 10-fold. Using 3 µl of cDNA sample in each reaction, the expression of selected genes was analyzed by quantitative PCR in the larger group of 15 patients in order to extend our observations from the oligonucleotide microarray study. Thus, the expression of YKL-40 (Hs00609691_m1) was measured on the ABI PRISM 7700 Sequence Detector System (Applied Biosystems [AB], Foster City, CA, USA). The gene expression levels of YKL-40 was determined by real-time RT-PCR and related to expression of the housekeeping gene β-actin. β-actin: Forward: CCT TTT TGT CCC CCA ACT TGA, Reverse: TGG CTG CCT CCA CCC A, Probe: ATG TAT GAA GGC TTT TGG TCT CCC TGG GA; YKL-40: Forward GCC TGG CAA GGG AAT TTC TT, Reverse: TGC CAA AAT GGT GTC CTT TG, Probe: AAC TCC CTG CCC CCT AGC CCT CC. Both probes were labeled with FAM (6-carboxy-fluorescein) as reporter in the 5′ end and the quencher TAMRA (6-carboxy-tetramethyl-rhodamine) in the 3′ end. Both Primer sets spanned introns to reduce the risk of amplifying genomic DNA. Primers were purchased from Invitrogen, Denmark and probes from MWG, Germany. All the reactions were 30 µl (reaction mixture: 15 µl Platium QPCR supermix-UDG supplemented with + Rox reference dye, 1.5 µl primer forward (10 µM) and 1.5 µl primer reverse (10 µM), 0.4 µl probe (0.625 µM for β-actin and 2.5 µM for YKL-40), 10.6 µl H2O and 2.0 µl cDNA), and each sample was run in duplicate. Two “no-template-control” samples were included for each run. The thermal cycling conditions included 2 minutes at 50°C and 10 minutes at 95°C followed by 45 cycles of 95°C for 15 seconds and 58°C for 1 minute. All reactions were performed on an Mx3000 (Stratagene, AH-diagnostics, Denmark). Collection and analysis of data were done with the Mx3000 version 2.0 software for Windows (Stratagene, AH-diagnostics, Denmark). All assays have been tested in logarithmic dilutions of template to verify that the assays have comparable affinity at high and low expression levels.
Statistical analysis
All results are expressed as means±SD or median and inter quartile range (IQR), where appropriate. Due to small sample size and a non-Gaussion distribution pattern of results, non-parametric testing was chosen. The gene expression data obtained from the microarray experiments were processed and normalized using the protocol and program provided by the manufacture as described in the method section. Comparison of plasma YKL-40 levels between different groups as well as comparison of gene expression between ischemic area and non-ischemic area were analysed by using Mann-Whitney's U test, one-way ANOVA, and Kruskal-Wallis test where appropriate. Sequential changes measured within the different area by using the Wilcoxon signed-ranks test for paired samples. Correlation coefficient was calculated by non-parametric Spearman test. When the distribution of the values was not normal, log transformed data were used for correlation studies. Analyses other than done with GeneSpring software were performed with the SPSS statistical analysis program (SPSS version 11.0, SPSS INC., Chicago, Il, USA). A p-value of < 0.05 was considered to be statistically significant.
Results
Patient characteristics
The demographic characteristics of the patients are presented in . All STEMI patients had the standard medication according to guidelines and complete reperfusion with Thrombolysis in Myocardial Infarction (TIMI) grade 2-3 flow after primary PCI with stent implantation. All patients with chronic stable coronary artery disease had severe stable angina pectoris and limited exercise capacity. These patients were treated with more than one anti-angina drug and had previously had at least one coronary artery by-pass operation and/or PCI.
Table I. Demographics and clinical data of controls, patients with chronic myocardial ischemia, patients with STEMI and the patients treated with CABG.
Plasma YKL-40 in acute and stable coronary artery disease
Plasma level of YKL-40 in STEMI patients (day 1), patients with stable coronary artery disease and controls are shown in . Plasma YKL-40 was significantly higher in patients with acute myocardial infarction (88 µg/l [44–132], median [25th/75th percentile]) and in patients with stable coronary artery disease (66 µg/l [49–102]) when compared to controls (16 µg/l [14–37]) (p < 0.01 for both). When compared with control subjects plasma YKL-40 was elevated 5 fold and 7 fold in patients with stable coronary artery disease and STEMI, respectively. In patients with STEMI, plasma YKL-40 was significantly increased on day 1 and decreased over time post STEMI (, p < 0.05 for all), but remained elevated (33 µg/l [24–55], p = 0.01) one month after STEMI when compared with controls. Furthermore, the median plasma YKL-40 at day 1 in the STEMI patients was non-significant when compared to the chronic ischemic group ().
Figure 1. Plasma concentrations of YKL-40 in patients with STEMI or stable chronic coronary artery disease, and in control subjects. The difference between plasma YKL-40 in the two patients groups was not statistically significant (p=0.57). Plasma YKL-40 concentrations in the patients were significantly higher than in controls. p < 0.01. Results are indicated as box and whiskers plots, median, range, 25th/75th percentile, round marker indicates outliers and the star-shaped points represent those extremes.
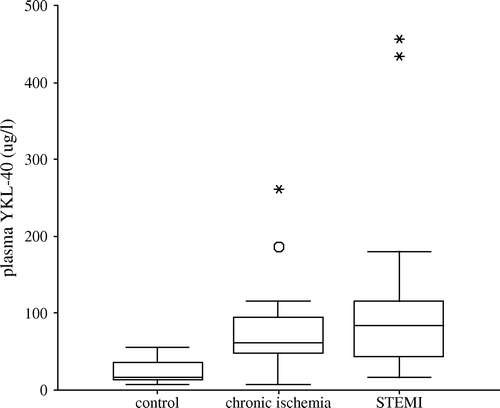
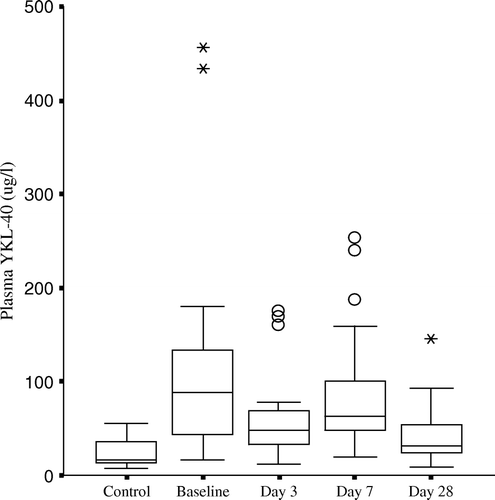
Figure 2. Plasma YKL-40 during the first month after acute STEMI in patients treated with primary PCI. Values are indicated as box and whiskers plots, median, range and 25th/75th percentile, the round markers indicate outliers and the star-shaped points represent those extremes. Plasma YKL-40 were significantly higher at baseline than day 3, day 7 and day 28, p < 0.05. Plasma YKL-40 concentrations remained elevated one month after STEMI when compared with controls.
Correlation between plasma YKL-40 and other biochemical markers of inflammation
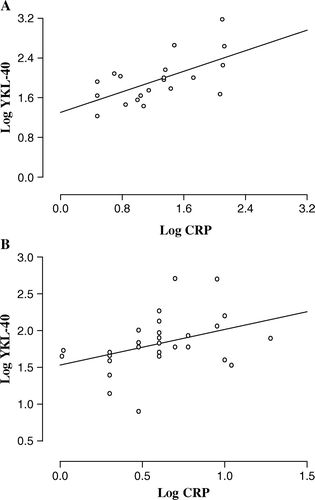
Plasma YKL-40 correlated significantly with serum c reactive protein (CRP) at baseline in STEMI patients (r = 0.53, p = 0.02, A) and in patients with stable coronary artery disease (r = 0.41, p = 0.031, B). There was no correlation between YKL-40 and CRP at later time points in patients with STEMI. In addition, there was no significant association in STEMI patients between baseline plasma YKL-40 and maximal serum creatine kinase fraction B (CK-MB), baseline erythrocyte sedimentation rate, age, diabetes and ejection fraction.
Figure 3. Correlation between plasma YKL-40 and serum C reactive protein (CRP) in STEMI patients A (r = 0.53, p=0.02) and in patients with stable coronary artery disease B (r = 0.41, p = 0.031) at baseline. Data are logarithmically transformed prior to correlation analysis and each point represents values from individual patients (A, n = 20; B, n = 28).
Expression of YKL-40 mRNA in myocardium
We performed cDNA microarray gene expression profiling of inflammatory cytokines in the myocardium, comparing the YKL-40 expression with the expression of 4 inflammatory genes IL-6, IL-8, MCP-1, and VCAM-1 in the described settings during CABG. These inflammatory genes have well-characterized inflammatory functions, which could be related to YKL-40 and which might exhibit an acute change in transcription. We found that the most differentially expressed genes in this study were IL-8 and IL-6 (). There was no significant difference in YKL-40 RNA expression between myocardium subtended by a stenotic artery and non-ischemic myocardium at baseline. In addition, there were no significant changes in response to acute ischemia and reperfusion in both two tissue areas (). Confirmative Real-time PCR could not demonstrate any significant differences inYKL-40 expression between the chronic ischemic and the normally perfused myocardium before or after acute ischemia and reperfusion ().
Figure 4. Real time PCR analysis showed relative expression of YKL-40 mRNA (2−▵▵Ct) during reperfusion compared with baseline in both chronic ischemic and normally perfused myocardium.
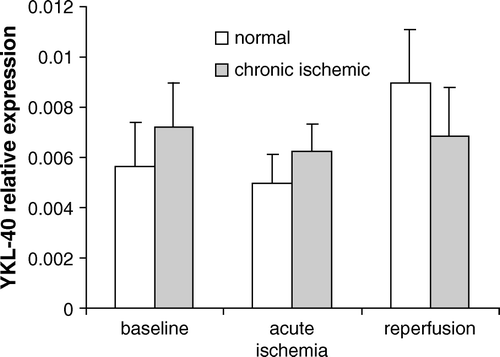
Table II. Expression of YKL-40 in two tissues region compared to non-ischemic samples (baseline) measured by oligonucleotide microarrays.
Correlation between YKL-40 and inflammatory genes expression
The expressions of YKL-40 and IL-6 genes correlated significantly (Spearman test: r = 0.81, p = 0.050) in chronic ischemic myocardium but not in normally perfused myocardium (Spearman test: r = − 0.34, p = 0.54) at baseline. Such a correlation was not found between YKL-40 and IL-8, MCP-1 or VCAM-1 in both chronic ischemic myocardium and normally perfused myocardium at baseline.
Discussion
The main observation is that plasma concentration of YKL-40 is significantly increased, not only in patients with ST-elevation myocardial infarction treated successfully with primary PCI, but also in patients with stable chronic coronary artery disease, when compared to healthy control subjects. However, the micro-array analyses of myocardial biopsies did not indicate, that the cardiomyocytes were the source for the measured increase in plasma YKL-40. In addition, plasma YKL-40 was significantly associated with serum CRP in both patient groups at admission, but not one month after the STEMI. Our findings seem to suggest that plasma YKL-40 is a new biomarker of the inflammatory processes and maybe in the regenerative process in both acute and chronic myocardial ischemic conditions. This observation is in accordance with an unpublished report showing elevated serum YKL-40 in patients with AMI treated with and without thrombolytic therapy Citation20. However, limitation in the interpretation of the data is the small number of patients included in the study.
YKL-40 is synthesized by and promotes vascular smooth muscle cells (VSMCs) attachment, spreading and migration Citation8, Citation9, suggesting that the protein has a role in the process of atherosclerotic plaque formation, where VSMC are induced to migrate through the intima in response to exogenous signals. YKL-40 also modulates vascular endothelial cell morphology by promoting the formation of branching tubules, indicating that YKL-40 has a role in angiogenesis by stimulating the migration and reorganization of vascular endothelial cells Citation8.
We found that plasma YKL-40 was elevated 7 fold in STEMI patients and 4 fold in patients with chronic ischemic heart disease compared to controls. These findings indicate that YKL-40 could play a role in both the acute inflammatory process eliciting the plaque instability and in the recovery and remodelling process after an acute STEMI by promoting the growth of new cardiomyocytes and inducing vasculogenesis. It could be speculated that YKL-40 may protect cardiomyocytes from undergoing apoptosis under ischemia since in cancer cells YKL-40 expression is up-regulated following hypoxia Citation21. It has recently been shown that serum YKL-40 is associated with the extent of coronary artery disease defined by the number of vessels with stenosis Citation17.
Inflammation plays an important role in atherogenesis and atherothrombotic events and is associated with the development of myocardial infarction, stroke and cardiovascular mortality Citation1–4. Serum YKL-40 has in non-cardiac diseases been suggested to be a new biomarker of acute and chronic inflammation and tissue remodeling Citation5, Citation10, Citation12–16, Citation19, Citation22 and YKL-40 is produced locally in tissues with inflammation by macrophages and neutrophils Citation8, Citation12, Citation16, Citation2431, 32). YKL-40 is a growth factor for fibroblasts and chondrocytes and acts synergistically with IGF-1 Citation23, is regulated by TNF Citation23 and IL-6 (personal observation). Furthermore YKL-40 binds specifically to types I, II and III collagen and modulates the rate of type I collagen fibril formation Citation24. These observations suggest that YKL-40 plays a protective role in inflammatory environments, limiting degradation of the extracellular matrix and thereby controlling tissue remodelling.
It is not known which type of cells that is the main source of the increased plasma level of YKL-40 in patients with acute and chronic ischemic heart disease. The lack of relation between maximal serum CK-MB and plasma YKL-40 in STEMI patients suggests that cardiomocytes are not the major and only source of this marker during acute ischemia. Others have found that the maximum serum YKL-40 was correlated with CK-MB in non-thrombolysed AMI patients but not in thrombolysed AMI patients Citation20. The thrombolytic therapy activates the inflammatory system and this could release YKL-40 from other cell types such as activated monocytes, macrophages and neutrophils.
In the present study plasma YKL-40 in patients with STEMI tended to be higher than in patients with chronic myocardial ischemia the day after the STEMI, but decreased in plasma YK-40 in the sub-acute phase after STEMI suggesting that the degree of inflammation and acute ischemia after STEMI progressively decreased after PCI treatment. The remained higher plasma YKL-40 one month after STEMI compared with controls may represent its continuous involvement in the regenerative process initiated by the irreversible myocardial damage resulting from the acute myocardial infarction. In accordance with other studies of patients with non-cardiac acute or chronic inflammation Citation10, Citation12–16, Citation22 we also found a significant correlation between plasma YKL-40 and the classical acute protein CRP in patients with both acute or chronic coronary artery disease. However, there was no correlation between YKL-40 and CRP one month after STEMI indicating a non-inflammatory function of YKL-40 at this time point. It could be speculated whether YKL-40 also could be a prognostic factor in myocardial ischemia independent of inflammation.
In patients with stable chronic ischemic heart disease the mRNA expressions of YKL-40 and IL-6 correlated significantly in chronic ischemic myocardium but not in normally perfused myocardium. This provides evidence, that at least a part of the increased plasma YKL-40 was induced by or collaborated with inflammatory cytokines during the sub-clinical systemic/myocardial inflammation. However, the present study was not designed to explore this relationship in details. The lack of correlation between YKL-40 mRNA expression and other markers of inflammation such as IL-8, MCP-1 and VCAM-1 in acute ischemia followed by reperfusion in normal or chronic ischemic myocardium may be due to, that the myocardial damaged induced by the present short-time total myocardial ischemic period during by-pass surgery was smaller in comparison to the mixture of irreversible changes and reversible dysfunctions “myocardial stunning” induced by a prolonged myocardial infarction Citation25.
In conclusion, our study demonstrated that plasma YKL-40 is increased, not only in STEMI patients, but also in patients with stable chronic coronary artery disease. The increase seemed not to be induced by an activation of the genes in the cardiomyocytes. Further studies are needed to define the mechanisms of YKL-40 release and the possible role of YKL-40 as a clinically useful biomarker in acute and stable coronary artery syndromes. YKL-40 could potentially be a new biomarker for myocardial ischemia, inflammation, remodelling and maybe a prognostic marker.
Acknowledgements
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Jialal I, Devaraj S. Role of C-reactive protein in the assessment of cardiovascular risk. Am J Cardiol. 2003; 91: 200–2
- Mueller C, Buettner HJ, Hodgson JM, Marsch S, Perruchoud AP, Roskamm H, et al. Inflammation and long-term mortality after non–ST-elevation acute coronary syndrome treated with a very early invasive strategy in 1042 consecutive patients. Circulation. 2002; 105: 1412–5
- Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: A comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001; 285: 2481–5
- Albert CM, Ma J, Rifai N, Stampfer MJ, Ridker PM. Prospective study of C-reactive protein, homocysteine, and plasma lipid levels as predictors of sudden cardiac death. Circulation. 2002; 105: 2595–9
- Johansen JS, Jensen BV, Roslind A, Nielsen D, Price PA. Review. Serum YKL-40, a new prognostic biomarker in cancer patients? Review. Cancer Epidemiol Biomarkers Prev. 2006; 15: 194–202
- Rehli M, Niller H-H, Ammon C, Langmann S, Schwarz-Fischer L, Andreesen R, et al. Transcriptional regulation of CHI3L1, a marker gene for late stages of macrophage differentiation. J Biol Chem. 2003; 278: 44058–67
- Volck B, Price PA, Johansen JS, Sørensen O, Benfield T, Calafat J, et al. YKL-40, a mammalian member of the chitinase family, is a matrix protein of specific granules in human neutrophils. Proc Assoc Am Phys. 1998; 110: 351–60
- Malinda KM, Ponce L, Kleinman HK, Shackelton LM, Millis AJT. Gp38k, a protein synthesized by vascular smooth muscle cells, stimulates directional migration of human umbilical vein endothelial cells. Exp Cell Res. 1999; 250: 168–73
- Nishikawa KC, Millis AJT. gp38k (CHI3L1) is a novel adhesion and migration factor for vascular cells. Exp Cell Res. 2003; 287: 79–87
- Volck B, Johansen JS, Stoltenberg M, Garbarsch C, Price PA, Østergaard M, et al. Studies on YKL-40 in knee joints of patients with rheumatoid arthritis and osteoarthritis. Involvement of YKL-40 in the joint pathology. Osteoarthritis Cartilage. 2001; 9: 203–14
- Boot RG, van Achterberg TAE, van Aken BE, Renkema GH, Jacobs MJHM, Aerts JMFG, et al. Strong induction of members of the chitinase family of proteins in atherosclerosis. Chitotriosidase and human cartilage gp-39 expressed in lesion macrophages. Arterioscler Thromb Vasc Biol. 1999; 19: 687–94
- Kronborg G, Østergaard C, Weis N, Nielsen H, Obel N, Pedersen SS, et al. Serum level of YKL-40 is elevated in patients with Streptococcus pneumoniae bacteremia and is associated to the outcome of the disease. Scand J Infect Dis. 2002; 34: 323–6
- Johansen JS, Krabbe K, Møller K, Pedersen BK. Circulating YKL-40 levels during human endotoxaemia. Clin Exp Immunol. 2005; 140: 343–8
- Vind I, Johansen JS, Price PA, Munkholm P. Serum YKL-40, a potential new marker of disease activity in patients with inflammatory bowel disease. Scand J Gastroenterol. 2003; 38: 599–605
- Johansen JS, Baslund B, Garbarsch C, Garbarsch C, Hansen M, Stoltenberg M, et al. YKL-40 in giant cells and macrophages from patients with giant cell arteritis. Arthritis Rheum. 1999; 42: 2624–30
- Rathcke CN, Johansen JS, Vestergaard H. YKL-40, a biomarker of inflammation, is elevated in patients with type 2 diabetes and is related to insulin resistance. Inflamm Res. 2006; 55: 53–9
- Kucur M, Isman FK, Karadag B, Vural VA, Tavsanoglu S. Serum YKL-40 levels in patients with coronary artery disease. Coronary Artery Dis. 2007; 18: 391–6
- Scheidl SJ, Nilsson S, Kalen M, Hellstrom M, Takemoto M, Hakansson J, Lindahl P. mRNA expression profiling of laser microbeam microdissected cells from slender embryonic structures. Am J Pathol. 2002; 160: 801–13
- Wang Y, Gabrielsen A, Lawler P, Brown LF, Paulsson-Berne G, Hansson GK, et al. Myocardial gene expression of angiogenic factors in human chronic ischemic heart disease-influence of acute ischemia/cardioplegia and reperfusion. Microcirculation. 2006; 13: 187–97
- Nøjgaard C, Høst NB, Christensen IJ, Poulsen SH, Egstrup K, Price PA, et al. Serum levels of YKL-40 increases in patients with acute myocardial infarction. Coronary Artery Dis. 2008;19:257–63.
- Junker N, Johansen JS, Hansen LT, Lund EL, Kristjansen PG. Regulation of YKL-40 expression during genotoxic or microenvironmental stress in human glioblastoma cells. Cancer Sci. 2005; 96: 183–90
- Johansen JS, Milman N, Hansen M, Garbarsch C, Price PA, Graudal N. Increased serum YKL-40 in patients with pulmonary sarcoidosis. A potential marker of disease activity?. Respir Med. 2005; 99: 396–402
- Recklies AD, White C, Ling H. The chitinase 3-like protein human cartilage 39 (HC-gp39) stimulates proliferation of human connective-tissue cells and activates both extracellular signal-regulated kinase-and protein kinase B-mediated signalling pathways. Biochem J. 2002; 365: 119–26
- Bigg HF, Wait R, Rowan AD, Cawston TE. The mammalian chitinase-like lectin, YKL-40, binds specifically to type I collagen and modulates the rate of type I collagen fibril formation. J Biol Chem. 2006; 281: 21082–95
- Braunwald E, Kloner RA. Myocardial reperfusion: A double edged sword. J Clin Invest. 1985; 76: 1713–9